Abstract
Objectives
To investigate the associations between the apolipoprotein E (APOE) ε4 allele, carbon dioxide (CO2) vasoreactivity, and cognitive performance and to explore the effect of CO2 vasoreactivity and hypertension on the associations between APOE and cognition.
Design
Observational.
Setting
Community.
Participants
Older adults (N=625) enrolled in the Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly of Boston Study
Measurements
Change in cerebral blood flow velocity in response to CO2 challenge (CO2 vasoreactivity) measured using transcranial Doppler ultrasonography, Trail-Making Test Part B – A (TMT), Hopkins Verbal Learning Test delayed recall (HVLT).
Results
APOE-ε4 was associated with lower CO2 vasoreactivity (p=.009) and poorer performance on the TMT (p<.001) and HVLT (p<.001). Having hypertension and APOE-ε4 was associated with worse cognitive and CO2 vasoreactivity measures than having neither or either alone (p<.001 for TMT and HVLT, p=.01 for CO2 vasoreactivity). The association between APOE-ε4 and cognition was only significant if it was present concurrent with low CO2 vasoreactivity, defined as below the median of the sample (APOE by CO2 vasoreactivity: p=.04 for TMT, p=.04 for HVLT). In hypertension, the association between APOE-ε4 and executive function was also only significant in participants with lower CO2 vasoreactivity (p=.005 for APOE by CO2 vasoreactivity)
Conclusion
Individuals at risk of Alzheimer’s disease (AD) because they have APOE-ε4 may have lower CO2 vasoreactivity, which in turn may be contributing to the observed lower cognitive performance associated with this allele. The cognitive effect of APOE-ε4 are magnified in hypertension and low CO2 vasoreactivity. This study offers evidence that APOE-ε4 may be associated with microvascular brain injury even in the absence of clinical AD.
Keywords: hypertension, apolipoprotein E, cognition, CO2 vasoreactivity
The ε4 allele of the gene that codes apolipoprotein E (APOE) has been consistently found to be a risk factor for Alzheimer’s disease (AD) and other cognitive disorders.1 APOE-ε4 may also be associated with poor cognitive performance in older adults without dementia2, 3 and is associated with hypertension, which in turn may increase the risk of cognitive impairment.4, 5 Prior studies suggest that hypertension and APOE have a cumulative effect on the risk of AD,6 but fewer studies have explored the combined effect on cognition in older adults without dementia. For example in the Honolulu–Asia Aging Study, midlife high systolic blood pressure had a stronger negative effect on global measures of cognition in participants with APOE-ε4.7
APOE is a protein involved in the maintenance of lipid homeostasis in the brain by taking up lipids produced after neuronal degeneration and redistributing them for cellular repair.8 Carriers of the APOE-ε4 allele have lower levels of APOE lipoprotein than carriers of the other alleles. 9 In addition to neurotoxic manifestations of this allele, recent evidence suggests possible harmful cerebrovascular effects. 10 In an in vitro blood–brain barrier (BBB) model, APOE was found to be involved in the regulation of the integrity of tight junctions in an isoform-dependent fashion.11 Mice models suggest that APOE-ε4 disrupts BBB integrity through cyclophilin A. 12 As reviewed recently, most of the observations regarding APOE and cerebral microvascular effects were derived from animal models or postmortem studies; few human in vivo studies exist.10 Measuring the cerebral blood flow response to changes in end-tidal carbon dioxide (CO2) (CO2 vasoreactivity) is a noninvasive in vivo method of assessing the integrity and function of the brain microcirculation.13 Low CO2 vasoreactivity has been observed in individuals with AD and vascular dementia.14, 15 Previous work suggests that CO2 vasoreactivity is low in individuals with hypertension and is linked to poor executive function.16, 17 It was hypothesized that APOE-ε4 carriers would have low CO2 vasoreactivity, which also might be related to cognitive performance.
