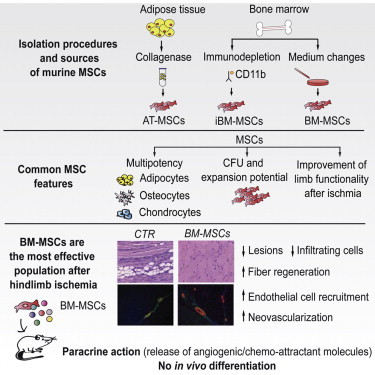
Summary
Over the last several years, mesenchymal stromal cells (MSCs) have been isolated from different tissues following a variety of different procedures. Here, we comparatively assess the ex vivo and in vivo properties of MSCs isolated from either adipose tissue or bone marrow by different purification protocols. After MSC transplantation into a mouse model of hindlimb ischemia, clinical and histological analysis revealed that bone marrow MSCs purified on adhesive substrates exerted the best therapeutic activity, preserving tissue viability and promoting formation of new arterioles without directly transdifferentiating into vascular cells. In keeping with these observations, these cells abundantly expressed cytokines involved in vessel maturation and cell retention. These findings indicate that the choice of MSC source and purification protocol is critical in determining the therapeutic potential of these cells and warrant the standardization of an optimal MSC isolation procedure in order to select the best conditions to move forward to more effective clinical experimentation.
Graphical Abstract
Highlights
-
•
MSCs were isolated from various tissues according to different protocols
-
•
All MSCs provided a therapeutic benefit in a hindlimb ischemia model
-
•
Adhesive MSCs from bone marrow were the most effective in preserving tissue viability
-
•
These cells promoted massive vascularization in a paracrine fashion
Zacchigna and colleagues compared the ex vivo and in vivo properties of mesenchymal stromal cells (MSCs) isolated from either adipose tissue or bone marrow by different protocols. Adhesive MSCs from bone marrow exerted the best therapeutic activity in a hindlimb ischemia model, preserving viability and inducing angiogenesis in a paracrine manner. The optimization of a standard MSC isolation procedure is required to improve clinical results.
Introduction
Mesenchymal stromal cells (MSCs) are defined as multipotent, self-renewing cells residing in several tissues, including the bone marrow, adipose tissue, umbilical cord blood, and placenta (Pittenger et al., 1999). These cells are defined as multipotent, as they are capable of generating different mesenchymal cell types, traditionally adipocytes, chondrocytes, and osteocytes, but also smooth muscle cells and cardiomyocytes (Makino et al., 1999; Pittenger et al., 1999). MSCs have been at the forefront of clinical research for the therapy of cardiovascular disorders for many years. In particular, cardiac and peripheral ischemia is a leading cause of morbidity and mortality in our aging society and suffers from a lack of curative therapies (Tendera et al., 2011). In this setting, MSC transplantation has been proposed as an innovative therapy for no-option ischemic patients. Originally, the therapeutic potential of these cells was thought to arise through their putative capacity to transdifferentiate, thereby directly contributing to vasculogenesis and tissue regeneration (Quevedo et al., 2009). This attractive hypothesis led to the prompt, perhaps immature transition of the results obtained in animal models to the clinics, with the ambitious goal to regenerate ischemic tissues (Hare et al., 2009; Tateishi-Yuyama et al., 2002). However, MSC plasticity has been later harshly questioned (Noiseux et al., 2006), and the therapeutic potential of these cells is currently considered to derive from the secretion of a variety of growth factors and cytokines exerting a paracrine, protective effect on ischemic cells (Gnecchi et al., 2012).
