Summary
Efficient differentiation of pluripotent cells to proximal and distal lung epithelial cell populations remains a challenging task. The 3D extracellular matrix (ECM) scaffold is a key component that regulates the interaction of secreted factors with cells during development by often binding to and limiting their diffusion within local gradients. Here we examined the role of the lung ECM in differentiation of pluripotent cells in vitro and demonstrate the robust inductive capacity of the native lung matrix alone. Extended culture of stem cell-derived definitive endoderm on decellularized lung scaffolds in defined, serum-free medium resulted in differentiation into mature airway epithelia, complete with ciliated cells, club cells, and basal cells with morphological and functional similarities to native airways. Heparitinase I, but not chondroitinase ABC, treatment of scaffolds revealed that the differentiation achieved is dependent on heparan sulfate proteoglycans and its bound factors remaining on decellularized scaffolds.
Graphical Abstract
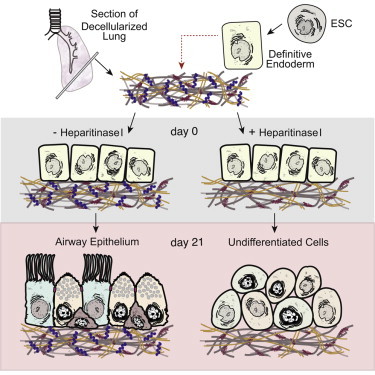
Highlights
-
•
Lung scaffolds direct ESC-derived endoderm differentiation to airway epithelia
-
•
ESC-derived airway epithelial cells are functional and resemble native airways
-
•
Differentiation by scaffolds is dependent on matrix heparan sulfate proteoglycans
In this article, Post and colleagues have demonstrated that functional airway epithelial cells (ciliated, club, and basal cells) can be generated from murine ESCs by culturing endoderm-induced cells on decellularized lung scaffolds without additional serum and growth factor supplementation. This differentiation was found to be dependent on heparan sulfate proteoglycans present on lung scaffolds.
Introduction
Lineage restriction of pluripotent stem cells (PSCs) is a dynamic process mediated by many environmental components that include growth factors, cell-matrix interactions, cell-cell signaling, and mechanical forces (Daley et al., 2008; Discher et al., 2009). Understanding how these components combine and control cell fate in vivo will allow recapitulation of niche microenvironments in vitro and support lineage-specific differentiation and generation of target cell populations. Recent reports have attempted to capture the lung developmental milieu with the addition of soluble growth factors in monolayer cultures. Success in achieving differentiation to lung epithelial cells has employed a stepwise lineage restriction strategy to first achieve definitive endoderm, followed by anterior foregut endoderm, and finally lung progenitor cells with positive expression for the homeodomain-containing transcription factor NKX2-1. NKX2-1+ lung progenitors were further differentiated to airway or alveolar epithelia with some success using continued supplementation of monolayer cultures with inductive factors (Ghaedi et al., 2013; Green et al., 2011; Huang et al., 2014; Jensen et al., 2012; Longmire et al., 2012; Mou et al., 2012; Wong et al., 2012). Repopulation of decellularized scaffolds has been used as an end-point assay to assess regenerative potential of predifferentiated cells (Ghaedi et al., 2013; Huang et al., 2014; Jensen et al., 2012; Longmire et al., 2012). Gilpin et al. (2014) recently reported the importance of the matrix environment for maintaining lung progenitor identity, but again using predifferentiated NKX2-1+ lung progenitor cells and growth factor-supplemented culture media, precluding assessment of the scaffolds alone on differentiation. To our knowledge, no reports have assessed the inductive capacity of the lung extracellular matrix (ECM) alone during early lung specification.
Here we present a strategy to examine the role of the lung ECM in differentiation of pluripotent cells in vitro and show the inductive capacity of decellularized lung scaffolds alone in directing differentiation to functional airway epithelial cells. Decellularized lung scaffolds were seeded with embryonic stem cell-derived endoderm under defined, serum-free conditions to investigate the sole potential of the lung ECM in promoting lineage-specific differentiation. We demonstrate the importance of a 3D matrix environment with site-specific cues that are bound to heparan-sulfate proteoglycans for achieving robust differentiation to mature and functional airway epithelial cells.
Results
Endodermal Cells Differentiate to NKX2-1+/SOX2+ Early Proximal Lung Progenitors with Culture on Decellularized Scaffolds
To investigate cell-ECM interactions during lung specification, we isolated decellularized lung scaffolds from adult rats. Rapid and complete decellularization was achieved using a 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)-based decellularization solution (Figure S1 available online). Tissue staining, electron microscopy (EM), tensile testing, and DNA and immunoblot analyses of decellularized scaffolds confirmed removal of all host cells and preservation of matrix proteins (Figures S1A–S1J).
During embryonic development, lung-specific endoderm progenitors originate from definitive anterior endoderm found in the developing foregut (Murry and Keller, 2008; Zorn and Wells, 2009). Therefore, we first generated definitive endoderm from mouse embryonic stem cells (ESCs) using activin A (Gouon-Evans et al., 2006; Kubo et al., 2004) and isolated an enriched population of endodermal cells by fluorescence-activated cell sorting for coexpression of CXCR4 and cKIT (Figures S2A and S2B). Sorted cells were seeded onto 350 μm thick sections of decellularized scaffolds and cultured in a supportive base media for up to 3 weeks without the addition of exogenous factors. To better recapitulate the lung microenvironment, we maintained cell-matrix constructs under air-liquid interface (ALI) culture conditions (Figure S2C). By 7 days of culture, seeded endodermal cells presented a pattern of organization reminiscent of the developing lung, lined by basement membrane proteins collagen IV and laminin (Figures S2D and S2E). Tubule structures were formed, and over half of the seeded population coexpressed pan-epithelial cell markers CDH1 and panKRT (Figure S2F). RT-PCR analysis showed maintenance of endoderm transcription factor Foxa2 expression for the duration of culture on scaffolds (Figure 1B). Nkx2-1 is an important transcriptional regulator of the lung that is one of the earliest markers for emergence of lung-specific endodermal cells (Kimura et al., 1996; Minoo et al., 1999). There was upregulation of Nkx2-1 after 7 days of culture on scaffolds, and expression was maintained for up to 21 days (Figure 1C); proximal (Sox2) and distal (Sox9) epithelial progenitor markers were both detected at the mRNA level, although Sox2 levels were greater (Figures 1D and 1E).