The associations between APOE, CO2 vasoreactivity, and cognitive function were examined in a population-based study of older adults, and the potential effect of CO2 vasoreactivity and hypertension on the relationship between APOE-ε4 and cognition was investigated.
METHODS
The current analyses used data collected during the baseline evaluations of the Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly of Boston Study (MOBILIZE Boston), which has been described previously.18 Briefly, it is a population-based prospective observational study funded by the National Institute on Aging. The institutional review board at Hebrew SeniorLife approved MOBILIZE Boston, and each participant provided a written informed consent. The institutional review board at the University of Southern California approved this analysis.
Participants
Eligibility criteria included aged 70 and older, able to speak and understand English, and living in the recruitment area for at least 2 years after enrollment. Exclusion criteria included a Mini-Mental State Examination (MMSE) score less than 18, hearing or visual impairment that interfered with communication, having a terminal illness, and inability to walk 20 feet without assistance. Participant assessments included anthropometric and blood pressure measurement, health habits, medical history, medication inventory, and cognitive evaluations. Hypertension was defined as high blood pressure (≥140/90 mm Hg) or receiving antihypertensive medication. Neuropsychological assessments included the Trail Making Test (TMT) Parts A and B19 and the Hopkins Verbal Learning Test (HVLT). The TMT is a benchmark test for executive function. TMT Part B minus Part A (B–A), which adjusts the test for the motor speed and dexterity of the participant, was calculated.20 HVLT scores included delayed recall and recognition abilities.
Cerebral Hemodynamics Cerebral hemodynamics were assessed on a subset of subjects using transcranial Doppler ultrasonography (TCD; MultiDop X4, DWL-Transcranial Doppler Systems, Inc., Sterling, VA). Subjects without TCD evaluations were of the same age but were more likely to be female and nonwhite and to have diabetes mellitus, hypertension, and lower MMSE than those with TCD evaluations, as reported previously.21 A heart rate and beat-to-beat arterial pressure monitor (Finapres, Ohmeda Monitoring Systems, Englewood, CO) was attached to subjects, as previously described.22 End-tidal CO2 was measured using a CO2 analyzer (Vacumed, Ventura, CA) attached to a nasal cannula. Mean blood flow velocity (BFV) was measured in the middle cerebral artery at a depth of 50 to 60 mm. A well-trained dedicated TCD technician performed TCD procedures. BFV in the middle cerebral artery was measured continuously while subjects inspired a gas mixture of 8% CO2, 21% oxygen, and 71% nitrogen for 2 minutes and then mildly hyperventilated to an end-tidal CO2 of approximately 25 mmHg for 2 minutes. CO2 itself may also affect blood pressure, so cerebrovascular conductance (cerebral blood flow/mean arterial blood pressure) was derived, and CO2 vasoreactivity was calculated as the slope of the change in cerebrovascular conductance versus the change in end-tidal CO2.23
Genotyping
Genotyping was conducted at the Harvard Medical School–Partners Healthcare Center for Genetics and Genomics. Multiplex polymerase chain reaction assays were designed using Sequenom Spectro DESIGNER software version 3.0.0.3 (San Diego, CA) by inputting sequences containing the single nucleotide polymorphism (SNP) site and 100 base pairs of flanking sequences on either side of the SNP. Quality control was conducted using a subsample (5%) of duplicate genotyping to identify any discordance in the results. APOE status was determined according to the genotypes at the rs429358 and rs7412 SNPs24 and was defined as APOE-ε4+ if the individual had at least one APOE-ε4 allele and APOE-ε4− if the individual had no APOE-ε4 alleles.
Statistical Analysis
Linear models were used to compare cognitive and cerebral hemodynamics between the two APOE-ε4 groups and between the following three groups: combination (hypertension and APOE-ε4+), either alone (hypertension or APOE-ε4+), and neither (normotension and APOE-ε4−). The modifying effects of the interactions between CO2 vasoreactivity and APOE and between hypertension and APOE on cognitive performance were explored by including an interaction variable (APOE by CO2 vasoreactivity and APOE by hypertension) in the models. A categorical variable was used for the interaction analysis (low and high CO2 vasoreactivity divided at the median measure of the sample).
