Abstract
Objective
Up to half of patients with amyotrophic lateral sclerosis (ALS) may have cognitive difficulty, but most cognitive measures are confounded by a motor component. Rare studies have related impaired cognition in ALS to disease in gray matter (GM) and white matter (WM). We evaluated a simple, untimed measure of executive functioning with minimal motor demands in ALS, and relate performance to structural disease.
Methods
Fifty-six patients with ALS and 29 matched healthy controls were assessed with the Visual-Verbal Test (VVT). This brief measure of cognitive flexibility first assesses an individual's ability to identify a shared feature in three of four simple geometric designs. Cognitive flexibility is challenged when individuals are next asked to identify a different shared feature in another three of the same four geometric designs. Regression analyses related performance to GM atrophy and reduced WM fractional anisotropy (FA) in a subset of patients.
Results
ALS patients were significantly impaired on this simple measure of cognitive flexibility (p<0.01). An error in cognitive flexibility was present in 48.2% of individual ALS patients. Regression analyses related impaired cognitive flexibility to GM atrophy in inferior frontal and insula regions, and to reduced FA in WM projections in inferior frontal-occipital and uncinate fasciculi and corpus callosum.
Conclusion
Patients with ALS have impaired cognitive flexibility on an untimed measure with minimal motor demands, and this is related in part to a large-scale frontal network that is degraded in ALS.
Keywords: Amyotrophic lateral sclerosis, cognitive flexibility, prefrontal, white matter
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a motor neuron disease associated with progressive degeneration of upper and lower motor neurons(Keirnan et al., 2011; Mitchell & Borasio, 2007). Pathologic studies indicate that ALS is a multisystem disorder associated overwhelmingly with TDP-43 pathology(Brettschneider et al., 2013; Geser et al., 2009). Histopathologic and genetic research has solidified the link between ALS and frontotemporal degeneration (FTD) by showing that TDP-43 pathology is also found in many cases of FTD, and repeat expansions associated with C9orf72 are found in families with both of these conditions(DeJesus-Hernandez et al., 2011; Irwin et al., 2013; Neumann et al., 2006; Renton et al., 2011). Finally, a spectrum of cognitive change paralleling deficits in FTD has been found in up to half of patients with ALS(Murphy et al., 2007; Phukan et al., 2012).
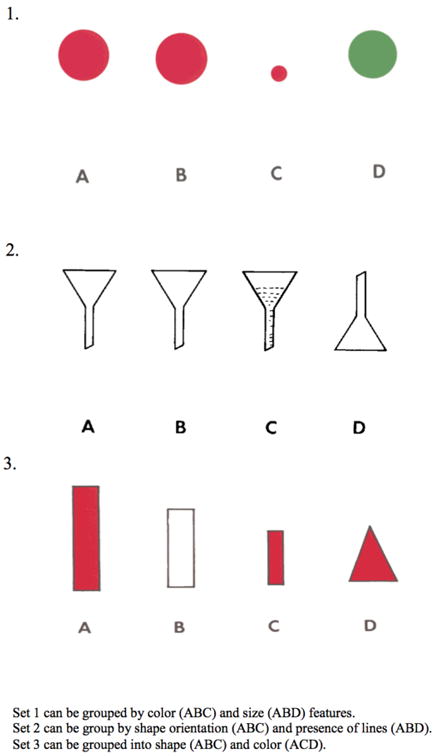
Several studies have demonstrated a limitation in executive control in ALS(Goldstein & Abrahams, 2013). The present study investigates cognitive flexibility. Cognitive flexibility is one facet of executive functioning that involves perceiving the sensory-motor and cognitive attributes of a pattern, inhibitory control over a previously established pattern, and mental switching to identify a new pattern. Perhaps the most common demonstration of a deficit in cognitive flexibility involves naming fluency difficulty when guided by a letter(Abrahams et al., 2000). However, the demonstration of executive deficits in ALS is often confounded by the motor disorder due to dependence on timed performance or a specific response modality that may be compromised. The recognition of these confounds led to an important innovation that controls in part for these by normalizing slowed speech on the letter fluency task with written copying of an individual's oral output(Abrahams et al., 2000). More recently, an untimed measure of cognitive flexibility was reported that minimizes dependence on timing and the response modality. This task used recognition performance to document difficulty on a categorization task in ALS(Libon et al., 2012; Taylor et al., 2013). Unfortunately, this measure is complex and time-consuming to administer. Thus, a concise, straightforward task is needed to assess cognitive flexibility in a clinical setting. Here we examine cognitive impairment in ALS with the Visual-Verbal Test (VVT)(Feldman & Drasgow, 1960). This task requires identifying a common perceptual attribute in three of four simple geometric designs, and then identifying another perceptual feature that is common to a different set of three designs (Figure 1). VVT thus is well suited for measuring cognitive flexibility in ALS because it is brief, nonsocial, nonverbal, and minimizes a confounding motoric component.
Figure 1. Examples of Stimuli from the Visual-Verbal Test.