Figure 1.
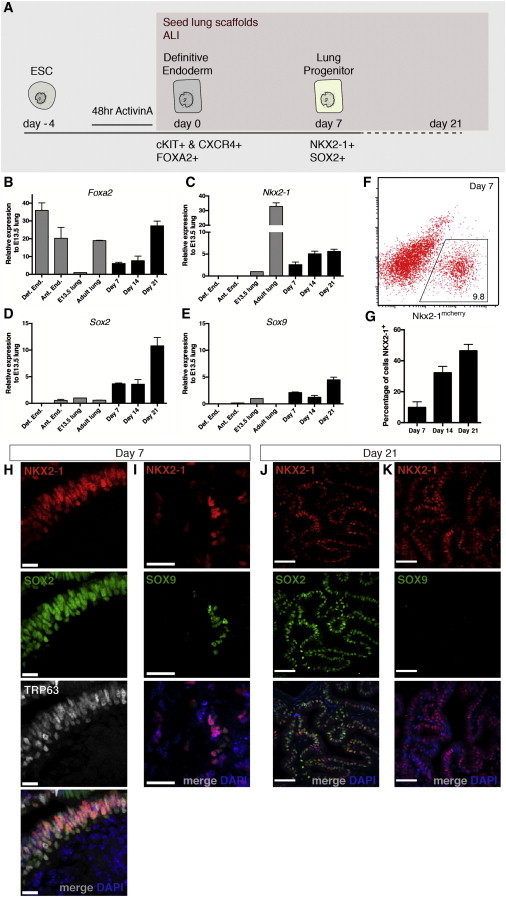
Seeded Endodermal Cells Differentiate to NKX2-1+/SOX2+ Proximal Lung Progenitors with Culture on Decellularized Scaffolds
(A) Schematic representation of differentiation to lung progenitor cells.
(B and C) Real-time PCR analysis reveals an upregulation of Foxa2 and lung progenitor marker Nkx2-1 expression. Expression is presented relative to E13.5 mouse lungs, mean ± SEM, n = 4 experiments.
(D and E) Proximal airway progenitor marker Sox2 and distal alveolar progenitor marker Sox9 were both detected, n = 4 experiments; with extended culture (day 21), Sox2 expression is upregulated.
(F and G) NKX2-1mcherry+ cells are quantified with flow cytometry. The proportion of NKX2-1+ cells increases with extended culture at day 21 to 46.42% ± 3.6%, n = 3 experiments.
(H and I) Immunostaining of day 7 cultures shows the presence of NKX2-1+/SOX2+/TRP63+ coexpressing airway basal stem cells. A small population of distal lineage NKX2-1+/SOX9+ progenitor cells is also present at this time point. (H) Scale bar represents 13 μm; (I) scale bar represents 25 μm.
(J and K) Immunostaining of day 21 cultures for NKX2-1, SOX2, and SOX9 reveals that the majority of NKX2-1+ cells coexpress SOX2, while SOX9 protein expression is rare. Scale bar represents 50 μm.
See also Figures S1 and S2.
Using an Nkx2-1mCherry ESC line with a nondisruptive mCherry reporter gene knockin (Bilodeau et al., 2014), we were able to capture the lung epithelial progenitor population as it emerged with culture on the scaffolds. Flow cytometric quantification revealed that 9.8% of cells expressed mCherry at day 7, and this percentage increased to 46.4% with extended culture on scaffolds at day 21 (Figures 1F and 1G). Elastase treatment was used to retrieve cells from scaffolds for flow cytometric analysis. Approximately 9.43 × 105 cells (n = 3) were recovered from each day 21-scaffold culture using this method, and each seeded scaffold culture generated approximately 4.38 × 105 NKX2-1+ cells (n = 3) at day 21 (see Supplemental Experimental Procedures).
NXK2-1 is not specific to lung development as it is also a transcriptional regulator during thyroid and forebrain formation (Kimura and Deutsch, 2007). However, relative to expression of adult tissue, thyroid lineage markers Tg and Pax8 and neuroectoderm marker Olig2 were hardly detected in seeded scaffold cultures (Figure S2G). Expression of posterior endoderm lineage markers such as Alb (liver), Pdx1 (pancreas), and Foxa3 (posterior endoderm) was also not noted (Figure S2G). Immunofluorescent (IF) confocal analysis showed the presence of NKX2-1+/SOX2+/TRP63+ airway basal stem cells in day 7 cultures (Figure 1H). A smaller population of distal lineage NKX2-1+/SOX9+ progenitor cells was also detected (Figure 1I). By day 21, majority of the emerging NKX2-1+ cells coexpressed SOX2, while staining of SOX9 was not detected, indicating lineage restriction to the proximal airway (Perl et al., 2005; Que et al., 2009) (Figures 1J and 1K).
We conclude that decellularized lung scaffolds are able to support the adherence and organization of seeded endodermal cells and promote the upregulation of lung progenitor marker NKX2-1, with preference for the proximal airway lineage.
Prolonged Culture of Endodermal Cells on Lung Scaffolds Promotes Differentiation to Ciliated, Club, and Basal Epithelial Cells
To determine whether NKX2-1+/SOX2+ cells cultured on lung scaffolds alone were differentiating further to mature epithelia, seeded cells were examined for expression of proximal and distal lung epithelial cell markers (Figure 2A). RT-PCR analysis showed upregulated gene expression of proximal airway epithelia Foxj1 (ciliated cell), Scgb1a1 (club cell), Trp63 (basal cell), and Cftr (Figure 2B), while expression of distal lung epithelial cell markers Aqp5 (type I alveolar cell marker), Sftpb, and Sftpc (type II alveolar cell markers) were much lower (Figure 2C).
Figure 2.
