Abstract
Several taxa of simultaneously hermaphroditic land snails exhibit a conspicuous mating behaviour, the so-called shooting of love darts. During mating, such land snail species transfer a specific secretion by stabbing a mating partner's body with the love dart. It has been shown that sperm donors benefit from this traumatic secretion transfer, because the secretions manipulate the physiology of a sperm recipient and increase the donors' fertilization success. However, it is unclear whether reception of dart shooting is costly to the recipients. Therefore, the effect of sexual conflict and antagonistic arms races on the evolution of traumatic secretion transfer in land snails is still controversial. To examine this effect, we compared lifetime fecundity and longevity between the individuals that received and did not receive dart shooting from mating partners in Bradybaena pellucida. Our experiments showed that the dart-receiving snails suffered reduction in lifetime fecundity and longevity. These results suggest that the costly mating behaviour, dart shooting, generates conflict between sperm donors and recipients and that sexually antagonistic arms races have contributed to the diversification of the morphological and behavioural traits relevant to dart shooting. Our findings also support theories suggesting a violent escalation of sexual conflict in hermaphroditic animals.
Keywords: sexual conflict, traumatic secretion transfer, mate manipulation, pulmonate land snails, simultaneous hermaphrodites
1. Introduction
In species with multiple matings and sperm storage by females, males face sperm competition for fertilization after mating as well as competition in the phase before mating [1,2]. There is increasing evidence that sperm competition can cause conflict between the sexes, as can pre-copulatory male–male competition (reviewed in [3,4]). Theoretical studies have suggested that in the post-copulatory phase, sexually antagonistic arms races driven by such conflict can escalate more drastically in hermaphroditic animals (i.e. species in which an individual has both male and female functions) than in species with separate sexes [5–7]. Indeed, various prominent and violent reproductive behaviours have been reported in hermaphrodites (e.g. [8,9]) and, indeed, a few of them have been shown to be advantageous to sperm competition (e.g. dart shooting in land snails [10]; body piercing in earthworms [11]). However, it remains unclear whether such behaviours actually impose costs on sperm recipients (i.e. individuals that play a female role and receive the seemingly damaging behaviour) and, therefore, whether sexual conflict occurs via the behaviours. To better understand sexual conflict and its effects on the evolution of reproductive traits in hermaphrodites, it is important to examine the economics (the relationship between costs and benefits) involved in such reproductive behaviours [12,13].
Here, we focus on the so-called dart shooting behaviour in simultaneously hermaphroditic land snails. This example is one of the best-known cases of extraordinary reproductive traits in hermaphroditic animals. Dart shooting is a behaviour during which a snail drives its love dart(s) into its mating partner (i.e. through its partner's body wall). Love darts are hard and sharp objects, composed of a crystalline form of calcium carbonate called aragonite [14], and are everted using a muscular dart sac. Dart-bearing species are known from at least nine families of stylommatophoran land snails [15]. Several studies on functional aspects of dart shooting have been performed on species within the families Helicidae and Bradybaenidae. During dart penetration in Cornu aspersum, a specific mucus is transferred from glands associated with the love dart into the recipient's blood [16]. Because the dart does not deliver sperm, this behaviour is categorized as ‘traumatic secretion transfer’ [17]. The dart's mucus causes conformational changes in the female reproductive system, closing off the route to the bursa copulatrix, a gametolytic organ (in C. aspersum [18] and in Euhadra peliomphala [19]). The mucus also induces peristaltic movements in the bursa tract diverticulum, a dead-end duct that branches off from the tract to the bursa copulatrix (in C. aspersum [18]). Because the spermatophore is transferred into the bursa tract diverticulum in C. aspersum, it has been hypothesized that the conformational change and the peristalsis would delay the digestion of the donated sperm and promote sperm storage. Subsequent studies have found that either successful dart shooting or experimental injection of the dart mucus alone increased sperm storage and paternity of the sperm donor in C. aspersum [10,20,21]. Furthermore, a recent study has reported that the mucus decreases the likelihood of remating by darted individuals in Euhadra quaesita [22]. Therefore, dart shooting is considered to be a reproductive strategy for sperm competition. Indeed, there is increasing evidence that such simultaneously hermaphroditic land snails have various sperm competition strategies other than dart shooting (e.g. [23,24]), suggesting an intense influence of sperm competition in hermaphroditic land snails. However, it is still unclear whether this traumatic secretion transfer with the love dart is costly to stabbed snails, as proposed in the previous theoretical model [7,25]. Moreover, although a comparative phylogenetic study showed a coevolutionary pattern between male reproductive traits including the dart's characteristics and female traits [26], it remains to be tested whether sexual conflict and antagonistic arms races have led to this diversification of reproductive traits.
