Abstract
Chemical reactions with unsaturated phospholipids in the respiratory tract lining fluid have been identified as one of the first important steps in the mechanisms mediating environmental ozone toxicity. As a consequence of these reactions, complex mixtures of oxidized lipids are generated in the presence of mixtures of non-oxidized naturally occurring phospholipid molecular species, which challenge methods of analysis. Untargeted mass spectrometry and statistical methods were employed to approach these complex spectra. Human bronchoalveolar lavage (BAL) was exposed to low levels of ozone and samples, with and without derivatization of aldehydes, were analyzed by liquid chromatography electrospray ionization tandem mass spectrometry. Data processing was carried out using principal component analysis (PCA). Resulting PCA score plots indicated an ozone dose-dependent increase, with apparent separation between BAL samples exposed to 60 ppb ozone and non-exposed BAL samples, and a clear separation between ozonized samples before and after derivatization. Corresponding loadings plots revealed that more than 30 phosphatidylcholine (PC) species decreased due to ozonation. A total of 13 PC and 6 phosphatidylglycerol oxidation products were identified with the majority being structurally characterized as chain-shortened aldehyde products. This method exemplifies an approach for comprehensive detection of low abundance, yet important, components in complex lipid samples.
Keywords: pulmonary surfactant, phospholipids, oxidized lipids, mass spectrometry, lung, ozone, principal component analysis
Introduction
Ambient ozone is a common air pollutant produced from photochemical reactions between nitrogen oxides and volatile organic compounds that originate from both anthropogenic and natural sources. The lung toxic properties of ozone were recognized more than a century ago [1] and the detrimental health effects, mainly with regard to pulmonary function, are today well described [2]. Challenges remain to understand the complex biological response and the chemical mechanisms mediating these effects [3]. It is known that the toxicity of ozone in the lungs partly involves reaction with unsaturated fatty acids esterified to glycerophospholipids present in cell membranes of the lung airway cells and the lung surfactant. The lung surfactant is vital for maintaining airway patency but is also involved in the airways’ barrier and defense against inhaled toxic components [4–5]. Removal of unsaturated phospholipids by oxidation has been shown to affect the ability of the surfactant to decrease surface tension [6]. In addition, oxidation of these lipids leads to production of potentially harmful oxidative species, lipid-ozone reaction products. The mechanism of formation of these products starts with the chemical reaction of ozone with the fatty acid double bond leading to the formation of an ozonide. The ozonide decomposes into an aldehyde and an intermediate hydroxyl hydroperoxide product that further decomposes to a second aldehyde and hydrogen peroxide [7–8] (scheme provided as Supplemental Figure 1). In this context, lipid-ozone products such as 1-palmitoyl-2-(9′-oxo-nonanoyl)-sn-glycero-3-phosphocholine (PC 16:0/9:0al) and 1-hydroxy-1-hydroperoxynonane have been shown to act as signal transduction molecules inducing both pro-inflammatory cytokine and chemokine production [9–10].
Previous studies of ozone and surfactant lipids typically employed rather high concentration of ozone (200–2000 ppb) with the aim to detect major products [11–12]. The detection of oxidized phospholipids was usually based on predicted structures, often made with the help of previous studies of similar pure compounds [13] or aimed at isolating active compounds of interest and elucidating the structure using tandem mass spectrometry (MS/MS) [9]. In human lung surfactant, phosphatidylcholine (PC) is the dominating lipid class (about 80% of the total lipid) with saturated dipalmitoylphosphatidylcholine making up about 45% of the PC pool [14]. However, the diversity of surfactant lipids is significant. When different chain lengths and number of double bonds are taken into consideration, there are more than 20 documented unsaturated diacyl phospholipids in the lung surfactant counting the most abundant species of PC, phosphatidylglycerol (PG), phosphatidylethanolamine (PE) and phosphatidylinositol (PI) [15]. In addition, plasmanyl and plasmenyl species of PC and PE also exist [16]. An approach with the ability to detect small changes after exposure to relevant concentrations of ozone, similar to the average and peak concentrations observed in ambient air today, in such a complex biological sample is therefore attractive.
The use of mass spectrometry (MS) for comprehensive and untargeted lipid profiling in biological samples generates a large amount of data. In recent years, to simplify data processing, multivariate analysis techniques such as principal component analysis (PCA) and partial least squares of latent structures (PLS) have been used extensively in MS applications with the aim to find quantitative or qualitative differences between groups [17–18].
Here, we present a new approach to identify components for further structural studies using electrospray ionization MS/MS in combination with PCA for global and unbiased data processing. Several oxidized phospholipids in bronchoalveolar lavage (BAL) were identified from the mass spectral data of samples exposed to low concentrations of ozone.
Materials and Methods
Materials
1-palmitoyl(D31)-2-hydroxy-sn-glycero-3-phosphocholine were from Avanti Polar Lipids, Inc (Alabaster, AL, USA). Methoxylamine hydrochloride was from Acros organics (Geel, Belgium). Solvents (methanol, acetonitrile, methylene chloride) and ammonium acetate were from Fisher Scientific (Fair Lawn, NJ, USA).
Isolation and determination of total lavage phospholipids
Healthy, non-smoking volunteers, between 21–65 years of age, were recruited for bronchoalveolar lavage using a protocol reviewed and approved by the National Jewish Health Institutional Review Board. Lavage was performed with a bronchoscope wedged in a segment of the right middle lobe, and four-60 ml aliquots of sterile saline were instilled and sequentially recovered, with a recovery of approximately 70% of starting volume. The crude lavage fluid was centrifuged at 200 × g for 10 minutes to remove cells. The resultant supernatant was typically frozen at −20°C and subsequently processed for analysis of pulmonary surfactant phospholipids. The phospholipids were extracted from thawed lavage supernatants using a Bligh and Dyer procedure [19]. The total phospholipid content of the lipid extract was determined by measuring the inorganic phosphate produced after perchloric acid digestion of the sample [20]. The phospholipid concentration of the recovered lavage was 20 nmole/mL. The bronchoalveolar lavage was pooled for LC-MS analysis.
