Abstract
Obesity is a major public health concern. This condition results from a constant and complex interplay between predisposing genes and environmental stimuli. Current attempts to manage obesity have been moderately effective and a better understanding of the etiology of obesity is required for the development of more successful and personalized prevention and treatment options. To that effect, mouse models have been an essential tool in expanding our understanding of obesity, due to the availability of their complete genome sequence, genetically identified and defined strains, various tools for genetic manipulation and the accessibility of target tissues for obesity that are not easily attainable from humans. Our knowledge of monogenic obesity in humans greatly benefited from the mouse obesity genetics field. Genes underlying highly penetrant forms of monogenic obesity are part of the leptin-melanocortin pathway in the hypothalamus. Recently, hypothesis-generating genome-wide association studies for polygenic obesity traits in humans have led to the identification of 119 common gene variants with modest effect, most of them having an unknown function. These discoveries have led to novel animal models and have illuminated new biologic pathways. Integrated mouse-human genetic approaches have firmly established new obesity candidate genes. Innovative strategies recently developed by scientists are described in this review to accelerate the identification of causal genes and deepen our understanding of obesity etiology. An exhaustive dissection of the molecular roots of obesity may ultimately help to tackle the growing obesity epidemic worldwide.
Keywords: Polygenic obesity, Monogenic obesity, Genetics, Integrative biology, Human, Mouse, Genome-wide association study, Next generation sequencing, Knock-out, Transgenic
Introduction
Obesity is a worldwide epidemic affecting over 400 million adults (World Health Organization, 2011), and is defined as the accumulation of excess body fat to the extent that it results in other health complications and reduces life expectancy (Bahreini et al., 2013). Co-morbidities associated with obesity include psychological distress, osteoarthritis, type 2 diabetes mellitus, hypertension, hyperlipidemia, liver steatosis, cardiovascular disease and certain types of cancer (Switzer, Mangat & Karmali, 2013). The steady increase in life expectancy due to advanced medical treatment may be reversed by negative impacts of obesity on youth today in Westernized countries (Olshanky et al., 2005).
Unfortunately, attempts to prevent obesity have had limited success thus far. The individualistic lifestyle approach of “eat less, move more” has been ineffective as a preventive measure for obesity (Kirk et al., 2012). Effective obesity prevention and management is dependent on the acknowledgement that obesity goes beyond the individual behavior and is influenced by genetics, psychology, society and public policy (Kirk, Penney & McHugh, 2010).
The rise of obesity coincided with several major societal and environmental changes that are likely causal factors. The umbrella term ‘obesogenic’ has been applied to these changes, which include excessive consumption of energy dense foods, sedentary lifestyles, urbanization, and socioeconomic-dependent access to a healthy diet (Hill, 1998; Misra & Khurana, 2008; Must et al., 1999). Aside from environmental factors, considerable evidence from twin, adoption, and family studies indicates that 40 to 70% of BMI variation is due to genetic factors (Choquet & Meyre, 2011). Obesity is a heritable neurobehavioral condition that is highly sensitive to the environment (Ahmad, Varga & Franks, 2013; O’Rahilly & Farooqi, 2008). Understanding the molecular roots of obesity is an important prerequisite to improve both prevention and management of this condition (Bessesen, 2008). This has prompted considerable effort to identify the genes predisposing to obesity by conducting studies in rodents and humans. This review summarizes the progress in the elucidation of obesity genes focusing on the synergies developed between mouse and human obesity genetic fields. The paper also reviews the innovative strategies that synergize the two disciplines to comprehensively uncover the genetic architecture of obesity.
What Have Mouse Models Taught us About Human Obesity?
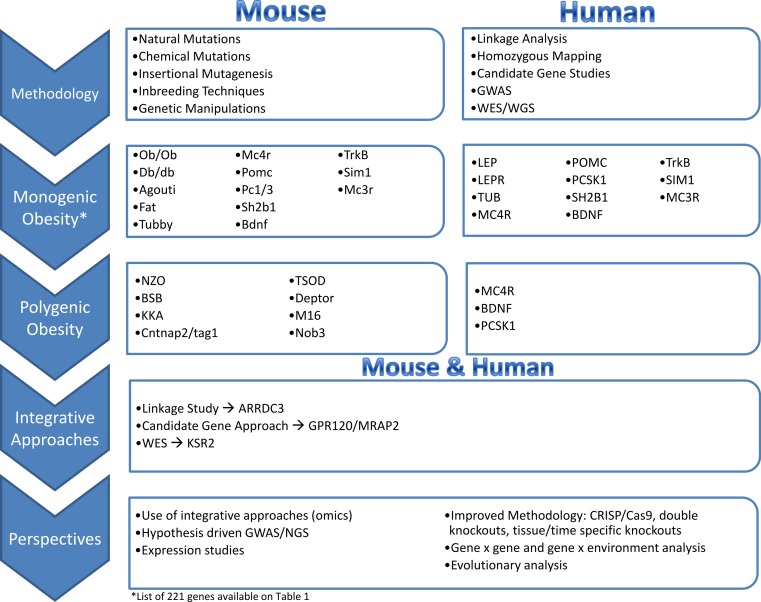
Mouse models are the most common experimental animals in obesity genetics. General advantages of mouse models include: low maintenance cost, small size, ease of breeding and short gestation period. They reach sexual maturity faster than other mammals and have a shorter life span (Lee & Cox, 2011; Rosenthal & Brown, 2007). The availability of genetically defined strains, the complete genome sequence of numerous strains, dense single nucleotide polymorphism (SNP) characterization of many others, and well-developed genetic manipulation tools facilitate sophisticated genetic analyses. Genetic manipulation techniques employed in mouse models, such as knock-out/knock-in, overexpression or tissue-specific expression methods can be used to test the functionality of genes associated with obesity (Cox & Church, 2011). Additionally, since homozygote null mutations are exceptionally rare in humans, knock-out mouse models are an attractive alternative to study such rare occurrences (Rees & Alcolado, 2005). Mice also exhibit obesity and metabolic phenotypes that are comparable to humans and can be measured with standardized diagnostic tests (Toye et al., 2004). More detailed phenotyping, such as direct metabolic measurements and assessment of body fat content, that are difficult and costly in large numbers of humans are also possible in mice (Butler & Kozak, 2010; Ellacott et al., 2010; Kaiyala et al., 2010). Importantly, environmental factors can be carefully controlled and specifically manipulated in mouse models (Ayala et al., 2010). Reducing environmental heterogeneity results in an increased power to link genetic variation to the phenotypic differences observed. Specific environmental manipulations allow direct testing of hypotheses to inform about gene-environment interactions. Mice also provide obesity-related tissues such as brain tissue that are otherwise difficult to obtain in humans (Tung et al., 2010).