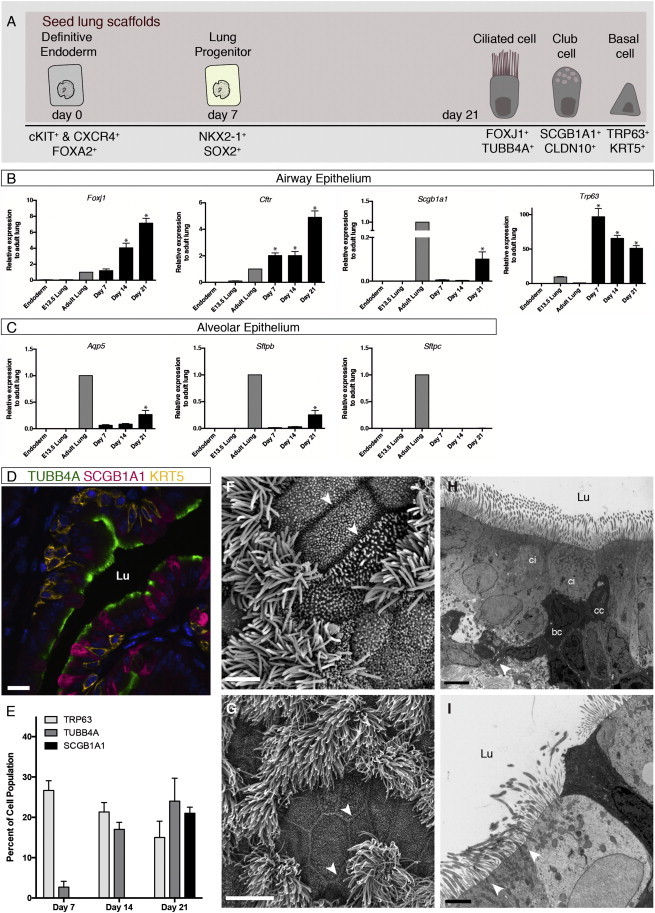
Extended Culture of Endodermal Cells on Lung Scaffolds Promotes Differentiation to Ciliated, Club, and Basal Airway Epithelial Cells
(A) Schematic representation of ESC differentiation to airway epithelial populations.
(B and C) RT-PCR analysis reveals the expression of airway epithelial genes Foxj1, Scgb1a1, TRP63, and Cftr. Alveolar epithelial markers Aqp5, Sftpb, and Sftpc were detected at much lower levels, although Aqp5 and Sftpb increased with extended culture. Gene expression is presented relative to adult lungs, mean ± SEM, n = 4 experiments, ∗p < 0.001.
(D) IF staining and confocal microscopic analysis of day 21 cell-matrix cultures showed mature airway epithelial populations: ciliated cells (TUBB4A+), club cells (SCGB1A1+), and basal cells (KRT5+). Scale bar represents 50 μm.
(E) Cell proportions were quantified as percent of total cell population at days 7, 14, and 21, mean ± SEM, n = 3 experiments.
(F and G) Scanning EM image of the surface epithelia of day 21 cultures (F, scale bar represents 2.5 μm) displays mature tight junction-coupled (white arrowhead) ciliated and nonciliated cells with similar morphology to native mouse airways (G, scale bar represents 5 μm).
(H and I) Transmission EM analysis of day 21 tissue sections confirms the presence of tight junction-coupled (white arrowhead) ciliated cells (ci), club cells (cc), and basal cells (bc). (H) Scale bar represents 4 μm. (I) Scale bar represents 2 μm.
See also Figures S2 and S3.
IF confocal microscopy of day 21 cell-matrix cultures revealed ciliated cells (FOXJ1+, TUBB4A+), club cells (CLDN10+, SCGB1A1+), and basal cells (TRP63+, KRT5+) (Figures 2D and S3A). Quantification of cells with positive immunostaining for lung lineage markers revealed 24.3% TUBB4A+, 21.1% SCGB1A1+, and 21.7% TRP63+ cells at 21 days of culture on scaffolds (Figure 2E). EM analysis was used for high-magnification morphological analysis of differentiated epithelia. Seeded cells resembled that of native mouse airways (Figures 2F and 2G), where mature multiciliated cells were present among nonciliated secretory cells with rounded apical surfaces. Scanning and transmission EM analysis revealed tight junction-coupled pseudostratified columnar epithelial sheets (Figures 2F, 2H, and 2I).
A reduction in the number of TRP63+ airway basal cells was observed by RT-PCR and by immunostaining at day 21 compared with day 7 (Figures 2B and 2E). This is consistent with previously published work on the progenitor activity of airway basal cells (Hong et al., 2004; Rock et al., 2009; Zuo et al., 2014), suggesting that multipotent NKX2-1+/SOX2+/TRP63+ basal stem cells in early cultures may be differentiating to club cells and ciliated cells lining the developing airway structures.
Decellularized scaffolds derived from mouse lungs seeded with definitive endodermal cells produced similar results to that obtained with rat lung scaffolds. Comparable cellular organization and differentiation was achieved using scaffolds generated from both the proximal and distal lung regions (Figures 3A and 3B). Tissue staining of day 21 culture sections obtained from various tissue depths demonstrated that decellularized scaffolds are completely repopulated after seeding (Figure S3B). Differentiation of definitive endoderm to airway epithelia using rat lung scaffolds was reproducible with two additional mouse ESC lines: G4 and 129/Ola (data not shown), as well as a rat ESC line (DAc8) (Figure S3C). Overall, decellularized adult lung scaffolds promote efficient differentiation of stem cell-derived definitive endoderm to airway epithelial cells.
Figure 3.