Ozonation of BAL
Ozone was generated from ambient air with an ozone calibrator source (Model 306, 2B technologies, Inc., Boulder, CO). Exposure of the pooled BAL sample to ozone was accomplished by bubbling the ozone flow, held at a concentration of approximately 60, 150 or 300 ppb, through 1 mL of BAL sample for 60 min. Outgoing ozone concentrations were measured using an ozone monitor (Model 202, 2B technologies, Inc.) before and after each exposure. Each 1 mL sample of BAL was added 0.14 μg (0.27 nmol) of 1-palmitoyl(D31)-2-hydroxy-sn-glycero-3-phosphocholine as internal standard before ozone exposure. After exposure, the BAL sample was immediately treated as described in the sample preparation section. The laboratory air concentration of ozone was 25–30 ppb during these experiments.
Sample preparation
Non-ozonized and ozonized BAL samples were either subject to direct lipid extraction or treated with methoxylamine prior to lipid extraction by adding 500 μL of 0.2 M methoxylamine to the BAL sample (samples exposed to 60, 150 or 300 ppb ozone). The samples were incubated in water bath overnight at 37°C. During this procedure the methoxylamine reacts with ketone or aldehyde groups present on the oxidized phospholipid and forms a methoxime (MOX) derivative [21]. Phospholipids in untreated and methoxylamine derivatized BAL samples were extracted using a modified Bligh and Dyer extraction [19] by adding 1.2 mL of methanol and 1.2 mL of dichloromethane. The sample was mixed thoroughly and centrifugated after which the dichloromethane phase was transferred to a glass test tube. The extraction was repeated with chloroform. The solution was mixed again and centrifugated. The organic phase with phospholipids was evaporated to dryness under N2 and resuspended in mobile phase A.
Electrospray ionization mass spectrometry
Reversed phase liquid chromatography (LC) and MS was performed on an AB Sciex API 3200 triple quadrupole mass spectrometer with an electrospray ionization source (AB Sciex, Concord, Canada). Chromatography was performed on a Shimadzu LC20-AD HPLC system equipped with a Gemini 5u C18 110A column (150×2.00 mm, 5 um, Phenomenex). For acquisition of full scan data, the gradient mobile phase was composed of A: 60/20/20 of methanol/acetonitrile/water v/v/v with 2 mM ammonium acetate and B: methanol with 2 mM ammonium acetate. The flow rate was 0.2 mL/min. Initial conditions was 40% A for 1 min, followed by a linear gradient from 40 to 100% B within 50 min, 100% B was then held for 5 min, followed by re-equilibration for 8 min. Each sample was injected in duplicate in order to improve the statistical analysis.
For untargeted analysis of lipids in BAL samples, mass spectra were acquired in full scan mode. Full scans were carried out in both positive and negative mode where a range of m/z 400–1000 was employed. The orifice was set at +58 and −50 V in positive and negative mode, respectively. Data acquisition was carried out by Analyst software 1.6.1.
Data processing and statistical analysis
Data extraction (peak finding and peak alignment) and PCA were performed on 14 separate LC-MS data sets using MarkerView software 1.2.1.1 (AB Sciex, Concord, Canada). The sample sets corresponded to 4 separate experiments with pooled BAL exposed to 0, 60, 150 and 300 ppb ozone before and after derivatization by methoximation, each of the eight samples run in duplicate, except for the sample not exposed to ozone. In order to obtain an adequate peak list (containing variables identified by m/z and retention time), parameters were optimized in a step-by-step manner. For acquired data, the final peak detection parameters were as follows: background subtraction offset for 10 scans, subtraction multiply factor of 1.3 and a minimum spectral peak width of 5 ppm. Minimum retention time peak width was 3 scans and noise threshold 100–20000 counts depending on type of scan. For alignment and filtering parameters, a retention time tolerance of 1.5 min and mass tolerance of 250 ppm were used. In this step, the option of using area integrated from raw data was employed. Ions present in only one sample were removed. Retention time correction and normalization were accomplished using internal standard and/or saturated PC species not affected by ozonation. For PCA, pareto scaling of data was applied. Pareto scaling is efficient for noisy data with large dynamic range and is the most commonly used approach in mass spectrometric applications [22]. The PCA analysis of these LC-MS data sets is presented in Supplemental Figure 2.
The feature principal component variable grouping (PCVG), available in the MarkerView software for grouping correlated variables, was applied to facilitate interpretation of loadings plots [22]. For PCVG, the first three principal components were used, the selected angle delta was 35 degrees and the minimum distance of origin was 0.015. A list of ions important for separation between untreated and samples derivatized with methoxylamine before and after ozonation was constructed based on score and loading plots from the PCA. Isotopes were removed manually.
LC tandem mass spectrometry and lipid identification
After tentative identification of the most important ions in the LC-MS data sets, a second set of experiments was carried out on a fresh aliquot of human surfactant in duplicate experiments, exposed to 0, 60, 150 and 300 ppb ozone for 60 min followed by reversed phase LC separation and tandem mass spectrometry (LC-MS/MS). Each sample was also derivatized by methoximation for a total of 16 LC-MS/MS analyses. Precursor ion scans for m/z 184 and neutral loss scans of 172 Da in positive mode were employed for identification of PC and PG, respectively [23]. LC conditions were the same as in previous analyses. Using PCA, a new list with ions important for the separation of untreated samples and samples derivatized by methoximation before and after ozonation was constructed for precursor ions scans of m/z 184. Neutral loss scans of 172 Da were examined manually. Structural characterization of listed ions was carried out on a separate set of samples exposed to 300 ppb ozone for 60 min before and after derivatization by collision-induced dissociation (CID) of the negative molecular ion species using an orifice potential of −80 V and collision energy of −45 V. In this way the fatty acyl groups in each targeted phospholipid could be identified [23]. Lipid species were annotated as previously recommended [24]. To this end, these separate exposure experiments served as a verification of results obtained in the PCA.