In this study, we conducted laboratory experiments to test the hypothesis that the snails stabbed with a love dart by mating partners suffer decreased lifetime fecundity in the dart-bearing land snail Bradybaena pellucida.
2. Material and methods
(a). Study species
In this study, we used the simultaneously hermaphroditic land snail B. pellucida (Gastropoda, Bradybaenidae). Over the course of a 1-year life cycle, B. pellucida grows determinately to an adult shell size of approximately 10–17 mm (shell diameter). When snails of this species reach sexual maturity, they form a reflected lip at the shell aperture. In a single mating event, two adult snails simultaneously reciprocally intromit their penises and exchange sperm in the form of a sperm mass [27]. A sperm mass is donated into an interior edge of a vagina (N = 15 matings: K.K. 2012, unpublished data). This sperm mass is removed into a gametolytic organ for less than one week (N = 15 matings: K.K. 2012, unpublished data), suggesting that only a small number of sperm can reach a sperm storage organ, as in C. aspersum [10]. Prior to sperm exchange, the two mating snails stab each other repeatedly with their love dart (figure 1; electronic supplementary material, movie S1). Although certain land snail species omit dart shooting (e.g. [28,29]), our preliminary investigation suggests that use of the dart during the mating process is most likely obligatory in B. pellucida (N > 20 matings: K.K. 2012, unpublished data) as in C. aspersum [30] and E. quaesita [22]. However, virgin snails of this species perform this mating behaviour without shooting their dart. Snails first develop their love dart after losing their virginity. Similar to many other land snail species, adults of B. pellucida experience multiple mating events with different partners, store the received sperm (allosperm) in an allosperm-storage organ and lay eggs several times.
Figure 1.

Mating of B. pellucida. Dart shooting is performed simultaneously by both mating snails. A bloated muscular sac containing a love dart was everted (arrow) and shot its dart into the body of a partner (see the electronic supplementary material, movie S1). (Online version in colour.)
Juvenile snails of B. pellucida were collected in the summer of 2012 in Hamamatsu, Japan, and were kept individually in plastic pots (180 ml) at 22°C and at approximately 65% relative humidity. All snails were maintained under the same photoperiod. The snails were fed oatmeal with a powder mixture consisting of proteins and calcium ad libitum, and the housing containers were cleaned every week. After sexual maturation in the laboratory (identified by formation of a reflected shell lip), the adult individuals were used in laboratory experiments.
(b). Effect of dart receipt on fecundity and longevity
Two groups of 25 focal snails were randomly selected from 250 virgin snails that matured in the laboratory. The two groups were categorized as the ‘dart-received (R)’ and ‘dart not received (NR)’ groups. The remaining 200 virgins were randomly separated into two groups. Fifty pairs of virgins were randomly selected from one of the groups of 100 snails and provided the opportunity to mate. All 50 pairs had a single successful mating. These snails were then isolated as they had been before this mating. The 100 snails of this group started to form a love dart after this initial mating and were categorized as shooting partners, while the other group of 100 snails was categorized as non-shooting partners without initial mating. Because our preliminary investigation revealed that it takes less than one week to form a love dart in our study species (N = 20: K.K. 2012, unpublished data), the following procedures were initiated more than one week after the initial mating. The focal snails of group R were provided the opportunity to mate with a different randomly selected shooting partner every week for four weeks (i.e. they ultimately mated with four shooting partners). The partners that had experienced mating with the focal snails were never used again as a mating partner. The focal snails of group NR had four matings that were arranged in the same way except that non-shooting partners were used rather than shooting partners. After the fourth mating, the focal snails of groups R and NR were separately cultured in pots to which a bed of moist soil was added to allow the snails to lay eggs. Egg laying was checked every day. Once laid, the eggs were cultured in separate pots containing moist soil to avoid desiccation prior to hatching. Because B. pellucida does not show intra-clutch cannibalism [27], the eggs from a single clutch were kept in a pot. We recorded the number of hatched eggs for each of the snails until they died and the length of life of the snails subsequent to the experimental matings. Photographs of the shell were taken with a Nikon COOLPIX P7000 camera. The shell diameter and height of all individuals were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Shell volume (shell diameter2 × shell height) was used as an indicator of body size.