However, it should be noted that mouse models of obesity are not without their limitations. Different conclusions may arise in mouse and human because of the use of different phenotypes in the study. For instance, BMI measurements are typical in human studies, whereas the direct measurement of percent body fat or body fat mass is more common in mouse models. Unlike humans, there is no defined threshold for obesity based on BMI in mice. Also, in comparison to humans, the secondary complications of obesity substantially depend upon the background strain (Clee & Attie, 2007). Moreover, some physiological differences between humans and mice make studying certain important genes or pathways difficult. For example, the role of β-MSH in control of energy balance was overlooked in humans mainly due to the fact that mouse models lack β-MSH (Lee et al., 2006).
Tools and Approaches Available in Mouse and Human
Human genetics approaches
Linkage analysis
This approach aims to map the location of a disease causing loci by looking for genetic markers that co-segregate with the disease within pedigrees (Teare et al., 2005). Different linkage approaches are applied depending on the type of the disease or trait. For example, parametric analysis is used if the disease is a Mendelian disease (Li & Meyre, 2014).
Homozygosity mapping
This is a powerful method to map genes responsible for recessive Mendelian disorders in consanguineous pedigrees. This approach requires less than a dozen of affected individuals, and no additional family members are required to identify the disease causing locus (Lander & Botstein, 1987).
Candidate gene studies
Candidate gene approach is hypothesis-driven and has been widely used before the rise of GWAS. Candidate genes have a known biological function that directly or indirectly influence the trait being investigated (Zhu & Zhao, 2007). The main disadvantage of this approach is that it is heavily reliant on the current level of knowledge of a specific gene (Hirschhorn et al., 2002). Candidate genes also have a low success rate overall, as consistent associations have been reported only for a selected few candidate genes (Vimaleswaran et al., 2012).
Genome-wide association studies
This approach exhaustively tests the genotype/phenotype associations across up to 4.8 million genetic markers and to date represents the most efficient way to identify common variants (MAF>1%) associated with complex diseases (Visscher et al., 2012).
Whole exome/whole genome sequencing
This relatively new approach is efficiently applied to identify rare variants associated with Mendelian or complex traits for a reasonable cost in comparison to classical approaches such as Sanger sequencing. It is powerful because it detects mutations in novel genes not previously detected by candidate gene approaches. The main challenge is to identify a causal gene analyzing the large sequencing dataset (Li & Meyre, 2014). With advances in sequencing technology, it is now possible to sequence approximately 95% of all protein-coding bases of all known genes (the “exome”) at a cost that is comparable to sequencing a single gene by the Sanger method (Shendure, 2011; Singleton, 2011). Despite the fact that whole-genome sequencing experiments are more expensive than whole-exome sequencing experiments, they are more and more used to identify genetic variants associated with Mendelian and complex traits (Morrison et al., 2013; Styrkarsdottir et al., 2014).
Mouse genetic approaches
Natural mutations
Naturally occurring mutations are spontaneous mutations in mice that could be linked to the trait of interest. Natural mutations can range from simple single nucleotide substitution to complex rearrangements (Justice, Siracusa & Stewart, 2011). They occur by chance and transmission from parent to offspring results in fixation of these mutations within a population (Justice, Siracusa & Stewart, 2011). These mutations are often studied by quantitative trait loci (QTLs), which link a chromosomal region to the trait of interest (Chiu et al., 2006; Diament, Fisler & Warden, 2003).
Although studying natural variants may be appealing, regrettably the spontaneity of their appearance is often matched by their impromptu disappearance (Stanford, Cohn & Cordes, 2001). Furthermore, studying obesity genes in mouse models with natural mutations may be a more time consuming approach compared to chemically induced mutations.
Chemically induced mutations
Chemical mutagenesis increases frequency and variety of mutations for functional genetic studies. Furthermore, with the use of inbreeding techniques, chemical mutagenesis can create a genetic variant that is identical to parent strain except for the induced mutation that may be responsible for phenotypic diversity compared to parent strain (Svenson, Bogue & Peters, 2003). This approach creates a set of mutants that differ minimally in genotype from parental strain, but differ robustly in phenotype, making it a promising approach in functional genetic studies (Svenson, Bogue & Peters, 2003).
Successful genetic manipulation requires DNA modifications of germ-line cells so that the modification is heritable (Strachan & Read, 1999). Target cells usually can differentiate into different cells or give rise to germ-line cells, which makes embryonic stem cells (ES) ideal because they can differentiate to somatic and germ line cells (Strachan & Read, 1999).
X-ray mutagenesis
This method induces mutations 20–100 times greater than spontaneous mutations. It causes chromosomal rearrangements which can range from simple deletions, inversions and translocations to complex rearrangements (Silver, 1995). In this approach, several genes are affected by chromosomal rearrangements, therefore this method adds complexity to genetic studies and makes it difficult to dissect individual gene function (Stanford, Cohn & Cordes, 2001).
Chlorambucil
Chlorambucil (CAB) induces similar chromosomal translocations and multigene deletions to X-ray (Russell et al., 1989). CAB is an alkalyting agent that impacts cell division and results in aneugenic activity (Efthimiou et al., 2007). CAB causes smaller deletions and translocations in comparison to X-ray mutagenesis, but it does not lead to single-gene identification and therefore, is not used in high-throughput approaches (Stanford, Cohn & Cordes, 2001).
Ethylnitrosourea
Ethylnitrosourea (ENU) is an alkylating agent that induces point mutations in the DNA of spermatogonial stem cells via single-base mismatching to the unrepaired alkylated base (Svenson, Bogue & Peters, 2003). ENU is advantageous since it is easy to administer, results in higher mutagenesis rate and is amendable to high-throughput screening (Justice, 2000). Through international collaborative efforts several archives of DNA, embryonic stem (ES) cells or typically sperm, from mutagenized mice have been created and catalogued along with some standardized phenotypic data and the listing of mutations they contain. The corresponding ES cells/sperm can be ordered and used to regenerate mice harboring the mutation of interest (Acevedo-Arozena et al., 2008).