Results and discussion
Albeit protective antioxidants such as glutathione and ascorbic acid present in the respiratory tract lining fluid react rapidly with inhaled ozone [25], unsaturated surfactant phospholipids remain vulnerable targets leading to formation of lipid ozonation products. Identification of the various oxidation products originating from the ozonation of phospholipids is an important part of understanding the complex mechanisms for ozone toxicity in the airways. Previous studies have mostly focused on the effect of predicted structures originating from 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (PC 16:0_18:1) [9–10, 28]. Considering the complexity of human surfactant, additional products may be of importance. However, a comprehensive identification of oxidation products in a complex mixture like surfactant where unsaturated phospholipids are minor components can be challenging, particularly when exposing at low ozone concentrations reflecting the actual average or peak concentrations reported in ambient air today. Options for data analysis include both univariate (eg. ANOVA and student’s t-test) and multivariate methods. Univariate methods are designed for studies dealing with few independent variables and a larger set of samples. These methods are thus not optimal in MS studies where the conditions usually are the opposite and noise variables may be abundant. Multivariate statistical analysis, including both supervised (PLS-DA) and unsupervised (PCA) methods, reduces the data complexity and separates real signals from noise. Here, we used PCA to identify changes and lipid oxidation products originating from ozonation of surfactant phospholipids in BAL.
LC-MS analysis of ozonized surfactant
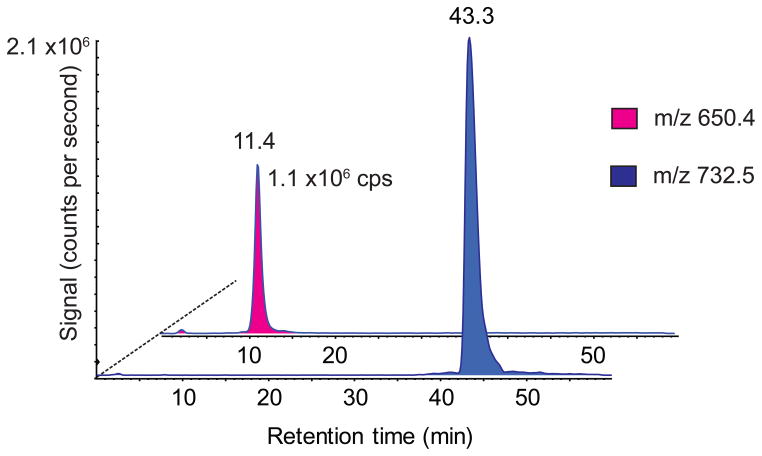
Human BAL was exposed to various concentrations of ozone for 60 minutes. LC-MS was performed to study changes between BAL samples before and after ozonation and with and without treatment with methoxylamine. Reversed phase chromatography separates according to lipophilicity and for separation of phospholipids, retention time depended on number of carbon atoms in the fatty acid chains and degree of unsaturation as well as presence of different functional groups on the fatty acids. As expected, diacyl phosphatidylcholine and phosphatidylglycerol species, known to be present in human surfactant, dominated the mass spectra of extracted lipids from BAL. Signals from late-eluting (> 40 min) phospholipids, such as the positive ion m/z 732 (Figure 1) derived from PC 16:1_16:0, decreased after ozonation, while new peaks appeared in the first half of the chromatogram, indicating presence of truncated and/or less lipophilic phospholipids. This is illustrated by the appearance of m/z 650 for PC 16:0_9:0al (Figure 1).
Figure 1.
Elution profiles of m/z 732 in an untreated (blue trace) and m/z 650 in an ozonized (red trace) BAL sample in positive mode. The ozonized sample was exposed to 300 ppb ozone for 60 min. then subjected to LC-MS analysis (positive electrospray ionization) and these ion traces extracted from the LC-MS data.
Principal component analysis of full scan data
PCA is a descriptive, unsupervised method used to reveal groupings, patterns and outliers in the data. It is a data reduction method where data sets with a large number of correlated variables are reduced to a few principal components that will account for most of the variance. Results are typically displayed in a score plot and a corresponding loadings plot. The loadings plot is directly compared to the score plot showing the contribution of all variables to the grouping or pattern displayed in the score plot. Initially, PCA was performed on full scan data from in total of 14 samples (BAL exposed to 0–300 ppb ozone), including duplicates. Separate PCA models were constructed for positive and negative full scan data, see Supplemental Figure 2 for resulting scores and loadings plot for full scan data in positive ion mode. In the score plot, the first principal component (PC1), explaining the largest variation in the data, separated untreated from ozonized BAL and the second principal component (PC2) separated samples before and after treatment with methoxylamine. Despite the low number of samples and small difference in the ozone dose, score plots indicated a clear separation, suggesting a dose-response relationship with ozone. A noticeable difference was indicated already after exposure to 60 ppb of ozone. The score plot for negative spectra had a similar pattern (Supplemental Figure 3). The patterns obtained in the PCA plots of these 16 LC-MS data sets were directly related to experimental conditions. No outliers or other unrelated patterns were detected, excluding the need for discriminant analysis to extract differentiating variables. The corresponding loadings plot for positive spectra confirmed that the intensity of known unsaturated phospholipids, like mono- and di-unsaturated species of PC (m/z 732 and 760) decreased after ozonation while the intensity from lower mass ions, such as m/z 650, increased. The strongest differentiating ions for ozonized BAL treated with methoxylamine were m/z 679, 651 and 707, all shifted +29 in mass compared to the strongest ions in ozonized BAL (m/z 650, 622 and 678). Collision induced dissociation of these ions yielded a strong m/z 184 product ion, characteristic for PC species. The corresponding loadings plot for negative spectra revealed that known unsaturated PGs and isobaric bis(monoacylglycero)phosphate (BMP) (m/z 747 and 773) were important discriminating ions. Important ions also included acetate adducts and [M−15]− ions of PC species (eg. m/z 790 and 716 in untreated BAL and m/z 808 and 634 in ozonized BAL).
