Abstract
BACKGROUND
Optimal resuscitation of hypotensive trauma patients has not been defined. This trial was performed to assess the feasibility and safety of controlled resuscitation (CR) versus standard resuscitation (SR) in hypotensive trauma patients.
METHODS
Patients were enrolled and randomized in the out-of-hospital setting. 19 EMS systems in the Resuscitation Outcome Consortium participated. Eligible patients had an out-of-hospital systolic blood pressure (SBP) ≤ 90 mmHg. CR patients received 250 cc of fluid if they had no radial pulse or a SBP < 70 mmHg and additional 250 cc boluses to maintain a radial pulse or a SBP ≥ 70 mmHg. SR group patients received 2 liters initially and additional fluid as needed to maintain a SBP ≥ 110 mmHg. The crystalloid protocol was maintained until hemorrhage control or 2 hours after hospital arrival.
RESULTS
192 patients were randomized (97 CR and 95 SR). The CR and SR groups were similar at baseline. Average crystalloid volume administered during the study period was 1.0 liter (SD 1.5) in the CR group and 2.0 liters (SD 1.4) in the SR group, a difference of 1.0 liter (95% CI: 0.6 to 1.4). ICU-free days, ventilator-free days, renal injury and renal failure did not differ between groups. At 24 hours after admission, there were 5 deaths (5%) in the CR group and 14 (15%) in the SR group (adjusted odds ratio 0.39 [95% CI: 0.12, 1.26]). Among patients with blunt trauma, 24-hour mortality was 3% (CR) and 18% (SR) with an adjusted OR of 0.17 (0.03, 0.92). There was no difference among patients with penetrating trauma: 9% vs 9%, adjusted OR 1.93 (0.19, 19.17).
CONCLUSION
Controlled resuscitation is achievable in out-of-hospital and hospital settings and may offer an early survival advantage in blunt trauma. A large-scale, Phase III trial to examine its effects on survival and other clinical outcomes is warranted.
Keywords: Controlled resuscitation, hypotension, hemorrhage control
BACKGROUND
Trauma is the leading cause of death for persons between the ages of 1 and 44 years.1 Approximately 80% of trauma deaths are from central nervous system injury, hemorrhage or a combination of the two.2 Over 50% of trauma deaths occur in the first 12 hours after injury, and the most common cause of preventable death after trauma is exsanguinating hemorrhage.3 In addition to control of the airway and treatment of breathing related problems, the primary out-of-hospital treatments of hemorrhage are to stop bleeding from external sources and restore perfusion with fluid resuscitation. Methods of controlling cavitary, non-compressible hemorrhage do not currently exist. Field management is limited to volume resuscitation strategies and rapid transport to a trauma center.
The traditional treatment of trauma patients is aggressive fluid resuscitation to restore effective circulating volume and systolic blood pressure (SBP)4–7 as shock and tissue hypoperfusion are strong independent risk factors for poor outcomes in trauma patients.8–12 This approach to resuscitation is based on the controlled hemorrhage animal models popularized by Wiggers13 and Shires.14 These studies conclude that a large quantity of crystalloid and blood is required to replace intravascular and extravascular fluid losses. These concepts are endorsed by the American College of Surgeons Advanced Trauma Life Support course. The current guideline is to initiate resuscitation of trauma patients with 1–2 liters of crystalloid.15,16
Over the last two decades, the practice of aggressively resuscitating hemorrhagic shock utilizing crystalloid has been reexamined. Data from both animal15–17 and human3–5 studies show that massive intravenous fluid therapy prior to hemorrhage control leads to increased bleeding; this was recognized as early as 1918 by Walter Cannon and coworkers.18 There is also increasing evidence that early aggressive resuscitation of hemorrhagic shock with predominately saline-based regimens may be associated with cardiac dysfunction, abdominal compartment syndrome, harmful inflammation, acute respiratory distress syndrome, multiple organ dysfunction syndrome and increased mortality.19–25
High chloride containing crystalloid solutions infused at room temperature may also contribute to the lethal triad of hypothermia, coagulopathy and acidosis.26 Recent studies have identified significant early coagulopathy as a primary response to injury in 25% of severely injured civilian trauma patients on arrival to the hospital independent of fluid resuscitation and hypothermia.27–29 Severe tissue injury and shock causes activation of coagulation, producing a consumptive coagulopathy and exhaustion of the hemostatic system.27 Furthermore, hyperfibrinolysis (most likely caused by release of tissue plasminogen activator from damaged tissue) may lead to subsequent disruption of newly formed clots exacerbating hemorrhage. This leads to tissue hypoperfusion, resulting in the release of activated protein C into the systemic circulation contributing to the acute traumatic coagulopathy (ATC).30 The identification of the ATC magnifies the importance of identifying fluid regimens that minimize further exacerbation of coagulopathy.
This out-of-hospital, prospective, randomized pilot trial was performed to assess the feasibility and safety of controlled resuscitation (CR) for the early resuscitation of patients with traumatic shock due to blunt or penetrating mechanisms. We hypothesized that controlled resuscitation would result in a significant reduction in the quantity of early fluid administration without an increase in morbidity or mortality. This study was not powered to determine the superiority of one therapy compared to the other.
METHODS
The study was conducted by the Resuscitation Outcomes Consortium (ROC), a collaboration of 10 regional sites in the United States and Canada and a Data Coordinating Center. ROC is charged with the task of conducting clinical trials in patients with life threatening trauma and cardiac arrest. It has existed for 10 years and has a long history of successfully performing both out-of-hospital and in-hospital trauma trials.31,32 Patients were enrolled and randomized in the out-of-hospital setting. Nineteen Emergency Medical Service systems and 10 hospitals in 6 regions of the ROC participated. These EMS agencies were selected for their ability to perform out-of-hospital clinical trials and had previously participated in the ROC hypertonic saline studies. Advanced life support personnel enrolled patients and the study protocol was incorporated into the standing orders of all participating agencies. Eligible trauma cases were tracked through an active surveillance effort. Regular feedback was provided to EMS agencies regarding missed opportunities and training included both didactic and practical components.
The study was performed utilizing exception from informed consent required for emergency research as outlined in regulation 21CFR50.24 of the Food and Drug Administration (FDA) and article 2.8 of the Canadian Tri-Council Agreement. All fluids were licensed products approved by the FDA, but whose use in clinical research mandates the trial’s conduct under an Investigational New Drug application as well as a Clinical Trial Application with Health Canada. The protocol was approved by the institutional review board at each institution. As soon as it was feasible, an attempt was made to notify the patient or legal representative of enrollment and allow an opportunity to withdraw from the study or obtain consent for continued participation. The study was registered with ClinicalTrials.gov (NCT01411852) and monitored by an independent Data and Safety Monitoring Board.