(c). Male and female aspects of the focal snails
To test whether the focal snails occupied male and female roles equally when they mated with shooting and non-shooting partners, we examined the sperm mass that the focal snails donated and received in the first and fourth matings. Two groups of 21 focal virgins, 21 shooting partners and 21 non-shooting partners were established as described above. The focal virgins were provided the opportunity to mate with a randomly selected shooting or non-shooting partner. The resulting mated pairs were frozen with liquid nitrogen soon after mating. The frozen snails were dissected after defrosting and the sperm masses that the focal virgins donated and received in this first mating were extracted. The sperm masses were dried in an oven at 60°C for more than 24 h, and their weights were then measured. Following this procedure, the weights of the sperm masses in the fourth mating were also measured using two groups of 21 focal snails that had already had three matings with shooting or non-shooting partners. The inter-mating interval was determined as a one-week period. Because a sperm mass remains in place for less than a week following donation, the focal sperm masses were measured without being blended with other sperm masses.
(d). Statistical analyses
Generalized linear models (GLMs) with a Poisson distribution and log link function were used to evaluate the effect of dart shooting on the number of eggs (i) and clutches (ii) of the stabbed individuals. (i) The lifetime fecundity of the focal snail was treated as the dependent variable. Whether the snail was stabbed with the dart (i.e. group R or NR), the volume of the snail and the two-way interaction terms were treated as fixed effects. (ii) Clutch number was treated as the dependent variable. Dart receipt by the snail, snail volume and the two-way interaction terms were treated as fixed effects. Similarly, generalized linear mixed models (GLMMs) with a Poisson distribution and log link function were used to evaluate the effect of dart shooting on the number of eggs per clutch. Clutch size was treated as the dependent variable. Dart receipt by the snail, snail volume and the two-way interaction terms were treated as fixed effects. The order of clutches was treated as a random effect to consider the difference between early and late clutches. Snail volume was log (natural) transformed prior to the GLM and GLMM analyses. The significance of the fixed effects was assessed with likelihood ratio tests using a χ2 approximation. The models were simplified by stepwise deletion of non-significant (p > 0.05) fixed effects starting with the effect showing the highest p-value [31]. To evaluate the effect of dart shooting on longevity of the stabbed individuals, the elapsed time until the focal snails died after the four experimental matings was compared between groups R and NR using a log-rank test with Kaplan–Meier estimates.
Linear mixed models (LMMs) were used to assess aspects of the male and female roles of the focal snails. For the male aspect, the dry weight of sperm mass that the snails donated was treated as the dependent variable. Dart receipt by the snail, snail volume and the two-way interaction terms were treated as fixed effects. The number of matings that the snail experienced (i.e. first or fourth mating) was treated as a random effect. For the female aspect, the dry weight of sperm mass that the snails received was treated as the dependent variable. Dart receipt by the snail, snail volume and the two-way interaction terms were treated as fixed effects. The number of matings that the snail experienced was treated as a random effect. The models were simplified by stepwise deletion as above. All analyses were conducted in R v. 3.1.1 [32].
3. Results
(a). Effect of dart receipt on fecundity and longevity
We collected 211 clutches that the 50 focal snails from the R and NR groups laid during their lifetime. Lifetime fecundity and clutch size for each group are shown in figure 2. The stepwise deletion of the fixed effects from the GLMs for lifetime fecundity produced a model including two effects: whether the snail received dart shooting by a mating partner and snail volume (table 1). For clutch number (group R: mean ± s.e. = 3.76 ± 0.16, group NR: 4.68 ± 0.22), we found a slight influence of dart reception and snail volume (table 1). The analyses for clutch size selected a model including both dart reception and snail volume as in the analyses for fecundity (table 1). The survival rate of the snails after the four experimental matings is shown in figure 3. The longevity of the snails differed significantly between the R and NR groups (group R: mean ± s.e. = 44.00 ± 3.45 days; n = 25, group NR: 60.28 ± 4.88 days; n = 25, p = 0.003).
Figure 2.