Insertional mutagenesis
Pronuclear injection
This approach involves microinjection of DNA into fertilized oocytes to affect the function of an endogenous gene. This approach requires the cloning of cDNA (coding sequence of a selected gene). The sequence is then inserted in frame with a constitutively active promoter that drives transcription (Gordon & Ruddle, 1981; Harbers, Jahner & Jaenisch, 1981). This method disturbs endogenous gene expression and can generate chromosomal rearrangements and deletions (Belizário et al., 2012). Pronuclear injections are labor intensive and highly technical, thus they are not used in high-throughput screening (Stanford, Cohn & Cordes, 2001).
Gene targeting
Targeted mutagenesis by homologous recombination in embryonic stem cells is used to efficiently target a single gene (Sun, Abil & Zhao, 2012). Gene targeting was first pioneered by Thomas & Capecchi (1987) who were able to exchange the endogenous gene with a mutated copy in cultured mammalian cells by using a homologous sequence of the gene. This approach is able to produce specific alterations to the mouse genome to analyze targeted gene function (Menke, 2013).
Gene trapping
Gene trapping is a vector insertion that disrupts the regular transcription of endogenous genes (O’Kane & Gehrin, 1987). Gene trapping includes enhancer, promoter and exon traps. The enhancer trap is used for gene identification, and it involves the introduction of a reporter construct that requires a cis-acting DNA to activate gene expression. Genes are then identified depending on the expression information (Yamamura & Araki, 2008). The promoter and the exon traps are mainly used for mutagenesis. The promoter trap contains the coding sequence of the reporter gene and can interfere with normal coding capacity of endogenous genes and create a mutation. The exon trap is designed to create spliced fusion transcripts between the reporter and the endogenous gene (Yamamura & Araki, 2008). Gene trapping is an efficient system for simultaneous studies of gene function, sequence and expression (Stanford, Cohn & Cordes, 2001). Many of the targeted ES cells produced by the international consortia include gene-trap vectors.
Lentivirus vectors
Viral vectors are a stable, long-term gene delivery system of genetic information to host cells. The system depends on replicating viruses that have the genetic information for the targeted gene instead of their own coding regions (Kootstra & Verma, 2003). Lentiviral vectors are often preferred over other viral vectors because they are more efficient at delivering complex gene expression cassettes (May et al., 2000), they can mediate long term gene expression (Seppen et al., 2001) and they are relatively safe (Brown et al., 2010). They provide high control over the manipulated gene, and are ideal for studying gene function in small populations (Osten, Dittgen & Licznerski, 2006). Other viral vectors that are commonly used, particularly adeno-associated virus (AAV) vectors, have different tissue tropisms that can be used for the over-expression of genes of interest even within the central nervous system, that may be of particular relevance for obesity (Lentz, Gray & Samulski, 2012). The development of novel viral vectors is ongoing (Huang & Kamihira, 2013). Recent advances in materials and nanotechnologies may also facilitate non-viral methods of direct gene delivery (Yin et al., 2014), although these are not yet routinely used.
Inbreeding methods
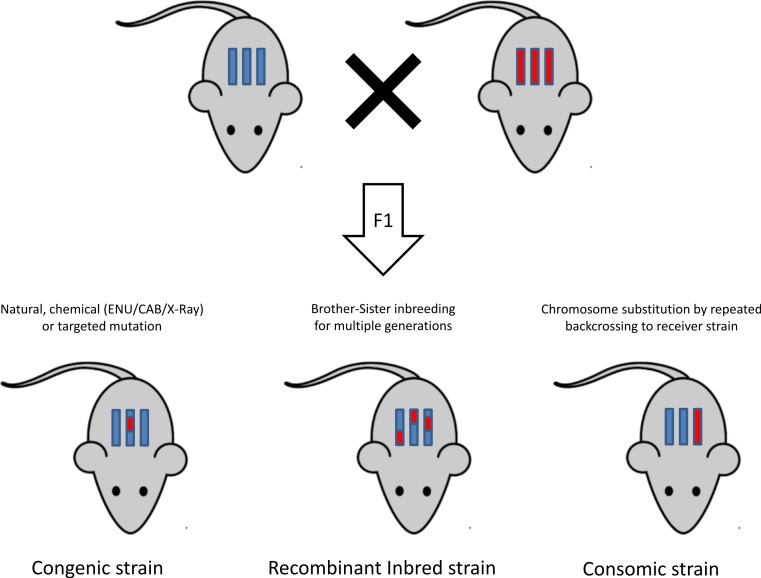
Once a mutation is successfully induced, a mutant model is obtained and the next step is to dissect the genetic roots of the phenotype. Figure 3 is a graphical representation of different inbreeding techniques (Rogner & Avner, 2003). This section provides an overview of the different inbreeding approaches used to reach this objective.
Figure 3. General overview of mutagenesis and inbreeding in mice.
Congenic, recombinant inbred and consomic mice are obtained when part of the genome of one mouse strain is transferred to another strain by backcrossing the donor mouse to the receiver strain. In congenic mouse, the offspring resembles the parent strain except for the mutated chromosomal segment, whereas in consomic strain, the offspring carries an entire chromosome from the donor strain. Recombinant inbred strains are obtained by cross breeding of inbred mice to increase their genotypic diversity and carrying a series of brother-sister mating for multiple generations.
Genetic cross
Genetic cross is a classical cross where two inbred strains are mated and their offspring are either mated to each other (an intercross F2 design) or to a progenitor strain (a backcross design) (Flint & Eskin, 2013). Second-generation offspring are then phenotyped and genotyped, and linkage analysis is carried out to identify a region that is associated with the trait of interest (Silver, 1995).
The classical inbred strains
This method provides a higher mapping resolution than the genetic cross, since the inbred mouse strains are separated from their founders by more generations, thus increasing the recombination events between the genomes of the founding strains (McClurg et al., 2007). These strains are commercially available for purchase from vendors, and no breeding steps are required in this approach.
The recombinant inbred lines
Recombinant inbred lines are created through cross breeding of inbred mice, amplifying the genotypic/phenotypic diversity found across inbred mice. The power of recombinant inbred lines are in that they represent a fixed polygenic model that can be phenotyped deeply, in multiple environments (Zou et al., 2005). This fixed model is established via crossing two different inbred parental strains to produce F1 offspring, and then generating a series of brother-sister mating for at least 20 generations. This produces fully inbred strains which are homozygous at all loci for a unique combination of the original parental genomes (Pomp, 2007). An example of recombinant inbred lines is the Hybrid Mouse Diversity Panel (HMDP). This is a panel of approximately 100 strains that are phenotyped and association is carried out after correcting for population structure using efficient mixed-model association and made available to the scientific community. The combined populations in the HMDP provide a high statistical power and a high resolution (Flint & Eskin, 2013), which makes this model ideal for systems-level analysis of gene by environment interactions (Parks et al., 2013).