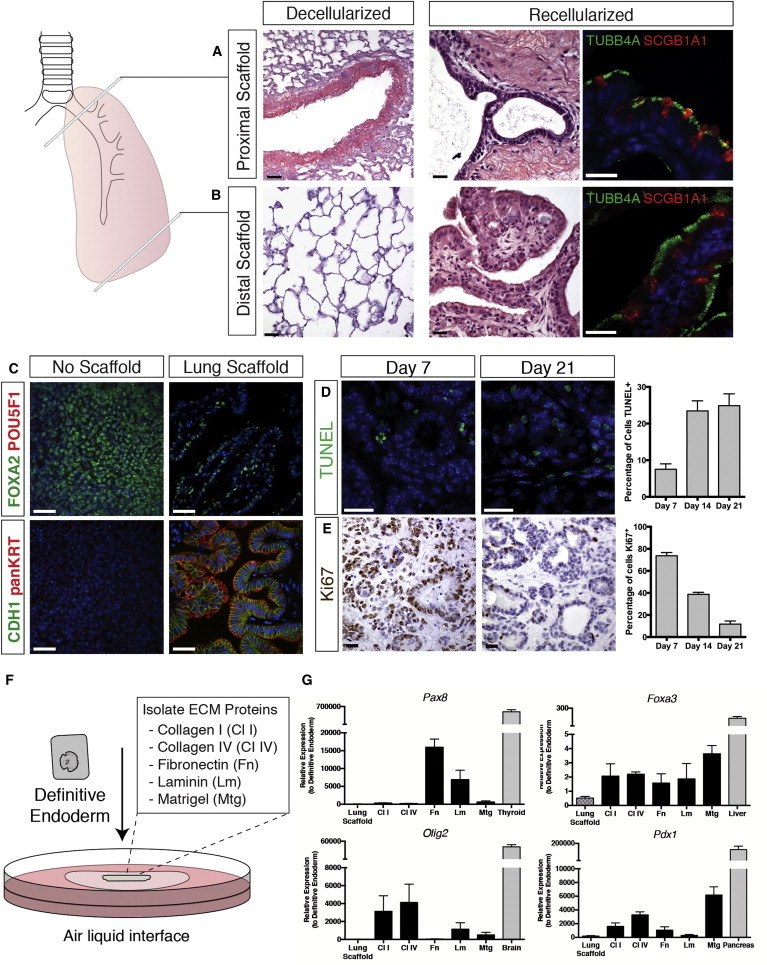
Proximal and Distal Lung Scaffolds Both Promote Airway Differentiation, while Isolate Matrix Proteins Alone Do Not Support Lung Lineage Commitment of Seeded Endodermal Cells
(A and B) Decellularized sections from both the proximal and distal regions of the lungs were generated and seeded with sorted endodermal cells. Tissue and IF staining show that both matrix sources promoted differentiation to airway ciliated (TUBB4A) and club (SCGB1A1) cells. Scale bars represent 25 μm.
(C) Immunostaining analysis demonstrates that endodermal cells seeded on lung scaffolds maintain definitive endoderm marker FOXA2 and do not express pluripotency marker POU5F1. Luminal structures are positive for epithelial cell markers CDH1 and panKRT. In contrast to cells seeded on scaffolds, endodermal cells cultured at ALI without the support of the lung matrix do not form organized structures and do not express epithelial cell markers. Scale bar represents 25 μm.
(D) TUNEL staining for identification of apoptotic cells on tissue sections was completed for different time points. Cell counts of immunostained sections shows the percentage of apoptotic (TUNEL+) cells at days 7, 14, and 21 cultures. Scale bars represent 25 μm. Mean ± SEM, n = 3 experiments.
(E) Immunostaining for Ki67 shows a decline in proliferation with progressive differentiation of seeded cells to airway epithelia from day 7 to day 21 (scale bar represents 25 μm); mean ± SEM, n = 3 experiments.
(F) Schematic representation of isolated matrix-protein recellularization. Sorted endodermal cells are seeded on specific matrix proteins or Matrigel and cultured at ALI in base supportive media.
(G) Real-time PCR analysis reveals upregulation of other endodermal lineage (Pax8, Foxa3, Pdx1) and neuroectoderm (Olig2) markers with culture of definitive endoderm on isolated matrix proteins compared with lung scaffolds. Gene expression is presented relative to definitive endoderm; mean ± SEM, n = 3 experiments.
See also Figures S3 and S4.
Day 21 scaffold cultures were negative for pluripotency marker POU5F1, while maintaining definitive endoderm transcription factor FOXA2 expression (Figure 3C). In contrast to seeded scaffolds, endodermal cells cultured alone at ALI did neither form epithelial structures nor express any lung epithelial cell markers (Figure 3C). There was an increase in the number of apoptotic cells at day 21 compared with day 7, as apparent with transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining (Figure 3D) and flow cytometric analysis of recovered cells from scaffolds (data not shown). Apoptosis of cells that are not committed to a lung lineage with continued culture on scaffolds can partially explain the increase in the proportion of NKX2-1+ cells at day 21 (Figure 1G). An increase in the number of differentiated cells with extended culture occurred in parallel with a decline in cell proliferation, as apparent with a reduction in Ki67+ cells (Figure 3E).
To determine whether individual matrix proteins can promote lung-lineage differentiation with ALI culture, we seeded endodermal cells on individual matrix proteins: collagen I, collagen IV, fibronectin, laminin, and matrix substitute Matrigel (Figure 3F). RT-PCR analysis showed the upregulation of all endodermal lineage markers Pax8, Foxa3, Pdx1, and neuroectoderm marker Olig2 on single matrix proteins compared with decellularized lung scaffolds (Figure 3G). Tissue staining and high-magnification transmission EM showed that no individual matrix protein or Matrigel was able to promote organization and lung lineage differentiation comparable to that achieved with lung scaffolds (Figures S4B and S4C). To determine whether the ability of scaffolds to promote lung lineage specification is organ specific, we seeded decellularized kidney scaffolds (mesoderm germ layer origin) with definitive endoderm and cultured them at ALI. However, definitive endoderm seeded at varied densities did not adhere and proliferate on kidney sections. This suggests that kidney-derived ECM is distinct from lung-derived ECM in its ability to support and promote endoderm-lineage specification (Figure S4D).