Prefrontal cortex is an early locus of pathological burden in ALS(Brettschneider et al., 2013). Impairments on measures of executive control involving cognitive flexibility are thought to be associated in part with prefrontal disease in ALS(Abrahams et al., 1996; Abrahams et al., 2004; Kew et al., 1993; Libon et al., 2012), although most of these measures were difficult and involved a motor component. fMRI work in healthy adults associated inferior frontal cortex (Goel & Vartanian, 2005) and insula (Chang, Yarkoni, Khaw, & Sanfey, 2013) with cognitive flexibility and set switching. Disease in white matter (WM) tracts also has been implicated in impaired executive performance(Abrahams et al., 2005), although the associated cognitive measures included a motor component. Impaired category naming fluency was related to reduced FA in the cingulum(Sarro et al., 2011), presumably linking simple cognitive switching supported by ventral frontal regions with more dorsal regions that modulate this decision-making process. A recent study used a largely motor-free measure of dual-tasking to relate executive functioning to reduced fractional anisotropy (FA) in several WM regions of interest in the frontal lobe(Pettit et al., 2013). We evaluated the neuroanatomic basis for a deficit on an executive measure with minimal motor confounds by relating VVT performance in ALS with MRI of GM atrophy and WM reduced FA. We hypothesized that patients with ALS would be impaired on VVT, and this would be related to disease in a large-scale GM network involving inferior prefrontal and insula regions integrated by frontal WM projections into a prefrontal decision-making network.
Methods
Subjects
Fifty-six patients meeting El-Escorial-revised criteria for ALS(Brooks, Miller, Swash, & Munsat, 2000) (El-Escorial-revised criteria were unavailable in 3 ALS-FTD cases) and 29 elderly controls were recruited from the out-patient Department of Neurology of the University of Pennsylvania. Onset was bulbar in 11, cervical in 20, and lumbosacral in 21 (motor onset was unavailable in 4 ALS-FTD cases). As summarized in Table 1, patients were examined on average early in their course – about 6 months after diagnosis. Patients were significantly younger than controls, although age did not correlate with VVT performance [r(53)=0.17; p>0.3]. Clinical assessment includes a Mini-Mental Status Examination (MMSE) as a general reflection of cognitive functioning, and patients had significantly lower MMSE scores than controls, even though we scored the MMSE proportionately to take into account points for items that ALS patients could not perform because of a motor deficit. Nevertheless, ALS average MMSE scores were within the normal range. None of the participants was taking centrally-acting medications that could compromise cognitive functioning. The ALS cohort was divided into two categories, ALS or ALS-FTD, based on physician evaluation using published diagnostic criteria(Rascovsky et al., 2011; Strong et al., 2009). The ALS-FTD cohort (n=9) was diagnosed with co-occurring motor neuron disease and behavioral variant FTD. All ALS-FTD cases presented with FTD before ALS. The ALS cohort (n=47) exhibited motor neuron disease with mild or no cognitive impairment using a brief cognitive battery that included letter-guided category naming fluency (FAS), MMSE, and digit span. Table 1 summarizes digit span forward and reverse, a measure of working memory; and letter-guided category naming fluency, a measure of cognitive flexibility. All participants completed a written informed consent procedure approved by the University of Pennsylvania Institutional Review Board.
Table 1. Mean (+S.D.) Demographic and Cognitive Performance.
| ALS | ALS-FTD | ALS Total | Control | |
|---|---|---|---|---|
| n | 47 | 9 | 56 | 29 |
| Age (years) | 58.19 (13.04) | 62.44 (9.81) | 58.88 (12.59)* | 67.76 (7.67) |
| Education (years) | 15.09 (2.80) | 14.11 (3.41) | 14.93 (2.89) | 16.07 (2.67) |
| Gender (M/F) | 29/18 | 6/3 | 35/21 | 13/16 |
| Disease Duration (months) | 6.85 (8.66) | 5.40 (6.62) | 6.71 (8.44) | |
| Functional Rating Scale (n=52) | 34.13 (8.48) | 38.57 (3.05) | 34.73 (8.10) | |
| MMSE (n=56) | 27.85 (3.23) | 24.89 (6.33)* | 27.38 (4.00)* | 29.25 (1.11) |
| Visual Verbal Test (VVT) | ||||
| Initial Categorization Failures | 0.04 (0.20) | 0.22 (0.67) | 0.07 (0.32) | 0.00 (0.00) |
| Cognitive Flexibility (max=10) | 8.55 (2.40)* | 6.67 (4.03)* | 8.25 (2.77)* | 9.41 (1.15) |
| Letter Fluency (n=47)1 | ||||
| Words/minute Total | 11.63 (5.31)* | 6.81 (1.26)ˆ* | 10.91 (5.21)* | 14.31 (5.20) |
| Digit Span (n=45)2 | ||||
| Digits Forward | 6.72 (1.24) | 6.80 (0.84) | 6.69 (1.10) | 6.95 (1.17) |
| Digits Backwards | 4.24 (1.763) | 4.20 (1.30) | 4.33 (1.49) | 5.09 (1.74) |
Notes
We combined the number of F words/minute obtained from a generation task (FAS task, n=30) where subjects are required to provide as many words that begin with the letters F, A, or S as possible within sixty seconds (Spreen & Struss, 1990) and a similar measure task is used where subjects only provide words that begin with the letter F (F words generation task, n=17). This was necessitated by having changed our procedure for collecting letter fluency, and we maximized available data by focusing on category letter fluency for F words.
In digit span forward, subjects are required to repeat a series of number sequences that become progressively longer; backward digit span requires subjects to repeat the number sequences in the reverse order of which they are presented.