All models were adjusted for demographic characteristics, education, body mass index (BMI), blood pressure, and stroke. P-values were adjusted for multiple testing using simulation-based multiple comparison.25 The results are presented in the tables and figures as least square means (adjusted for the covariates) to account for confounding in gene-association studies.26 SAS version 9.2 (SAS Institute, Inc., Cary, NC) was used for the analyses.
RESULTS
The sample included 625 participants who were evaluated at baseline (mean age 78, 80% white, 63% female, mean MMSE score 27, mean 14.5 years of education). As shown in Table 1, APOE-ε4− participants were more likely to be white, have lower cholesterol, and score higher on the MMSE. Of the 625 participants, 74% had hypertension, and 24% had at least one APOE-ε4 allele. (Eight participants were homozygous APOE-ε4+.) Cerebral hemodynamic data were gathered on 374 (60%) participants. There was no difference in the genotypic distribution between those with (26% APOE-ε4+) and without (22% APOE-ε4+) hemodynamic data (p=.17), but those without hemodynamic data had lower cognitive performance on the TMT Part B–A (p<.001) and HVLT delayed recall (p=.01).
Table 1.
Demographic, Social, and Clinical Characteristics According to Apolipoprotein E (APOE)-ε4 Status of Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly of Boston Study Participants
| Characteristic | APOE-ε4−, n=478 | APOE-ε4+, n=147 | P-Value |
|---|---|---|---|
| Age, mean±SD | 78.2±5.5 | 77.2±4.7 | .08 |
| Female, % | 61% | 67% | .18 |
| White, % | 82% | 73% | .02 |
| Body mass index, kg/m2, mean±SD | 27.7±5.2 | 27.3±4.8 | .46 |
| Alcohol nondrinker, % | 25% | 29% | .34 |
| Never smoked, % | 42% | 43% | .19 |
| Systolic blood pressure, mmHg, mean±SD | 129.1±17.7 | 130.5±18.4 | .42 |
| Diastolic blood pressure, mmHg, mean±SD | 69.7±8.8 | 70.7±8.6 | .28 |
| Stroke, % | 10% | 9% | .76 |
| Hypertension, % | 74% | 73% | .97 |
| Antihypertensive therapy, % | 68% | 65% | .58 |
| Heart disease, % | 27% | 24% | .53 |
| Congestive heart failure, % | 5% | 5% | .96 |
| Diabetes mellitus, % | 17% | 16% | .64 |
| Low density lipoprotein, mg/dL, mean±SD | 106.4±33 | 112.1±35.7 | .08 |
| Cholesterol, mg/dL, mean±SD | 183.3±39.7 | 191.6±37.5 | .03 |
| Mini-Mental State Examination score, mean±SD | 27.4±2 | 26.7±3 | .003 |
P-values were obtained from the t-test or chi-square test comparing the two APOE groups.
SD=standard deviation.
APOE, Cognition and CO2 Vasoreactivity
After adjustment for demographic characteristics, BMI, systolic blood pressure, educational level, and prior history of stroke, the APOE-ε4 allele was associated with lower CO2 vasoreactivity (p=.009) and poorer performance on the TMT part B (p<.001) and Part B–A (p<.001) and the HVLT delayed recall (P<.001) and recognition (p.006). There was no difference in resting BFV between the two APOE-ε4 groups (P=.24) (Table 2).
Table 2.