Despite the common definition of MSCs and the modest but real therapeutic effect exerted by these cells in various experimental and clinical settings, there is no universal agreement on the optimal source and method for MSC isolation and culture (Soleimani and Nadri, 2009; Sung et al., 2008; Yamamoto et al., 2007). This importantly limits the possible comparison of the observed results in terms of MSC characterization and therapeutic activity. Most of the studies that compared different MSC types are based on the analysis of surface markers, multipotency, and angiogenic assays ex vivo (Lee et al., 2004; Peng et al., 2008). A few studies also compared MSC activity in vivo, in animal models of cardiac and limb ischemia, but they considered either different tissue sources (van der Bogt et al., 2009) or different purification methods (Seeger et al., 2007) and never the combination of the two parameters. Additional relevant variables in these studies are the species from which MSCs were isolated and the specific animal model used to ascertain their therapeutic properties. This again prevents the direct comparison of most of the existing studies and warrants the prospective characterization of MSCs derived from various tissues and purified according to different purification protocols.
Here, we provide evidence that murine MSCs, harvested from either adipose tissue or bone marrow and following different purification procedures, behave differently and, most importantly, exhibit different therapeutic potential in a mouse model of critical limb ischemia (CLI). This underlines the need to adopt optimal and standardized methods for MSC processing in future preclinical and clinical trials.
Results
MSCs Isolated from Different Sources and according to Different Procedures Show a Common Phenotype
To identify the optimal population of MSCs to treat hindlimb ischemia, cells were purified from either the adipose tissue or the bone marrow of mice using three different protocols. Adipose tissue (AT) MSCs were isolated by tissue digestion followed by centrifugation, while bone marrow (BM)-derived MSCs were obtained by either frequent medium changes (BM-MSCs) or immunodepletion (iBM-MSCs) (Figure 1A). In all three cases, the first MSC colonies appeared after a few days and cells were cultured until they reached 80% confluence (within ∼10 days for AT-MSCs and ∼6 weeks for both types of BM-derived cells). When assessing their proliferative potential, iBM-MSCs appeared to grow significantly faster than the other populations (Figure 1B). When seeded at a low number, all three MSC cell types formed a similar number of colonies, confirming their clonogenic potential (Figures 1C and 1D). Despite their high proliferative capacity, MSCs did not become immortal but started to express the senescence-associated β-galactosidase (SA-βgal) marker at passage 12 (Figure S1A available online) and stopped multiplying after an additional three to six passages.
Figure 1.
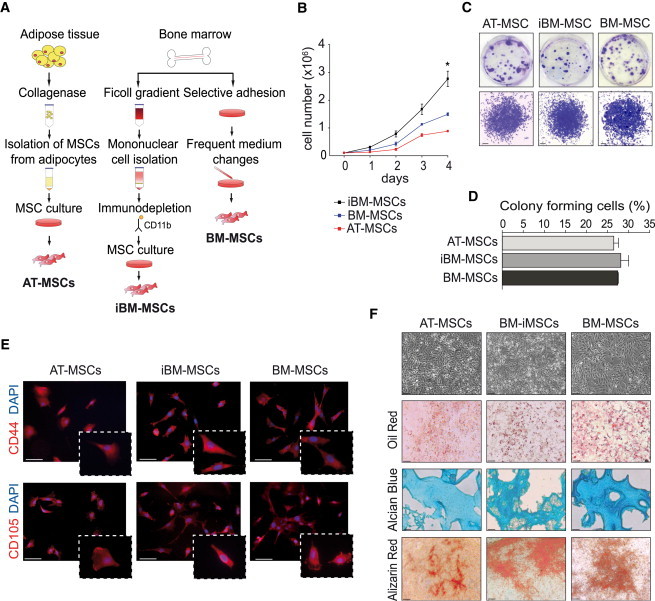
Characterization of MSCs Isolated according to Three Different Procedures
(A) Schematic overview of MSCs isolation procedures.
(B) Counting of AT-MSCs, BM-MSCs, and iBM-MSCs for 4 consecutive days. Data (n = 3 biological replicates) are presented as mean ± SEM (∗p < 0.05).
(C) Crystal violet staining of MSCs showing colonies after 10 days of culture (upper panels) and a representative colony (lower panels). Scale bar, 100 μm.
(D) Quantification of colonies formed after 10 days of cell culture. Data (n = 3 biological replicates) are presented as mean ± SEM.