PCA of LC-MS/MS Data and Identification of PC oxidation products in ozonized BAL
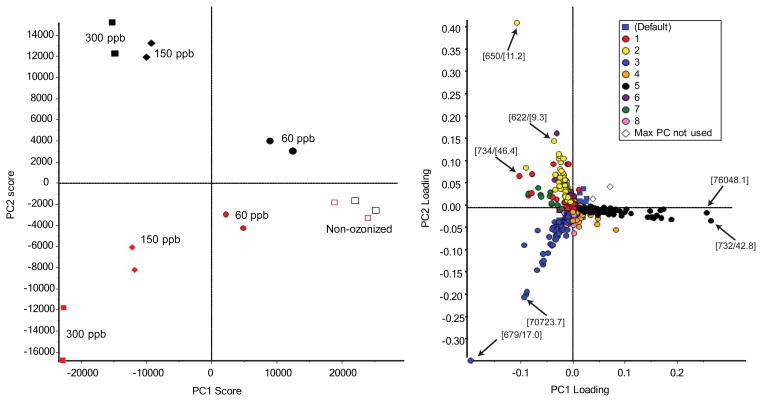
To get a detailed view of PC and for a more feasible detection of smaller components related to PC, new BAL samples were ozonized and analyzed by tandem mass spectrometry for precursors of m/z 184 in the positive ion mode. The experimental design was the same as in the previous experiment with the only difference being the addition of a non-ozonized BAL sample treated with methoxylamine. This sample was included in the data set in order to exclude the risk of potential components originating from methoxylamine that could be misinterpreted as oxidized products. PCA was repeated on the new data and in agreement with previous experiments, sample separation in PC1 suggested a dose-response relationship. Non-ozonized BAL samples with and without methoxylamine treatment clustered together, indicating that the methoxylamine itself did not contribute to the results, see Figure 2. Following PCA, PCVG was applied to enhance interpretation. PCVG identifies variables that display similar behavior across all samples and groups them [22]. Here, PCVG generated 10 groups, see Figure 2. The first and fourth group of variables, displayed as red and orange circles, respectively in the loading plot included variables that were in high abundance, including both m/z 706 and m/z 734. These ions corresponded to PC 16:0_14:0 and PC 16:0_16:0, respectively. These ions did not seem to be directly related to exposure, but rather have high variability due to unsatisfactory normalization, probably due to saturation of the signal, which was expected since these lipids were the most abundant in surfactant. Group 2 and possibly also group 6 (yellow and purple circles, respectively) included variables that were high in ozonized samples, while blue circles (group 3) encompassed variables that were high in ozonized samples treated with methoxylamine. Variables that had a high intensity in non-ozonized samples were represented as black circles (group 5). Here, using PCVG, interesting lipid molecular species, including low-abundance components, that correlated to the different treatments could be extracted and components with a high variation that were not directly related to ozonation and/or treatment with methoxylamine could be easily removed. Lipid molecular species in group 7 were high in ozonized samples both with and without methoxylamine treatment which would be expected for oxidized products not containing an aldehyde or a ketone and that had not been derivatized. A closer look revealed however that these components were in fact different co-eluting lipid constituents with overlapping isotope distribution, for example the monoisotopic mass of [M+H]+ of m/z 609 in samples treated with methoxylamine and the +1 isotope of [M+H]+ m/z 608 in ozonized samples.
Figure 2.
Principal component analysis of ozonized samples (duplicate samples, n=16) with and without derivatization analyzed by LC-MS/MS (precursors of m/z 184). A) Score plot for samples analyzed in full scan positive ion mode. White squares represent non-ozonized samples, circles represent samples exposed to 50 ppb ozone, diamonds 150 ppb and filled in squares 300 ppb, respectively. Symbols in red represent samples treated with methoxylamine. Here, the first principal component (PC1) separated the samples according to ozone exposure and the second principal component (PC2) separated the untreated samples and samples treated with methoxylamine. PC1 explained 65.8% and PC2 20.1% of the variation in the data. B) Loadings plot showing all variables explaining the separation of samples in the score plot. Principal component variable grouping (PCVG) groups the variables that display the same behavior across samples and was here based on the first 3 principal components, together explaining 91.4 % of the variation in the data. Labeled variables show the m/z and retention time.
All electrospray generated ions differing between samples (group 2, 3, 5, 6 and 7) were examined manually in spectra. After manual removal of isotopes, a total of 39 lipid molecular species were identified to decrease and 28 were found to increase after ozone exposure. A total of 23 lipid molecular species were increased in ozonized samples treated with methoxylamine compared to non-ozonized and ozonized samples (Supplementary Tables 3–5). Collision induced dissociation in the negative ion mode on [M−15]− ions of the most important PC species enabled characterization of the fatty acyl groups esterified to the PC head group, see Table 1 (non-ozonized) and Table 2 (ozonized) which contains several phosphatidylcholines not previously identified as ozonolysis products.
Table 1.
List of the most important identified unsaturated phosphatidylcholines (precursors of m/z 184) that decreased in intensity after exposure to ozone according to PCA loadings plot. A complete list of all m/z is included in supplemental data. MS/MS (product ion scan) was performed in the negative ion mode. Relative abundance (mean±standard error of the mean) is based on 9 measurements (3 separate experiments).