Differentiated Airway Epithelial Cultures on Lung Scaffolds Consist of Mature Ciliated and Club Cells with Cystic Fibrosis Transmembrane Conductance Regulator Function
Fundamental to airway mucociliary function, day 21 differentiated ciliated epithelial cells contained the appropriate 9+2 dynein arms of respiratory cilia (Figures 4A and 4B) and showed coordinated beating in culture (Movie S1). High-magnification analysis of day 21 differentiated club cells with transmission EM showed characteristic secretory droplets (El-Gawad and Westfall, 2000; Peão et al., 1993) (Figure 4C), and SCGB1A1 (club/Clara cell secretory protein) was detected by western blot analysis in the culture media (Figure 4D), suggesting that mature club cells are synthesizing and secreting SCGB1A1 into the airway lumen. Close examination of mature cultures also revealed the presence of pit structures reminiscent of emerging submucosal glands that function to adjust the flow of fluid and mucus secretions in the proximal segments of the respiratory tracts (Engelhardt et al., 1995; Smolich et al., 1978). Scaffold cultures showed an upregulation of mucin-producing cell marker Muc5ac with prolonged culture (Figure S3D). Numerous gland-like orifices were discovered with scanning EM analysis, and a strong PAS-positive stain was apparent in the developed submucosal tubules (Figures S3E and S3F).
Figure 4.
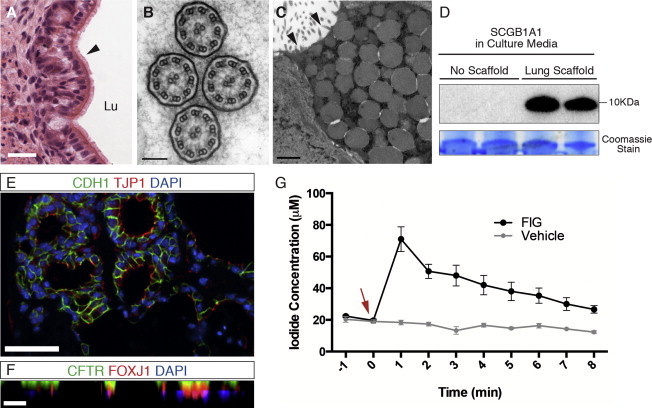
Differentiated Airway Epithelial Cultures Consist of Mature Airway Epithelia with Functional CFTR Protein
(A) H&E staining of day 21 scaffold cultures shows a sheet of ciliated cells lining a luminal structure. Scale bar represents 25 μm.
(B) Transmission EM image of cilia shows formation of the appropriate 9+2 dynein arms of motile respiratory cilia. Scale bar represents 100 nm.
(C) Transmission EM of day 21 cultures shows characteristic secretory vesicles inside differentiated club cells with microvilli lining the cell surface (black arrowheads). Scale bar represents 1 μm.
(D) Western blot of d21 culture media shows secreted SCGB1A1, while it is not detected in endodermal cultures without the lung scaffold. Coomassie stain is used as loading control; presented band is at 55 kDa.
(E) IF staining of cell cultures shows mature epithelial sheets with established tight junctions, represented by positive TJP1 staining of CDH1+ epithelial cells. Scale bar represents 50 μm.
(F) Stacked confocal images (X-Z plane) show polarized CFTR expression on the apical cell membranes. Scale bar represents 25 μm.
(G) Iodide flux was measured after cAMP agonist-induced CFTR activity in day 21 scaffold cultures. cAMP agonists, forskolin and 3-isobutyl-1-methylxanthine, and a CFTR potentiator, genistein (together as forskolin, 3-isobutyl-1-methylxanthine [IBMX], and genistein) (FIG), were added (0 min, red arrow) and replaced periodically for 8 minutes while iodide flux was measured every minute. Peak efflux, 70.6 ± 7.7 μM, was reached immediately with addition of FIG at 1 min. No efflux was detected with added vehicle control (DMSO). n = 3 experiments; mean ± SEM.
See also Movie S1.
Mature differentiated epithelial sheets had established junctional complexes that were clearly identified with EM analysis (Figures 2F and 2I) and IF staining for tight junction-associated protein, TJP1 (Figure 4E). Stacked confocal images showed polarized cystic fibrosis transmembrane conductance regulator (CFTR) expression on the apical cell membranes, a characteristic feature of functional airway epithelial cells required for chloride and water transport in the airways (Figure 4F). CFTR channel activity was assessed using a cAMP-stimulated halide flux assay. Iodide efflux from scaffold cultures after cAMP agonist stimulation was measured periodically to assess channel activity. Differentiated day 21 cells showed peak iodide efflux within the first minute of CFTR channel stimulation, demonstrating robust expression of functional CFTR protein in the day 21 scaffold cultures (Figure 4G).
Organization and Differentiation of Endodermal Cells Is Dependent on Heparan Sulfate Proteoglycans and Its Bound Factors Remaining on Decellularized Lung Scaffolds
To better understand the ECM inductive signals present in decellularized lungs, we selectively cleaved and removed two major growth factor-binding matrix proteins, heparan sulfate (HS) and chondroitin sulfate (CS) (Shannon et al., 2003; Thompson et al., 2007, 2010; Toriyama et al., 1997). Decellularized scaffolds were subjected to enzymatic degradation with heparitinase I or chondroitinase ABC to selectively cleave HS or CS and release any bound factors. Tomato-lectin immunostaining of scaffolds and spectral UV analysis of incubation solution after heparitinase treatment confirmed HS cleavage (Figures S5A and S5B), while immunoblot analysis of chondroitinase ABC-treated scaffolds confirmed CS cleavage (data not shown). Basement membrane proteins laminin and collagen IV were preserved with both treatments (Figures S5C and S5D). Enzyme-treated scaffolds were seeded with definitive endoderm and cultured under ALI conditions as before for up to 21 days.
Scaffolds treated with chondroitinase-ABC did not show a significant difference in promoting lung-lineage differentiation of endodermal cells, while treatment with heparitinase I resulted in a complete loss of organization and differentiation (Figure 5A). SEM analysis showed a lack of epithelial morphology and tight junction coupling of seeded cells in heparitinase I-treated scaffolds (Figure 5B).
Figure 5.