Patients were eligible for the study if they suffered blunt or penetrating trauma, were ≥ 15 years old or were estimated to weigh ≥ 50 kg if their age was unknown, had an out-of-hospital SBP ≤ 90 mmHg and absence of evidence of a severe head injury or a Glasgow Coma Scale score > 8. Evidence of a severe head injury included an obvious skull fracture, unequal pupils, non-reactive pupils, history of seizure, otorrhea or rhinorrhea, Battle’s sign or raccoon’s eyes. Exclusion criteria included receipt of > 250 cc of fluid prior to randomization, out-of-hospital cardiopulmonary resuscitation by EMS, drowning or asphyxia due to hanging, burns > 20% total body surface area, time of call received at dispatch to study intervention > 4 hours, prisoner status and evidence of pregnancy. Due to concerns about early high enrollment of minimally injured patients, ground level falls were added as an exclusion criterion during the study. Bilateral paralysis was also added as an exclusion criterion due to concerns about exacerbating spinal cord injury in the CR group.
Participating transport vehicles carried pre-randomized, sealed, numbered, non-transparent containers of fluid. The containers either housed a 1000 cc bag of normal saline (randomized to the SR) or two 250 cc bags of normal saline and a 500 cc bottle of water (randomized to the CR group). The bottle was added so that EMS personnel could not determine the randomization due to weight differences prior to opening the container. After confirming patients met criteria for entry into the study, the container was opened. Once the container was opened, the patient was considered to be randomized. The study fluid had to be initiated in the out-of-hospital setting for the patient to be considered an eligible enrollment.
Patients in the SR group received 2 liters of fluid as an initial bolus. Following the initial bolus, additional fluid was given as needed to maintain a SBP of 110 mmHg for the duration of the study period, which extended from out-of-hospital enrollment until two hours into the hospital stay or until hemorrhage control was achieved, whichever occurred first. Patients in the CR group received a 250 cc bolus of fluid only if their SBP was < 70mmHg or they had no palpable radial pulse. These patients received additional 250 cc boluses to maintain a SBP of 70 mmHg or a palpable pulse as needed. If the patient had a SBP ≥ 70 mmHg and/or a palpable pulse, fluid was administered only to keep the vein open. Hemorrhage control was defined as ligation of a bleeding vessel, successful embolization of a bleeding vessel, removal of an injured solid organ or successful packing of a bleeding site. Blood products could be administered to both groups at any point during the protocol at the discretion of the provider.
In an attempt to minimize variability in the subsequent care of the study patients, all sites agreed to encourage the implementation of trauma resuscitation and critical care guidelines, which are supported by evidence based medicine. These were adapted from protocols already developed by the NIH funded multi-center Glue Grant (www.gluegrant.org).
The primary feasibility endpoint was early crystalloid volume (ECV) defined as crystalloid infused from EMS arrival at the scene until the end of the study period. Crystalloid infused during this period was included in ECV regardless of whether the protocol algorithm was followed as designed. The treatment difference in mean ECV was calculated, and its 95% confidence interval was computed allowing for unequal variances in the two arms. The primary safety endpoint, 24-hour mortality, was evaluated using a test for difference in proportions. The primary analyses were conducted on all randomized patients utilizing the concept of intent-to-treat except for one patient who was enrolled while in police custody. One patient who withdrew from further medical records review approximately two hours after ED admission was included and was assumed to have survived beyond 24 hours. This patient’s ECV was recorded as all crystalloid infused up until the time of withdrawal.
A sample size with more than adequate power to detect meaningful differences in the feasibility endpoint was selected in order to obtain a better assessment of the safety of the CR protocol. The planned sample size of 200 patients, 100 in each treatment group, had power greater than 99% to detect a true difference in ECV between the groups of 50% assuming a SR group mean of 4.6 L and a standard deviation of 3.2 L, but little power to detect all but large differences in survival (e.g. 80% power for a difference between 88.2% and 98.9%). The trial was stopped at 192 patients because of a sharp decline in enrollment when several sites switched their participation to a new ROC trial.
Secondary analyses of the primary endpoints were conducted on the two pre-specified subgroups with adequate sample sizes: patients who had experienced blunt trauma and patients who had experienced penetrating trauma. Results are also presented for five other subgroups of interest: patients with Injury Severity Score (ISS) > 15, eligible patients, eligible patients with blunt trauma, eligible patients with penetrating trauma, and eligible patients with ISS > 15.
Additionally, adjusted treatment comparisons were made using the full analysis population and all subgroups noted above. The adjusted treatment difference in mean ECV was estimated using linear regression analysis including the following pre-randomization variables: regional site (6 categories), age (linear spline with knot at 45 years), penetrating mechanism (yes/no), and ISS (linear). The adjusted odds ratio comparing 24-hour mortality for the two treatments was estimated using logistic regression including the same variables as the ECV model except regional site, since three sites had no deaths within 24 hours. ISS was multiply imputed for one ineligible SR group patient with blunt trauma, who suffered early death, using linear regression with all analysis model variables in the imputation model. Results from the multiply imputed datasets were combined using Rubin’s method.33
Additional secondary outcomes include 24-hour fluid volumes, in-hospital mortality, admission vital signs and blood chemistries, admission hematologic assays, renal performance as measured by the RIFLE classification34, ICU-free days, ventilator-free days, out of hospital days and protocol violations. Six patients, 4 in the SR group and 2 in the CR group, were excluded from outcomes measured beyond 24 hours due to withdrawal of consent (3) or being taken into police custody (3). Treatment arm differences (means for continuous measures and proportions for binary measures) and their 95% confidence intervals are presented for each outcome. Unequal variances are allowed for continuous measures. Analyses were conducted using R v3.0135 and the R packages mice v2.16 and mitools v2.2.
RESULTS
Between March 2012 and April 2013, 295 patients evaluated by participating EMS agencies were eligible for the study. One-hundred ninety two patients were randomized, 95 to the SR group and 97 to the CR group. Thirty-three ineligible patients were enrolled (17%). The fluid intervention was discontinued prematurely in 36 patients in the SR group and in 25 patients in the CR group. Circumstances for failure to enroll, ineligible enrollments and premature discontinuation of the study protocol are summarized in Figure 1. The most common reason for failure to enroll patients was EMS personnel not thinking about the study. The majority of patients who were inappropriately enrolled met other entry criteria but had no recorded out-of-hospital SBP ≤ 90 mmHg. Approximately two-thirds of enrollment-related protocol deviations occurred either within the first month of the enrolling agency’s participation or before the enrolling agency had enrolled 5 patients. The rate of enrolling ineligibles among agencies that had been participating for at least a month and had enrolled at least 5 patients was 12 out of 127 (9%).