(E) MSC immunophenotyping at passage 3. CD44 or CD105, red; DAPI, blue; scale bar, 50 μm.
(F) All MSCs exhibited a similar morphology and became positive for oil red (adipocytic differentiation; scale bar, 50 μm), alcian blue (chondrocytic differentiation; scale bar 50 μm), and alizarin red (osteocytic differentiation; scale bar, 80 μm).
Regardless of their different origin and isolation protocol, the three cell populations exhibited a very similar marker profile. They abundantly expressed the MSC-defining CD44, CD105, CD29, and CD90 markers, whereas they scored negative for endothelial (CD31), myeloid (CD11b, GR-1), or hematopoietic (CD45 and CD34) antigens (Boxall and Jones, 2012). Low levels of expression were also found for SCA-1, c-KIT, TIE-2, CXCR4, and TER-119 (Figures 1E and S1B; Table S1).
Finally, AT-MSCs, iBM-MSCs, and BM-MSCs were probed for their capacity to differentiate into adipocytes, chondrocytes, and osteocytes. Adipogenic medium induced the appearance of lipid vacuoles, positive to oil red staining; chondrogenic medium forced the cells to accumulate alcian blue-positive proteoglycans; and osteogenic medium dramatically induced accumulation of intracellular calcium deposits, reactive to Alizarin staining (Figure 1F). These results confirm the multipotency of the three MSC populations.
Transplantation of AT-MSCs, iBM-MSCs, and BM-MSCs Markedly Reduces Severe Hindlimb Ischemia
We assessed MSC therapeutic potential in a mouse model of hindlimb ischemia induced by removal of the entire femoral artery. The day following surgery, the animals received 2 × 105 cells (or medium as control) in the limb homolateral to ischemia (Figure 2A). Clinical follow-up over 3 weeks showed a significant improvement in the group injected with iBM-MSCs, but the most significant improvement was seen in the group injected with BM-MSCs (Figure 2B). Histological analysis of muscle sections at day 21 showed extensive necrosis, adipose substitution, and marked infiltration by inflammatory cells in control animals. In contrast, the muscles injected with any MSC population showed a statistically significant reduction of the inflammatory infiltrate (Figures 2C and 2D), associated with a smaller lesion area (Figures 2C and 2E). In addition, the number of regenerating fibers, characterized by central nuclei, was significantly higher in animals treated with cells of BM origin, in particular in the case of BM-MSCs (Figures 2C and 2F). Thus, postischemic administration of any MSC type, but mostly of BM-MSCs, remarkably improves functional and structural recovery of the ischemic limb.
Figure 2.
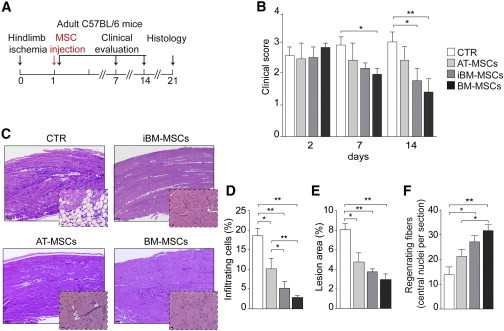
Morphological and Functional Evaluation of the Therapeutic Effect of MSCs
(A) CLI experimental flow chart. Mice were injected with AT-MSCs, BM-MSCs, or iBM-MSCs at passage 3–5 or medium (n = 10 animals per group).
(B) Limb function evaluation at days 2, 7, and 21 after CLI according to the criteria described in Table S2.
(C) Representative hematoxylin-eosin staining of ischemic muscle from untreated and treated animals at day 21. Scale bar represents 100 μm for lower magnification and 10 μm for higher magnification.
(D) Percentage of infiltrating cells at the site of ischemia.
(E) Percentage of muscle affected by ischemic damage.
(F) Quantification of central nuclei as a hallmark of muscle regeneration.