Another illustration of recombinant lines is the Collaborative Cross, which is a large-scale effort to create a set of recombinant inbred strains that are specifically designed for mapping traits (Aylor et al., 2011). By using wild derived strains, a substantial amount of genetic diversity is introduced, giving the collaborative cross the advantage of covering more genetic variations compared to other approaches (Philip et al., 2011).
Chromosome substitution strains or consomic strains
Chromosome substitution occurs when a single, full-length chromosome from one inbred strain has been transferred onto the genetic background of a second strain by repeated backcrossing (Nadeau et al., 2000). The method involves construction of chromosome substitution strains (CSS) between a donor and a host strain, which partitions the variation between two strains and becomes a resource for studying genetic control of phenotype variation (Singer et al., 2004).
Long-term selection lines
Long-term selection lines are developed through selective breeding for a wide variety of phenotypes (Paigen, 2003). Several of these lines can be joint and inbred to characterize different metabolic traits together and develop models for gene mapping. For example, the body fat phenotype in mice were developed by long-term selection of fat (F) and lean (L) mice in over 60 generations (Horvat et al., 2000). A genome-wide quantitative trait locus (QTL) analysis of a cross between F and L lines revealed QTLs that mapped to regions that were previously described as obesity QTLs (Horvat et al., 2000).
The heterogeneous stock
The heterogeneous stock (HS) uses outbred mice to increase statistical power compared to recombinant inbred strains. The outbred mice are similar to F2 animals from a cross, but they have ancestry from eight founder strains instead of only two, and the population is bred for more generations (Valdar et al., 2006). Commercial outbred stock animals have been maintained for many generations, and they provide high-resolution mapping (Flint & Eskin, 2013).
Genetic manipulations in mice
Gene manipulation techniques allow for definitive alterations of specific genes at the systemic level. They also enable gene alterations in a time or tissue specific manner (Speakman et al., 2007).
Over-expression of target genes
This method involves cloning of a full-length coding sequence downstream of a promoter, which may provide a global or tissue-specific overexpression of a target gene in the transgenic offspring (Speakman et al., 2007). This technique is straightforward and inexpensive, but the extent of changes in gene and protein expression is not always predictable. It can also disturb endogenous gene expression (Justice, Siracusa & Stewart, 2011; Stanford, Cohn & Cordes, 2001).
Knock-out models
Knock-out models involve the total ablation of the target gene in all tissues, but the ablation of target genes could reveal unpredicted effects (Davey & MacLean, 2006). For example, homozygous knock-outs may result in embryonic death (eg: Sim1−/−) (Michaud et al., 2001), or developmental compensation (eg: Npy) (Erickson, Hollopeter & Palmiter, 1996; Erikson, Clegg & Palmiter, 1996) in which no particular phenotype will be observed. In the example of Sim1, heterozygous knock outs of this gene survive, but develop severe obesity associated with increase in food intake without measurable energy expenditure. This is indicative of how Sim1 plays a role in energy homeostasis (Ramachandrappa et al., 2013).
SiRNA/shRNA
Conditional gene knockdown is a powerful tool for studying gene function, and methods of gene knockdowns are in constant evolution (Brown et al., 2010). The discovery of small interfering RNA (siRNA) as a viable mechanism for eliciting RNA interference (RNAi) expanded opportunities for functional genetic studies (Elbashir et al., 2001). Vector directed siRNA technology allows for rapid generation of large number of knockdown mice in any strain of interest (De Souza et al., 2006), which produces strong and specific suppression of gene expression with no cytotoxicity (Elbashir et al., 2001).
Short hair pin RNAs (shRNA) provide a more straightforward approach for down regulation of gene expression, because unlike siRNAs, they can be stably expressed within the cell and are not lost with cell division (Brown et al., 2010). Lentivirus vectors containing polymerase III promoters for shRNA expression and polymerase II promoter for fluorescent protein expression (for labeling the cell) can be used in order to knock-down endogenous genes (Dittgen et al., 2004). An example of this application is silencing of the leptin receptor gene related protein (OB-RGPR) that was accomplished via lentiviral vector encoding a shRNA directed against OB-RGPR in the hypothalamus (Couturier et al., 2007). Silencing of OB-RGPR in hypothalamus prevents the development of diet-induced obesity in mice fed high-fat diet (HFD) (Couturier et al., 2007). Expression of microRNAs (miRNA) can also be used to modulate gene expression (Casola, 2010).
Knock-in models
These models involve the replacement of the endogenous gene with the mutated form and are used to study more specific roles of the changes in protein function (Speakman et al., 2007). This technique could also be used to confirm the impact of target mutations on phenotype of the disease. For example, humans with dominant negative PPARγ L466A mutation display severe insulin resistance, dyslipidemia and hypertension (Freedman et al., 2005). Further studies of the mutation in mice revealed that mice with Pparγ knock-in L466A mutation exhibit lipodystrophy, decrease in adipogenic genes, high circulating free fatty acids (FFAs), and low adiponectin. Human studies of this mutation, coupled with the animal experiments confirm the importance of PPARγ in adipose tissue maintenance (Freedman et al., 2005)
The Cre/loxP system
This is a tool for tissue-specific and time specific knock out genes. When Cre is expressed in mice with a loxP containing gene, the desired gene is excised (Kühn & Torres, 2002). The expression of Cre can be driven either through a transgenic under a tissue specific and/or temporally regulated promoter, or by direct delivery to cells. Depending on the specific tissue or time of Cre expression, modifications can be restricted to a certain cell type or a development stage (Kühn & Torres, 2002). Delivery of Cre via viral vectors provides a more specific gene delivery in the nervous system (Kaspar et al., 2002), particularly if performed by targeted injection.
Newer gene expression manipulation techniques such as zinc fingers and TALENs are also used in obesity genetics field, albeit less frequently than Cre/loxP. Zinc fingers are a versatile DNA recognition domain that have been combined in a modular fashion to generate fusion proteins that recognize unique DNA sites in complex eukaryotic genomes (Urnov et al., 2010). Similarly, transcription activator-like effector nuclease (TALENs) from pathogenic bacterium Xanthomonas can be engineered to virtually bind to any DNA sequence (Boch, 2011). Each TALEN can travel to the nucleus, bind to the promoters of target genes and induce transcription based on their specific DNA-binding site (Boch, 2011).