FIGURE 1.
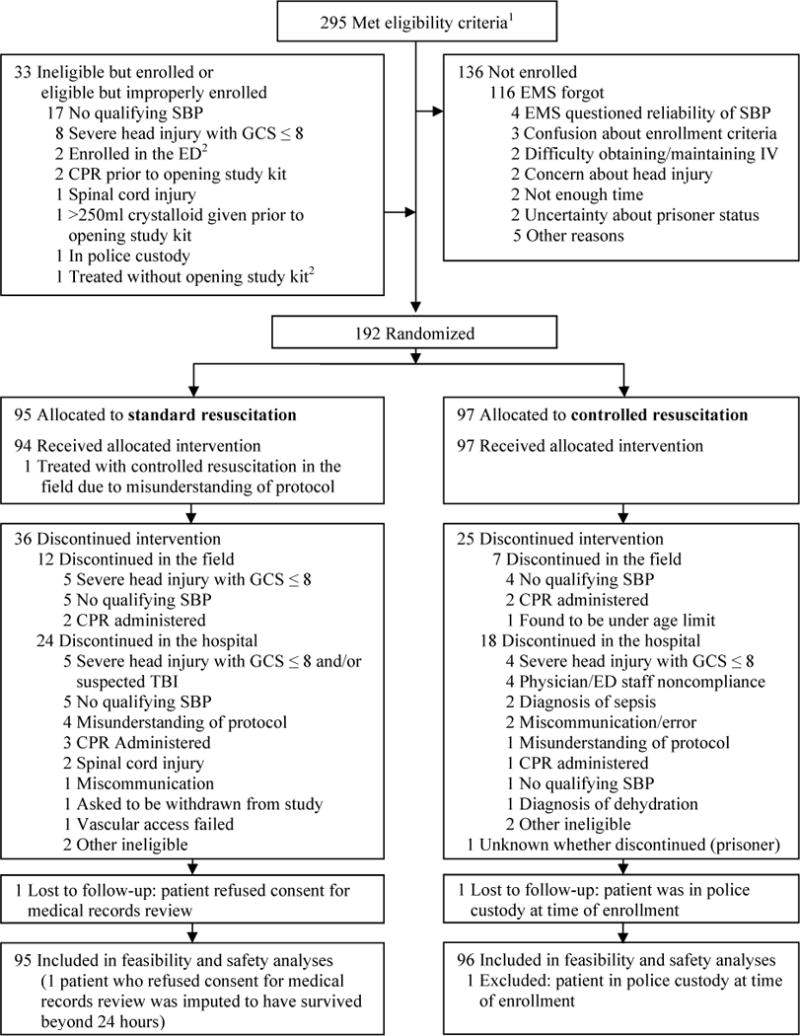
Patient flow diagram. Abbreviations: CPR, cardiopulmonary resuscitation; ED, emergency department; EMS, Emergency Medical Services; GCS, Glasgow Coma Scale; SBP, systolic blood pressure; TBI, traumatic brain injury. 1Excludes 3 patients who were at one point eligible but were improperly enrolled. 2Eligible but improperly enrolled.
As shown in Table 1, the SR and CR groups were similar at baseline with respect to age, gender, mechanism of injury, injury severity, physiology and transport characteristics. The mean transport time was < 15 minutes and the mean total out-of-hospital time was < 45 minutes in both groups. The qualifying SBP was lower in the CR group. During the study period, patients randomized to the SR group received on average 2.0 liters (L) (SD 1.4) of ECV and patients randomized to the CR group received 1.0 L (SD 1.5), a difference of 1.0 L (95% CI: 0.6 – 1.4). Similar differences were observed in the adjusted and subgroup analyses for all but one subgroup, patients with ISS > 15 (Table 2). Boxplots of ECV by treatment arm for all patients, eligible patients, patients with ISS > 15 and eligible patients with ISS > 15 are presented in Figure 2. The smaller difference in ECV in the ISS > 15 subgroup compared to the eligible & ISS > 15 subgroup was driven in part by two ineligible patients who received 5 and 7 liters of ECV. In general, ineligible patients were identified early and the randomized study algorithm was stopped in favor of local resuscitation practices. For example, the two previously noted ineligible patients received 0 and 0.35 liters of ECV at the time that they were identified as ineligible and the study algorithm was stopped. There was a notable difference in the number of patients found to be ineligible in the SR group (n = 24, 25%) compared to the CR group (n = 9, 9%), which can be explained, in part, by an error in study kit sequencing by the distribution center to one site (the kits were mistakenly sequenced by treatment arm rather than study ID number, thus at that site the higher proportion of enrollment errors occurring early in the conduct of the study took place mainly for patients assigned to the SR group). The most frequent problem with compliance with the assigned resuscitation procedure was failure to complete the initial 2 liter bolus after patients arrived to the hospital in the SR group.
TABLE 1.
Demographic, Injury, and Out-of-Hospital Care Characteristics
| Characteristic | n | Standard Resuscitation | n | Controlled Resuscitation1 | P value2 |
|---|---|---|---|---|---|
| Age, mean (SD), y | 95 | 41.8 (19.2) | 96 | 41.9 (20.2) | 0.96 |
|
| |||||
| Male sex, No. (%) | 95 | 74 (77.9) | 96 | 72 (75.0) | 0.64 |
|
| |||||
| Penetrating trauma, No. (%) | 95 | 33 (34.7) | 96 | 32 (33.3) | 0.84 |
|
| |||||
| ISS, median (IQR) | 94 | 9 (2–24.2) | 96 | 9.5 (1.8–19.2) | 0.77 |
| >15, No. (%) | 94 | 32 (34.0) | 96 | 33 (34.4) | 0.96 |
|
| |||||
| Initial SBP, median (IQR), mmHg | 95 | 84 (74.5–94) | 96 | 81.5 (72–92) | 0.25 |
|
| |||||
| Initial GCS, mean (SD) | 94 | 12.4 (4.1) | 95 | 13.0 (3.6) | 0.25 |
|
| |||||
| Initial HR, mean (SD), b/min | 92 | 91.9 (24.2) | 96 | 94.7 (22.9) | 0.41 |
|
| |||||
| RTS, mean (SD) | 93 | 6.4 (1.7) | 94 | 6.5 (1.4) | 0.64 |
|
| |||||
| Qualifying SBP3, median (IQR) | 95 | 82 (72–89) | 96 | 78 (69.8–85) | 0.03 |
|
| |||||
| Advanced airway, No. (%) | 95 | 12 (12.6) | 96 | 7 (7.3) | 0.22 |
|
| |||||
| Time from dispatch call to first IV/IO, mean (SD), min | 93 | 22.1 (9.9) | 96 | 21.0 (8.7) | 0.41 |
|
| |||||
| Post-randomization measures | |||||
| Transport time, mean (SD), min | 93 | 13.5 (6.8) | 95 | 14.6 (8.1) | 0.30 |
|
| |||||
| Total out-of-hospital time, mean (SD), min | 94 | 41.0 (13.8) | 96 | 42.7 (16.5) | 0.44 |
|
| |||||
| Air transport, No. (%) | 94 | 6 (6.4) | 96 | 5 (5.2) | 0.73 |
Abbreviations: GCS, Glasgow Coma Scale; HR, heart rate; IQR, interquartile range; ISS, Injury Severity Score; IV/IO, intravenous/intraosseous access; RTS, Revised Trauma Score; SBP, systolic blood pressure.