Data in (B) and (D)–(F) are expressed as mean ± SEM (n = 10 animals per group; ∗p < 0.05, ∗∗p < 0.01).
MSCs Induce Neovascularization in Ischemic Muscles
To assess the angiogenic potential of MSCs, muscles were double labeled with lectin, which stains endothelial and mononuclear cells, and antibodies against α-smooth muscle actin (α-SMA), which marks smooth muscle cells (SMCs) surrounding arterial vessels (Figure 3A). Whereas no significant difference in the number of lectin-positive cells was detected, all three MSC populations stimulated a massive formation of 10–100 μm arterioles (Figure 3B). This effect was particularly pronounced in the case of BM-MSCs.
Figure 3.
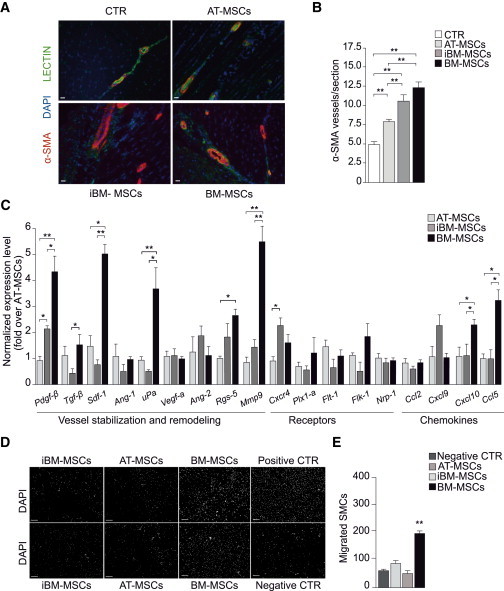
MSC Treatment Effectively Induces Neovascularization in Ischemic Muscles
(A) Representative immunostaining for vascular structures at day 21. α-SMA, red; lectin, green; DAPI, blue; scale bar, 50 μm.
(B) Number of α-SMA positive vessels. Data (n = 10 animals per group) are expressed as mean ± SEM (∗p < 0.05, ∗∗p < 0.01).
(C) Quantification of mRNA levels of factors involved in angiogenesis, vessel stabilization, and remodeling (n = 3 biological replicates). Expression of each gene was first normalized over Gapdh and then over AT-MSCs.
(D) Representative images of murine SMCs migrated in response to the various MSCs. SMCs were seeded on the upper chamber and stained with DAPI after migration to the bottom side of the filter. MSCs were seeded in the lower chamber (n = 3 biological replicates). Serum-free medium and serum-rich medium were used as negative and positive controls; scale bar, 100 μm.
(E) Quantification of SMCs migrated in response to the various MSCs, expressed as number of migrated cells per 100× field.
Data in (B), (C), and (E) are expressed as mean ± SEM (∗p < 0.05, ∗∗p < 0.01).
Lead by recent literature supporting the paracrine activity of MSCs (Gnecchi et al., 2012; Mirotsou et al., 2011), we analyzed the mRNA levels of a series of molecules involved in blood vessel formation and maturation (Figure 3C). BM-MSCs were found to express particularly abundant levels of factors required for vessel stabilization, SMC migration, and matrix remodeling, such as Tgf-β, Pdgf-β, and Mmp9. In addition, these cells also expressed high levels of Ccl5 and Sdf-1α, two chemokines known to be involved in the recruitment and retention of proangiogenic macrophages and MSCs themselves (Abbott et al., 2004; Chen et al., 2008). Thus MSCs, and in particular BM-MSCs, express a cocktail of soluble factors able to enhance their retention in an autocrine manner and also attract proangiogenic cells. To confirm the latter concept experimentally, we performed a migration assay and found that BM-MSCs were the only cell type able to attract murine SMCs (Figures 3D and 3E), similar to 10% fetal bovine serum (used as positive control) and consistent with their abundant expression of Pdgf-β at the mRNA and protein level (Figures 3C and S2) (Gerthoffer, 2007). The ample production of proangiogenic cytokines by the various MSCs suggested that the same promigratory effect could be achieved by the delivery of their conditioned medium (CM). To explore this possibility, we injected the medium harvested from the same number of AT-MSCs, iBM-MSCs, and BM-MSCs (2 × 105) used in the previous experiment, at days 1, 3, and 7 after surgery. This treatment resulted in a clear trend toward improvement in all the morphological and functional parameters analyzed (Figure S3).
