Abstract
Background
Early ampullary cancers present with good prognosis. Pancreaticoduodenectomy (PD) has been standard treatment for ampullary cancers, but it remains high rate of postoperative complications. So there raises a discussion on the role of local ampullectomy for early ampullary cancers (mainly focusing on pT1).
Methods
89 patients with pT1 ampullary cancer who underwent surgical treatment between 1978 and 2010 were retrospectively studied.
Results
Rate of postoperative complications, especially post-operative pancreatic fistula (P = 0.009), after PD was higher than after local ampullectomy, . Multivariate analysis showed that tumor size (HR 2.204; P = 0.014), lymph node metastasis (HR 4.362; P < 0.001), lymph vascular invasion (HR 4.258; P < 0.001), and perineural invasion (HR 4.467; P < 0.001), gross morphology (HR 2.536; P = 0.004) and tumor grade (HR 4.213; P = 0.001) were independent risk factors for long-term survival, as well as risk factors for failure of ampullectomy in early ampullary cancer. For patients absent of these factors, local ampullectomy would achieve a good prognosis.
Conclusions
Because of high rate of lymph node metastasis, PD should be preferably performed for radical resection. Local ampullectomy could be an alternative for patients in high operative risk; and would achieve a good outcome in patients whose tumors were well differentiated and showed polypoid gross morphology and size ≤1 cm.
Keywords: Ampullary cancer, pT1 stage, Local ampullectomy, Lymph node metastasis
Background
Ampullary cancer was the second common peri-ampullary malignancy. Recent literatures reported a 5-year survival rate ranging from 32% to 65%.[1-5] Pancreatoduodenectomy (PD), the standard surgical strategy for ampullary cancer, was still associated with high rate of postoperative complications, reaching to 33%-52%.[5,6] Therefore, local ampullectomy had been attempted to be an alternative to PD for early cancer .
Local ampullectomy, first described by Halsted in 1899, was generally accepted in treatment of small benign tumors; but controversy still remained about expanding the indications to early ampullary cancers (mainly focusing on pT1) because of the high rate of recurrence.[6-10] Furthermore, because of the limited number of ampullary cancer patients, as regards to the surgical mode of early ampullary cancers, indications for performing local ampullectomy were not very clear and well-accepted.
In this study, we collected patients with pT1 ampullary cancers who underwent a surgical treatment, including PD and local ampullectomy; and further analysis were done to determine the feasibility of local ampullectomy.
Methods
Patients
There were 89 patients with a pT1 ampullary adenocarcinoma who underwent surgical resection at the First Affiliated Hospital of Zhengzhou University between January, 1978 and December, 2010. Carcinomas of the distal bile duct, pancreas, or duodenum, as well as carcinoid tumors of the ampulla, were excluded. The pT1 stage meant that according to the seventh edition of the American Joint Committee on Cancer (AJCC), the tumor was confined to ampulla of Vater, As present study sought to examine the outcomes following surgical management of ampullary cancers, patients who had endoscopic excision of ampullary neoplasm were also excluded from this study. All the patients did not receive adjuvant therapy. The following data were collected: demographics, operation details, postoperative complications, tumor size, lymph node metastasis, lymphovascular invasion. Specific complications such as pancreatic fistula and delayed gastric emptying were defined according to the International Study Group of Pancreatic Surgery definition.[11,12] This research was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Pre-operative staging
All the patients had a magnetic resonance (MR) examination before operation. Endoscopic ultrasound (EUS) was used to determine the stage of 82 patients after 1990.
Operative approach
Following were rules for choosing operation approach: 1. If the tumor size was >4 cm, PD would be performed. 2. For patients with enlarged abdominal lymph node identified by pre-operative imaging examination, PD would be performed. 3. For patients who were older than 70 years and refused to receive PD, local ampullectomy was performed.
The technique of pancreaticoduodenectomy was performed as previously described.[2,11] Drains were routinely placed intraoperatively near the pancreatic and biliary anastomoses. Local ampullectomy consisted of local resection of the ampulla through a transduodenal approach followed by a pancreaticobiliary sphincteroplasty.[9] Lymphadenectomy was not conducted.