Data not available for one patient who was in police custody prior to enrollment.
P value calculated from t-test allowing for unequal variances for means, Mood’s median test for medians, and test of proportions for binary outcomes.
Includes SBPs for patients who did not have a qualifying SBP (i.e. SBP > 90 mmHg).
TABLE 2.
Primary Feasibility Outcome – Early Crystalloid Volume (ECV)
| Subgroup | Standard Resuscitation | Controlled Resuscitation | Difference [Standard−Controlled] |
Adjusted Difference1 [Standard−Controlled] |
||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | (95% CI) | (95% CI) | |
| All patients | 95 | 2.04 (1.38) | 962 | 1.04 (1.46) | 1.00 (0.59, 1.41) | 0.92 (0.54, 1.31) |
|
| ||||||
| Blunt trauma | 62 | 1.73 (1.26) | 63 | 0.83 (1.07) | 0.90 (0.48, 1.31) | 0.84 (0.48, 1.21) |
|
| ||||||
| Penetrating trauma | 33 | 2.64 (1.42) | 32 | 1.47 (1.99) | 1.16 (0.30, 2.02) | 1.27 (0.35, 2.20) |
|
| ||||||
| ISS > 15 | 33 | 2.46 (1.68) | 33 | 1.89 (1.98) | 0.57 (−0.34, 1.47) | 0.64 (−0.35, 1.64) |
|
| ||||||
| Eligible3 patients | 71 | 2.18 (1.32) | 88 | 0.92 (1.28) | 1.26 (0.85, 1.67) | 1.26 (0.87, 1.65) |
|
| ||||||
| Eligible3 w/ blunt trauma | 44 | 1.84 (1.11) | 57 | 0.71 (0.91) | 1.13 (0.72, 1.54) | 1.17 (0.82, 1.51) |
|
| ||||||
| Eligible3 w/ penetrating trauma | 27 | 2.72 (1.48) | 30 | 1.32 (1.74) | 1.40 (0.54, 2.25) | 1.67 (0.80, 2.54) |
|
| ||||||
| Eligible3 w/ ISS > 15 | 22 | 2.71 (1.64) | 28 | 1.58 (1.76) | 1.12 (0.15, 2.09) | 1.16 (0.07, 2.24) |
Abbreviations: CI, confidence interval; ISS, Injury Severity Score.
Models adjust for regional site, age (linear spline with knot at 45 years), penetrating vs. blunt or no trauma (one patient had neither blunt nor penetrating trauma), and Injury Severity Score (linear). ISS is multiply imputed for one ineligible standard arm patient in the “All patients” and “Blunt trauma” populations.
One patient with a GI bleed did not have a blunt or penetrating traumatic mechanism.
Three patients were eligible but not properly enrolled: two were enrolled while in the emergency department and one was treated with controlled resuscitation without a study kit being opened. These patients are not included in the eligible subgroups.
FIGURE 2.
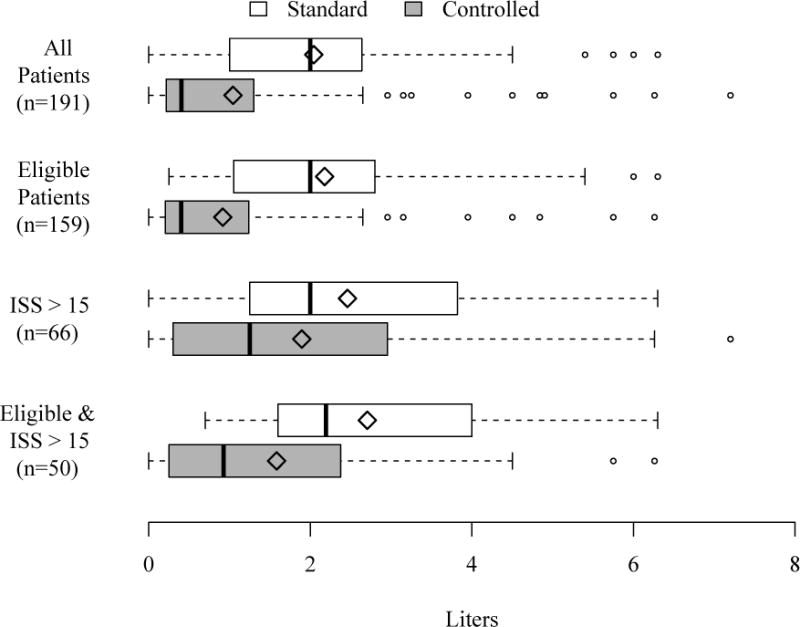
Early crystalloid volume by treatment group. The left and right sides of boxes are drawn at the 25th and 75th percentiles. The median is drawn as a thick solid line and the mean as a diamond. Outliers, outcomes > 75th percentile + 1.5 × IQR, are drawn as open circles where IQR is the interquartile range.
Crystalloid, blood product volumes and total fluids given over time are shown in Table 3. Patients who were randomized to the CR group received significantly less crystalloid in the out-of-hospital setting and from 0 to 2 hours per protocol. No patient in this study received out-of-hospital blood products. Patients in the CR group received more packed red blood cells (PRBCs) and total blood products during the period from hospital arrival until 2 hours later. There was no statistically significant difference between groups in total crystalloid, PRBC volume or total blood products at 24 hours.
TABLE 3.