BM-MSCs Stimulate Functional Neovascularization but Are Not Incorporated in the Newly Formed Vasculature
Functional muscle perfusion was evaluated by planar scintigraphy (Figures 4 and S4). In control animals, a large perfusion deficit was evident at day 1 and was still present 2 weeks after ischemia. A statistically significant improvement that was evident starting at day 7 after injection was detected in the animals treated with BM-MSCs (Figures 4B and 4C). A moderate recovery was detected in iBM-MSC-treated animals at 1 week, while AT-MSCs did not provide any benefit compared to controls. Thus, BM-MSCs were the most effective cells in inducing functional vascularization.
Figure 4.
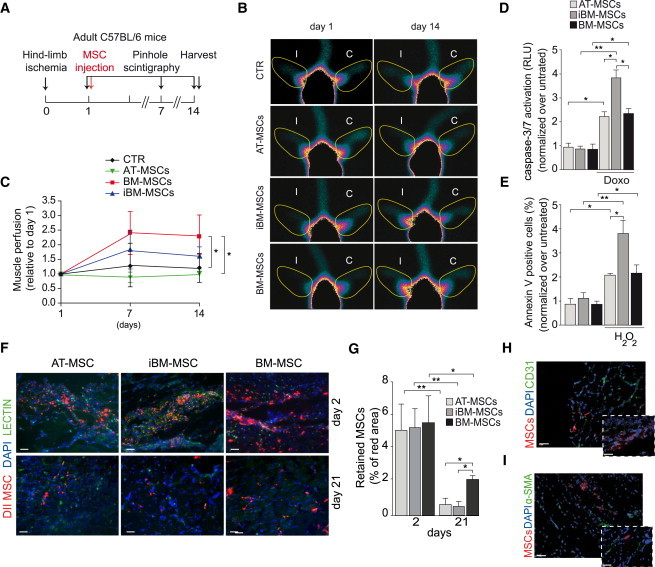
MSCs Induce Functional Vascularization but Do Not Differentiate into Vascular Structures In Vivo
(A) Planar scintigraphy experimental flow chart (n = 8 animals per group).
(B) Representative images showing the regions of interest (yellow line) used to quantify muscle perfusion on the ischemic (I) and control (C) limb at the indicated time points.
(C) Quantification of muscle perfusion, normalized over the perfusion measured at day 1.
(D) Level of caspase activation in MSCs exposed to doxorubicin, normalized over untreated cells (n = 9, 3 biological and 3 technical replicates).
(E) Percentage of Annexin V+ cells after exposure to H2O2, normalized over untreated cells (n = 9, 3 biological and 3 technical replicates).
(F) Representative images of MSC engraftment at days 2 and 21. DiI-labeled MSCs, red; lectin, green; DAPI, blue; scale bar, 50 μm.
(G) Quantification of cell engraftment at the indicated time points (n = 6 animals per group).
(H) Representative images of DiI-labeled BM-MSCs (red) stained for CD31 (green). Nuclei are counterstained with DAPI (blue). Scale bar represents 25 μm for lower magnification and 40 μm for higher magnification.
(I) Representative images of DiI-labeled BM-MSCs (red) stained for α-SMA (green). Nuclei are counterstained with DAPI (blue). Scale bars as in (H).
Data in (C)–(E) and (G) are expressed as mean ± SEM (∗p < 0.05, ∗∗p < 0.01).