ALS differs from controls p<0.05;
ALS differs from ALS-FTD p<0.05
The Visual Verbal Test
Participants were administered the VVT as part of a standard neuropsychological protocol. The study consisted of 10 untimed trials, each on its own card. Participants were given two practice trials to ensure that they understood the task. Briefly, the subject is presented with a card displaying four simple geometric designs (Figure 1). These designs can be grouped into two sets of three based on visual features of color, size, shape, orientation, or line pattern. Subjects are asked first to identify a set of three designs with a shared feature. They are then asked to identify a second set of three designs with a different shared feature. Grouping the first set of three designs shows that subjects are able to perceive the visual-perceptual designs and conceptually group a subset of items based on a shared feature. Patients could respond verbally or manually. We scored the number of times that a patient could not identify a shared feature across the geometric designs. A score of 0 on this part of the task indicates perfect performance. Disengaging from the first set and identifying the second set of shared-feature designs requires inhibitory control and set-switching, components of cognitive flexibility, and we counted the number of times that subjects were able to switch from the first set to the second set as a measure of cognitive flexibility. A score of 10 on this portion of the task indicates successful identification of all second sets and intact cognitive flexibility. Although there may be differences in the saliency of particular visual features, task difficulty does not progress over the course of successive trials. Statistical analyses, including analysis of variance (ANOVA), t-test and Pearson correlation, were performed using SPSS v.21.
Imaging Acquisition and Analysis
A subset of ALS patients (n=17, including ALS=11 and ALS-FTD=6) had high resolution T1-weighted MRI to assess GM atrophy and diffusion weighted imaging (DWI) to analyze WM reduced FA. Imaged ALS patients matched the entire ALS cohort in age, gender, disease duration, and education, and matched the entire cohort in VVT performance (all p-values>0.1). A Siemens 3.0T Trio scanner with an 8-channel head coil collected images. T1-weighted 3-dimensional spoiled gradient-echo images were obtained with TR=1620msec, TE=3msec, flip angle=15°, matrix=192×256, slice thickness=1mm, and in-plane resolution=1.0×1.0mm. A 30-directional DWI sequence was collected on 15 patients using a single-shot, spin-echo, diffusion-weighted echo planar imaging sequence (matrix size=128×128; number of slices=70; voxel size=2mm isotropic; TR=8100ms; TE=83ms; fat saturation). Thirty volumes with diffusion weighting (b=1000s/mm2) were collected along 30 non-collinear directions, and one volume was collected per subject without diffusion weighting (b=0s/mm2). Four additional b=0 volumes were collected for eight subjects. Two patients had DWI images collected using a 12-directional sequence with a single-shot, spin-echo, diffusion-weighted echo planar imaging sequence (matrix size=128×128, number of slices=40, voxel size=3mm isotropic; TR=6500ms, TE=99ms), acquiring 12 non-collinear, non-coplanar, isotropic diffusion gradients. Imaging was also collected in a control group of 36 healthy seniors (28 30-directional, 8 12-directional), comparable in age and other demographic characteristics to the patient group (all p-values>0.5).
As described previously(Grossman et al., 2013), images were normalized to a standard space and segmented using the PipeDream interface (http://sourceforge.net/projects/neuropipedream/) to the ANTS toolkit (http://www.picsl.upenn.edu/ANTS/)(Avants, Epstein, Grossman, & Gee, 2008). A local 1mm3-resolution T1 template consisted of 25 healthy seniors and 25 FTD patients. Subject images were registered to the local template, transformed into MNI space, and down-sampled to 2mm3-resolution. Images were smoothed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) using a 5mm full-width half-maximum (FWHM) isotropic Gaussian kernel to minimize individual gyral variations. GM atrophy relative to controls used a whole-brain voxel-wise analysis in SPM8 with height threshold=q<0.05 (false discovery rate (FDR)-corrected), minimum cluster size=50 voxels, and peak voxel threshold=p<0.001. VVT cognitive flexibility performance was related to GM atrophy using the multiple regressions module in SPM8, constrained to regions of GM atrophy. Clusters survived height threshold=p<0.005 (uncorrected), minimum cluster size=40 voxels and peak voxel threshold=p<0.001.
PipeDream and ANTS preprocessed DWIs as above. Motion and distortion artifacts were removed using affine co-registration of each DWI to the unweighted (b=0) image(s). A linear least-squares algorithm(Salvador et al., 2005) implemented in Camino(Cook et al., 2006) computed diffusion tensors (DTs). Tensors were reoriented using the preservation of principal directions method(Alexander, Pierpaoli, Basser, & Gee, 2001). The DT image was used to compute FA for each subject. Each FA image was registered to the T1 image. The symmetric diffeomorphic procedure in ANTS warped T1 images to the local template, and each FA image was warped to MNI space by applying the T1-to-template and template-to-MNI warps. FA images were smoothed using a 4mm FWHM isotropic Gaussian kernel. The two-sample t-test module in SPM8 compared FA differences between ALS and controls, constrained to white matter by averaging all patient and control FA images and generating a mask consisting of voxels with FA of greater than or equal to 0.25. Comparisons of patients to controls were performed at FDR-corrected height threshold=q<0.01 with 200-voxel extent. Using deterministic tractography in Camino, WM fibers were tracked in a healthy elderly template generated using the 30-directional DTI sequence described above. Fiber tracts passing through voxels of reduced FA, as determined by reduced FA in ALS compared to controls, were retained to define the mask for regression analyses. The multiple regression module of SPM8 related VVT cognitive flexibility to WM tracts with significantly reduced FA in ALS using height threshold=p<0.005, 50-voxel extent, and peak voxel threshold=p<0.001.