Statistical analysis
Categorical variables were compared using Fisher’s exact test. Continuous variables were compared using the Mann–Whitney sum test. Actuarial survival was estimated using the non-parametric product limit method (e.g. Kaplan–Meier) and differences in survival were examined by the log-rank test. Multivariate Cox proportional hazard models were employed to determine clinicopathological factors which were associated with long-term survival. The most parsimonious model was created using a step-wise approach, which included factors with statistically significance (e.g.P ≤ 0.10) in univariate analysis. Averages were provided as median values and statistical significance was designated as P < 0.05. All statistical analysis were performed using SPSS 17.0 software (SPSS Inc. Chicago, IL, USA).
Results
Pre-operative staging
As indicated in Table 1, the accuracy of EUS in assessing the depth of carcinoma extension was 87.8% (72/82), superior to the MR (67.0%, P < 0.001); The results of EUS in N staging of ampullary carcinomas were 65.9% (54/82), inferior to the MR (80.90%, P = 0.024).
Table 1.
Accuracy of EUS, MR in the detection and staging of ampullary tumors
| EUS | MR | P | |
|---|---|---|---|
| T staging | 87.8% (72/82) | 70.8% (63/89) | 0.001 |
| Overstaging | 9.8% (8/82) | 12.4% (11/89) | |
| Understaging | 2.4% (2/82) | 22.5% (20/89) | |
| N staging | 65.9% (54/82) | 85.4% (76/89) | 0.024 |
| Overstaging | 23.1% (19/82) | 14.6% (13/89) | |
| Understaging | 11.0% (9/82) | 5.6% (5/89) |
Surgical details and post-operative complications
Among the 89 patients with pT1 ampullary cancer who underwent surgical resection, PD were performed in 63 (70.7%) patients, while local ampullectomy were did in 26 (29.2%) patients. Intraoperative and postoperative data were summarized in Table 2. Mean blood loss was higher in patients who underwent PD than in patients who underwent local ampullectomy (281.28 ml vs 97 ml; P < 0.001). Similarly, PD took longer operative time than local ampullectomy (307.16 min vs 214.46 min; P < 0.001). As expected, patients with PD had more post-operative complications than patients with local ampullectomy. There were 3 in-hospital deaths caused by postoperative complications in patients with PD, while there were no in-hospital death in patients with local ampullectomy. Post-operative pancreatic fistula occurred in 21 patients with PD and in 2 patients with local ampullectomy (P = 0.009). Other complications, such as wound infection, delayed gastric emptying, bile leakage and pleural effusion showed no significant differences between the patients with PD and with local ampullectomy.
Table 2.
Intra- and postoperative data on patients undergoing resection of ampullary cancers stratified by procedure type
| Local ampullectomy ( n = 26) | Pancreaticodudenectomy ( n = 63) | P | |
|---|---|---|---|
| Blood lost, mean ± SD (ml) | 97.16 ± 36.87 | 281.28 ± 201.18 | <0.001 |
| Intra-operative transsfusion | 0 (0.0%) | 10 (15.9%) | <0.001 |
| Operation time,mean ± SD (min) | 214.46 ± 23.76 | 307.16 ± 28.08 | <0.001 |
| POPF | 2 (7.6%) | 21 (33.3%) | 0.009 |
| Intra-abdominal infection | 8 (30.7%) | 18 (28.6%) | 0.513 |
| Abdominal abscess formation | 0 (0.0%) | 7 (11.1%) | 0.080 |
| Anastomotic leakage | |||
| Bile leakage | 1 (3.8%) | 5 (7.9%) | 0.431 |
| GJ anastomosis leakage | _ | 2 | _ |
| DGE | 0 (0.0%) | 5 (7.9%) | 0.169 |
| Wound infection | 1 (3.8%) | 3 (4.8%) | 0.667 |
| Pleural effusion | 0 (0.0%) | 3 (4.8%) | 0.350 |
| Reoperation | 0 (0.0%) | 5 (7.9%) | 0.169 |
| In-hospital death | 0 (0.0%) | 3 (4.8%) | 0.350 |
POPF: postoperative pancreatic fistula; GJ: gastrojejunostomy; DGE: delayed gastric emptying.