We then explored the possible mechanisms responsible for the superior performance of BM-MSCs. We observed that BM-MSCs and AT-MSCs resisted doxorubicin- or hydrogen-peroxide-induced apoptosis more than iBM-MSCs, as evaluated by caspase-3/7 activation and Annexin V staining (Figures 4D and 4E). When analyzing their persistence into ischemic muscles in vivo, we found that the engraftment of BM-MSCs was significantly higher at day 21 compared to the other two MSC types (Figures 4F and 4G). To investigate whether these cells directly contributed to the formation of new vessels by transdifferentiation, we evaluated the colocalization of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)-labeled MSCs with endothelial (CD31) and SMC (α-SMA) markers. The complete lack of colocalization (Figures 4H and 4I) further supports a paracrine mechanism responsible for their angiogenic properties.
Discussion
This study provides a comparison of the therapeutic properties of three MSC populations isolated from two different tissues (BM and AT) according to different purification protocols. Our results show that (1) the three MSC types acquire similar phenotypic and functional properties after the first passages, (2) any MSC type provides therapeutic benefit after CLI, (3) BM-MSCs perform better than the other types in preserving tissue viability and inducing neovascularization, and (4) BM-MSCs do not transdifferentiate in vivo into vascular structures but persist longer and act in a paracrine manner to promote vascular formation.
The first objective of this study was the definition of an optimal protocol for MSC isolation. We intentionally focused on BM and AT, as these are the most accessible tissues for MSC recovery. Current protocols for AT-MSC isolation are almost universally based on tissue digestion; for the BM, instead, we selected the two most frequently used methods, namely isolation based on MSC adhesive properties (BM-MSCs) (Soleimani and Nadri, 2009) and density gradient centrifugation, followed by immunodepletion (iBM-MSCs) (Sung et al., 2008).
Despite presenting a similar behavior in cell culture, the three MSC types showed a different therapeutic potential once injected in vivo in a CLI model, reducing necrosis and inflammation and stimulating muscle regeneration, although at a different extent. They also prompted the formation of functional arterioles, in accordance with previous reports (Cho et al., 2009; Iwase et al., 2005). Interestingly, this was better accomplished by BM-MSCs, which abundantly secreted soluble cytokines involved in vessel remodeling and stabilization (such as PDGF-β) and induced the migration of vascular SMCs. This contradicts the findings of Kim et al., who attributed a higher angiogenic and therapeutic potential to AT-MSCs in comparison to MSCs of BM origin (Kim et al., 2007). A few differences can explain this discrepancy. First, their BM-MSCs were purified by density gradient centrifugation, while our results clearly indicate that the therapeutic potential strictly depends on the isolation protocol. Second, they transplanted human cells into nude mice, which did not allow for the assessment of the immune-modulatory action of MSCs.
Although multiple evidence points toward vascular endothelial growth factor (Vegf) as the main angiogenic cytokine secreted by MSCs (Kinnaird et al., 2004), we did not observe significant differences in Vegf expression among the three MSC types. Instead, the major differences occurred in genes involved in SMC recruitment and matrix remodeling (Tgf-β, Pdgf-β, and Mmp9), which are two essential events for the proper maturation of arterial vessels (Zacchigna et al., 2008). Can this arteriogenic effect be recapitulated by the delivery of the MSC supernatant? Although not reaching statistical significance, probably due to the relatively low number of animals analyzed, the repeated injection of CM from the three MSC types resulted in a trend toward improvement, fully consistent with the results observed upon cell transplantation. This supports the conclusion that the MSC secretome could recapitulate the effect of MSC injection (Ranganath et al., 2012), but its effectiveness is most likely dampened by the short half-life of its molecules. These results leave at least two open questions. First, CM and MSC transplantation were not performed on the same set of animals, and thus we cannot exclude interanimal variability when comparing the efficacy of the two treatments. Second, we did not investigate whether the injection of higher CM amounts or the administration of more frequent doses would result in a better outcome.
An additional reason for the higher therapeutic activity of BM-MSCs could be related to the longer engraftment and survival of the cells in vivo. In any case, it appears important to remark that our MSCs did not become immortalized, as they underwent cellular senescence after passage 12 and we never observed formation of tumor masses during long-term follow-up.