Results
Behavioral Analyses
We found impaired cognitive flexibility in ALS. A one-way ANOVA thus showed that ALS and controls do not differ in their ability to identify an initial set of shared-feature designs [F(1,83)=1.416; p=0.237]. However, ALS and controls differed in their ability to identify a second set of shared-feature designs, consistent with a deficit in cognitive flexibility [F(1,83)=4.674; p=0.034]. The subgroup of ALS patients without FTD [t(74)=6.928; p=0.04] and the subgroup with FTD [t(36)=3.342; p=0.002] each differed from controls. A cognitive flexibility error was present in 27 (48.2%) of 56 individual ALS patients, including 9 (69.2%) of 13 patients with autopsy-proven ALS. A similar error was present in only 9 controls. Cognitive flexibility measured by VVT correlated with performance on letter-guided category naming fluency [r(45)=0.350; p=0.016], reverse digit span [r(43)=0.573; p<0.001] and MMSE [r(54)=0.660; p<0.001]. There was no difference between subgroups with bulbar, cervical and lumbosacral onsets (all p-values>0.3).
Image Analyses
Figure 2 Panel A shows significant GM atrophy primarily in a frontal distribution bilaterally, extending into anterior temporal and parietal lobes. Peak foci of atrophy are summarized in Table 2, and these correspond to areas of GM atrophy illustrated in Figure 2. Regression analysis, illustrated in Figure 2 Panel B and summarized in Table 2, shows that cognitive flexibility deficits were related to areas of significant atrophy in bilateral inferior frontal and left insula regions.
Figure 2. Gray Matter Atrophy and Reduced White Matter Fractional Anisotropy, and Regression Analyses Relating Performance to Atrophy and Fracrional Anisotropy1.
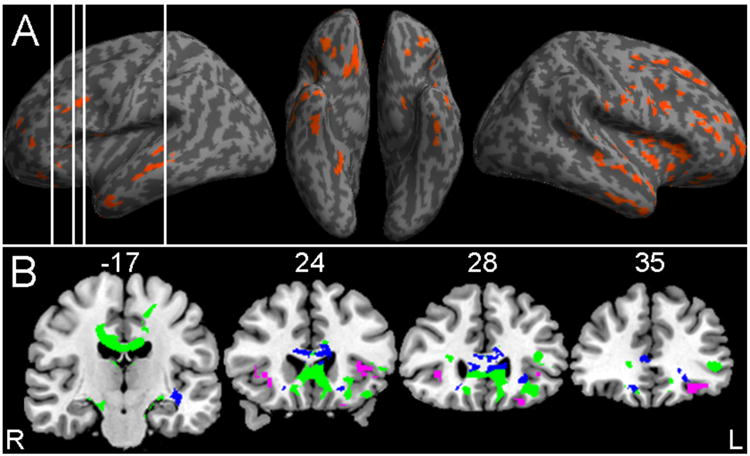
Note: 1. Panel A: Significant gray matter atrophy relative to healthy controls (red); vertical white lines indicate locations provided in Panel B; PANEL B shows coronal slices illustrating gray matter regressions relating gray matter atrophy to impaired cognitive flexibility performance (purple), reduced white matter fractional anisotropy (green), and regressions relating reduced fractional to impaired cognitive flexibility performance (blue).
Table 2. Anatomic Locations of Significant Gray Matter Atrophy in ALS Relative to Controls, and Regressions Relating Impaired Cognitive Flexibility to Atrophy in ALS.
| Anatomic Locus (Brodmann Area) | MNI Coordinates | Z score | Cluster Size (voxels) | ||
|---|---|---|---|---|---|
| ALS GRAY MATTER ATROPHY | |||||
| X | Y | Z | |||
| R primary motor (4) | 44 | -8 | 36 | 3.86 | 109 |
| R premotor (6) | 34 | -2 | 50 | 4.25 | 73 |
| R inferior frontal (44) | 42 | 8 | 30 | 5.74 | 266 |
| R middle frontal (10) | 28 | 50 | 6 | 4.56 | 227 |
| R middle frontal (46) | 38 | 34 | 22 | 3.97 | 141 |
| R cingulate (32) | 12 | 34 | 24 | 5.2 | 247 |
| R medial frontal (9) | 20 | 28 | 36 | 4.74 | 165 |
| R medial frontal (11) | 12 | 34 | -20 | 4.4 | 147 |
| R orbital frontal (11) | 24 | 32 | -14 | 4.38 | 145 |
| R insula | 40 | 0 | -4 | 5.11 | 710 |
| L inferior frontal (47) | -40 | 24 | -2 | 3.71 | 135 |
| L middle frontal (10) | -34 | 46 | 6 | 3.89 | 73 |
| L middle frontal (9) | -36 | 16 | 30 | 3.49 | 83 |
| L cingulate (24) | -12 | 38 | 12 | 4.67 | 546 |
| L orbital frontal (11) | -28 | 36 | -14 | 3.68 | 106 |
| L insula | -42 | 2 | 6 | 3.63 | 90 |
| R superior temporal (22) | 50 | -42 | 6 | 3.49 | 50 |
| R superior temporal (38) | 48 | 14 | -20 | 3.34 | 73 |
| R middle temporal (21) | 50 | -2 | -18 | 4.08 | 65 |
| R middle temporal (21) | 52 | -14 | -12 | 3.86 | 115 |
| R inferior temporal (20) | 52 | -6 | -32 | 4.52 | 201 |
| R fusiform (20) | 40 | -18 | -28 | 4.77 | 127 |
| R lingual (19) | 22 | -44 | -10 | 4.35 | 76 |