Clinicopathological characteristics
As illustrated in Table 3, of the 89 patients inclued in this study, 42 are males and 47 are females; Several clinicopathological parameters such as jaundice (local ampullectomy vs PD, 2/26 vs 31/63, P < 0.001), gross morphology (local ampullectomy vs PD, 24/26 vs 38/63, P = 0.002) and tumor size (local ampullectomy vs PD, 7/26 vs 33/63, P = 0.024) shows statistically significant differences between the patients with PD and with local ampllectomy, while other clinicopathological parameters, such as, age, gender, tumor grade shows no. In addition, because lymph node and perineural dissection was not performed in ampullectomy, it is nonsense to compare the difference in lymph node metastasis, lymphovascular invasion and perineural invasion between the two groups.
Table 3.
Patient Characteristics of 89 PatientsWith pT1 Ampullary Cancer
| Local ampullectomy ( n = 26) | Pancreaticoduodenectomy ( n = 63) | P | |
|---|---|---|---|
| Age, mean ± SD | 66.25 ± 14.149 | 67.16 ± 11.658 | 0.942 |
| Gender | 0.543 | ||
| Female | 14 | 33 | |
| Male | 12 | 30 | |
| Jaundice | <0.001 | ||
| Positive | 2 | 31 | |
| Negative | 24 | 32 | |
| Gross morphology | 0.002 | ||
| Polypoid | 24 | 38 | |
| Ulcerative | 2 | 25 | |
| Tumor size | 0.024 | ||
| ≤1 cm | 7 | 33 | |
| >1 cm | 19 | 30 | |
| Tumor grade | 0.128 | ||
| Well | 10 | 15 | |
| Moderate/Poor | 16 | 48 | |
| Lymph node metastasis | |||
| Positive | 22 | ||
| Negative | ___ | 41 | ___ |
| Lymphovascular invasion | |||
| Positive | ___ | 25 38 | ___ |
| Negative | |||
| Perineural invasion | |||
| Positive | 6 | ||
| Negative | ___ | 57 | ___ |
Survival analysis
A number of clinicopathological factors were associated with survival in the patients with ampullary cancers,. Univariate analysis showed that gross morphology, tumor size, tumor grade, lymph node metastasis and lymphovascular invasion were significant predictors of poor survival. (Table 4) The overall survival showed no significant difference between patients with PD and with local ampullectomy. But patients with PD had longer disease-free survival. (Figure 1) Of patients with local ampullectomy, 10 patients were found to have recurrence within two year after surgery (2 in pancreatic, 6 in peri-pancreatic lymph nodes, 2 in liver). No recurrence was found in patients with PD. Multivariate analysis showed that tumor size, lymph node metastasis, lymphovascular invasion, grass morphology and tumor grade turned out to be independent risk factors which influenced patients’ survival (Table 4).
Table 4.