In conclusion, our findings indicate that BM-MSCs are the most effective MSC type to improve perfusion and functional recovery after hindlimb ischemia. In contrast to initial studies supporting MSC plasticity (Quevedo et al., 2009), these cells were not able to transdifferentiate into either endothelial cells or SMCs while exerting their effect through a paracrine mechanism.
Experimental Procedures
MSC Culture and In Vivo Experiments
MSCs were isolated from the AT or BM of C57/BL6 mice according to three adapted protocols (Soleimani and Nadri, 2009; Sung et al., 2008; Yamamoto et al., 2007). Cells (2 × 105) at passage 3–5 were injected into the hindlimb of syngeneic mice the day following resection of the femoral artery. Mice were monitored clinically and by planar scintigraphy and sacrificed at day 21 for histological analysis. Animal care and treatment were conducted in conformity with institutional guidelines, in compliance with national and international laws and policies (European Economic Community Council Directive 86/609, OJL 358, December 12th, 1987, and UE2010763), after institutional review board approval.
MSC Phenotypic Characterization
MSC-specific surface antigens were analyzed by flow cytometry and immunofluorescence. Clonogenic potential was evaluated upon staining with crystal violet. Differentiation was performed using a dedicated kit from LifeLine Cell Technologies.
Histology and Immunofluorescence
Muscle sections were stained with hematoxylin-eosin to perform morphometric analysis, with fluorescein isothiocyanate (FITC)-conjugated lectin and anti-α-SMA antibodies to stain the vasculature. Cells were labeled with DiI prior injection to track cell engraftment.
Real-Time PCR
Total RNA was extracted using TRIzol and reverse transcribed using hexameric random primers. All the amplifications were performed on a Bio-Rad real-time thermal cycler CFX96 using TaqMan probes (Applied Biosystems).
Migration Assay
Murine SMC migration in response to MSC supernatant was performed using 8 μm transwell supports (Costar, Corning).
Apoptosis Assay
MSC apoptosis was induced by hydrogen peroxide (H2O2) or doxorubicin and quantified either by flow cytometry using the Annexin V-FITC Apoptosis Detection Kit (Roche) or by using the Caspase-Glo 3/7 Assay System (Promega).
Statistical Analysis
All in vitro experiments were repeated at least three times and performed including at least three biological replicates. Data are presented as mean and SEM. Comparison within groups was analyzed by ANOVA (followed by Bonferroni post hoc analysis) for the evaluation based on tissue sections. In the case of experiments entailing the follow-up of the same animals over multiple time points, we used repeated-measures ANOVA to analyze statistical significance of the differences between groups over time. p < 0.05 was considered as statistically significant.
Additional details on the experimental procedures used in this work are available in Supplemental Experimental Procedures.
Author Contributions
F.B. performed purification and characterization of MSCs. L.U. performed the in vivo experiments. V.R. and V.M. contributed to MSC purification and culture. B.P. and F.D. performed scintigraphy experiments. G.R. and M.G. critically evaluated the research approach and results. S.Z. planned the experiments and wrote the manuscript.