| L middle temporal (21) | -54 | -12 | -14 | 4.14 | 299 |
| L middle temporal (21) | -50 | 2 | -32 | 3.63 | 73 |
| L fusiform (20) | -44 | -18 | -30 | 4.35 | 94 |
| L hippocampal (28) | -20 | -10 | -16 | 3.74 | 62 |
| R postcentral (2) | 62 | -18 | 28 | 3.68 | 68 |
| REGRESSIONS RELATING ALS GRAY MATTER ATROPHY TO IMPAIRED COGNITIVE FLEXIBILITY | |||||
| R inferior frontal (47) | 32 | 28 | 0 | 3.47 | 53 |
| L oribital frontal (11) | -32 | 34 | -12 | 3.26 | 57 |
| L insula | -36 | 20 | 4 | 3.64 | 42 |
Figure 2 Panel B also illustrates areas of significantly reduced FA in several WM tracts. Table 3 summarizes areas of reduced FA in WM, corresponding to areas visualized in Figure 2. This included corpus callosum, descending fibers from motor cortex, and projections involving inferior frontal regions including uncinate fasciculus, inferior frontal-occipital fasciculus, inferior longitudinal fasciculus, and cingulum. Regression analysis, also summarized in Table 3 and illustrated in Figure 2, shows that difficulty with cognitive flexibility is related to reduced FA in frontal WM including inferior frontal-occipital fasciculus and uncinate fasciculus, and in corpus callosum.
Table 3. Reduced White Matter Fractional Anisotropy in ALS Relative to Controls, and Regressions Relating Impaired Cognitive Flexibility to Reduced Fractional Anisotropy in ALS.
| Anatomic Locus (White Matter Tract) | MNI Coordinates | Z score | Cluster Size (voxels) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| REDUCED FRACTIONAL ANISOTROPY | |||||||||||
| X | Y | Z | |||||||||
| R inferior frontal WM | 15 | -8 | -13 | 5.23 | 951 | ||||||
| R orbital frontal WM | 10 | 23 | -12 | 5.2 | 418 | ||||||
| R inferior frontal-occipital fasciculus | 26 | 28 | 12 | 4.34 | 248 | ||||||
| R middle frontal-occipital fasciculus | 31 | 11 | 8 | 3.84 | 410 | ||||||
| B corpus callosum (frontal and body) | 0 | -16 | 29 | 5.55 | 11958 | ||||||
| B fornix | -2 | -6 | 17 | 5.59 | 1114 | ||||||
| L inferior frontal gyrus WM | -35 | 12 | 23 | 4.28 | 619 | ||||||
| L inferior frontal-occipital fasciculus | -44 | 35 | 2 | 4.52 | 630 | ||||||
| L inferior longitudinal fasciculus | -41 | 21 | -15 | 4.97 | 2000 | ||||||
| L inferior longitudinal fasciculus | -38 | -5 | -22 | 4.28 | 291 | ||||||
| L cingulum | -21 | -26 | -17 | 4.69 | 283 | ||||||
| L cerebral peduncle | -18 | -13 | -16 | 6.02 | 1446 | ||||||
| REGRESSIONS RELATING ALS REDUCED MATTER ATROPHY FRACTIONAL ANISOTROPY TO IMPAIRED COGNITIVE FLEXIBILITY | |||||||||||
| R uncinate fasciculus | 23 | 21 | -11 | 3.72 | 99 | ||||||
| R corpus callosum (frontal) | 17 | 55 | -6 | 5.02 | 1273 | ||||||
| R corpus callosum (frontal) | 11 | 30 | 7 | 4.18 | 5078 | ||||||
| R corpus callosum (frontal) | 19 | -7 | 50 | 3.2 | 69 | ||||||
| L uncinate fasciculus | -17 | 26 | -10 | 3.27 | 256 | ||||||
| L inferior frontal gyrus WM | -37 | 5 | 23 | 3.65 | 115 | ||||||
| L inferior frontal-occipital fasciculus | -38 | -17 | -5 | 3.92 | 455 | ||||||
| L corpus callosum (frontal) | -15 | 52 | -2 | 4.11 | 745 | ||||||
| L corpus callosum (frontal) | -14 | 44 | 23 | 3.51 | 268 | ||||||
| L corpus callosum (frontal) | -13 | 19 | 44 | 3.09 | 82 | ||||||
Discussion
Our findings suggest that the VVT is sensitive to impairments in cognitive flexibility in ALS. This is a simple, untimed measure that depends minimally on a specific response modality and requires minimal administration time. Therefore, this measure is well-suited for evaluating cognition in the clinic in ALS. Regression analyses related performance on this task to GM regions in prefrontal cortex, areas of known pathological burden in ALS, and interconnected frontal WM projections including uncinate and inferior frontal-occipital fasciculi and corpus callosum.