| [M+H]+ m/z | tR a | Relative abundance (%)b | [M−15]− m/z | CID of [M−15]− c | Molecular speciesd |
|---|---|---|---|---|---|
| 704 | 36.9 | 4.2±0.20 | 688 | 227 253 |
PC 14:0_16:1 |
| 730 | 38.4 | 5.5±0.18 | 714 | 227 251 253 255 279 |
PC 16:1_16:1 PC 14:0_18:2 PC 16:0_16:2 |
| 732 | 42.8 | 85±2.7 | 716 | 253 255 |
PC 16:0_16:1 |
| 746 | 45.7 | 7.1±0.19 | 730 | 241 253 255 267 269 281 |
PC 16:0_17:1 PC 15:0_18:1 PC 17:0_16:1 |
| 756 | 41.4 | 7.8±0.96 | 740 | 253 255 277 279 |
PC 16:1_18:2 PC 16:0_18:3 |
| 758 | 44.5 | 67±4.3 | 742 | 255 279 |
PC 16:0_18:2 |
| 760 | 48.1 | 100 | 744 | 255 281 |
PC 16:0_18:1 |
| 782 | 41.3 | 5.5±0.99 | 766 | 279 | PC 18:2_18:2 |
| 782 | 43.9 | 7.9±1.2 | 766 | 255 303 |
PC 16:0_20:4 |
| 784 | 45.3 | 19±2.7 | 768 | 255 279 281 305 |
PC 18:1_18:2 PC 16:0_20:3 |
| 786 | 49.8 | 28±2.7 | 770 | 255 279 281 283 307 |
PC 18:1_18:1 PC 18:0_18:2 PC 16:0_20:2 |
| 788 | 53.1 | 9.5±0.47 | 772 | 281 283 |
PC 18:0_18:1 |
Reversed phase retention time (min)
Relative abundance corresponding to the most abundant mass in the precursor ion scan of m/z 184
Product carboxylate ions
Molecular species suggested from tR, [M+H]+, [M−15]− and the carboxylate anions
Table 2.
Most abundant PC species unique for oxidized samples with and without treatment with methoxylamine based on the results from PCA. Shown here are also the product ions formed after collision induced dissociation (CID) of [M−15]− ions before and after methoxylamine derivatization and the tentative identification of oxidation products. Relative abundance (mean±standard error of the mean) is based on 9 measurements (3 separate experiments).
| [M+H]+ | tR a | Relative abundance (%)b | CID of [M−15]− c m/z | [M+H]+ d | CID of [M−15]− e | Molecular speciesh | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| m/zf | m/zg | ||||||
| 608i | 8.8 | 7.5±0.25 | 255 129 |
637 | 255 158 |
126 | PC 16:0_6:0al |
| 622 | 7.3 | 3.2±0.76 | 227 171 |
651 | 227 200 |
168 | PC 14:0_9:0al |
| 622i | 9.3 | 12±0.46 | 255 143 |
651 | 255 172 |
140 | PC 16:0_7:0al |
| 636 | 10.1 | 13±1.7 | 255 157 |
665 | 255 186 |
154 | PC 16:0_8:0al |
| 650 | 11.2 | 100 | 171 255 |
679 | 255 200 |
168 | PC 16:0_9:0al |
| 664 | 12.6 | 5.3±0.38 | 255 185 |
693 | 255 214 |
182 | PC 16:0_10:0al |
| 676 | 11.9 | 1.6±0.49 | 281 171 |
705 | 281 200 |
168 | PC 18:1_9:0al |
| 676 | 13.8 | 2.8±0.14 | 255 197 |
705 | 255 226 |
194 | PC 16:0_11:1al |
| 678 | 14.1 | 7.8±0.6 | 255 199 |
707 | 255 228 |
196 | PC 16:0_11:0al |
| 678 | 16.6 | 12 ±0.56 | 283 171 |
707 | 283 200 |
168 | PC 18:0_9:0al |
| 692 | 16.2 | 4.4±0.39 | 255 213 |
721 | 255 242 |
210 | PC 16:0_12:0al |
Reversed phase retention time (min)
Relative abundance corresponding to the most abundant mass in the precursor ion scan of m/z 184 after exposure to 300 ppb ozone
Product carboxylate ions before treatment with methoxylamine
MOX derivative
After treatment with methoxylamine
Product carboxylate ions
Product carboxylate ion -32
Molecular species suggested from tR, [M+H]+, [M−15]− and the carboxylate ions
Two isomers
The loadings plot in Figure 2 suggested that the most important ions for separation of the two groups with oxidized samples before and after treatment with methoxylamine were the [M+H]+ ion of m/z 650 eluting at 11.6 min and m/z 679, at 17.0 min, respectively. For the [M+H]+ ion at m/z 650, the CID spectrum of the corresponding [M−15]− ion at m/z 634 had abundant product ions at m/z 255 and m/z 171 which was consistent with the intact carboxylate ion of hexadecanoic acid (16:0) and a 9-carbon aldehyde, respectively. The most abundant ion at m/z 679 was consistent with the addition of 29 u to m/z 650 and the conversion of the terminal aldehyde to a methoxime. Accordingly, the CID spectrum of the corresponding negative [M−15]− ion at m/z 663 had product ions at m/z 255, 200 (9-MOX-nonanoate, m/z 171+29 u) and 168 (loss of 32 u, methanol from 9-MOX-nonanoate). These findings confirmed the presence of PC 16:0_9:0al as the most abundant product. This is the predicted product from ozonation of the three most abundant unsaturated phospholipids in human surfactant; PC 16:0_18:1, 1-palmitoyl-2-palmitoleoyl-sn-glycero-3-phosphocholine (PC 16:0_16:1) and 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PC 16:0_18:2), also important discriminators in the loadings plot for non-ozonized samples. As for untreated, oxidized BAL, exposure of oxidized BAL to methoxylamine resulted in several new products as identified in loadings plots deriving from PCA. In the CID spectrum of m/z 622 at least 2 different species were present, giving rise to product ion pairs at m/z 227 and 171 as well as m/z 255 and 143. The increasing retention times of the HPLC peaks were consistent with the increasing length of the saturated fatty acyl substituent, most likely at the sn-1 position. The same held true for m/z 676 and 678 that both had two isobaric species. As expected, the MOX derivatives, being more lipophilic, eluted later than their precursors. The conversion of terminal aldehydes to methoximes was observed for all oxidized products summarized in Table 2. Retention times and spectra were in line with the results obtained for PC 16:0_9:0al supporting the conclusion that these compounds also were chain-shortened aldehyde-PC. These reasonably abundant aldehydes presumably originate from 7 and 8 unsaturated fatty acids, such as dihomo-γ-linoleic (20:3), docosapentaenoic (22:5) or adrenic (22:4) fatty acids. A dihomo-γ-linoleic fatty acid could also be the precursor of the identified PC species containing a monounsaturated 11-carbon aldehyde. These fatty acids are however minor constituents in surfactant and thus, we cannot rule out possible complex chemistry of the more abundant 9 unsaturated fatty acids leading to the formation of these oxidation products. The unexpected finding of a 10-carbon aldehyde could be derived from such structural rearrangement. Two isobaric compounds (m/z 676) were found to contain non-ozonized double bonds. These products were however quickly further oxidized at 150 ppb, indicating that at low ozone concentrations different ozone products may be present although these differences were not visible in the score and loadings plots.