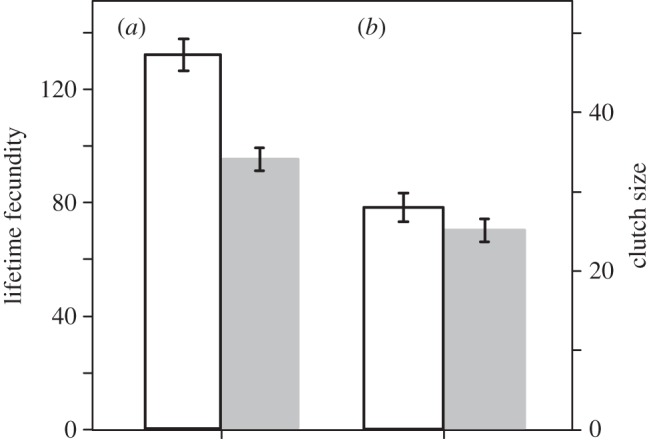
Lifetime fecundity (a) and clutch size (b) (mean ± s.e.) in the individuals that received dart shooting by mating partners (grey bars) and those that did not receive (white bars).
Table 1.
Results of stepwise deletion of effects from the initial model for the effect of dart reception on lifetime fecundity, clutch number and clutch size. Significant effect is in bold.
| variable | coefficient (±s.e.) | log-likelihood ratio | d.f. | p-value |
|---|---|---|---|---|
| fecundity | ||||
| intercept | 0.52 ± 0.33 | |||
| dart reception (A) | −0.37 ± 0.027 | 188.27 | 1 | <0.001 |
| snail volume (B) | 0.89 ± 0.067 | 176.00 | 1 | <0.001 |
| A × B | 1.36 | 1 | 0.24 | |
| clutch number | ||||
| intercept | −1.89 ± 1.72 | |||
| dart reception (A) | 3.28 | 1 | 0.070 | |
| snail volume (B) | 0.68 ± 0.35 | 3.73 | 1 | 0.053 |
| A × B | 0.015 | 1 | 0.90 | |
| clutch size | ||||
| intercept | −1.09 ± 0.53 | |||
| dart reception (A) | −0.31 ± 0.032 | 7.96 | 1 | 0.005 |
| snail volume (B) | −0.70 ± 0.067 | 107.56 | 1 | <0.001 |
| A × B | 0.17 | 1 | 0.68 | |
Figure 3.
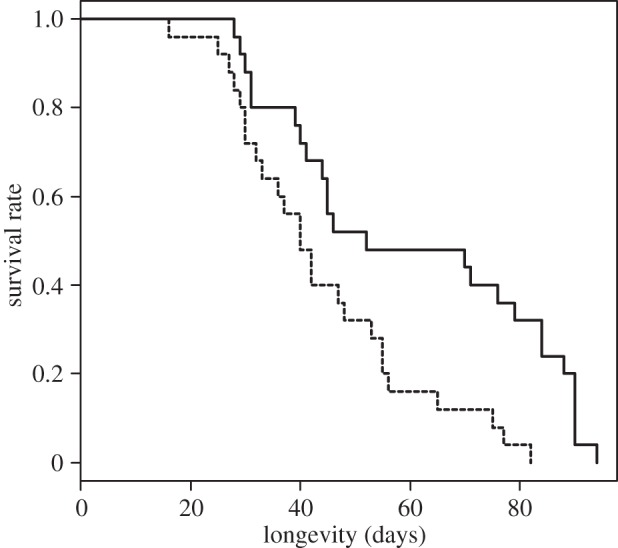
Kaplan–Meier plot of survival rate after the four experimental matings for the individuals that received dart shooting by mating partners (dashed line) and those that did not receive partners' dart (solid line).
(b). Male and female aspects of the focal snails
The weight of dried sperm mass that the focal snails donated when they mated with a shooting or non-shooting partner is shown in figure 4 (a-1 and a-2). The stepwise deletion of the fixed effects from the LMMs for the donated sperm mass weight produced a model including neither the type of mating partner nor snail volume (table 2). The sperm mass weight that the focal snails received is shown in figure 4 (b-1 and b-2). Similar to the analyses for the donated sperm mass weight, a model including no effect was selected for the received sperm mass weight (table 2).
Figure 4.