Fluid and Blood Resuscitation in Liters
| Standard Resuscitation (n = 95)1 Mean (SD) |
Controlled Resuscitation (n = 96) Mean (SD) |
Difference [Standard−Controlled] (95% CI) |
|
|---|---|---|---|
| Out-of-hospital | |||
| Crystalloid | 0.50 (0.35) | 0.23 (0.19) | 0.27 (0.18, 0.35) |
|
| |||
| 0 to 2 hrs from ED arrival2 | |||
| Crystalloid3 | 1.75 (1.57) | 0.99 (1.46) | 0.76 (0.33, 1.19) |
|
| |||
| Red blood cells | 0.27 (0.62) | 0.73 (1.73) | −0.46 (−0.83, −0.09) |
|
| |||
| All blood products4 | 0.40 (0.94) | 1.05 (2.62) | −0.65 (−1.21, −0.09) |
|
| |||
| Total fluids5 | 2.18 (2.26) | 2.07 (3.56) | 0.11 (−0.74, 0.96) |
|
| |||
| 0 to 24 hrs from ED arrival2 | |||
| Crystalloid | 3.50 (2.51) | 3.88 (3.99) | −0.38 (−1.33, 0.57) |
|
| |||
| Red blood cells | 0.58 (1.38) | 1.07 (2.17) | −0.50 (−1.02, 0.02) |
|
| |||
| All blood products4 | 0.84 (2.24) | 1.61 (3.53) | −0.77 (−1.61, 0.08) |
|
| |||
| Total fluids5 | 4.43 (4.02) | 5.61 (6.71) | −1.18 (−2.76, 0.40) |
Abbreviations: CI – confidence interval; ECV – Early Crystalloid Volume endpoint; ED – emergency department.
One ineligible standard arm patient died in the field and is not included in the 0 to 2 hrs and 0 to 24 hrs sections.
Does not include out-of-hospital crystalloid.
Note that crystalloid obtained after hemorrhage control but within 0 to 2 hours after ED arrival is included here but not in ECV.
Includes red blood cells, fresh frozen plasma, platelets, and cryoprecipitate.
Includes crystalloids, blood products, and colloids.
Admission vital signs, GCS and laboratory measurements are shown in Table 4. Despite patients receiving significantly more fluid in the SR group there was no statistically significant difference in admission vital signs, hemoglobin, standard coagulation assays or base deficit between groups. The platelet count in the SR group was lower but remained well within the normal range. Renal function by RIFLE classification, need for renal replacement therapy, ICU-free days, ventilator-free days, days out of hospital, need for major surgery within 24 hours and hemorrhage control procedures performed within 2 hours are shown in Table 4. Although the proportion of subjects in the RIFLE risk, injury and failure categories was higher in the CR group, there was no statistically significant difference between groups. Thirty-two (33.7%) patients in the SR group and 43 (44.8%) in the CR required a major surgical intervention (95% CI: −24.9, 2.7). The incidence of hemorrhage control procedures performed at 2 hours was 19/95 (20.0%) in the SR group and 29/96 (30.2%) in the CR group [95% CI −10.2 (−22.4, 2.0)]. The incidence of specific procedures for hemorrhage control at 2 hours was similar between groups.
TABLE 4.
Admissions Physiology and Hospital Outcomes
| n | Standard Resuscitation | n | Controlled Resuscitation | Difference [Standard−Controlled] (95% CI) |
|
|---|---|---|---|---|---|
| Admission Measure, Mean (SD) | |||||
| Systolic blood pressure1, mmHg | 91 | 105.0 (34.1) | 95 | 98.7 (32.5) | 6.3 (−3.3, 15.9) |
|
| |||||
| Heart rate, beats/m | 91 | 86.9 (24.7) | 94 | 92.9 (26.1) | −6.0 (−13.4, 1.3) |
|
| |||||
| Glasgow Coma Scale | 87 | 13.0 (3.8) | 92 | 13.1 (3.7) | −0.2 (−1.3, 1.0) |
|
| |||||
| Hemoglobin, g/dL | 86 | 12.6 (2.1) | 89 | 12.3 (2.3) | 0.4 (−0.3, 1.0) |
|
| |||||
| Prothrombin time, s | 68 | 14.4 (2.7) | 66 | 14.0 (2.9) | 0.4 (−0.6, 1.3) |
|
| |||||
| Partial thromboplastin time, s | 63 | 32.0 (25.0) | 64 | 27.4 (8.5) | 4.6 (−2.1, 11.2) |
|
| |||||
| INR | 74 | 1.18 (0.26) | 74 | 1.16 (0.25) | 0.02 (−0.06, 0.10) |
|
| |||||
| Platelets, 109/L | 83 | 219.5 (61.8) | 86 | 239.9 (82.4) | −20.4 (−42.5, 1.6) |
|
| |||||
| Base deficit, mmol/L | 55 | 6.4 (5.2) | 58 | 6.2 (5.7) | 0.2 (−1.8, 2.2) |
|
| |||||
| Procedures, No. (%) | |||||
| Hemorrhage control procedure within 2h | 95 | 19 (20.0) | 96 | 29 (30.2) | −10.2 (−22.4, 2.0) |
|
| |||||
| Vessel ligated | 95 | 10 (10.5) | 96 | 14 (14.6) | −4.1 (−13.4, 5.3) |
|
| |||||
| Vessel embolized | 95 | 0 (0.0) | 96 | 2 (2.1) | −2.1 (−4.9, 0.8) |
|
| |||||
| Organ packed | 95 | 2 (2.1) | 96 | 2 (2.1) | 0.0 (−4.0, 4.1) |
|
| |||||
| Organ removed | 95 | 2 (2.1) | 96 | 6 (6.3) | −4.1 (−9.8, 1.5) |
|
| |||||
| Laparotomy2 | 95 | 5 (5.3) | 96 | 2 (2.1) | 3.2 (−2.1, 8.5) |
|
| |||||
| Thoracotomy2 | 95 | 2 (2.1) | 96 | 7 (7.3) | −5.2 (−11.1, 0.8) |
|
| |||||
| Major surgery3 within 24h | 95 | 32 (33.7) | 96 | 43 (44.8) | −11.1 (−24.9, 2.7) |
|
| |||||
| Renal replacement therapy | 95 | 1 (1.1) | 96 | 1 (1.0) | 0.0 (−2.9, 2.9) |
|
| |||||
| RIFLE Classification4, No. (%) | |||||
| Risk5 | 24 | 3 (12.5) | 35 | 11 (31.4) | −18.9 (−39.2, 1.4) |
|
| |||||
| Injury6 | 24 | 3 (12.5) | 35 | 8 (22.9) | −10.4 (−29.6, 8.8) |
|
| |||||
| Failure7 | 24 | 1 (4.2) | 35 | 3 (8.6) | −4.4 (−16.6, 7.8) |
|
| |||||
| 28-day Measures, Mean (SD) | |||||
| ICU-free days | 91 | 23.0 (10.7) | 95 | 23.6 (9.8) | −0.6 (−3.6, 2.4) |
|
| |||||
| Ventilator-free days | 91 | 23.6 (10.7) | 95 | 24.5 (9.5) | −0.9 (−3.8, 2.1) |
|
| |||||
| Days out of the hospital | 91 | 18.6 (10.3) | 95 | 18.5 (10.5) | 0.1 (−2.9, 3.1) |
Abbreviations: CI, confidence interval; ICU, intensive care unit; INR, international normalized ratio for prothrombin time; RIFILE (Risk-Injury-Failure-Loss-End Stage Renal Disease), classification system for acute kidney injury; SBP, systolic blood pressure.