Using the PCVG approach that highlights all correlated variables across samples, low abundance oxidized products were easily retrieved from the data. Structural characterization of all these oxidation products was unfortunately not possible due to low abundance. The presence of corresponding MOX derivatives suggested that these also were chain-shortened aldehydes.
The two ions, m/z 780 and m/z 808, grouped together with other oxidation products (ie. their abundance was low in samples treated with methoxylamine and high in oxidized untreated samples) but seemed to lack a MOX derivative. A closer look at the relative abundance suggested that the pattern was different from other oxidation products with comparable intensity eg. m/z 580 and 608 that were absent after treatment with methoxylamine. The decrease after treatment could be due to stability of the molecule being compromised during the derivatization procedure. Detection of the [M−15]− ions in negative ion mode was not achieved due to low abundance and thus no information on fatty acyl chain composition was obtained. The observed [M+H]+ suggested that addition of oxygen to molecular species had occurred. CID in the positive ion mode produced product ions that correlated with previously published CID spectra of PC ozonides obtained by electrospray ionization tandem mass spectrometry [26]. The CID spectrum of the [M+H]+ ion of m/z 808 had product ions at m/z 184, 621, 650 and 666, which correlated to the 1,2,4-trioxolane structure (Criegee ozonide) being present at the 9-carbon at a 18-carbon chain (addition of three oxygen atoms (48 u) to PC 16:0_18:1, m/z 760) (Figure 3). The two HPLC peaks had no apparent differences in product ion spectra suggesting presence of cis and trans structures as observed previously [27].
Figure 3.
Analysis of a novel product observed in human surfactant after exposure to 300 ppb ozone for 60 min. (A) The elution profile of m/z 808.8 obtained in LC-MS/MS analysis revealed two closely eluting components at 27.2 and 28.2 min. (B) Collison induced decomposition of m/z 808 [M+H]+ from the component eluting at 28.2 min yielded an abundant product ion corresponding to phosphocholine (m/z 184) as well as product ions consistent with cleavage of an ozonide of 16:0_18:1-ozonide PC where the double bond is at carbon-9. The earlier eluting component had an identical product ion spectrum.
The other phospholipid (m/z 780) in the ozonized sample was also collisionally activated. The CID spectrum was similar to the CID spectrum of m/z 808, having abundant ions at m/z 184, 621, 650 and 666 correlating to the trioxolane group being located at the 9-carbon at a 16-carbon chain on a PC with palmitic acid presumably esterified at the sn-1 position (addition of 48 u to PC 16:0_16:1, m/z 732).
Identification of PG oxidation products in ozonized BAL
PG is less abundant than PC in human lung surfactant and has previously not been studied in lung surfactant exposed to low concentrations of ozone. The PG pool in human lung surfactant is largely monounsaturated and ozone exposure could therefore cause adverse effects simply by removing protective PG species [28]. In negative full scan spectra, multiple adducts of abundant PC species and PG species hampered interpretation and identification of PG oxidation products. By scanning for neutral loss of 172 Da, only PG species were identified. In contrast to the analysis in full scan mode where HPLC double peaks were identified to decrease after ozonation (e.g. m/z 747 and 773, see supplemental data), only single peaks were detected in chromatograms. Here, 7 PG species were identified to decrease after ozonation, see Table 3, where all of these lipid species have never been previously identified. Correspondingly, also the number of oxidation products was significantly lower than for PC species. Unlike PC, two species were shown to dominate. The most abundant ions were the [M+H]+ ions of 639 and 667, the expected aldehyde products of PG 16:0_16:1 and PG 18:0_18:1, respectively. The corresponding ions were identified in negative spectra as the [M−H]− ions of m/z 637 and 665. The CID spectra of these ions and corresponding MOX derivatives at m/z 666 and 694 respectively, confirmed the presence of terminal aldehydes and hence, the presence of palmitoyl-(9′-oxo-nonanoyl)-glycerophosphoglycerol (PG 16:0_9:0al) and stearoyl-(9′-oxo-nonanoyl)-glycerophosphoglycerol (PG 18:0_9:0al). Strong differentiators in the PCA analysis of full scan data were also m/z 669 and 697. These ions co-eluted with m/z 637 and 665, respectively and were consistent with the addition of 32 u and hence identified as hemiacetals formed with methanol in the mobile phase. Based on retention times and CID spectra, additional oxidation products were assigned aldehyde PG products; see Table 4. Previously, in model studies exposing PG 16:0/18:1 to high concentrations of ozone, hydroxyhydroperoxide and secondary ozonides products were observed by Kim et al [29]. These products were not observed here, possibly because of inherent instability.
Table 3.
List of identified novel PG species that changed in intensity after exposure to ozone. MS/MS (product ion scan) was performed in the negative ion mode. Relative abundance (mean±standard error of the mean) is based on 4 measurements (2 separate experiments).
| [M+H]+ m/z | tR a | Relative abundance (%)b | [M−H]− m/z | CID of [M−H]− c | Molecular speciesd |
|---|---|---|---|---|---|
| 721 | 35.4 | 14±1.2 | 719 | 253 255 |
PG 16:0_16:1 |
| 747 | 37.8 | 23±1.2 | 745 | 253 255 279 281 |
PG 16:0_18:2 PG 16:1_18:1 |
| 749 | 41.3 | 100 | 747 | 255 281 |
PG 16:0_18:1 |
| 773 | 38.5 | 7.9±0.44 | 771 | 279 281 |
PG 18:1_18:2 |
| 775 | 42.3 | 49±3.1 | 773 | 281 | PG 18:1_18:1 |
| 777 | 46.5 | 44±2.2 | 775 | 281 283 |
PG 18:0_18:1 |
Reversed phase retention time (min)
Relative abundance corresponding to the most abundant mass in the neutral loss scan of 172 Da
Product carboxylate ions
Molecular species suggested from tR, [M+H]+, [M−H]− and the carboxylate ions
Table 4.