The Golden Age of Mouse Obesity Genetics
Monogenic obesity mouse models and candidate gene studies in human
The identification of genes underlying monogenic obesity relied heavily on mouse genetic studies. By searching the available literature on mouse models of obesity, we collected 221 genes that have been linked to obesity or weight gain via knock-out or transgenic mice, or by utilizing techniques such as over-expression or Cre/loxP (Table 1). We conducted this literature review by searching key terms in PubMed and OMIM databases. We have focused on the obesity and weight gain phenotype and did not detail genes responsible for leanness phenotypes. Leanness may truly result from the manipulation of a gene important in energy balance (e.g., FTO inactivation leads to leanness, FTO overexpression leads to obesity). However, leanness may also be linked to toxicity and sickness of the animal due to genetic manipulations (Reed, Lawler & Tordoff, 2008). The study of monogenic obesity in mice pioneered our understanding of the mechanisms underlying the regulation of body weight in humans. The genes underlying monogenic forms of obesity in humans all encode members of these highly conserved pathways, which are essential in regulation of body weight and energy homeostasis (Fig. 1) (Choquet & Meyre, 2011; Farooqi & O’Rahilly, 2008). Since a detailed discussion of all the genes listed in Table 1 is not feasible for one review paper, we will focus on a subset of the genes that are all part of the leptin/melanocortin or paraventricular nucleus development pathways in the section below.
Table 1. List of genes experimented in mouse models for obesity or obesity-related phenotypes.
| Num | Cand. genes | Phenotypic details | Technique | Ref |
|---|---|---|---|---|
| 1 | ACADVL | Adult-onset fat mass gain | Knock-out | Exil, VJ. 2003. Circ Res |
| 2 | ADAR2 | Obesity under HFD* | Transgenic | Singh, M. 2007. J Biol Chem |
| 3 | ADRA1B | Accelerated weight gain on HFD | Knock-out | Burcelin, R. 2004. J Biol Chem |
| 4 | ADRA2A | Obesity in homozygous mutation | Transgenic | Valet, P. 2000. J Biol Chem |
| 5 | ADRB1 | Obesity | Knock-out | Bachman, ES. 2002. Science |
| 6 | ADRB2 | Obesity | Knock-out | Soloveva, V. 1997. Mol Endocrinol |
| 7 | ADRB3 | Obesity on HFD | Knock-out | Susulic, VS. 1995. J Biol Chem |
| 8 | AEBP1 | Obesity in females | Transgenic | Zhang, L. 2005. Mol Med |
| 9 | AGRP | Elevated weight gain & obesity | Transgenic | Ollman, MM. 1997. Science |
| 10 | ALMS1 | Obesity | Knock-out | Collin, GB. 2005. Hum Mol Genet |
| 11 | ALP1 | Accelerated weight gain on HFD | Knock-out | Narisawa, S. 2003. Mol Cell Biol |
| 12 | ANGPTL6 | Obesity and insulin resistance | Knock-out | Oike, Y. 2005. Nat Med |
| 13 | ANKRD26 | Obesity in homozygotes | Transgenic | Bera, TK. 2008. Acad Sci USA |
| 14 | APOB | Increased BW* | Knock-out | Siri, P. 2009. J Biol Chem |
| 15 | APOC3 | Obesity on HFD | Transgenic | Jong, MC. 2001. J Lipid Res |
| 16 | APOE | Obesity | Knock-out | Zhang, T. 2013. Reproduction |
| 17 | AQP7 | Adult-onset obesity | Knock-out | Hibuse, T. 2005. Proc Natl Acad Sci USA |
| 18 | AR | Obesity, decreased energy expenditure | Cre/LoxP | Fan, W. 2005. Diabetes |
| 19 | ASIP | Increased BW & Fat mass – Obesity | Transgenic | Mynatt, RL. 1997. Natl Acad Sci USA |
| 20 | AT2R | Increase in BW in females only | Knock-out | Samuel, P. 2013. PLoS One |
| 21 | ATX | Increase in adiposity in fat-specific knockout under HFD | Cre/LoxP | Dusaulcy, R. 2011. J Lipid Res |
| 22 | ATXN2 | Obesity under HFD | Knock-out | Kiehl, T. 2006. Biochem Biophys Res Commun |
| 23 | BBS1 | Adult-onset obesity in 10% of mutants | Knock-out | Kulaga, HM. 2004. Nat Genet |
| 24 | BBS2 | Adult-onset fat mass gain | Knock-out | Nishimura, DY. 2004. Proc Natl Acad Sci USA |
| 25 | BBS4 | Adult-onset obesity | Knock-out | Mykytyn, K. 2004. Proc Natl Acad Sci USA |
| 26 | BBS7 | Obesity | Knock-out | Zhang, Q. 2013. J Cell Sci |
| 27 | BDNF | Adult-onset obesity in heterozygotes | Knock-out | Coppola, V. 2004. Neuroreport |
| 28 | BRD2 | Obesity | Knock-out | Wang, F. 2009. Biochem J |
| 29 | BRS3 | Obesity | Knock-out | Ohki-Hamazaki, H. 1997. Nature |
| 30 | CAPN10 | Increase in body weight | Knock-out | Cheverud, JM. 2010. J Lipid Res |
| 31 | CART | Adult-onset obesity | Knock-out | Wierup, N. 2005. Regul Pept |
| 32 | CAV3 | Increased adiposity | Knock-out | Capozza, F. 2005. Am J Physiol Cell Physiol |
| 33 | CB2R | Increase in body weight and hyperphagia | Knock-out | Agudo, J. 2010. Diabetologia |
| 34 | CCKBR | Obesity | Knock-out | Lavine, J. 2010. Endocrinol |
| 35 | CDH2 | Increased adiposity | Transgenic | Castro, CH. 2004. J Cell Sci |
| 36 | CDKN1A (B) | Increased adiposity | Knock-out | Naaz, A. 2004. FASEB J |