Survival analysis for 89 Patients With pT1 Ampullary Cancer
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| 5-year survival rate | 10-year survival rate | P | HR (95%CI) | ||
| Age | 0.756 | ______ | |||
| <65 | 69.0% | 44.1% | |||
| ≥65 | 67.0% | 41.0% | |||
| Gender | 0.880 | ______ | |||
| Female | 69.3% | 43.3% | |||
| Male | 67.1% | 40.6% | |||
| Jaundice | 0.683 | ______ | |||
| Positive | 59.1% | 39.8% | |||
| Negative | 68.3% | 49.1% | |||
| Gross morphology | 0.006 | 1 2.536 (1.351-4.860) | 0.004 | ||
| Polypoid | 75.8% | 48.0% | |||
| Ulcerative | 44.2% | 29.5% | |||
| Tumor size | 0.016 | 1 2.204 (1.171-4.148) | 0.014 | ||
| ≤1 cm | 78.8% | 50.9% | |||
| >1 cm | 54.7% | 37.7% | |||
| Tumor grade | 0.017 | 1 4.213 (1.868-9.504) | 0.001 | ||
| Well | 88.1% | 61.1% | |||
| Moderate/poor | 56.8% | 34.6% | |||
| Lymph node metastasis | <0.001 | 1 4.362 (2.126-8.952) | <0.001 | ||
| Negative | 74.4% | 49.4% | |||
| Positive | 32.1% | 0.0% | |||
| Lymphovascular invasion | <0.001 | <0.001 | |||
| Negative | 69.4% | 51.l% | 1 | ||
| Positive | 33.2% | 12.1% | 4.258 (2.013-8.861) | ||
| Perineural invasion | <0.001 | <0.001 | |||
| Negative | 72.1% | 45.6% | 1 | ||
| Positive | 36.8% | 15.3% | 4.467 (2.315-9.034) | ||
| Operation style | 0.639 | 0.549 | |||
| Pancreaticoduodenectomy | 65.6% | 38.7% | 1 | ||
| Local ampullectomy | 64.6% | 42.6% | 1.372 (0.854-1.762) | ||
Figure 1.
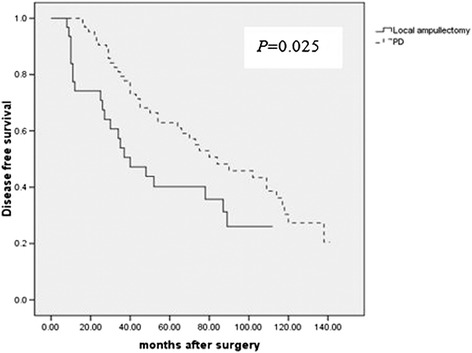
Patients in local ampullary group suffered from a poor disease-free survival than in PD group.
Predictability of lymph node metastasis
Of the above prognostic factors, lymph node metastasis was the key predictive factor that made local ampullectomy inappropriate. Therefore, we investigated the relationship between lymph node metastasis and other prognostic factors. As shown in Table 5, lymph node metastasis was identified in 22 (34.92%) of 63 patients who underwent PD; Patients with well-differentiated tumors showed a lower rate of positive lymph nodes metastasis (8%) than patients with moderate/poorly differentiated tumors (31.25%) (P = 0.022); In regard to tumor size, the patients with tumor size ≤1 cm showed a lower rate of positive lymph nodes metastasis than patients with tumor size >1 cm (15% vs 32.65%, respectively, P = 0.046); Similarly, lymph nodes metastasis tended to occur more frequently in patients with ulcerative morphology than patients with polypoid morphology (40.74% vs17.74%, respectively, P = 0.021). So tumor grade, morphology and size significantly correlated with lymph node metastasis (P < 0.05).
Table 5.
Correlation between pathological characteristics and lymph node metastasis
| Number of patients with lymph node metastasis | P | |
|---|---|---|
| Gross morphology | 0.021 | |
| Polypoid | 11/62 (17.74%) | |
| Ulcerative | 11/27 (40.74%) | |
| Tumor size | 0.046 | |
| ≤1 cm | 6/40 (15.00%) | |
| >1 cm | 16/49 (32.65%) | |
| Tumor grade | 0.022 | |
| Well | 2/25 (8.00%) | |
| Moderate/Poor | 20/64 (31.25%) |
Correlation between the risk factors of failure after ampullectomy and the indication factors of ampullectomy
As shown in Table 5, tumor size, tumor grade, and gross morphology were risk factors for ampullectomy in early ampullary cancer. In these patients, 4 patients absent of all these 3 risk factors, showed a survival of 57, 107,111, 142 months, respectively. Therefore, absence of these risk factors might be the indications for local ampullectomy.
Discussion
Early ampullary cancers limited to the ampulla of Vater (pT1) showed a fairly good prognosis. These tumors could be radically removed in all cases and showed a 5-year survival rate of 60% to 90% according to the previous reports.[5,13,14] In order to achieve such a good outcome, complete resection of the tumor was mandatory.[15,16].