While informal observation and clinical interview can ascertain impaired cognition in day-to-day performance to some extent, quantification of cognitive difficulty is essential to determining prognosis and progression, and may be helpful in evaluating response during a treatment trial. However, this has proven difficult on most standard cognitive measures such as MMSE and the Addenbrooke Cognitive Examination-Revised(Leyton, Hornberger, Mioshi, & Hodges, 2010) because of the motor component required to assess performance. Indeed, in an unpublished survey of our large clinical cohort, we find that only about 60% of cases are able to complete all components of the MMSE, in contrast to finding that 100% of ALS patients successfully completed the VVT. Measures of executive functioning such as letter-guided category naming fluency are sensitive to cognitive deficits in ALS. While this has been described in many reports(Goldstein & Abrahams, 2013), speech may be slowed in patients with a bulbar motor disorder, and this motor deficit potentially confounds performance on a cognitive task that is timed. An important innovation introduced a manual control task to account in part for the motor deficit, thereby defining more clearly an impairment on the cognitive component of this task(Abrahams et al., 2004). However, this control does not fully eliminate the confounding motor deficit. The basis for a deficit in cognitive flexibility is less confounded on the VVT than the Wisconsin Card Sorting Task, a lengthy measure of cognitive flexibility(Anderson, Damasio, Jones, & Tranel, 1991), because a patient is explicitly told on the VVT that a mental shift is required. Previous studies suggest that as many as 50% of ALS patients have cognitive difficulty(Murphy et al., 2007; Phukan et al., 2012), and the current finding of limited cognitive flexibility on the VVT in 48% of ALS patients is consistent with these prior reports.
ALS patients have been shown to have difficulty on a lengthy and demanding measure of cognitive flexibility(Libon et al., 2012; Taylor et al., 2013). In this work, ALS patients were asked to group a set of 16 cards based on a variety of shared features(Delis, Kaplan, & Kramer, 2001). ALS patients were significantly impaired on this task, and even though it was untimed, a motor component was needed to manipulate the cards into groups. A recognition procedure was also administered to minimize the motor component, and ALS patients remained significantly impaired on this task.
In the present study, we assessed performance on a brief, untimed measure of cognitive flexibility that does not depend on a specific response modality – the VVT. ALS patients had little difficulty initially perceiving a pattern of shared features across geometric designs, and thus perceptual categorization and visual perception of geometric features do not appear to play a major role in ALS patients' deficit. However, patients were significantly impaired on the subsequent measure of cognitive flexibility, since they had difficulty identifying a second set of three stimuli with a different shared feature. VVT performance is impaired in FTD(Eslinger et al., 2007), and our findings showed a significant impairment even when we ascertained performance in the subgroup of ALS patients without co-occurring FTD. As noted in the Results and in Table 1, performance was correlated with letter-guided category naming fluency and reverse digit span, traditional measures of executive functioning, although a caveat is that these latter measures are confounded by a specific response modality or entail a timed response.
Widespread GM and WM disease is now recognized in ALS(Agosta et al., 2007; Ellis, Suckling, & Amaro, 2001; Verstraete et al., 2012). This extends beyond primary motor cortex to include inferior and dorsolateral prefrontal cortical regions important for executive functioning, as well as anterior temporal and parietal GM areas. This corresponds to the anatomic distribution of pathology in ALS(Geser et al., 2008). Likewise, there is substantial disease in the associated WM corticospinal fibers and WM tracts implicated in the prefrontal projections that subserve large-scale cognitive networks thought to play a role in executive functioning(Mioshi et al., 2013), and this too corresponds to WM pathology in ALS(Douaud, Filippini, Knight, Talbot, & Turner, 2011).
In the present study, we observed disease in GM regions and WM tracts comparable to that reported in previous studies. Moreover, we were able to identify a compromised network of GM regions and WM tracts that is associated with impaired cognitive flexibility in ALS. A regression analysis thus implicated inferior frontal cortex in impaired cognitive flexibility. Previous fMRI work in healthy adults has demonstrated the contribution of inferior frontal cortex to inhibitory control and set switching(Goel & Vartanian, 2005). Others have used fMRI in healthy adults to demonstrate a role for the insula in cognitive flexibility and set-switching(Chang et al., 2013). Regression analysis in the present study implicated insula in cognitive flexibility difficulty in ALS as well. While previous work in ALS also associated dorsolateral prefrontal areas with impaired cognitive flexibility in ALS(Abrahams et al., 1996; Abrahams et al., 2004), we may not have observed this association for several reasons. For example, prior work may have combined complex cognitive challenges with the demands of a motor component in patients with a motor deficit. However, our previous study related category recognition performance to dorsolateral prefrontal GM atrophy on a task with minimal motor demands(Libon et al., 2012). This raises the possibility that the relative demands of the cognitive task itself may influence the anatomic distribution of prefrontal cortex implicated in cognitive flexibility, with more demanding tasks associated with dorsolateral prefrontal cortex and less demanding tasks associated with ventral frontal regions. fMRI studies in healthy adults have shown different patterns of prefrontal activation depending on the cognitive demands of the task(Badre & D'Esposito, 2007; Koechlin & Jubault, 2006). Additional work is necessary to examine the role of varying cognitive task demands explicitly in the GM basis for impaired cognitive flexibility in ALS.