| 37 | CEP19 | Obesity | Knock-out | Shalata, A. 2013. Am J Hum Genet |
| 38 | CHEMR23 | Adult-onset obesity | Knock-out | Rouger, L. 2013. J Endocrinol |
| 39 | CHGA | Increased adiposity | Knock-out | Bandyopadhyay, G. 2012. J Biol Chem |
| 40 | CHOP | Obesity under HFD | Knock-out | Grant, RW. 2014. J Biol Chem |
| 41 | CLOCK | Obesity | Knock-out | Turek, F. 2005. Science |
| 42 | CORIN | Increased bodyweight | Knock-out | Chan, JC. 2005. Proc Natl Acad Sci USA |
| 43 | CPE | Obesity | Knock-out | Cawley, NX. 2004. Endocrinology |
| 44 | CPT1 | Obesity under HFD | Knock-out | Gao, FX. 2009. Diabetologia |
| 45 | CRH | Excess fat accumulation & muscle atrophy | Transgenic | Stenzel-Poore, MP. 1992. Endocrinology |
| 46 | CRY (1/2) | Obesity under HFD | Knock-out | Barclay, JL. 2013. Am J Physiol Endorinol Metab |
| 47 | CSF2 | Adult-onset obesity | Knock-out | Reed, JA. 2005. J Clin Invest |
| 48 | CTRP9 | Increased bodyweight & adiposity | Knock-out | Wei, Z. 2014. Am J Physiol Endocrinol Metab |
| 49 | CYP19A1 | Elevated gonadal fat pad weight | Knock-out | Misso, ML. 2005. Horm Metab Res |
| 50 | D2 | Increased BW & adiposity | Knock-out | Marsili, A. 2011. PLoS One |
| 51 | DGAT1 | Increased gonadal but not subcutaneous fat | over-expression | Yamazaki, T. 2005. J Biol Chem |
| 52 | DPT | Increased subcutaneous fat | Knock-out | Takeda, U. 2002. J Invest Dermatol |
| 53 | DRD3 | Increased adiposity and obesity | Knock-out | McQuade, JA. 2004. Behav Brain Res |
| 54 | dup(17) | Obesity | Transgenic | Walz, K. 2003. Mol Cell Biol |
| 55 | ECSCR | Obesity under HFD | Transgenic | Akakabe, Y. 2013. Nat Commun |
| 56 | ESR1 | Obesity | Knock-out | Heine, PA. 2000. Proc Natl Acad Sci USA |
| 57 | FABP4 | Obesity in homozygotes under HFD | Knock-out | Hotamisligil, GS. 1996. Science |
| 58 | FATP4 | Obesity in homozygotes under HFD | Knock-out | Lenz, LS. 2011. J Biol Chem |
| 59 | FKBP51 | Increase in body weight under HFD | Transgenic | Yang, L. 2012. Am J Physiol Endocrinol Metab |
| 60 | FOXA2 | Heterozygotes develop obesity under HFD | Knock-out | Wolfrum, C. 2003. J Clin Invest |
| 61 | FOXO1 | Obesity | Transgenic | Kamei, Y. 2004. J Biol Chem |
| 62 | FOXO3A | Obesity | Knock-out | Fang, C. 2008. Am J Physiol |
| 63 | FSHR | Obesity | Knock-out | Danilovich, N. 2000. Endocrinology |
| 64 | FTO | Obesity | Over-expression | Church, C. 2010. Nat Genet |
| 65 | GAL-3 | Late-onset obesity | Knock-out | Pang, J. 2013. PLoS One |
| 66 | GAST | Obesity | Knock-out | Cowey, SL. 2005. Cancer |
| 67 | GCK | Increased BW under HFD | Transgenic | Ferre, T. 2003. Diabetologia |
| 68 | GDF3 | Increased BW under HFD | Over-expression | Wang, W. 2004. Biochem Biophys Res Commun |
| 69 | GFPT1 | Increased adiposity | Transgenic | McClain, DA. 2005. Am J Physiol Endocrinol Metab |
| 70 | GH | Obesity | Transgenic | Pomp, D. 1996. Transgenic Res |
| 71 | GHR | Increased adiposity in males | Knock-in | Rowland, JE. 2005. Mol Cell Bio |
| 72 | GHRH | Increased adiposity | Transgenic | Cai, A. 1999. Endocrinology |
| 73 | GIRK4 | Increased BW & adiposity | Knock-out | Perry, CA. 2008. Proc Natl Acad Sci USA |
| 74 | GNAS | Maternal inheritance of mutant allele leads to obesity | Knock-out | Germain-Lee, EL. 2005. Endocrinology |
| 75 | GNB3 | Increased BW and adiposity | Transgenic | Goldlust, S. 2013. Proc Natl Acad Sci USA |
| 76 | GPD2 | Increased BW & adiposity in females | Knock-out | Alfadda, A. 2004. Am J Physiol Regul Integr Comp Physiol |
| 77 | GPR10 | Adult-onset obesity | Knock-out | Ishii, M. 2003. Proc Natl Acad Sci USA |
| 78 | GPR120 | Obesity under HFD | Knock-out | Hirasawa, A. 2005. Nat Med |
| 79 | GPR26 | Obesity | Knock-out | Chen, D. 2012. PLoS One |
| 80 | GPR39 | Obesity | Knock-out | Moechars, D. 2006. Gastroenterology |
| 81 | GPR7 | Adult-onset obesity | Knock-out | Gu, W. 2004. J Mol Neurosci |
| 82 | GPX1 | Increased BW & adiposity | Transgenic | McClung, JP. 2004. Proc Natl Acad Sci USA |
| 83 | GRM8 | Increased adiposity | Knock-out | Duvoisin, RM. 2005. Eur J Neurosci |
| 84 | GRP | Resistant to diet-induced obesity | Knock-out | Ye, R. 2010. Diabetes |
| 85 | GRPR | Reduced food intake | Knock-out | Hampton, LL. 1998. Proc Natl Acad Sci USA |
| 86 | GSK3B | Increased BW & adiposity in males | Transgenic | Pearce, NJ. 2004. Metabolism |
| 87 | HDC | Increased BW & adiposity | Knock-out | Hara, J. 2001. Neuron |
| 88 | HIF1α | Obesity | Transgenic | Zhang, X. 2010. J Biol Chem |
| 89 | HRH1 | Late onset obesity | Knock-out | Masaki, T. 2004. Diabetes |
| 90 | HRH3 | Increased BW & adiposity | Knock-out | Takahashi, K. 2002. J Clin Invest |
| 91 | HSD1-11β | Obesity | Transgenic | Zhang, L. 2012. Transgenic Res |