Cognitive (n=625) and Cerebral Hemodynamic (n=374) Measures According to Apolipoprotein E (APOE)-ε4 Status in the Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly of Boston Study
| Measure | APOE-ε4− | APOE-ε4+ | P-Valueb |
|---|---|---|---|
| Least Square Mean (Standard Error)a | |||
| CO2 vasoreactivity, cm/s per mmHg/partial pressure of CO2mmHg x10−2 | 1.10 (0.05) | 0.95 (0.07) | .009 |
| Blood Flow Velocity, cm/s | 43.19 (1.26) | 42.13 (1.54) | .24 |
| Trail-Making Test, seconds | |||
| Part A | 62.5 (2.4) | 65.7 (3.0) | .36 |
| Part B | 165.4 (5.6) | 187.7 (7.0) | <.001 |
| Part B–A | 105.8 (4.8) | 124.7 (6.0) | <.001 |
| Hopkins Verbal Learning Test | |||
| Delayed recall | 5.8 (0.3) | 4.7 (0.3) | <.001 |
| Recognition | 11.6 (0.2) | 10.9 (0.2) | .006 |
Results obtained from generalized linear models adjusted for age, sex, race, educational level, body mass index, systolic blood pressure, and stroke.
Obtained from model comparing the two APOE groups adjusted for the same covariates. CO2=carbon dioxide.
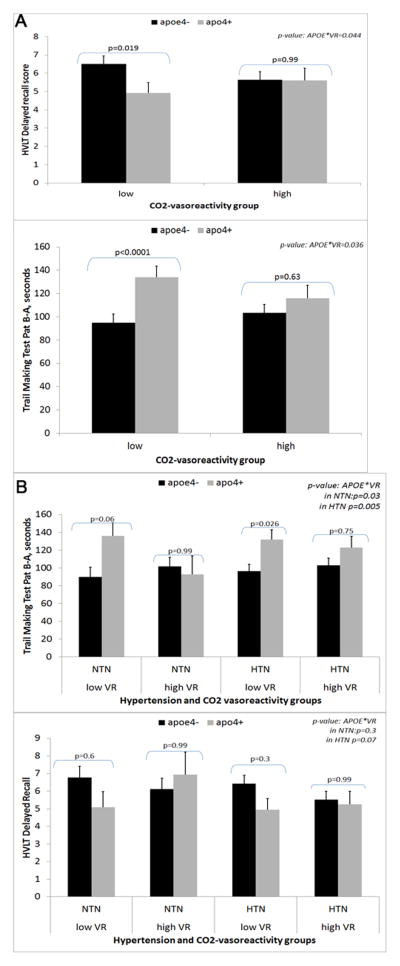
When the effect of CO2 vasoreactivity on the associations between APOE-ε4 and cognition was investigated, it was observed that participants with APOE-ε4 and lower (below the median of the sample) CO2 vasoreactivity had worse cognition (APOE by CO2 vasoreactivity: p=.04 for TMT Part B–A, p=.04 for HVLT delayed recall, after adjusting for covariates). In participants with higher CO2 vasoreactivity, APOE-ε4 had no significant association with cognitive performance (APOE effect: p=.63 for TMT Part B–A, p=.99 for HVLT delayed recall; Figure 1A).
Figure 1.
Cognitive scores in the two APOE-ε4 groups according to (A) carbon dioxide (CO2) vasoreactivity status (low and high) and (B) CO2 vasoreactivity (VR) and hypertension (HTN) status in the Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly of Boston Study. All p-values adjusted for multiple testing using simulation approach; scores (least square means) and p-values adjusted for age, sex, race, body mass index, stroke history, educational level, and systolic blood pressure. P-value for the interaction between APOE and CO2 vasoreactivity obtained from the parameter for APOE by CO2 vasoreactivity in the corresponding models. HVLT=Hopkins Verbal Learning Test; NTN=normotension.
APOE, hypertension, and CO2 vasoreactivity
Participants with hypertension and APOE-ε4 had poorer performance on executive function and memory tests (APOE by hypertension for TMT Part B–A and HVLT delayed recall, p <.001) and lower CO2 vasoreactivity (p=.01) than those with neither or either alone. There were no differences in BFV between the three groups. These results with pairwise comparisons are shown in Table 3.
Table 3.