Patients with “not detectable” SBP were assigned a value of 0. There were 5 such patients on the hypotensive arm at admission, all of them eligible. There were 4 at admission on the standard arm, one of them was ineligible. At 1h there was one hypotensive arm patient with a not detectable SBP.
For some patients a laparotomy and/or thoracotomy was recorded, but the specific hemorrhage control procedure was not listed.
Includes tracheostomy, laparotomy/laparoscopy, thoracotomy/sternotomy/VATS, major non-abdominal vascular repair, open fixation of fracture (includes fasciotomy for extremity compartment syndrome), craniotomy, neck exploration, angiographic control of hemorrhage, and ligation of bleeding vessel.
Loss (L) and End Stage Renal Disease (E) are not included since the follow-up period did not extend beyond 28 days. Classification here is the worst category obtained during the ICU stay. Only patients with at least 2-day ICU stay assessed.
Increased plasma creatinine > 1.5 × reference measure (ED admission). Urine criteria is based on 6-hour periods for this level of the RIFLE and cannot be assessed since study data are collected for 24-hour periods. This row includes patients who met the “Injury” and “Failure” criteria as well.
Increased plasma creatinine > 2 × reference measure (ED admission) or urine output < 0.5 mL/kg/h × 24h. The RIFLE urine criterion for this level actually specifies a 12 hour period of assessment but study data are collected for 24-hr periods. This row includes patients who met the “Failure” criteria as well.
Increased plasma creatinine > 3 × reference measure (ED admission) or acute plasma creatinine = 350 umol/L or acute rise = 44 umol/L or urine output < 0.3 mL/k/h × 24h.
Twenty-four hour mortality was 5/96 (5.2%) in the CR group and 14/95 (14.7%) in the SR group [adjusted odds ratio (aOR) 0.39 (95% CI: 0.12, 1.25)]. Patients in the blunt trauma sub-population randomized to the CR group had decreased mortality compared to those randomized to SR: 2/63 (3.2%) vs. 11/62 (17.7%), aOR 0.17 (0.03, 0.92). This mortality difference was not seen in the penetrating trauma subgroup [CR 3/32 (9.4%) vs SR 3/33 (9.1%), aOR 1.92 (0.19, 19.11) (P for interaction: 0.10)]. Overall in-hospital mortality did not differ between groups [CR 8/95 (8.4%), SR 15/91 (16.5%), aOR 0.62 (0.22, 1.77)]. The most frequent cause of death in both groups was exsanguination [CR 5/8 (62%), SR 9/15 (60%)]. Traumatic brain injury was the next most common cause of death [CR 2/8 (25%), SR 4/15 (20%)]. The remaining patient in the CR group died from a tension pneumothorax. One patient in the SR group died from sepsis and the final death in the SR group occurred in the field from an unknown cause.
DISCUSSION
The primary finding of this prospective randomized pilot trial was that controlled resuscitation, defined as delivering small crystalloid boluses for either a SBP < 70mmHg or an absent radial pulse, is feasible and safe for the initial resuscitation of hypotensive trauma patients. Our preliminary findings indicate that compared to SR, CR results in approximately one liter less volume of fluid resuscitation being delivered during early resuscitation defined as up to 2 hours after hospital admission. Despite the smaller volume of fluid delivered, there were no clinically relevant differences in admission vital signs, GCS, hematologic labs or base deficit. There were also no statistically significant differences in the need for major surgical procedures, renal function, ICU-free days, ventilator-free days or days discharged from the hospital. Twenty-four hour mortality did not differ between groups.
The controlled resuscitation philosophy tested in this analysis was first promulgated by the Tactical Combat Casualty Care Committee of the US Military and has been utilized throughout the conflicts in Iraq and Afghanistan.36 Injured warfighters at risk for hemorrhage are evaluated by medics and only receive fluid boluses if they have an absent or diminished radial pulse or decreased mental status in the absence of traumatic brain injury. The goal is to avoid artificially elevating the blood pressure and displacing tenuous clots after bleeding stops or diminishes in hypotensive trauma patients. We attempted to duplicate this battlefield strategy in the civilian setting.
Despite receiving a relatively small volume of fluid in the pre-hospital setting, patients in both groups experienced substantial elevations in blood pressure at the time of admission. The phenomenon of elevation in blood pressure in trauma patients receiving minimal or no fluid has been reported in other trials of delayed or hypotensive resuscitation.17,37,38 This phenomenon has been related to changes in systemic vascular resistance in animal models of uncontrolled hemorrhagic shock.39
Resuscitation with smaller volumes of crystalloid in the CR group was associated with transfusion of a higher volume of PRBCs and other blood products during the period from hospital arrival until 2 hours later. Transfusion of blood products was at the discretion of the patients’ caregivers and the inability to infuse more fluid in the CR group may have resulted in greater use of blood products. Alternatively patients in the CR group may have experienced more bleeding. Finally, the higher number of early deaths in the SR group may have resulted in reduced blood usage in that group (Supplemental Digital Content 1). Admission vital signs, hematologic assays and base deficit were similar between groups so the reason for this difference remains unclear in this pilot trial.
The use of more blood products and less crystalloid is consistent with damage control resuscitation (DCR). The DCR philosophy dictates that immediate hemorrhage control is emphasized, crystalloid infusion is limited permitting a lower blood pressure until bleeding is controlled and blood component transfusions are given in a 1:1:1 ratio of plasma:platelets:PRBCs. This strategy has been associated with improved survival in critically injured trauma patients.40,41
The trend of a higher proportion of subjects in the RIFLE risk, injury and failure categories in the CR group suggests the possibility that early under perfusion may have had a negative impact on renal function. The other possibility is the effect of survival bias wherein a greater proportion of critically injured patients in the SR group who would have had evidence of renal dysfunction died prior to exhibiting it (Supplemental Digital Content 1).