| 92 | HSD11β2 | Increased adiposity | Transgenic | Masuzaki, H. 2001. Science |
| 93 | HTR2C | Late onset obesity | Knock-out | Nonogaki, K. 2003. Diabetes |
| 94 | ICAM1 | Late onset obesity/accelerated under HFD | Knock-out | Gregiore, FM. 2002. AM J Physiol Endocrinol Metab |
| 95 | IDH1 | Obesity | Transgenic | Koh, HJ. 2004. J Biol Chem |
| 96 | IFRD1 | Increased adiposity | Transgenic | Wang, Y. 2005. J Biol Chem |
| 97 | IL18 | Increased BW | Knock-out | Netea, M. 2006. Nature Medicine |
| 98 | IL18R | Increased BW | Transgenic | Netea, M. 2006. Nature Medicine |
| 99 | IL-1RI | Adult-onset obesity | Knock-out | McGillicuddy, FC. 2013. Am J Physiol Endocrinol Metab |
| 100 | IL6 | Increased BW & adiposity | Knock-out | Wallenius, V. 2002. Nat Med |
| 101 | INSR | Increased adiposity & obesity | Cre/LoxP | Cariou, B. 2004. Endocrinol |
| 102 | IRS1 | Increase weight gain | Knock-out | Shirakami, A. 2002. J Endocrinol |
| 103 | IRS2 | Increased adiposity | Cre/LoxP | Lin, X. 2004. J Clin Invest |
| 104 | JAK2 (Adipose) | Increased adiposity | Cre/LoxP | Sy, S. 2014. Diabetalogia |
| 105 | KCNJ11 | Increased BW & adiposity | Knock-out | Kanezaki, Y. 2004. Endocr J |
| 106 | KDM3A | Obesity | Knock-out | Okada, Y. 2010. J Androl |
| 107 | KRAS | Obesity under HFD | Transgenic | Dawson, DW. 2013. Cancer Prev Res |
| 108 | KSR2 | Obesity | Knock-out | Revelli, JP. 2011. Obesity |
| 109 | LEP | Obesity | Knock-out | D’Souza, AM. 2014. Endocrinol |
| 110 | LEPR | Obesity | Knock-in | Bates, SH. 2003. Nature |
| 111 | LH (B) | Obesity in females | Transgenic | Kero, JT. 2003. Am J Physiol Endocrinol Metab |
| 112 | LIPC | Increased adiposity | Knock-out | Farahani, P. 2004. Obes Res |
| 113 | LPIN1 | Obesity due to increased fat storage | Transgenic | Phan, J. 2005. Cell Metab |
| 114 | LRH-1 | Mild obesity | Knock-out | Hattori, T. 2014. Endocr J |
| 115 | LSR | Obesity in heterozygotes | Transgenic | Yen, FT. 2008. J Biol Chem |
| 116 | MAGEL2 | Increased BW & adiposity | Knock-out | Bischof, JM. 2007. Hum Mol Genet |
| 117 | MAGP-1 | Increased BW & adiposity | Knock-out | Weinbaum, JS. 2008. J Biol Chem |
| 118 | MAS | Increased adiposity | Knock-out | Santos, SH. 2008. Diabetes |
| 119 | MC3R | Obesity | Knock-out | Butler, AA. 2000. Endocrinolopgy |
| 120 | MC4R | Obesity | Knock-out | Huszar, D. 1997. Cell |
| 121 | MED13 | Obesity | Cre/LoxP | Grueter, C. 2012. Cell |
| 122 | MEST | Increased adiposity | Transgenic | Takahashi, M. 2005. Am J Physiol Endocrinol Metab |
| 123 | MKKS | Obesity | Knock-out | Fath, MA. 2005. Hum Mol Genet |
| 124 | MMP11 | Obesity | Knock-out | Andarawewa, KL. 2005. Cancer Res |
| 125 | MMP19 | Accelerated weight gain on HFD | Knock-out | Pendas, AM. 2004. Mol Cell Biol |
| 126 | MPO | Increased BW | Transgenic | Castellani, LW. 2006. J Lipid Res |
| 127 | MRAP2 | Obesity | Knock-out | Asai, M. 2013. Science |
| 128 | MT1A (B) | Adult-onset obesity | Knock-out | Beattie, JH. 1998. Proc Natl Acad Sci USA |
| 129 | MT-HGH | Obesity | Transgenic | Wolf, E. 1991. Growth Dev Aging |
| 130 | NBEA | Increased BW & adiposity in heterozygotes | Knock-out | Olszewski, P. 2012. PLoS Genet |
| 131 | NEIL1 | Obesity | Knock-out | Sampath, H. 2011. Am J Physiol Endocrinol Metab |
| 132 | NEP | Adult-onset obesity | Knock-out | Becker, M. 2010. PLoS One |
| 133 | NGN3 | Obesity | Knock-out | Anthwal, N. 2013. Dis Model Mech |
| 134 | NHLH2 | Adult-onset obesity | Knock-out | Jing, E. 2004. Endocrinology |
| 135 | NMU | Increased BW & adiposity | Knock-out | Handa, R. 2004. Nat Med |
| 136 | NPB | Mild obesity | Knock-out | Kelly, MA. 2005. Proc Natl Acad Sci USA |
| 137 | NPC1 | Dose-dependent weight gain under HFD | Knock-out | Jelinek, D. 2010. Obesity |
| 138 | NPY | Obesity under high-sucrose diet | Transgenic | Kaga, T. 2001. Diabetes |
| 139 | NPY1R | Obesity | Knock-out | Kushi, A. 1998. Proc Natl Acad Sci USA |
| 140 | NPY2R | Obesity | Knock-out | Lin, D. 2006. Endocrinol |
| 141 | NPY5R | Increased adiposity | Knock-out | Marsh, DJ. 1998. Nat Med |
| 142 | NR5A1 | Adult-onset obesity | Knock-out | Majdic, G. 2002. Endocrinol |
| 143 | NTSR1 | Adult-onset obesity | Knock-out | Remaury, A. 2002. Brain Res |
| 144 | OGG1 | Increased adiposity in HFD | Knock-out | Sampath, H. 2012. PLoS One |
| 145 | OMA1 | Obesity | Knock-out | Quiros, PM. 2012. EMBO |
| 146 | OSMRβ | Increase in BW and hyperphagia | Knock-out | Gotardo, EM. 2013. J Nutr Sci Vitaminol |
| 147 | OXT | Obesity | Knock-out | Nishimori, K. 2008. Prog Brain Res |
| 148 | P62 | Adult-onset obesity and hyperphagia | Knock-out | Harada, H. 2013. J Neurosci |
| 149 | PARP1 | Adult-onset obesity | Knock-out | Devalaraja-Narashimha, K. 2010. J Endocrinol |
| 150 | PC1/3 | Increased adiposity in heterozygotes | Knock-out | Zhu, X. 2002. Proc Natl Acad Sci USA |