There was no doubt that the standard operation for ampullary cancer should be PD. But PD showed high rate of postoperative complications, so local ampullectomy had been an alternative for early ampullary cancer and showed comparable survival as PD [17-20]. In this study, we identified patients with pT1 ampullary cancer treated with local ampullectomy and standard PD, and analysed the clinical data of these patients. The results showed that patients treated with local ampullectomy showed overall survival equal to those patients treated with PD; but the disease-free survival time turned to be shorter in patients treated with local ampullectomy than that in patients treated with PD. Among the clinicopathological factors, lymph node metastasis was the main one which caused ampullectomy fail.
Therefore, the indications for local ampullectomy treatment should and must be fully explained and strictly executed. Botsios [18] et al. recommended ampullectomy for T1 cancer and Yoon et al. [5] recommended it for pTis cancers or pT1 cancers with size≦ 1.0 cm in patients with a high operative risk. However, because of lack of a large multi-center clinical studies, a generally accepted standard was still lacking.
Various of investigations were available for staging ampullary tumors, including CT scanning, magnetic resonance (MR) imaging, endoscopic ultrasound (EUS) and transpapillary intraductal ultrasound. Although EUS could accurately define the depth of invasion in 75% to 83% of cases, the reported accuracy of EUS for detecting lymph node metastasis ranged from 54% to 68%. [21-23] CT scanning had been compared with EUS in staging utility in a number of studies and shown a lower agreement for T and N staging with the histopathology when compared with EUS.[23-25] MR imaging had been reported that it harbored a sensitivity of 46% to 93.3% [26-28] in T staging; and one study presented a sensitivity of 77% for nodal detection [26]. In these patients included in this study, EUS was superior to MR in T staging, and achieved an accuracy of 87.8%; and only 2.4% patients were under-staged. So, EUS was strongly recommended before operation.
Previous studies showed that lymph nodes metastasis was associated with tumor size and tumor grade. Bottger and Junginger [29] reported that lymph node metastases was not found in tumors smaller than 0.6 cm, or in well-differentiated tumors; so local ampullectomy for ampullary cancer might be used in such cases. Brown [30] reported 10 cases of pT1 ampullary cancers with no lymph node metastasis. Winter et al. [6] also reported that tumor size ≥ 1 c m, poor histological grade, perineural invasion, microscopic vessel invasion and T stage were significantly associated with lymph node metastasis. Lymph node metastases were present in nearly 30% of patients with T1 diseases. In present study, we explored the relationship between lymph node metastasis and the clinicalpathological factors. The result showed that patients with pT1 cancers had a 34.92% (22/63) rate of lymph node metastasis. Rate of lymph node metastasis in patients with well-differentiated tumors was 8%,; In patients with tumor size ≤1 cm it was 15% and in patients with ulcerative tumor morphology it was 40.74%. This meant that well-differentiated tumors with polypoid gross morphology and size≦1 cm might be the indications of local ampullectomy in high operative risk patients.
However, there were some disadvantages in our study. Firstly, this study was retrospectively conducted, resulting in less strong evidence. Secondly, this was a single center study, the number of patients were limited. Therefore, a large scale, multi-center RCT study should be performed in the future to verify the feasibility of local ampullectomy in high risk patients.
Conclusions
Because of high rate of lymphovascular invasion, PD should be preferably performed for radical resection. Local ampullectomy could be an alternative in high operative risk patients and could achieve a good outcome when the tumors were well differentiated and showed polypoid gross morphology and size ≤1 cm.
Acknowledgements
This research was supported by the Joint Program of National Natural Science Foundation of China (NSFC) and Science and Technology Agency of Henan province (NSFC- Henan Province Joint Talent Training Program, No: U1204818)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JS, ZL and HL were the key authors for the conception, design, coordination, and drafting of the manuscript, as well as the analysis and interpretation of the data. CY, YS and CW participated in the design and interpretation of the data and helped in drafting the manuscript. YS and CW contributed substantively by revising the manuscript critically for intellectual content and participating in the interpretation of data and the revision of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Junmin Song, Email: songjunmin99@163.com.