WM disease has been observed consistently enough in ALS to warrant the proposal that this may serve as a biomarker of ALS(Foerster et al., 2013). We too observed significant WM disease in corpus callosum and WM tracts in the frontal lobe. Rare studies have related WM disease directly to motor deficits in ALS(Verstraete, Veldink, Mandl, van den Berg, & van den Heuvel, 2011). Previous studies related impaired letter-guided category naming fluency to WM volume in ALS(Abrahams et al., 2005) and to reduced FA in the cingulum(Sarro et al., 2011). More recent work related FA in several WM regions of interest to performance on a largely motor-free dual task but not to measures of information processing speed(Pettit et al., 2013). In the present study, regressions related WM projections in the frontal lobe to impaired cognitive flexibility of ALS. This included inferior-frontal-occipital fasciculus, linking prefrontal and posterior temporal regions. Temporal regions presumably contribute to the perception of the visual stimuli, while inferior frontal regions maintain control over processing the perceived visual sets. We also found that uncinate fasciculus is implicated in cognitive flexibility. This links inferior frontal and anterior temporal regions that may be related to inhibitory control. Finally, the regression analysis implicated anterior corpus callosum in cognitive flexibility, and this may be important in coordinating performance across left and right frontal regions. Our work thus describes a potential structural connectome critically implicated in cognitive flexibility deficits in ALS.
Several caveats should be kept in mind when considering our findings. While behavioral data were available on a large number of ALS patients, we were able to obtain imaging studies only in a subgroup of these patients because of respiratory limitations when prone for a prolonged period of time in the magnet bore. The precise role of each frontal region and WM projection in cognitive flexibility cannot be ascertained in detail with the materials and procedure used in this study. Although we report an unvalidated MMSE score that adjusts for items that could not be completed because of motor difficulty, we include this measure to provide the reader with a rough clinical guide of overall cognitive functioning. With these caveats in mind, our findings are consistent with the hypothesis that many patients with ALS have a deficit in cognitive flexibility even when using a simple, untimed task that minimizes motor confounds, and this deficit appears to be associated with disease in a large-scale neural network involving GM and WM regions in non-motor portions of the frontal lobe.
Acknowledgments
This work was supported in part by the National Institutes of Health (AG032953, AG017586, AG038490, NS044266, NS053488, AG043503), the ALS Association and the Wyncote Foundation
References
- Abrahams S, Goldstein LH, Kew JJM, Brocis DJ, Lloyd CM, Frith CD, Leigh PN. Frontal lobe dysfunction in amyotrophic lateral sclerosis: A PET study. Brain. 1996;119:2105–2120. doi: 10.1093/brain/119.6.2105. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Goldstein LH, Simmons A, Brammer M, Williams SCR, Giampietro V, Leigh PN. Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain. 2004;127:1507–1517. doi: 10.1093/brain/awh170. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Goldstein LH, Suckling J, Ng V, Simmons A, Chitnis X, et al. Leigh PN. Frontotemporal white matter changes in amyotrophic lateral sclerosis. Journal of Neurology. 2005;252(3):321–331. doi: 10.1007/s00415-005-0646-x. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS) Neuropsychologia. 2000;38:734–747. doi: 10.1016/s0028-3932(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Agosta F, Pagani E, Rocca MA, Caputo D, Perini M, Salvi F, et al. Filippi M. Voxel-based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Human Brain Mapping. 2007;28(12):1430–1438. doi: 10.1002/hbm.20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Partial transformations of diffusion tensor magnetic resonance images. IEEE Transactions for Medical Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Anderson S, Damasio H, Jones R, Tranel D. Wisconsin card sorting test performance as a measure of frontal lobe damage. Journal of Clinical and Experimental Neuropsychology. 1991;13:909–922. doi: 10.1080/01688639108405107. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional Magnetic Resonance Imaging Evidence for a Hierarchical Organization of the Prefrontal Cortex. Journal of Cognitive Neuroscience. 2007;19(12):2082–2099. doi: 10.1162/jocn.2007.19.12.2082. Article. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, et al. Trojanowski JQ. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of Neurology. 2013;74(1):20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cerebral Cortex. 2013;23(3):739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PA, Bai Y, Nadjati-Gilani S, Seunarine KK, Hall MG, Parker GJM, Alexander DC. Camino: Open-source diffusion-MRI reconstruction and processing; Paper presented at the International Society for Magnetic Resonance in Medicine; Berkeley. 2006. [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Rademakers R. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function Battery. Texas: Psychology Press; 2001. [Google Scholar]
- Douaud Gl, Filippini N, Knight S, Talbot K, Turner MR. Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain. 2011;134(12):3467–3476. doi: 10.1093/brain/awr279. [DOI] [PubMed] [Google Scholar]
- Ellis CM, Suckling J, Amaro E. Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology. 2001;57:1571–1578. doi: 10.1212/wnl.57.9.1571. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Troiani V, Antani S, Cross K, Kwok S, Grossman M. Oops! Resolving social dilemmas in frontotemporal dementia. Journal of Neurology, Neurosurgery and Psychiatry. 2007;78:457–460. doi: 10.1136/jnnp.2006.098228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman MJ, Drasgow J. The Visual-verbal Test: Manual. Western Psychological Services; 1960. [Google Scholar]