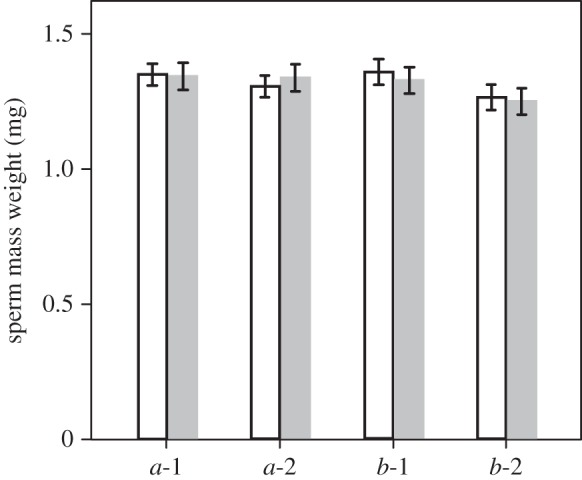
The weight of dried sperm mass that the focal snails donated in the first mating (a-1) and the fourth mating (a-2), and received in the first mating (b-1) and the fourth mating (b-2) when they mated with a shooting (grey bars) and non-shooting (white bars) partners.
Table 2.
Results of LMM analyses to quantitatively assess male and female investments that the focal snails made when they mated with shooting partners (the individuals that had already experienced a single mating) and non-shooting partners (virgins).
| variable | coefficient (±s.e.) | log-likelihood ratio | d.f. | p-value |
|---|---|---|---|---|
| donated sperm mass | ||||
| intercept | ||||
| mating partner type (A) | 0.0017 | 1 | 0.97 | |
| snail volume (B) | 2.44 | 1 | 0.12 | |
| A × B | 1.51 | 1 | 0.22 | |
| received sperm mass | ||||
| intercept | ||||
| mating partner type (A) | 0.21 | 1 | 0.65 | |
| snail volume (B) | 1.80 | 1 | 0.18 | |
| A × B | 0.10 | 1 | 0.75 | |
4. Discussion
Although various violent reproductive behaviours have been reported in hermaphrodites [8–11], as far as we know, there is no previous experiment testing whether these traumatic behaviours are actually costly for the individuals that receive it. A novel and noteworthy finding in this study is that the snails stabbed with the love dart of their mating partners laid fewer eggs than those that did not receive dart shooting (figure 2a and table 1). Although the analyses for the difference in clutch number between the snails of group R and NR appeared to be at a borderline significance level, it is at least unequivocal that the dart-received snails did not lay more clutches than the snails that received no darts. Instead, we found that the dart-received snails reduced their clutch size (figure 2b and table 1). Therefore, these results suggest that this reduction in clutch size results in the reduction in lifetime fecundity in the dart-received snails.
There are three possible explanations for this fecundity and clutch size reduction. First, the male investment (the amount of sperm and seminal fluids) by the focal snails may have differed between groups R and NR. In many animals, males can discriminate between a virgin and non-virgin female and adjust their investment according to the female's mating status (reviewed in [33]). Although the generality of such an adjustment of ejaculates is unclear in simultaneously hermaphroditic land snails [24,34], our study species may use a strategy that serves to adjust and transfer a larger ejaculate into a non-virgin partner rather than a virgin. If so, male investment could be higher in the dart-received snails than in the snails that had received no darts because the former mated with snails that had already had a single mating, whereas the latter mated with virgins. Therefore, the observed fecundity reduction in the dart-received snails could result from an increase in male investment and a subsequent decrease in the resources available for egg production. However, our results suggest that this is not the case. The dart-received snails and the snails that received no darts donated the same amount of sperm mass to a mating partner regardless of the mating status of their partners (figure 4(a-1,a-2) and table 2). This pattern of male investment could occur because B. pellucida does not adopt the strategy of ejaculate adjustment or because the individuals having only one mating, i.e. the shooting partners used in our experiments, are equivalent in their female role to virgins. Second, the amount of sperm and/or seminal fluids that the focal snails received may have differed between matings with a shooting and non-shooting partner. A decrease in fecundity could be found in the dart-received snails if the shooting partners provided a smaller amount of ejaculate than did the non-shooting snails. This second possibility can also be excluded, however, because we found no quantitative difference between the ejaculates that the focal snails received from shooting and non-shooting partners (figure 4(b-1,b-2) and table 2). Third, the physical and/or chemical stimuli (i.e. stabbing with the hard dart and transfer of the dart mucus) via dart shooting may cause damage to the snails receiving a dart from a partner. If so, such damage could be expressed as a decrease in fecundity. Although further experiments are needed to investigate shooting and non-shooting partners from various perspectives, the rejection of the first two possibilities suggests that the key difference between the two types of mating partners is the occurrence of dart shooting. Moreover, we found that the snails of group R died earlier than those of group NR (figure 3), indicating that serious damage was caused by dart reception. These findings, therefore, support the idea that the fecundity decrease found in this study was caused by dart reception.