The preliminary finding of improved mortality in the blunt trauma subgroup of the CR group is interesting. The landmark paper published by Bickell et al. in the New England Journal of Medicine in 199417 revealed a survival advantage in hypotensive penetrating torso trauma patients in whom resuscitation was delayed until hemorrhage was controlled. Patients in the delayed resuscitation group received a mean of 375 cc and patients in the immediate resuscitation group received a mean of 2478 cc prior to going to the operating room. The same group performed a prospective randomized trial in hypotensive inpatients requiring emergent surgery for hemorrhage control.42 They found reduced early mortality in the hypotensive group and a non-significant trend toward decreased mortality at 30 days in the combined cohort of blunt and penetrating patients. In a secondary analysis of the Prospective Observational Multicenter Massive Transfusion Study, Hampton et al. showed that a moderate out-of-hospital crystalloid resuscitation of 700 cc was associated with improved survival compared to no resuscitation.43 In the in-hospital study performed by Dutton et al., there was no difference in mortality between patients resuscitated with a hypotensive resuscitation strategy compared to those given standard resuscitation.38 It is important to note that our study was not powered to detect important survival differences between treatment groups and that as a subgroup analysis, the finding of increased survival in the blunt trauma cohort is by no means definitive.
Several very important lessons were learned during the conduct of this pilot trial that will become important in a larger pivotal trial. Approximately two-thirds of the patients enrolled in the study had an ISS < 15 suggesting that SBP ≤ 90 mmHg is not an adequate criterion to consistently exclude minimally injured patients in the pre-hospital setting. We attempted to address this during the trial by adding an exclusion criterion that omitted patients who suffered ground level falls. This patient population was found to consistently have a low ISS. Due to the fact that the study was prospectively designed as an intent-to-treat trial, all enrolled patients were included in the analysis.
Although it was always our intention to exclude patients with significant TBI, the importance of providing a specific definition of severe TBI for EMS personnel became obvious as the trial progressed. An additional exclusion was added for patients with bilateral paralysis in an attempt to avoid enrolling patients with spinal cord injury due to the theoretical harm of spinal hypoperfusion in the CR group. Finally, it was clear that compliance with the protocol improved as EMS agencies became more experienced. It may be beneficial for agencies to undergo a run-in phase prior to enrolling patients in future trials.
In summary, this pilot study demonstrates that a controlled resuscitation strategy can be successfully and safely implemented in a civilian environment beginning in the out-of-hospital setting and extending into early hospital care. This strategy resulted in a reduction of early crystalloid resuscitation volume of approximately one liter and an increase in early blood product transfusion. This study provides strong motivation for a large Phase III trial of controlled resuscitation versus standard resuscitation to determine if the CR strategy improves survival.
Supplementary Material
Acknowledgments
Funding/Support
The ROC is supported by a series of cooperative agreements to six regional clinical centers and one Data Coordinating Center (5U01 HL077863-University of Washington Data Coordinating Center, HL077866-Medical College of Wisconsin, HL077871-University of Pittsburgh, HL077873-Oregon Health & Science University, HL077881-University of Alabama at Birmingham, HL077885-Ottawa Hospital Research Institute, HL077887-University of Texas SW Medical Ctr/Dallas) from the National Heart, Lung and Blood Institute in partnership with the U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) – Institute of Circulatory and Respiratory Health, Defense Research and Development Canada, the Heart, Stroke Foundation of Canada and the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Footnotes
LEVEL OF EVIDENCE: Prospective randomized clinical trial, level I
AUTHOR CONTRIBUTIONS: Dr. Schreiber and Mr. Meier had full access to all of the study data and take responsibility for the integrity of the data and the accuracy of the analysis.
Study design: Schreiber, Tisherman, Kerby, Newgard, Brasel, Daya, Kannas, May, McKnight, Hoyt; Literature search: Schreiber, Meier, Kannas, May, McKnight; Data collection: Schreiber, Meier, Tisherman, Kerby, Witham, Williams, Daya, Beeson, Wheeler, Kannas; Data analysis: Meier; Data interpretation: Schreiber, Meier, Tisherman, Kerby, Newgard, Brasel, Kannas, May, McKnight; Writing: Schreiber, Meier, McCully; Critical revision: Meier, Tisherman, Kerby, Newgard, Brasel, Egan, Brasel, Witham, Williams, Daya, Beeson, McCully, Wheeler, Kannas, May, McKnight.
Contributor Information
Martin A. Schreiber, Email: schreibm@ohsu.edu.
Eric N. Meier, Email: emeier11@uw.edu.
Samuel A. Tisherman, Email: stisherman@umm.edu.
Jeffrey D. Kerby, Email: jkerby@uabmc.edu.
Craig D. Newgard, Email: newgardc@ohsu.edu.
Karen Brasel, Email: kbrasel83@gmail.com.
Debra Egan, Email: debrae@nhlbi.nih.gov.
William Witham, Email: withamwr@sbcglobal.net.
Carolyn Williams, Email: cswilliams@uabmc.edu.
Mohamud Daya, Email: dayam@ohsu.edu.
Jeff Beeson, Email: jbeeson@medstar911.org.
Belinda H. McCully, Email: houghtob@ohsu.edu.
Stephen Wheeler, Email: drswheeler@me.com.
Delores Kannas, Email: deloresk@uw.edu.
Susanne May, Email: sjmay@uw.edu.
Barbara McKnight, Email: bmck@uw.edu.
David B. Hoyt, Email: dhoyt@facs.org.