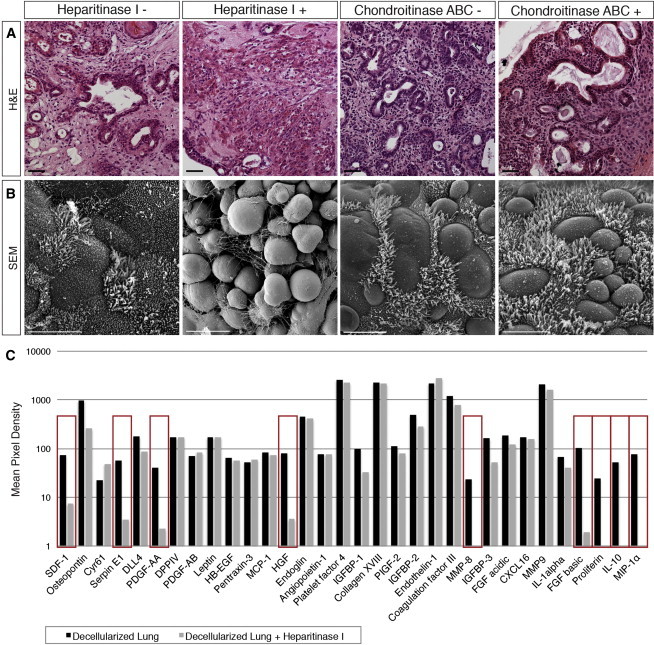
Organization and Differentiation of Endodermal Cells is Dependent on HS Proteoglycans on Decellularized Lung Scaffolds
(A) Acellular scaffolds were recellularized after heparitinase I or chondroitinase ABC treatment. H&E staining of day 21 seeded scaffold cultures show limited organization and differentiation in the heparitinase I-treated group, while chondroitinase ABC-treated cultures resemble control groups. Scale bar represents 50 μm.
(B) Scanning EM analysis of cultures show a lack of epithelial morphology and tight junction coupling of seeded cells in heparitinase I-treated scaffolds, where cells appear rounded with no resemblance to a lung phenotype. Scale bar represents 10 μm.
(C) Proteome profiler antibody array detects 31 proteins from the array profile that are remaining on lung scaffolds (black). Comparison of the protein profile from decellularized scaffolds treated with or without heparitinase I revealed several HS-bound proteins that are removed from scaffolds and found in the wash supernatant after enzyme treatment (red rectangles): CXCL12, serpinE1, PDGF-AB, HGF, MMP8, FGF2, proliferin, IL10, and CCL3. Data presented are average of two arrays from separate experiments.
See also Figure S5.
Using a commercially available proteome profiler antibody array, we detected 31 proteins from the array profile that remained on lung scaffolds after decellularization (Figure 5C). As an initial screen, a comparison of the protein profile from scaffolds treated with or without heparitinase I identified several candidate HS-bound proteins that were removed and found in the wash supernatant after enzyme treatment: CXCL12, serpinE1, PDGF-AB, HGF, MMP8, FGF2, proliferin, IL10, and CCL3 (Figure 5C). This revealed that ECM-inductive cues essential for organization and lung-lineage differentiation are dependent on HS proteoglycans and factors that may be bound to HS on decellularized scaffolds.
Discussion
Lineage restriction of pluripotent cells is dependent on the interaction of niche components in the microenvironment, where growth-factor signaling, cell-cell contact, and cell-matrix interactions combine and control stem cell fate. Growth factors are a salient component of directing cellular identity, and the ECM plays an important role in controlling growth factor availability by often binding and regulating local concentrations in space and time (Alberti et al., 2008; Discher et al., 2009; Murry and Keller, 2008; Peerani et al., 2007; Thompson et al., 2010). Because of the complex nature of these interactions during lung development, in vitro differentiation of PSC to functional airway epithelial cell populations remains a challenging task.
Here we report the robust differentiation of embryonic stem cell-derived endoderm to mature, functional airway epithelial cells using defined, serum-free culture on decellularized lung scaffolds at air liquid interface. Decellularized scaffolds alone directed differentiation of definitive endoderm to primarily NKX2-1+/SOX2+/TRP63+ lung progenitor cells as early as 7 days of culture. This progenitor population expanded with longer duration and formed fully differentiated airway luminal structures with basal cells, club cells, and beating ciliated cells. This was achieved with notable morphological and functional resemblance to native airway epithelia, providing a 3D in vitro platform to better understand cell-matrix signaling during lung development and an efficient approach for generating mature airway epithelia from PSC. Although few NKX2-1+/SOX9+ cells were detected after 7 days of culture, they disappeared with longer duration of culture, suggesting that differentiation to the distal lineage requires additional inductive factors after NKX2-1+ specification on scaffolds.
Recent reports have had success with promoting differentiation of PSC first to NKX2-1+ lung progenitor cells and further to airway (Gilpin et al., 2014; Gouon-Evans et al., 2006; Huang et al., 2014; Kubo et al., 2004; Mou et al., 2012; Wong et al., 2012) or alveolar (Ghaedi et al., 2013; Huang et al., 2014; Jensen et al., 2012; Kimura et al., 1996; Longmire et al., 2012; Minoo et al., 1999) epithelial cells using monolayer cultures and supplementation with inductive factors. However, these monolayer protocols achieved lung differentiation to a limited repertoire of functional epithelial cells with unclear efficiencies, potential contamination from other endodermal lineages, lacking relevant 3D structure and in some cases with undefined culture conditions using fetal bovine serum. To our knowledge, there have been no previous reports demonstrating the efficient generation of stem cell-derived mature airway structures with CFTR function differentiated with extended culture on decellularized lung scaffolds alone.
The ability to repopulate decellularized scaffolds in culture has been used as an assay to assess the regenerative potential of differentiated cells in previous reports, where primary cells or predifferentiated cells derived from PSC have been seeded onto rat or human decellularized lungs (Bilodeau et al., 2014; Ghaedi et al., 2013; Gilpin et al., 2014; Huang et al., 2014; Jensen et al., 2012; Longmire et al., 2012; Ott et al., 2010; Petersen et al., 2010). Successful repopulation and maturation of seeded cells on lung scaffolds achieved by these studies reinforces the importance of a 3D ECM setting for supporting cell adherence, organization, and maturation. However, in these models, cells were seeded after specification to the lung lineage using inductive factors in monolayer cultures, and supplementation continued after seeding on scaffolds, therefore hindering assessment of the influence of lung ECM solely on promoting lineage-specific differentiation.