Most abundant PG species unique for oxidized samples with and without treatment with methoxylamine based on the results from PCA. Shown here are also the product ions formed after collision induced dissociation (CID) of [M−H]− ions before and after derivatization with methoxylamine and the tentative identification of oxidation products. Relative abundance (mean±standard error of the mean) is based on 4 measurements (2 separate experiments).
| [M+H]+ | tR a | Relative abundance (%)b | [M−H]− | CID of [M−H]− c m/z | [M−H]− d | CID of [M−H]− e | Molecular speciesh | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| m/zf | m/zg | |||||||
| 625 | 7.4 | 16±1.9 | 623 | 157 255 |
652 | 255 186 |
154 | PG 16:0_8:0al |
| 639 | 8.2 | 100 | 637 | 255 171 |
666 | 255 200 |
168 | PG 16:0_9:0al |
| 653 | 10.2 | 15±0.88 | 651 | 157 171 269 283 |
680 | 186 200 269 283 |
154 168 |
PG 17:0_9:0al PG 18:0_8:0al |
| 665 | 9.4 | 5.2±0.87 | 663 | 171 281 |
692 | 281 200 |
168 | PG 18:1_9:0al |
| 667 | 12.4 | 88±4.7 | 665 | 171 283 |
694 | 283 200 |
168 | PG 18:0_9:0al |
Reversed phase retention time (min)
Relative abundance corresponding to the most abundant mass in the neutral loss scan of 172 Da after exposure to 300 ppb ozone.
Products carboxylate ions before treatment with methoxylamine
MOX derivative
After treatment with methoxylamine
Product carboxylate ions
Product carboxylate ion -32
Molecular species suggested from tR, [M+H]+, [M−H]− and the carboxylate ions
The biological effects of ozonized phospholipid products have mainly focused on PC 16:0_9:0al. In cell studies it has been known to possess proinflammatory properties [9, 30]. The biological activity of the new PC and PG oxidation products discovered here remains to be elucidated. Previous studies have shown that inflammatory effects are observed even at relatively low levels (80 ppb) of ozone (background levels of ozone in the troposphere normally vary between 20 and 40 ppb) highlighting the importance of documenting the oxidation products formed during these conditions [31]. The results presented here suggest that studies of biological effects of lipid oxidation products should be conducted at lower ozone concentrations and take the large diversity among lipid oxidation products into account.
Conclusion
This study demonstrated the utility of PCA in mass spectrometry for identification of unknown species in the analysis of complex mixtures of lipids, specifically oxidized phospholipids. PCA in combination with predictions based on known ozone chemistry provided a tool for a comprehensive discovery of oxidation products related to phospholipid ozonation at low exposure concentrations relevant to human exposure. Previously unknown ozonation products derivatized with methoxylamine could easily be extracted from the data and paired to non-derivatized ozonation products in order to strengthen identification. Several chain-shortened aldehyde oxidation products were found after ozonation of BAL, most of which have never been identified before including an unexpected 10-carbon aldehyde PC and an 11-carbon aldehyde PC. This method is a straightforward approach to detect important alterations in a complex lipid mixture when exposed to subtle changes of a reactive agent such as ozone.
Supplementary Material
Acknowledgments
This work was supported by The Swedish Research Council Formas (210-2011-1606) and a grant from the National Institutes of Health HL 34303. The authors acknowledge Ed D. Chan, MD, Dept Medicine, National Jewish Health for performing lavage and Pitchaimani Kandasamy, PhD, Dept Medicine, National Jewish Health for lavage processing and phosphate determination.
Abbreviations
- PCA
Principal component analysis
- BAL
Bronchoalveolar lavage
- PC
Phosphatidylcholine
- PG
Phosphatidylglycerol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schonbein CF. On some Secondary Physiological Effects produced by Atmospheric Electricity. Med Chir Trans. 1851;34:205–220. doi: 10.1177/095952875103400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mudway IS, Kelly FJ. Ozone and the lung: a sensitive issue. Mol Aspects Med. 2000;21:1–48. doi: 10.1016/s0098-2997(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hegelan M, Tighe RM, Castillo C, Hollingsworth JW. Ambient ozone and pulmonary innate immunity. Immunol Res. 2011;49:173–191. doi: 10.1007/s12026-010-8180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiba H, Piboonpocanun S, Mitsuzawa H, Kuronuma K, Murphy RC, Voelker DR. Pulmonary surfactant proteins and lipids as modulators of inflammation and innate immunity. Respirology. 2006;11(Suppl):S2–6. doi: 10.1111/j.1440-1843.2006.00797.x. [DOI] [PubMed] [Google Scholar]
- 5.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 6.Thompson KC, Jones SH, Rennie AR, King MD, Ward AD, Hughes BR, Lucas CO, Campbell RA, Hughes AV. Degradation and rearrangement of a lung surfactant lipid at the air-water interface during exposure to the pollutant gas ozone. Langmuir. 2013;29:4594–4602. doi: 10.1021/la304312y. [DOI] [PubMed] [Google Scholar]
- 7.Santrock J, Gorski RA, O’Gara JF. Products and mechanism of the reaction of ozone with phospholipids in unilamellar phospholipid vesicles. Chem Res Toxicol. 1992;5:134–141. doi: 10.1021/tx00025a023. [DOI] [PubMed] [Google Scholar]
- 8.Pryor WA, Squadrito GL, Friedman M. The cascade mechanism to explain ozone toxicity: the role of lipid ozonation products. Free Radic Biol Med. 1995;19:935–941. doi: 10.1016/0891-5849(95)02033-7. [DOI] [PubMed] [Google Scholar]