References
- 1.Bonnie R, Fulco C, Liverman C. Anonymous. Washington D.C.: National Acadamy Press; 1999. Reducing the Burden of Injury Advancing Prevention and Treatment. [PubMed] [Google Scholar]
- 2.Stewart RM, Myers JG, Dent DL, Ermis P, Gray GA, Villarreal R, Blow O, Woods B, McFarland M, Garavaglia J, et al. Seven hundred fifty-three consecutive deaths in a level I trauma center: The argument for injury prevention. J Trauma. 2003;54:66–70. doi: 10.1097/00005373-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 3.MacLeod JB, Cohn SM, Johnson EW, McKenney MG. Trauma deaths in the first hour: Are they all unsalvageable injuries? Am J Surg. 2007;193:195–199. doi: 10.1016/j.amjsurg.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Champion HR. Combat fluid resuscitation: Introduction and overview of conferences. J Trauma. 2003;54:S7–12. doi: 10.1097/01.TA.0000056154.34788.C6. [DOI] [PubMed] [Google Scholar]
- 5.Takaori M, Safar P. Treatment of massive hemorrhage with colloid and crystalloid solutions. studies in dogs. JAMA. 1967;199:297–302. [PubMed] [Google Scholar]
- 6.Runyon DE, Bruttig SP, Dubick MA, Clifford CB, Kramer GC. Resuscitation from hypovolemia in swine with intraosseous infusion of a saturated salt-dextran solution. J Trauma. 1994;36:11–19. doi: 10.1097/00005373-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg. 1995;32:925–1002. doi: 10.1016/s0011-3840(05)80008-5. [DOI] [PubMed] [Google Scholar]
- 8.Crowell JW, Smith EE. Oxygen deficit and irreversible hemorrhagic shock. Am J Physiol. 1964;206:313–316. doi: 10.1152/ajplegacy.1964.206.2.313. [DOI] [PubMed] [Google Scholar]
- 9.Weil MH, Afifi AA. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock) Circulation. 1970;41:989–1001. doi: 10.1161/01.cir.41.6.989. [DOI] [PubMed] [Google Scholar]
- 10.Vitek Vladimir, ScD PhD, Cowley RA., MD Blood lactate in the prognosis of various forms of shock. Ann Surg. 1971;173:308–313. doi: 10.1097/00000658-197102000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop MH, Shoemaker WC, Appel PL, Meade P, Ordog GJ, Wasserberger J, Wo C, Rimle DA, Kram HB, Umali R, et al. Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation endpoints in severe trauma. J Trauma. 1995;38:780–787. doi: 10.1097/00005373-199505000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Siegel JH, Fabian MS, Joyce A, Kingston EP, Steele KA, Wells MR., Dvm Oxygen debt criteria quantify the effectiveness of early partial resuscitation after hypovolemic hemorrhagic shock. J Trauma. 2003;54:862–880. doi: 10.1097/01.TA.0000066186.97206.39. [DOI] [PubMed] [Google Scholar]
- 13.Wiggers CJ. Physiology of Shock. Cambridge Massachussetts: Harvard University: Commonwealth Fund; 1950. [Google Scholar]
- 14.Shires T, Coln D, Carrico J, Lightfoot S. Fluid therapy in hemorrhagic shock. Arch Surg. 1964;88:688–693. doi: 10.1001/archsurg.1964.01310220178027. [DOI] [PubMed] [Google Scholar]
- 15.Committee on Trauma, American College of Surgeons. ATLS: Advanced Trauma Life Support Program for Doctors. Chicago: American College of Surgeons; 2008. [Google Scholar]
- 16.Kortbeek JB, Al Turki SA, Ali J, Antoine JA, Bouillon B, Brasel K, Brenneman F, Brink PR, Brohi K, Burris D, et al. Advanced trauma life support, 8th edition, the evidence for change. 2008;64:1638–1650. doi: 10.1097/TA.0b013e3181744b03. [DOI] [PubMed] [Google Scholar]
- 17.Bickell WH, Wall MJ, Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 18.Cannon WB, Fraser J, Cowell EM. The preventive treatment of wound shock. JAMA. 1918;70:618–621. [Google Scholar]
- 19.Ng KF, Lam CC, Chan LC. In vivo effect of haemodilution with saline on coagulation: A randomized controlled trial. Br J Anaesth. 2002;88:475–480. doi: 10.1093/bja/88.4.475. [DOI] [PubMed] [Google Scholar]
- 20.Walker J, Criddle LM. Pathophysiology and management of abdominal compartment syndrome. Am J Crit Care. 2003;12:367–371. [PubMed] [Google Scholar]
- 21.Rodas EB, Malhotra AK, Chhitwal R, Aboutanos MB, Duane TM, Ivatury RR. Hyperacute abdominal compartment syndrome: An unrecognized complication of massive intraoperative resuscitation for extra-abdominal injuries. Am Surg. 2005;71:977–981. doi: 10.1177/000313480507101113. [DOI] [PubMed] [Google Scholar]
- 22.Cotton BA, Guy JS, Morris JA, Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–121. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205:541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: Its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055–1064. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- 25.Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Holcomb JB, Ware DN, Moore FA. Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am J Surg. 2002;184:538–543. doi: 10.1016/s0002-9610(02)01050-4. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care. 2005;11:590–597. doi: 10.1097/01.ccx.0000186374.49320.ab. [DOI] [PubMed] [Google Scholar]
- 27.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 28.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 29.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 30.Ledgerwood AM, Lucas CE. A review of studies on the effects of hemorrhagic shock and resuscitation on the coagulation profile. J Trauma. 2003;54:S68–74. doi: 10.1097/01.TA.0000064513.59253.70. [DOI] [PubMed] [Google Scholar]
- 31.Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, Brasel KJ, Tisherman SA, Coimbra R, Rizoli S, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: A randomized, placebo controlled trial. Ann Surg. 2011;253:431–441. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baraniuk S, Tilley BC, del Junco DJ, Fox EE, van Belle G, Wade CE, Podbielski JM, Beeler AM, Hess JR, Bulger EM, et al. Pragmatic randomized optimal platelet and plasma ratios (PROPPR) trial: Design, rationale and implementation. Injury. 2014;45:1287–1295. doi: 10.1016/j.injury.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin D, Little R. Statistical analysis with missing data. Hoboken, NJ: J Wiley & Sons; 2002. [Google Scholar]
- 34.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team. A language and environment for statistical computing. R foundation for statistical computing; vienna, austria: 2013. Available at: http://www.R-project.org/ [Google Scholar]
- 36.Butler FK, Jr, Blackbourne LH. Battlefield trauma care then and now: A decade of tactical combat casualty care. J Trauma Acute Care Surg. 2012;73:S395–402. doi: 10.1097/TA.0b013e3182754850. [DOI] [PubMed] [Google Scholar]
- 37.Dutton RP. Low-pressure resuscitation from hemorrhagic shock. Int Anesthesiol Clin. 2002;40:19–30. doi: 10.1097/00004311-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: Impact on in-hospital mortality. J Trauma. 2002;52:1141–1146. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 39.Phillips CR, Vinecore K, Hagg DS, Sawai RS, Differding JA, Watters JM, Schreiber MA. Resuscitation of hemorrhagic shock with normal saline vs. lactated ringer’s: Effects on oxygenation, extravascular lung water, and hemodynamics. Crit Care. 2009;13:R30. doi: 10.1186/cc7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg. 2014 doi: 10.1001/jamasurg.2014.940. [DOI] [PubMed] [Google Scholar]
- 41.Kaafarani HM, Velmahos GC. Damage control resuscitation in trauma. Scand J Surg. 2014;103:81–88. doi: 10.1177/1457496914524388. [DOI] [PubMed] [Google Scholar]
- 42.Morrison CA, Carrick MM, Norman MA, Scott BG, Welsh FJ, Tsai P, Liscum KR, Wall MJ, Jr, Mattox KL. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: Preliminary results of a randomized controlled trial. J Trauma. 2011;70:652–663. doi: 10.1097/TA.0b013e31820e77ea. [DOI] [PubMed] [Google Scholar]
- 43.Hampton DA, Fabricant LJ, Differding J, Diggs B, Underwood S, De La Cruz D, Holcomb JB, Brasel KJ, Cohen MJ, Fox EE, et al. Prehospital intravenous fluid is associated with increased survival in trauma patients. J Trauma Acute Care Surg. 2013;75:S9–15. doi: 10.1097/TA.0b013e318290cd52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