Interestingly, this distinct ability of decellularized lung to differentiate endoderm into airway epithelia was further restated by the lack of differentiation achieved with seeded isolated matrix proteins (fibronectin, laminin, collagen I, collagen IV), ECM substitute Matrigel, and decellularized kidney scaffolds. Differentiation on lung scaffolds was replicated using mouse-derived lung scaffolds and rat-derived ES cells. This demonstrates the conserved nature of this interaction between mouse and rat lung scaffolds with cell lines derived from both species.
Matrix-associated HS and CS proteoglycans are major modulators of growth factor binding and signaling on the ECM surface and function by stabilizing FGF/FGFR complexes, increasing local gradients, and promoting FGF internalization and processing (Izvolsky et al., 2003; Kimura and Deutsch, 2007; Shannon et al., 2003; Thompson et al., 2010). Specification to the airway lineage in our model was found to be dependent on HS proteoglycans and bound factors remaining on scaffolds. Using a proteome profiler antibody array as an initial screen, we showed that numerous proteins remain on scaffolds after decellularization. A list of potential candidates implicated in lung specification was identified using this method, although the array profile is not an exhaustive list and a more in-depth analysis is required to parse out the HS-bound proteins present on scaffolds that are essential for differentiation.
Given the role of the ECM in integrating and mediating inductive signals in a 3D setting and our success with the efficient differentiation of embryonic stem cell-derived endodermal cells to functional airway epithelia, decellularized lung scaffolds are an attractive in vitro platform for lung airway engineering. This differentiation strategy generates a renewable source of functional airway epithelial cells in a 3D matrix setting that can be further examined for regenerative potential in vivo. Seeded decellularized lung scaffolds from human or xenogeneic sources can be used for generation of lung epithelia that may be valuable for airway repair and regeneration and can serve as a platform for drug discovery in human airway-related diseases such as cystic fibrosis.
Experimental Procedures
ESC Culture and Endoderm Induction
Mouse ESC lines (R1 (Nkx2-1mCherry) (Bilodeau et al., 2014; Perl et al., 2005; Que et al., 2009), G4 (dsRed-MST), and129/Ola (Bry-GFP/Foxa2-hCD4), and rat ESC line (DAc8) were maintained in the pluripotent state under feeder-free, serum-free culture using 2i media conditions (Hong et al., 2004; Rock et al., 2009; Ying et al., 2008). Endoderm induction was achieved in serum-free differentiation media (SFDM) supplemented with activin A (El-Gawad and Westfall, 2000; Gouon-Evans et al., 2006; Peão et al., 1993). Cells were sorted for dual expression of definitive endoderm markers cKIT and CXCR4 after induction. A detailed protocol is provided in the Supplemental Experimental Procedures.
Decellularization and Recellularization
Animal husbandry and procedures were approved and carried out in accordance with the Animal Care Committee guidelines of the Hospital for Sick Children. Mouse and rat lungs were decellularized using a CHAPS-based decellularization solution followed by a rapid wash and decontamination protocol to remove cellular material. Thick sections (350 μM) of lung scaffolds were generated using a vibratome and placed on hydrophobic floating membranes in culture (Whatman #110614) to achieve ALI culture conditions. Scaffolds were seeded with 100,000 sorted definitive endoderm cells (cKIT+/CXCR4+) and maintained for up to 21 days using SFDM, with no additional growth factor supplementation. Details of the procedure are outlined in the Supplemental Experimental Procedures.
Characterization of Decellularized Scaffolds
Removal of cellular material from decellularized lungs was confirmed using tissue staining, EM, and DNA assay analysis. Preservation of matrix proteins and properties was confirmed using IF staining for matrix proteins, Hart’s elastin stain, immunoblot analysis, immuno-TEM, and tensile strength testing. Detailed protocols for scaffold characterization are outlined in the Supplemental Experimental Procedures.
qPCR
Quantitative amplification of template cDNA was done using SYBR GreenER qPCR SuperMix (Life Technologies). Gene expression was normalized to RNA Polymerase II and expressed relative to selected positive (adult tissue, E13 lung) or negative controls (definitive endoderm). Details of the protocol are outlined in the Supplemental Experimental Procedures.
Immunostaining
Seeded scaffold cultures were fixed with 4% paraformaldehyde, paraffin embedded, and sectioned at 5 μm. Sample sections were rehydrated, and heat-induced epitope retrieval with citrate buffer was performed. Slides were then blocked for 1 hr with 5% normal donkey serum and 1% BSA in PBS for 1 hr at room temperature. Samples were then incubated with primary antibodies at 4°C overnight in a humidified chamber and detected using appropriate secondary antibodies (Invitrogen) for 1 hr at room temperature. For immunofluorescence, nuclei were counterstained using 4′,6-diamidino-2-phenylindole (Invitrogen). Details of antibodies and imaging are provided in the Supplemental Experimental Procedures.
EM
Samples were fixed in 2.5% gluteraldehyde in 0.1 M phosphate buffer (pH 7.4). Sample processing and microscopy details are described in the Supplemental Experimental Procedures.
Iodide Flux Assay
CFTR channel activity was assessed using a cAMP-stimulated halide flux assay to measure iodide flux across the apical membrane of day 21 differentiated epithelial cultures. Details of the assay are provided in the Supplemental Experimental Procedures.
Scaffold Enzymatic Treatment
HS or CS proteoglycans were cleaved from decellularized lung scaffolds using treatment with heparitinase I (Amsbio #100704) or chondroitinase ABC (Amsbio #100330-1A), respectively. Details of the enzymatic treatment are provided in the Supplemental Experimental Procedures.
Protein Profiler Array
A protein antibody array (R&D Systems, ARY015) was used to identify candidate proteins remaining on decellularized scaffolds with or without heparitinase I treatment, as per manufacturer’s instructions. Details of the array protocol are provided in the Supplemental Experimental Procedures.