Cognitive and Cerebral Hemodynamic Measures According to Apolipoprotein E (APOE)-ε4 and Hypertension Status in the Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly of Boston Study
| Measure | Normotension and APOE-ε4−, n=126 | Hypertension or APOE- ε4+, n=391 | Hypertension and APOE- ε4+, n=108 | APOE by Hypertension, P-Valueb |
|---|---|---|---|---|
| Least Square Means (Standard Error)a | ||||
| Trail-Making Test, seconds | ||||
| Part A | 59.9 (3.4)c | 62.9 (2.4)c | 66.2 (3.3)c | .03 |
| Part B | 156.6 (7.7) | 169.7 (5.7) | 185.4 (7.6) | <.001 |
| Part B–A | 98.5 (6.5) | 109.6 (4.8) | 122.1 (6.4) | <.001 |
| Hopkins Verbal Learning Test | ||||
| Delayed recall | 6.1 (0.4)c | 5.6 (0.3)c | 4.8 (0.4) | <.001 |
| Recognition | 11.8 (0.3)c | 11.4 (0.2)c | 11.0 (0.3) | .002 |
| Blood flow velocity, cm/s | 42.6 (1.6)c | 42.9 (1.3)c | 42.0 (1.7)c | .61 |
| CO2 vasoreactivity, cm/s per mmHg/partial pressure of CO2mmHg x10−2 | 1.06 (0.07)c | 1.09 (0.05)c | 0.97 (0.07) | .01 |
Adjusted for age, sex, race, body mass index, stroke, systolic blood pressure, and educational level.
Obtained from models adjusted for the same covariates with the interaction parameter (APOE by hypertension).
Not significantly different from each other (after adjusting for pairwise multiple testing).
When investigating the effect of CO2 vasoreactivity on the associations between APOE-ε4 and cognition according to hypertension status it was observed that, in individuals with hypertension, only those with low CO2 vasoreactivity demonstrated the negative association between APOE-ε4 and executive function (APOE effect in those with low CO2 vasoreactivity p =.03 vs high CO2 vasoreactivity p=.75, p=.005 for APOE by CO2 vasoreactivity; Figure 1B). There was no interaction between hypertension, APOE, and CO2 vasoreactivity with respect to the memory measure.
DISCUSSION
Older adults with APOE-ε4 may have lower CO2 vasoreactivity and poorer executive function and memory performance compared to those without. Concurrent hypertension and low CO2 vasoreactivity with the APOE-ε4 allele is linked to poor cognitive performance in older adults. Multiple neuropathological findings have been reported to be relevant to the APOE-ε4-related risk of AD and cognitive impairment, including low amyloid clearance and high aggregation, high tau phosphorylation, synaptic deficits, mitochondrial dysfunction, and neuroinflammation. (For a review, see 27.) Prior studies have also demonstrated that APOE-ε4 may be linked to disruption of BBB integrity and endothelial dysfunction.28, 29 APOE-ε4 is associated with low peripheral endothelial-mediated vasoreactivity.30 CO2 vasoreactivity is linked to low peripheral endothelial function and is low in individuals with AD and prodromal AD.14, 31 The results of the current study extend these observations to suggest that individuals at risk of developing AD by virtue of their APOE-ε4 allele have impaired CO2 vasoreactivity function. This may serve as an early vascular biomarker for the risk of cognitive decline related to APOE-ε4. To the knowledge of the authors of the current study, this is the first evidence that APOE-ε4 carriers may have lower CO2 vasoreactivity than noncarriers.
As in previous studies, we demonstrated that the combination of APOE-ε4 and hypertension is associated with worse cognitive performances. 7 We also found that this combination is associated with decreased CO2 vasoreactivity suggesting an increased impact on the cerebral microvasculature.