Acknowledgments
This work was supported by Advanced Grant 250124 from the European Research Council (ERC), the Fondation Leducq’s Transatlantic Networks of Excellence Program, projects FIRB RBAP11Z4Z9 and PRIN 2010RNXM9C from the MIUR (Italy), and project CTC from the Fondazione CRTrieste (Italy) to M.G. The authors are grateful to Suzanne Kerbavcic for precious editorial assistance.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information
References
- Abbott J.D., Huang Y., Liu D., Hickey R., Krause D.S., Giordano F.J. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- Boxall S.A., Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012;2012:975871. doi: 10.1155/2012/975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tredget E.E., Wu P.Y., Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.H., Kim Y.J., Kim J.T., Song J.S., Shin K.K., Bae Y.C., Jung J.S. The role of chemokines in proangiogenic action induced by human adipose tissue-derived mesenchymal stem cells in the murine model of hindlimb ischemia. Cell. Physiol. Biochem. 2009;24:511–518. doi: 10.1159/000257495. [DOI] [PubMed] [Google Scholar]
- Gerthoffer W.T. Mechanisms of vascular smooth muscle cell migration. Circ. Res. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- Gnecchi M., Danieli P., Cervio E. Mesenchymal stem cell therapy for heart disease. Vascul. Pharmacol. 2012;57:48–55. doi: 10.1016/j.vph.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Hare J.M., Traverse J.H., Henry T.D., Dib N., Strumpf R.K., Schulman S.P., Gerstenblith G., DeMaria A.N., Denktas A.E., Gammon R.S. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase T., Nagaya N., Fujii T., Itoh T., Murakami S., Matsumoto T., Kangawa K., Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc. Res. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Kim Y., Kim H., Cho H., Bae Y., Suh K., Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell. Physiol. Biochem. 2007;20:867–876. doi: 10.1159/000110447. [DOI] [PubMed] [Google Scholar]
- Kinnaird T., Stabile E., Burnett M.S., Shou M., Lee C.W., Barr S., Fuchs S., Epstein S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- Lee R.H., Kim B., Choi I., Kim H., Choi H.S., Suh K., Bae Y.C., Jung J.S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- Makino S., Fukuda K., Miyoshi S., Konishi F., Kodama H., Pan J., Sano M., Takahashi T., Hori S., Abe H. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirotsou M., Jayawardena T.M., Schmeckpeper J., Gnecchi M., Dzau V.J. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell. Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiseux N., Gnecchi M., Lopez-Ilasaca M., Zhang L., Solomon S.D., Deb A., Dzau V.J., Pratt R.E. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Peng L., Jia Z., Yin X., Zhang X., Liu Y., Chen P., Ma K., Zhou C. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008;17:761–773. doi: 10.1089/scd.2007.0217. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Quevedo H.C., Hatzistergos K.E., Oskouei B.N., Feigenbaum G.S., Rodriguez J.E., Valdes D., Pattany P.M., Zambrano J.P., Hu Q., McNiece I. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath S.H., Levy O., Inamdar M.S., Karp J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger F.H., Tonn T., Krzossok N., Zeiher A.M., Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur. Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- Soleimani M., Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- Sung J.H., Yang H.M., Park J.B., Choi G.S., Joh J.W., Kwon C.H., Chun J.M., Lee S.K., Kim S.J. Isolation and characterization of mouse mesenchymal stem cells. Transplant. Proc. 2008;40:2649–2654. doi: 10.1016/j.transproceed.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Tateishi-Yuyama E., Matsubara H., Murohara T., Ikeda U., Shintani S., Masaki H., Amano K., Kishimoto Y., Yoshimoto K., Akashi H., Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- Tendera M., Aboyans V., Bartelink M.L., Baumgartner I., Clément D., Collet J.P., Cremonesi A., De Carlo M., Erbel R., Fowkes F.G., European Stroke Organisation. ESC Committee for Practice Guidelines ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the task force on the diagnosis and treatment of peripheral artery diseases of the European Society of Cardiology (ESC) Eur. Heart J. 2011;32:2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- van der Bogt K.E., Schrepfer S., Yu J., Sheikh A.Y., Hoyt G., Govaert J.A., Velotta J.B., Contag C.H., Robbins R.C., Wu J.C. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Akamatsu H., Hasegawa S., Yamada T., Nakata S., Ohkuma M., Miyachi E., Marunouchi T., Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J. Dermatol. Sci. 2007;48:43–52. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Zacchigna S., Pattarini L., Zentilin L., Moimas S., Carrer A., Sinigaglia M., Arsic N., Tafuro S., Sinagra G., Giacca M. Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice. J. Clin. Invest. 2008;118:2062–2075. doi: 10.1172/JCI32832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