Statistical Analysis
Statistical comparisons were performed using unpaired t tests, unless specified otherwise. For multiple comparisons of more than two groups, one-way ANOVA was used with Dunnett’s test for significance.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information
References
- Alberti K., Davey R.E., Onishi K., George S., Salchert K., Seib F.P., Bornhäuser M., Pompe T., Nagy A., Werner C., Zandstra P.W. Functional immobilization of signaling proteins enables control of stem cell fate. Nat. Methods. 2008;5:645–650. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- Bilodeau M., Shojaie S., Ackerley C., Post M., Rossant J. Identification of a proximal progenitor population from murine fetal lungs with clonogenic and multilineage differentiation potential. Stem Cell Rep. 2014;3:634–649. doi: 10.1016/j.stemcr.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley W.P., Peters S.B., Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- Discher D.E., Mooney D.J., Zandstra P.W. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gawad M.A., Westfall J.A. Comparative ultrastructure of Clara cells in neonatal and older cattle. J. Morphol. 2000;244:143–151. doi: 10.1002/(SICI)1097-4687(200005)244:2<143::AID-JMOR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Engelhardt J.F., Schlossberg H., Yankaskas J.R., Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]
- Ghaedi M., Calle E.A., Mendez J.J., Gard A.L., Balestrini J., Booth A., Bove P.F., Gui L., White E.S., Niklason L.E. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J. Clin. Invest. 2013;123:4950–4962. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin S.E., Ren X., Okamoto T., Guyette J.P., Mou H., Rajagopal J., Mathisen D.J., Vacanti J.P., Ott H.C. Enhanced lung epithelial specification of human induced pluripotent stem cells on decellularized lung matrix. Ann. Thorac. Surg. 2014;98:1721–1729. doi: 10.1016/j.athoracsur.2014.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V., Boussemart L., Gadue P., Nierhoff D., Koehler C.I., Kubo A., Shafritz D.A., Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat. Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- Green M.D., Chen A., Nostro M.-C., d’Souza S.L., Schaniel C., Lemischka I.R., Gouon-Evans V., Keller G., Snoeck H.-W. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K.U., Reynolds S.D., Watkins S., Fuchs E., Stripp B.R. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- Huang S.X.L., Islam M.N., O’Neill J., Hu Z., Yang Y.-G., Chen Y.-W., Mumau M., Green M.D., Vunjak-Novakovic G., Bhattacharya J., Snoeck H.W. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2014;32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izvolsky K.I., Shoykhet D., Yang Y., Yu Q., Nugent M.A., Cardoso W.V. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev. Biol. 2003;258:185–200. doi: 10.1016/s0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- Jensen T., Roszell B., Zang F., Girard E., Matson A., Thrall R., Jaworski D.M., Hatton C., Weiss D.J., Finck C. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng. Part C Methods. 2012;18:632–646. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Deutsch G.H. Key mechanisms of early lung development. Pediatr. Dev. Pathol. 2007;10:335–347. doi: 10.2350/07-06-0290.1. [DOI] [PubMed] [Google Scholar]
- Kimura S., Hara Y., Pineau T., Fernandez-Salguero P., Fox C.H., Ward J.M., Gonzalez F.J. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kubo A., Shinozaki K., Shannon J.M., Kouskoff V., Kennedy M., Woo S., Fehling H.J., Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Longmire T.A., Ikonomou L., Hawkins F., Christodoulou C., Cao Y., Jean J.C., Kwok L.W., Mou H., Rajagopal J., Shen S.S. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P., Su G., Drum H., Bringas P., Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(-/-) mouse embryos. Dev. Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Mou H., Zhao R., Sherwood R., Ahfeldt T., Lapey A., Wain J., Sicilian L., Izvolsky K., Musunuru K., Cowan C., Rajagopal J. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C.E., Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Ott H.C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L., Kotton D., Vacanti J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- Peão M.N., Aguas A.P., de Sá C.M., Grande N.R. Anatomy of Clara cell secretion: surface changes observed by scanning electron microscopy. J. Anat. 1993;182:377–388. [PMC free article] [PubMed] [Google Scholar]
- Peerani R., Rao B.M., Bauwens C., Yin T., Wood G.A., Nagy A., Kumacheva E., Zandstra P.W. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A.-K.T., Kist R., Shan Z., Scherer G., Whitsett J.A. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis. 2005;41:23–32. doi: 10.1002/gene.20093. [DOI] [PubMed] [Google Scholar]
- Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J., Luo X., Schwartz R.J., Hogan B.L.M. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y., Randell S.H., Hogan B.L.M. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J.M., McCormick-Shannon K., Burhans M.S., Shangguan X., Srivastava K., Hyatt B.A. Chondroitin sulfate proteoglycans are required for lung growth and morphogenesis in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L1323–L1336. doi: 10.1152/ajplung.00226.2003. [DOI] [PubMed] [Google Scholar]
- Smolich J.J., Stratford B.F., Maloney J.E., Ritchie B.C. New features in the development of the submucosal gland of the respiratory tract. J. Anat. 1978;127:223–238. [PMC free article] [PubMed] [Google Scholar]
- Thompson S.M., Connell M.G., Fernig D.G., Ten Dam G.B., van Kuppevelt T.H., Turnbull J.E., Jesudason E.C., Losty P.D. Novel ’phage display antibodies identify distinct heparan sulfate domains in developing mammalian lung. Pediatr. Surg. Int. 2007;23:411–417. doi: 10.1007/s00383-006-1864-8. [DOI] [PubMed] [Google Scholar]
- Thompson S.M., Jesudason E.C., Turnbull J.E., Fernig D.G. Heparan sulfate in lung morphogenesis: The elephant in the room. Birth Defects Res. C Embryo Today. 2010;90:32–44. doi: 10.1002/bdrc.20169. [DOI] [PubMed] [Google Scholar]
- Toriyama K., Muramatsu H., Hoshino T., Torii S., Muramatsu T. Evaluation of heparin-binding growth factors in rescuing morphogenesis of heparitinase-treated mouse embryonic lung explants. Differentiation. 1997;61:161–167. doi: 10.1046/j.1432-0436.1997.6130161.x. [DOI] [PubMed] [Google Scholar]
- Wong A.P., Bear C.E., Chin S., Pasceri P., Thompson T.O., Huan L.-J., Ratjen F., Ellis J., Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 2012;30:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.-L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn A.M., Wells J.M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W., Zhang T., Wu D.Z., Guan S.P., Liew A., Yamamoto Y., Wang X., Lim S.J., Vincent M., Lessard M. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2014 doi: 10.1038/nature13903. Published online November 12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