CO2 vasoreactivity may be contributing to the association between APOE and cognition. In particular, when CO2 vasoreactivity is not impaired, APOE-ε4 was not linked to lower cognitive performance. Stated differently, in this sample of older adults, CO2 vasoreactivity above the median was protective against the negative effect of APOE-ε4 on cognition. This observation is novel and supports the hypothesis that microvascular injury may be an important mechanism by which APOE leads to cognitive impairment, but because of the cross-sectional nature of this analysis, this observation does not imply causality. Nevertheless, it is possible to deduce a mechanistic role of CO2 vasoreactivity in associations between APOE and cognition because a genetic marker by definition precedes CO2 vasoreactivity and cognitive performance. Because other potential mechanisms, such as amyloid deposition, mitochondrial dysfunction, and inflammation, were not measured, this role of CO2 vasoreactivity needs to be confirmed in a more-comprehensive risk factor assessment study.
An association between APOE and resting BFV was not found. One possible explanation is that the effect of APOE-ε4 may be exerted on some but not all regions of the brain.32 It was not possible to assess regional variations in cerebral blood flow velocity using transcranial Doppler. Results of this study should be interpreted in the context of its limitations, which include the cross-sectional nature of the phenotypic measures (cognition and hemodynamics) and the small number of subjects with available CO2 vasoreactivity data. The latter limitation was accounted for by adjusting for factors associated with the lack of CO2 vasoreactivity data (age, education, sex, race). A difference in the APOE-ε4 allele distribution was not observed according to presence or absence of cerebral hemodynamic data.
CONCLUSION
Individuals at risk for AD because they have the APOE-ε4 allele have low CO2 vasoreactivity, and the effects of this allele on cognition and CO2 vasoreactivity may be greater when concurrently present with hypertension. Having higher CO2 vasoreactivity may offer a protective mechanism against cognitive impairment in individuals with the APOE-ε4 allele. This study provides in vivo evidence that APOE-ε4 carriers have cerebral microcirculatory dysfunction, which may play a role in its cognitive manifestations
Acknowledgments
IH was supported by Grants AG030057 and AG042127 from the National Institute on Aging. FAS was supported by AG030967 and NS085002 from the National Institutes of Health. MOBILIZE Boston was funded by a grant from the National Institute on Aging to LAL (AG004390 and AG025037).
Footnotes
Conflict of Interest: The authors disclose no conflict of interest.
Author Contributions: Hajjar: study concept, data analysis, interpretation, manuscript preparation and revisions. Sorond: study concept, data acquisition (for MOBILIZE Boston), data interpretation, critical revision of manuscript for important intellectual content. Lipsitz: study concept, data acquisition, data interpretation, critical revision of the manuscript for important intellectual content, study supervision
Sponsor’s role: None.
References
- 1.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RS, Schneider JA, Barnes LL, et al. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 3.Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiol Aging. 2011;32:63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Stoumpos S, Hamodrakas SJ, Anthopoulos PG, et al. The association between apolipoprotein E gene polymorphisms and essential hypertension: A meta-analysis of 45 studies including 13 940 cases and 16 364 controls. J Hum Hypertens. 2013;27:245–255. doi: 10.1038/jhh.2012.37. [DOI] [PubMed] [Google Scholar]
- 5.Paglieri C, Bisbocci D, Caserta M, et al. Hypertension and cognitive function. Clin Exp Hypertens. 2008;30:701–710. doi: 10.1080/10641960802563584. [DOI] [PubMed] [Google Scholar]
- 6.Qiu C, Winblad B, Fastbom J, et al. Combined effects of APOE genotype, blood pressure, and antihypertensive drug use on incident AD. Neurology. 2003;61:655–660. doi: 10.1212/wnl.61.5.655. [DOI] [PubMed] [Google Scholar]
- 7.Peila R, White LR, Petrovich H, et al. Joint effect of the APOE gene and midlife systolic blood pressure on late-life cognitive impairment: The Honolulu-Asia aging study. Stroke. 2001;32:2882–2889. doi: 10.1161/hs1201.100392. [DOI] [PubMed] [Google Scholar]