- 9.Uhlson C, Harrison K, Allen CB, Ahmad S, White CW, Murphy RC. Oxidized phospholipids derived from ozone-treated lung surfactant extract reduce macrophage and epithelial cell viability. Chem Res Toxicol. 2002;15:896–906. doi: 10.1021/tx010183i. [DOI] [PubMed] [Google Scholar]
- 10.Kafoury RM, Hernandez JM, Lasky JA, Toscano WA, Jr, Friedman M. Activation of transcription factor IL-6 (NF-IL-6) and nuclear factor-kappaB (NF-kappaB) by lipid ozonation products is crucial to interleukin-8 gene expression in human airway epithelial cells. Environ Toxicol. 2007;22:159–168. doi: 10.1002/tox.20246. [DOI] [PubMed] [Google Scholar]
- 11.Pryor WA, Das B, Church DF. The ozonation of unsaturated fatty acids: aldehydes and hydrogen peroxide as products and possible mediators of ozone toxicity. Chem Res Toxicol. 1991;4:341–348. doi: 10.1021/tx00021a014. [DOI] [PubMed] [Google Scholar]
- 12.Frampton MW, Pryor WA, Cueto R, Cox C, Morrow PE, Utell MJ. Ozone exposure increases aldehydes in epithelial lining fluid in human lung. Am J Respir Crit Care Med. 1999;159:1134–1137. doi: 10.1164/ajrccm.159.4.9807057. [DOI] [PubMed] [Google Scholar]
- 13.Adachi J, Yoshioka N, Funae R, Nushida H, Asano M, Ueno Y. Determination of phosphatidylcholine monohydroperoxides using quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;806:41–46. doi: 10.1016/j.jchromb.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Shelley SA, Balis JU, Paciga JE, Espinoza CG, Richman AV. Biochemical composition of adult human lung surfactant. Lung. 1982;160:195–206. doi: 10.1007/BF02719293. [DOI] [PubMed] [Google Scholar]
- 15.Postle AD, Heeley EL, Wilton DC. A comparison of the molecular species compositions of mammalian lung surfactant phospholipids. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:65–73. doi: 10.1016/s1095-6433(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 16.Wynalda KM, Murphy RC. Low-concentration ozone reacts with plasmalogen glycerophosphoethanolamine lipids in lung surfactant. Chem Res Toxicol. 2010;23:108–117. doi: 10.1021/tx900306p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan EL, Pettine SM, Hickey MS, Hamilton KL, Miller BF. Lipidomic analysis of human plasma reveals ether-linked lipids that are elevated in morbidly obese humans compared to lean. Diabetol Metab Syndr. 2013;5:24. doi: 10.1186/1758-5996-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo BC, Kong SY, Jang SG, Kim KH, Ahn SA, Park WS, Park S, Yun T, Eom HS. Identification of hypoxanthine as a urine marker for non-Hodgkin lymphoma by low-mass-ion profiling. BMC Cancer. 2010;10:55. doi: 10.1186/1471-2407-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 20.Rouser G, Siakotos AN, Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966;1:85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- 21.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 22.Ivosev G, Burton L, Bonner R. Dimensionality reduction and visualization in principal component analysis. Anal Chem. 2008;80:4933–4944. doi: 10.1021/ac800110w. [DOI] [PubMed] [Google Scholar]
- 23.Murphy RC, Axelsen PH. Mass spectrometric analysis of long-chain lipids. Mass Spectrom Rev. 2011;30:579–599. doi: 10.1002/mas.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebisch G, Vizcaino JA, Kofeler H, Trotzmuller M, Griffiths WJ, Schmitz G, Spener F, Wakelam MJ. Shorthand notation for lipid structures derived from mass spectrometry. J Lipid Res. 2013;54:1523–1530. doi: 10.1194/jlr.M033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behndig AF, Blomberg A, Helleday R, Duggan ST, Kelly FJ, Mudway IS. Antioxidant responses to acute ozone challenge in the healthy human airway. Inhal Toxicol. 2009;21:933–942. doi: 10.1080/08958370802603789. [DOI] [PubMed] [Google Scholar]
- 26.Harrison KA, Murphy RC. Direct mass spectrometric analysis of ozonides: application to unsaturated glycerophosphocholine lipids. Anal Chem. 1996;68:3224–3230. doi: 10.1021/ac960302c. [DOI] [PubMed] [Google Scholar]
- 27.Pryor WA, Wu M. Ozonation of methyl oleate in hexane, in a thin film, in SDS micelles, and in distearoylphosphatidylcholine liposomes: yields and properties of the Criegee ozonide. Chem Res Toxicol. 1992;5:505–511. doi: 10.1021/tx00028a008. [DOI] [PubMed] [Google Scholar]
- 28.Numata M, Kandasamy P, Voelker DR. Anionic pulmonary surfactant lipid regulation of innate immunity. Expert Rev Respir Med. 2012;6:243–246. doi: 10.1586/ers.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HI, Kim H, Shin YS, Beegle LW, Goddard WA, Heath JR, Kanik I, Beauchamp JL. Time resolved studies of interfacial reactions of ozone with pulmonary phospholipid surfactants using field induced droplet ionization mass spectrometry. J Phys Chem B. 2010;114:9496–9503. doi: 10.1021/jp102332g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Induction of inflammatory mediators in human airway epithelial cells by lipid ozonation products. Am J Respir Crit Care Med. 1999;160:1934–1942. doi: 10.1164/ajrccm.160.6.9902025. [DOI] [PubMed] [Google Scholar]
- 31.Alexis NE, Lay JC, Hazucha M, Harris B, Hernandez ML, Bromberg PA, Kehrl H, Diaz-Sanchez D, Kim C, Devlin RB, Peden DB. Low-level ozone exposure induces airways inflammation and modifies cell surface phenotypes in healthy humans. Inhal Toxicol. 2010;22:593–600. doi: 10.3109/08958371003596587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.