In the experiment on the effect of dart reception on lifetime fecundity, the focal snails were kept in conditions that allowed them to lay eggs only after the four experimental matings. Although this was an unusual situation, our preliminary investigation revealed that even if snails were always allowed egg-laying, they laid only 0–2 clutches before the fourth mating was achieved (N = 25, K.K. 2012, unpublished data). Therefore, even if fewer than four instances of dart reception are not especially costly for dart-received snails, clutches laid after the fourth mating decrease in size as shown in our laboratory experiments. Moreover, our preliminary investigation revealed that B. pellucida has 4–15 matings in its lifetime under laboratory conditions (N = 25, K.K. 2012, unpublished data). Assuming that individuals experience similar numbers of matings in the field, these findings suggest that the damage incurred by dart reception escalates so seriously that dart-received snails actually suffer reductions of lifetime fecundity in natural populations of B. pellucida.
This study explicitly shows the direct cost caused by the conspicuous mating behaviour of dart shooting in simultaneously hermaphroditic land snails. Although further experiments are needed to examine indirect benefits that dart-received individuals might receive through ‘Fisherian runaway’ or ‘good genes' processes [35], it has been theoretically suggested that such indirect effects are generally weaker than direct effects [36]. Therefore, these findings suggest that the cost of dart reception has generated sexual conflict and produced sexually antagonistic arms races between sperm donors and recipients, leading to the diversification of dart morphology [26] and shooting behaviour [37]. Moreover, our findings support previous theoretical work that suggested the potentially intense effect of sexual conflict in such hermaphrodites [7].
Although the snails receiving dart shooting obviously suffer the fecundity reduction, this reduction may also have a negative effect on the fitness of the shooter snails themselves. This is because the total number of eggs that the shooters can fertilize through a particular mating partner may also decrease even though they increase their short-term fertilization success via promoting sperm storage [20–21]. If so, however, dart shooting is difficult to be maintained evolutionarily. Promoting short-term egg laying in a mating partner, which is a well-known sperm competition strategy (e.g. [38,39]), can contribute to overcoming this potential fitness loss in dart shooters, although it is still unclear whether snails adopt such strategy [22,40]. This viewpoint of long-term fitness in shooter individuals needs to be examined in future research.
The influence and importance of sexual conflict have been highlighted mainly in separate-sex species (e.g. fruit flies [41], bed bugs [42,43], muscovy ducks [44]). However, theories have predicted greater escalation of post-copulatory sexual conflict in simultaneous hermaphrodites [5–7]. The first and essential step is testing for the actual cost in various organisms, as provided here, in order to understand the relevance of the prediction, and the effect of reproductive systems (separate-sex species and hermaphrodites) on the evolutionary consequences of sexual conflict in organisms.
Acknowledgements
We express our sincere gratitude to Y. Yusa, T. Asami, Y. Kameda, Y. Nakadera and Y. Morii for helpful comments on this study, J. M. Koene for discussion on the dart shooting, and N. Hirano for collecting materials. We are also grateful to M. Schilthuizen and G. Holwell for providing valuable and constructive comments on an earlier version of this manuscript.
Data accessibility
Data available from the Dryad digital repository (doi:10.5061/dryad.qg258).
Author contributions
K.K. designed the study, carried out the laboratory experiments, data analyses and drafted the manuscript. S.C. participated in the design of the study and drafted the manuscript.
Funding statement
This study was financially supported by the Environment Research and Technology Development Fund of the Ministry of the Environment (4–1402).
Competing interests
No competing interests declared.