- 8.Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: From lipid transport to neurobiology. Prog Lipid Res. 2011;50:62–74. doi: 10.1016/j.plipres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddell DR, Zhou H, Atchison K, et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zlokovic BV. Cerebrovascular effects of apolipoprotein E: Implications for Alzheimer disease. JAMA Neurol. 2013;70:440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishitsuji K, Hosono T, Nakamura T, et al. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradac GB, Simon RS, Heidsieck CH. Angiographically verified transient alteration of the intracranial arteries and veins in dependence of different CO2 tensions. Neuroradiology. 1976;10:257–262. doi: 10.1007/BF00327574. [DOI] [PubMed] [Google Scholar]
- 14.Cantin S, Villien M, Moreaud O, et al. Impaired cerebral vasoreactivity to CO2 in Alzheimer’s disease using BOLD fMRI. Neuroimage. 2011;58:579–587. doi: 10.1016/j.neuroimage.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 15.Vicenzini E, Ricciardi MC, Altieri M, et al. Cerebrovascular reactivity in degenerative and vascular dementia: A transcranial Doppler study. Eur Neurol. 2007;58:84–89. doi: 10.1159/000103642. [DOI] [PubMed] [Google Scholar]
- 16.Hajjar I, Zhao P, Alsop D, et al. Hypertension and cerebral vasoreactivity: A continuous arterial spin labeling magnetic resonance imaging study. Hypertension. 2010;56:859–864. doi: 10.1161/HYPERTENSIONAHA.110.160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajjar I, Marmarelis V, Shin DC, et al. Assessment of cerebrovascular reactivity during resting state breathing and its correlation with cognitive function in hypertension. Cerebrovascular Dis. 2014;38:10–16. doi: 10.1159/000365349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: Design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon NG. The Trail Making Test in neuropsychological diagnosis. J Clin Psychol. 1972;28:167–169. doi: 10.1002/1097-4679(197204)28:2<167::aid-jclp2270280212>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the Trail Making Test. J Clin Psychol. 1987;43:402–409. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Sorond FA, Galica A, Serrador JM, et al. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston Study. Neurology. 2010;74:1627–1633. doi: 10.1212/WNL.0b013e3181df0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: Design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claassen JA, Zhang R, Fu Q, et al. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol. 2007;102:870–877. doi: 10.1152/japplphysiol.00906.2006. [DOI] [PubMed] [Google Scholar]
- 24.Rebeck GW, Reiter JS, Strickland DK, et al. Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 25.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43:913–928. [PubMed] [Google Scholar]
- 26.Graybill FA. An Introduction to linear Statistical Models. New York: McGraw-Hill; 1961. [Google Scholar]
- 27.Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer’s disease: An update. Annu Rev Neurosci. 2014;37:79–100. doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- 28.Hafezi-Moghadam A, Thomas KL, Wagner DD. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am J Physiol Cell Physiol. 2007;292:C1256–C1262. doi: 10.1152/ajpcell.00563.2005. [DOI] [PubMed] [Google Scholar]
- 29.Folin M, Baiguera S, Guidolin D, et al. Apolipoprotein-E modulates the cytotoxic effect of beta-amyloid on rat brain endothelium in an isoform-dependent specific manner. Int J Mol Med. 2006;17:821–826. [PubMed] [Google Scholar]
- 30.Guangda X, Linshuang Z, Jie H, et al. Apo e4 allele is associated with endothelium-dependent arterial dilation in women with type 2 diabetes. Diabetes Res Clin Pract. 2006;72:155–161. doi: 10.1016/j.diabres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Algotsson A, Nordberg A, Winblad B. Apolipoprotein E varepsilon4 allele has an impact on vascular reactivity in Alzheimer’s disease. Arch Gerontol Geriatr. 2000;31:221–231. doi: 10.1016/s0167-4943(00)00082-0. [DOI] [PubMed] [Google Scholar]
- 32.Kim SM, Kim MJ, Rhee HY, et al. Regional cerebral perfusion in patients with Alzheimer’s disease and mild cognitive impairment: Effect of APOE epsilon4 allele. Neuroradiology. 2013;55:25–34. doi: 10.1007/s00234-012-1077-x. [DOI] [PubMed] [Google Scholar]