Hongxiang Liu, Email: liuhongxiang98@163.com.
Zhen Li, Email: lizhen5666@163.com.
Chao Yang, Email: yangchao96666@163.com.
Yuling Sun, Email: sunyuling566@163.com.
Chaojie Wang, Email: wangchaojie566@163.com.
References
- 1.Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998;228(1):87–94. doi: 10.1097/00000658-199807000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatzaras I, George N, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17(4):991–997. doi: 10.1245/s10434-009-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norero E, Vinuela E, Baez S, Martinez C, Reyes J, Kusanovic R, et al. [Results of pancreaticoduodenectomy in the treatment of periampullary tumors] Rev Med Chil. 2011;139(8):1015–1024. doi: 10.4067/S0034-98872011000800006. [DOI] [PubMed] [Google Scholar]
- 4.Shinkawa H, Takemura S, Kiyota S, Uenishi T, Kaneda K, Sakae M. Long-term outcome of surgical treatment for ampullary carcinoma. Hepatogastroenterology. 2012;59(116):1010–1012. doi: 10.5754/hge10788. [DOI] [PubMed] [Google Scholar]
- 5.Yoon YS, Kim SW, Park SJ, Lee HS, Jang JY, Choi MG, et al. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann Surg. 2005;242(1):92–100. doi: 10.1097/01.sla.0000167853.04171.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter JM, Cameron JL, Olino K, Herman JM, de Jong MC, Hruban RH, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Astrointestinal Surg Official J Soc Surg Alimentary Tract. 2010;14(2):379–387. doi: 10.1007/s11605-009-1080-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Wang H, Fan Y, Zhang L, Ding J, Cai L, et al. Pancreas-sparing duodenectomy with regional lymphadenectomy for pTis and pT1 ampullary carcinoma. Surgery. 2012;151(4):510–517. doi: 10.1016/j.surg.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Bottger TC, Boddin J, Heintz A, Junginger T. Clinicopathologic study for the assessment of resection for ampullary carcinoma. World J Surg. 1997;21(4):379–383. doi: 10.1007/PL00012257. [DOI] [PubMed] [Google Scholar]
- 9.Nikfarjam M, Muralidharan V, McLean C, Christophi C. Local resection of ampullary adenocarcinomas of the duodenum. ANZ J Surg. 2001;71(9):529–533. doi: 10.1046/j.1440-1622.2001.02185.x. [DOI] [PubMed] [Google Scholar]
- 10.Lindell G, Borch K, Tingstedt B, Enell EL, Ihse I. Management of cancer of the ampulla of Vater: does local resection play a role? Dig Surg. 2003;20(6):511–515. doi: 10.1159/000073647. [DOI] [PubMed] [Google Scholar]
- 11.Bassi C1, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142(5):761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Mori K, Ikei S, Yamane T, Yamaguchi Y, Katsumori T, Shibata Y, et al. Pathological factors influencing survival of carcinoma of the ampulla of Vater. European J Surg Oncol J European Soc Surg Oncol British Assoc Surg Oncol. 1990;16(3):183–188. [PubMed] [Google Scholar]
- 14.Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134(5):526–532. doi: 10.1001/archsurg.134.5.526. [DOI] [PubMed] [Google Scholar]
- 15.Su CH, Shyr YM, Lui WY, P’Eng FK. Factors affecting morbidity, mortality and survival after pancreaticoduodenectomy for carcinoma of the ampulla of Vater. Hepatogastroenterology. 1999;46(27):1973–1979. [PubMed] [Google Scholar]
- 16.Monson JR, Donohue JH, McEntee GP, McIlrath DC, van Heerden JA, Shorter RG, et al. Radical resection for carcinoma of the ampulla of Vater. Arch Surg. 1991;126(3):353–357. doi: 10.1001/archsurg.1991.01410270099016. [DOI] [PubMed] [Google Scholar]