References
- 1.Parker GA. 1970. Sperm competition and its evolutionary consequences in insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. London, UK: Academic Press. [Google Scholar]
- 3.Arnqvist G, Rowe L. 2005. Sexual Conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Edward D, Stockley P, Hosken D. 2014. Sexual conflict and sperm competition. Cold Spring Harb. Perspect. Biol. ( 10.1101/cshperspect.a017707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charnov E. 1979. Simultaneous hermaphroditism and sexual selection. Proc. Natl Acad. Sci. USA 76, 2480–2484. ( 10.1073/pnas.76.5.2480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greeff J, Michiels N. 1999. Sperm digestion and reciprocal sperm transfer can drive hermaphrodite sex allocation to equality. Am. Nat. 153, 421–430. ( 10.1086/303184) [DOI] [PubMed] [Google Scholar]
- 7.Michiels N, Koene J. 2006. Sexual selection favors harmful mating in hermaphrodites more than in gonochorists. Integr. Comp. Biol. 46, 473–480. ( 10.1093/icb/icj043) [DOI] [PubMed] [Google Scholar]
- 8.Michiels NK, Newman LJ. 1998. Sex and violence in hermaphrodites. Nature 391, 647 ( 10.1038/35527) [DOI] [Google Scholar]
- 9.Lange R, Werminghausen J, Anthes N. 2013. Cephalo-traumatic secretion transfer in a hermaphrodite sea slug. Proc. R. Soc. B 281, 20132424 ( 10.1098/rspb.2013.2424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers D, Chase R. 2001. Dart receipt promotes sperm storage in the garden snail Helix aspersa. Behav. Ecol. Sociobiol. 50, 122–127. ( 10.1007/s002650100345) [DOI] [Google Scholar]
- 11.Koene J, Pförtner T, Michiels N. 2005. Piercing the partner's skin influences sperm uptake in the earthworm Lumbricus terrestris. Behav. Ecol. Sociobiol. 59, 243–249. ( 10.1007/s00265-005-0030-y) [DOI] [Google Scholar]
- 12.Fricke C, Perry J, Chapman T, Rowe L. 2009. The conditional economics of sexual conflict. Biol. lett. 5, 671–674. ( 10.1098/rsbl.2009.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schärer L, Janicke T, Ramm SA. 2014. Sexual conflict in hermaphrodites. Cold Spring Harb Perspect Biol ( 10.1101/cshperspect.a017673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunt S. 1979. The structure and composition of the love dart (gypsobelum) in Helix pomatia. Tissue Cell 11, 51–61. ( 10.1016/0040-8166(79)90005-3) [DOI] [PubMed] [Google Scholar]
- 15.Davison A, Mordan P. 2007. A literature database on the mating behavior of stylommatophoran land snails and slugs. Am. Malacol. Bull. 23, 173–181. ( 10.4003/0740-2783-23.1.173) [DOI] [Google Scholar]
- 16.Adamo SA, Chase R. 1990. The love dart of the snail Helix aspersa injects a pheromone that decreases courtship duration. J. Exp. Zool. 255, 80–87. ( 10.1002/jez.1402550111) [DOI] [Google Scholar]
- 17.Lange R, Reinhardt K, Michiels N, Anthes N. 2014. Functions, diversity, and evolution of traumatic mating. Biol. Rev. 88, 585–601. ( 10.1111/brv.12018) [DOI] [PubMed] [Google Scholar]
- 18.Koene JM, Chase R. 1998. Changes in the reproductive system of the snail Helix aspersa caused by mucus from the love dart. J. Exp. Biol. 201, 2313–2319. [DOI] [PubMed] [Google Scholar]
- 19.Kimura K, Chiba S, Koene J. 2014. Common effect of the mucus transferred during mating in two dart-shooting snail species from different families. J. Exp. Biol. 217, 1150–1153. ( 10.1242/jeb.095935) [DOI] [PubMed] [Google Scholar]
- 20.Landolfa MA, Green DM, Chase R. 2001. Dart shooting influences paternal reproductive success in the snail Helix aspersa (Pulmonata, Stylommatophora). Behav. Ecol. 12, 773–777. ( 10.1093/beheco/12.6.773) [DOI] [Google Scholar]
- 21.Chase R, Blanchard K. 2006. The snail's love-dart delivers mucus to increase paternity. Proc. R Soc. B 273, 1471–1475. ( 10.1098/rspb.2006.3474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura K, Shibuya K, Chiba S. 2013. The mucus of a land snail love-dart suppresses subsequent matings in darted individuals. Anim. Behav. 85, 631–635. ( 10.1016/j.anbehav.2012.12.026) [DOI] [Google Scholar]