- Foerster BR, Dwamena BA, Petrou M, Carlos RC, Callaghan BC, Churchill CL, et al. Pomper MG. Diagnostic Accuracy of Diffusion Tensor Imaging in Amyotrophic Lateral Sclerosis: A Systematic Review and Individual Patient Data Meta-Analysis. Academic Radiology. 2013;20(9):1099–1106. doi: 10.1016/j.acra.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, et al. Trojanowski JQ. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66(2):180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM, et al. Trojanowski JQ. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathologica. 2008;145:115–133. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- Goel V, Vartanian O. Dissociating the Roles of Right Ventral Lateral and Dorsal Lateral Prefrontal Cortex in Generation and Maintenance of Hypotheses in Set-shift Problems. Cereb Cortex. 2005;15(8):1170–1177. doi: 10.1093/cercor/bhh217. doi:10.1093/cercor/bhh217. [DOI] [PubMed] [Google Scholar]
- Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. The Lancet Neurology. 2013;12(4):368–380. doi: 10.1016/S1474-4422(13)70026-7. http://dx.doi.org/10.1016/S1474-4422(13)70026-7. [DOI] [PubMed] [Google Scholar]
- Grossman M, Peelle JE, Smith EE, McMillan CT, Cook PA, Powers JM, et al. Burkholder L. Category-specific semantic memory: Converging evidence from bold fMRI and Alzheimer's disease. Neuroimage. 2013;68(0):263–274. doi: 10.1016/j.neuroimage.2012.11.057. http://dx.doi.org/10.1016/j.neuroimage.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, McMillan CT, Brettschneider J, Libon DJ, Powers JM, Rascovsky K, et al. Grossman M. Cognitive decline and reduced survival in C9orf72 expansion frontotemporal degeneration and amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery & Psychiatry. 2013;84(2):163–169. doi: 10.1136/jnnp-2012-303507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirnan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Zoing MC. Amyotrophic lateral sclerosis. The Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- Kew JJM, Goldstein LH, Leigh PN, Abrahams S, Cosgrave N, Passingham RE, et al. Brooks DJ. The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis: A neuropsychological and positron emission tomography study. Brain. 1993;116:1399–1423. doi: 10.1093/brain/116.6.1399. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Jubault T. Broca's Area and the Hierarchical Organization of Human Behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Hornberger M, Mioshi E, Hodges JR. Application of Addenbrooke's Cognitive Examination to Diagnosis and Monitoring of Progressive Primary Aphasia. Dementia and Geriatric Cognitive Disorders. 2010;29(6):504–509. doi: 10.1159/000313980. [DOI] [PubMed] [Google Scholar]
- Libon DJ, McMillan C, Avants B, Boller A, Morgan B, Burkholder L, et al. Grossman M. Deficit in concept formation in amyotrophic lateral sclerosis. Neuropsychology. 2012;26:422–429. doi: 10.1037/a0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioshi E, Lillo P, Yew B, Hsieh S, Savage S, Hodges JR, et al. Hornberger M. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology. 2013;80(12):1117–1123. doi: 10.1212/WNL.0b013e31828869da. [DOI] [PubMed] [Google Scholar]
- Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. The Lancet. 2007;369:2031–2041. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Henry RG, Langmore S, Kramer JH, Miller BL, Lomen-Hoerth C. Continuum of Frontal Lobe Impairment in Amyotrophic Lateral Sclerosis. Arch Neurol. 2007;64(4):530–534. doi: 10.1001/archneur.64.4.530. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micseny MC, Chou TT, et al. Lee VMY. Ubiquinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclereosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Pettit LD, Bastin ME, Smith C, Bak TH, Gillingwater TH, Abrahams S. Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain. 2013;136(11):3290–3304. doi: 10.1093/brain/awt243. [DOI] [PubMed] [Google Scholar]
- Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, et al. Hardiman O. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. Journal of Neurology, Neurosurgery & Psychiatry. 2012;83(1):102–108. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, SimÛn-S·nchez J, Rollinson S, Gibbs JR, et al. Traynor BJ. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Pena A, Menon DK, Carpenter TA, Pickard JD, Bullmore ET. Formal characterization and extension of the linearized diffusion tensor model. Human Brain Mapping. 2005;24:144–155. doi: 10.1002/hbm.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro L, Agosta F, Canu E, Riva N, Prelle A, Copetti M, et al. Filippi M. Cognitive Functions and White Matter Tract Damage in Amyotrophic Lateral Sclerosis: A Diffusion Tensor Tractography Study. American Journal of Neuroradiology. 2011;32(10):1866–1872. doi: 10.3174/ajnr.A2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Struss EA. Compendium of neuropsychological tests. New York: Oxford University Press; 1990. [Google Scholar]
- Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Figlewicz D. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis. 2009;10(3):131–146. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- Taylor LJ, Brown RG, Tsermentseli S, Al-Chalabi A, Shaw CE, Ellis CM, et al. Goldstein LH. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? Journal of Neurology, Neurosurgery & Psychiatry. 2013;84(5):494–498. doi: 10.1136/jnnp-2012-303526. [DOI] [PubMed] [Google Scholar]
- Verstraete E, Veldink JH, Hendrikse J, Schelhaas HJ, van den Heuvel MP, van den Berg LH. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery & Psychiatry. 2012;83(4):383–388. doi: 10.1136/jnnp-2011-300909. [DOI] [PubMed] [Google Scholar]
- Verstraete E, Veldink JH, Mandl RCW, van den Berg LH, van den Heuvel MP. Impaired Structural Motor Connectome in Amyotrophic Lateral Sclerosis. PLoS ONE. 2011;6(9):e24239. doi: 10.1371/journal.pone.0024239. [DOI] [PMC free article] [PubMed] [Google Scholar]