- 17.Yoon SM, Kim MH, Kim MJ, Jang SJ, Lee TY, Kwon S, et al. Focal early stage cancer in ampullary adenoma: surgery or endoscopic papillectomy? Gastrointest Endosc. 2007;66(4):701–707. doi: 10.1016/j.gie.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 18.Botsios D, Zacharakis E, Lambrou I, Tsalis K, Christoforidis E, Kalfadis S, et al. Our local experience with the surgical treatment of ampullary cancer. Int Seminars Surg Oncol. 2005;2:16. doi: 10.1186/1477-7800-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Dong J, Huang X, Zhang W, Jiang K: Prognostic factors for survival of patients with ampullary carcinoma after local resection. ANZ J Surg 2014 16. [Epub ahead of print]. [DOI] [PubMed]
- 20.Zhao XQ, Huang XQ, Zhang WZ, Liu Z. Comparison between two types of local resection in the treatment of ampullary cancer. ANZ J Surg. 2014;84(4):255–259. doi: 10.1111/ans.12047. [DOI] [PubMed] [Google Scholar]
- 21.Fusaroli P, Manta R, Fedeli P, Maltoni S, Grillo A, Giovannini E, et al. The influence of endoscopic biliary stents on the accuracy of endoscopic ultrasound for pancreatic head cancer staging. Endoscopy. 2007;39(9):813–817. doi: 10.1055/s-2007-966590. [DOI] [PubMed] [Google Scholar]
- 22.Kimura W, Nagai H. Study of surgical anatomy for duodenum-preserving resection of the head of the pancreas. Ann Surg. 1995;221(4):359–363. doi: 10.1097/00000658-199504000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wee E, Lakhtakia S, Gupta R, Anuradha S, Shetty M, Kalapala R, et al. The diagnostic accuracy and strength of agreement between endoscopic ultrasound and histopathology in the staging of ampullary tumors. Indian J Gastroenterol. 2012;31(6):324–332. doi: 10.1007/s12664-012-0248-3. [DOI] [PubMed] [Google Scholar]
- 24.Maluf-Filho F, Sakai P, Cunha JE, Garrido T, Rocha M, Machado MC, et al. Radial endoscopic ultrasound and spiral computed tomography in the diagnosis and staging of periampullary tumors. Pancreatology. 2004;4(2):122–128. doi: 10.1159/000078150. [DOI] [PubMed] [Google Scholar]
- 25.Takao S, Aikou T, Shinchi H, Uchikura K, Kubo M, Imamura H, et al. Comparison of relapse and long-term survival between pylorus-preserving and Whipple pancreaticoduodenectomy in periampullary cancer. Am J Surg. 1998;176(5):467–470. doi: 10.1016/S0002-9610(98)00243-8. [DOI] [PubMed] [Google Scholar]
- 26.Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50(1):27–33. doi: 10.1016/S0016-5107(99)70340-8. [DOI] [PubMed] [Google Scholar]
- 27.Sugita R, Furuta A, Ito K, Fujita N, Ichinohasama R, Takahashi S. Periampullary tumors: high-spatial-resolution MR imaging and histopathologic findings in ampullary region specimens. Radiology. 2004;231(3):767–774. doi: 10.1148/radiol.2313030797. [DOI] [PubMed] [Google Scholar]
- 28.Young Kon Kim M, Young Min H, Chong Soo K. Usefulness of fat-suppressed T1-weighted MRI using orally administered superparamagnetic iron oxide for revealing ampullary carcinomas. J Comput Assist Tomogr. 2007;31:519–525. doi: 10.1097/01.rct.0000250106.01047.4b. [DOI] [PubMed] [Google Scholar]
- 29.Bottger TC, Junginger T. Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World J Surg. 1999;23(2):164–171. doi: 10.1007/PL00013170. [DOI] [PubMed] [Google Scholar]
- 30.Brown KM, Tompkins AJ, Yong S, Aranha GV, Shoup M. Pancreaticoduodenectomy is curative in the majority of patients with node-negative ampullary cancer. Arch Surg. 2005;140(6):529–532. doi: 10.1001/archsurg.140.6.529. [DOI] [PubMed] [Google Scholar]