- 23.Kimura K, Chiba S. 2013. Delayed spermatophore removal in the land snail Euhadra peliomphala. Biol. J. Linn. Soc. 108, 806–811. ( 10.1111/bij.12008) [DOI] [Google Scholar]
- 24.Kimura K, Chiba S. 2013. Strategic ejaculation in simultaneously hermaphroditic land snails: more sperm into virgin mates. BMC Evol. Biol. 13, 264 ( 10.1186/1471-2148-13-264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schilthuizen M. 2005. The darting game in snails and slugs. Trends Ecol. Evol. 20, 581–584. ( 10.1016/j.tree.2005.08.014) [DOI] [PubMed] [Google Scholar]
- 26.Koene J, Schulenburg H. 2005. Shooting darts: co-evolution and counter-adaptation in hermaphroditic snails. BMC Evol. Biol. 5, 25 ( 10.1186/1471-2148-5-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura K, Hirano T, Chiba S. In press. Assortative mating with respect to size in the simultaneously hermaphroditic land snail Bradybaena pellucida. Acta Ethol. ( 10.1007/s10211-014-0211-7) [DOI] [Google Scholar]
- 28.Giusti F, Lepri A. 1980. Aspetti morfologici ed etologici dell'accoppiamento in alcune specie della famiglia Helicida (Gastropoda: Pulmonata). Accademia della Scienze di Sienna delta de’ Fisiocritici 1980, 11–71. [Google Scholar]
- 29.Baminger H, Locher R, Baur B. 2000. Incidence of dart shooting, sperm delivery, and sperm storage in natural populations of the simultaneously hermaphroditic land snail Arianta arbustorum. Can. J. Zool. 78, 1767–1774. ( 10.1139/z00-113) [DOI] [Google Scholar]
- 30.Chase R, Vaga K. 2005. Independence, not conflict, characterizes dart-shooting and sperm exchange in a hermaphroditic snail. Behav. Ecol. Sociobiol. 59, 732–739. ( 10.1007/s00265-005-0103-y) [DOI] [Google Scholar]
- 31.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GS. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 32.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 33.Kelly C, Jennions M. 2011. Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol. Rev. 86, 863–884. ( 10.1111/j.1469-185X.2011.00175.x) [DOI] [PubMed] [Google Scholar]
- 34.Baur B, Locher R, Baur A. 1998. Sperm allocation in the simultaneously hermaphroditic land snail Arianta arbustorum. Anim. Behav. 56, 839–845. ( 10.1006/anbe.1998.0855) [DOI] [PubMed] [Google Scholar]
- 35.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 36.Cameron E, Day T, Rowes L. 2003. Sexual conflict and indirect benefits. J. Evol. Biol. 16, 1055–1060. ( 10.1046/j.1420-9101.2003.00584.x) [DOI] [PubMed] [Google Scholar]
- 37.Koene JM, Chiba S. 2006. The way of the samurai snail. Am. Nat. 168, 553–555. ( 10.1086/508028) [DOI] [PubMed] [Google Scholar]
- 38.Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. 2003. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl Acad. Sci. USA 100, 9923–9928. ( 10.1073/pnas.1631635100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamane T, Miyatake T. 2010. Induction of oviposition by injection of male-derived extracts in two Callosobruchus species. J. Insect Physiol. 56, 1783–1788. ( 10.1016/j.jinsphys.2010.07.008) [DOI] [PubMed] [Google Scholar]
- 40.Koene JM, Chase R. 1998. The love dart of Helix aspersa Müller is not a gift of calcium. J. Mollus. Stud. 64, 75–80. ( 10.1093/mollus/64.1.75) [DOI] [Google Scholar]
- 41.Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory-gland products. Nature 373, 241–244. ( 10.1038/373241a0) [DOI] [PubMed] [Google Scholar]
- 42.Morrow EH, Arnqvist G. 2003. Costly traumatic insemination and a female counter-adaptation in bed bugs. Proc. R. Soc. Lond. B 270, 2377–2381. ( 10.1098/rspb.2003.2514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhardt K, Naylor R, Siva-Jothy MT. 2003. Reducing a cost of traumatic insemination: female bedbugs evolve a unique organ. Proc. R. Soc. Lond. B 270, 2371–2375. ( 10.1098/rspb.2003.2515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennan PLR, Clark C, Prum RO. 2010. Explosive eversion and functional morphology of the duck penis supports sexual conflict in waterfowl genitalia. Proc. R. Soc. B 277, 1309–1314. ( 10.1098/rspb.2009.2139) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad digital repository (doi:10.5061/dryad.qg258).