| 151 | PCK1 | Obesity | Transgenic | Franckhauser, S. 2002. Diabetes |
| 152 | PCSKIN | Adult-onset obesity | Transgenic | Wei, S. 2004. J Endocrinol |
| 153 | PCYT2 | Obesity | Knock-out | Fullerton, MD. 2009. J Biol Chem |
| 154 | PEG3 | Obesity | Knock-out | Curley, JP. 2005. FASEB J |
| 155 | PGC-1α | Obesity | Knock-out | Leone, TC. 2005. PLoS Biol |
| 156 | PGDS | Obesity | Knock-out | Tanaka, R. 2009. Biochem Biophys Res Commun |
| 157 | PGP | Increased BW & adiposity | Knock-out | Foucaud-Vignault, M. 2011. PLoS One |
| 158 | PHB | Obesity | Transgenic | Ande, SR. 2014. Diabetes |
| 159 | PI3K (p110α) | Increased adiposity, hyperphagia | Knock-in | Foukas, L. 2006. Nature |
| 160 | PLAC 8 | Increase in adiposity | Knock-out | Jimenez-Preitner, M. 2011. Cell Metab |
| 161 | PLSCR1 | Increased adiposity | Knock-out | Zhou, Q. 2002. Blood |
| 162 | PLSCR3 | Increased BW & adiposity | gene-trap | Wiedmer, T. 2004. Proc Natl Acad Sci USA |
| 163 | POMC | Obesity under HFD | Knock-out | Challis, BG. 2004. Proc Natl Acad Sci USA |
| 164 | PPARA | Increase in adiposity | Knock-out | Miyazaki, M. 2004. J Biol Chem |
| 165 | PPARG2 | Obesity under HFD | Knock-in | Heikkinen, S. 2009. Cell Metab |
| 166 | PPARGC1A | Increased adiposity in young females & old males | Knock-out | Leone, TC. 2005. PLoS Biol |
| 167 | PPARδ | Obesity under HFD | Knock-out | Kocalis, H. 2012. PLoS One |
| 168 | PPIF | Late-onset obesity | Knock-out | Luvisetto, S. 2008. Neurosci |
| 169 | PPIR3A | Increased BW & adiposity | Knock-out | Delibegovic, M. 2003. Diabetes |
| 170 | PPKAA2 | Increased adiposity | Knock-out | Villena, JA. 2004. Diabetes |
| 171 | PREF1 | Obesity | Knock-out | Moon, YS. 2002. Mol Cell Biol |
| 172 | PRKCQ | Obesity | Transgenic | Serra, C. 2003. J Cell Physiol |
| 173 | PRL | Increased BW | Knock-out | Perez-Villamil, B. 1992. J Endocrinol |
| 174 | PROX1 | Obesity in heterozygotes | Knock-out | Harvey, NL. 2005. Nat Genet |
| 175 | PRRP | Increased BW | Knock-out | Lawrence, C. 2002. Endocrinol |
| 176 | PTPN11 | Obesity | Knock-out | Zhang, EE. 2004. Proc Natl Acad Sci USA |
| 177 | PYY | Obesity | Knock-out | Batterham, R. 2002. Nature |
| 178 | RAGE | Increased BW | Knock-out | Leuner, B. 2012. Z Gerentol Geriatr |
| 179 | RAI1 | Obesity in heterozygotes | Knock-out | Bi, W. 2005. Hum Mol Genet |
| 180 | REN | Adult-onset obesity | Transgenic | Uehara, S. 2003. Int J Mol Med |
| 181 | RETN | Increased adiposity | Transgenic | Kim, KH. 2004. Proc Natl Acad Sci USA |
| 182 | RPGRIP1L | Obesity | Knock-out | Vadnais, C. 2013. BMC Genomics |
| 183 | RSC1A1 | Obesity | Knock-out | Osswald, C. 2005. Mol Cell Biol |
| 184 | RSL1 | Females prone to diet-induced obesity | Transgenic | Krebs, CJ. 2014. Mol Cell Biol |
| 185 | SAR1B | Obesity under HFD | Transgenic | Levy, E. 2014. J Nutr Biochem |
| 186 | SDC1 | Adult-onset obesity | Transgenic | Reizes, O. 2001. Cell |
| 187 | SELM | Increased BW & adiposity | Transgenic | Pitts, MW. 2013. J Biol Chem |
| 188 | SFRP1 | Increase in BW and adiposity under HFD | Knock-out | Gauger, KJ. 2013. PLoS One |
| 189 | SH2B | Obesity | Knock-out | Ren, D. 2005. Cell Metab |
| 190 | SHP | Obesity and increased adiposity | Transgenic | Tabbi-Anneni, I. 2010. Am J Physiol Endocrinol Metab |
| 191 | SIM1 | Obesity in heterozygotes | Knock-out | Michaud, JL. 2001. Hum Mol Genet |
| 192 | SIRT6 | Adult-onset obesity | Transgenic | Schwer, B. 2010. PNAS |
| 193 | SLC2A4 | Increased adiposity | Transgenic | Carvalho, E. 2005. Am J Physiol Endocrinol Metab |
| 194 | SLC6A1 | Obesity | Transgenic | Ma, YH. 2000. Cell Res |
| 195 | SOCS1 | Liver degeneration, obesity | Knock-out | Starr, R. 1998. Proc Natl Acad Sci USA |
| 196 | SOCS3 | Obesity under HFD | Knock-out | Sachithanandan, N. 2010. Hepatology |
| 197 | SPARC | Increased adiposity | Knock-out | Bradshaw, AD. 2003. Proc Natl Acad Sci USA |
| 198 | SPONDIN 2 | Obesity | Knock-out | Zhu, LH. 2014. J Hepatol |
| 199 | SRC-1 | Obesity | Knock-out | Picard, F. 2002. Cell |
| 200 | STAT3 | Obesity | Cre/LoxP | Cui, Y. 2004. Mol Cell Biol |
| 201 | STAT5B | Increased adiposity | Knock-out | Gao, Q. 2004. Proc Natl Acad Sci USA |
| 202 | TAp63 | Obesity | Knock-out | Su, X. 2012. Cell Metab |
| 203 | T-BET | Obesity | Knock-out | Kim, K. 2013. J Nutr Biochem |
| 204 | THRA | Increased BW & adiposity | Knock-out | Udy, GB. 1997. Proc Natl Acad Sci USA |
| 205 | TIMP-2 | Obesity and hyperphagia | Knock-out | Stradecki, HM. 2011. J Neuroendocrinol |
| 206 | TIS7 | Increased BW & adiposity | Transgenic | Wang, Y. 2005. J Biol Chem |
| 207 | TNF | Increased BW & adiposity | Transgenic | Liu, YY. 2003. J Biol Chem |
| 208 | TNF-α | Increased BW & adiposity | Knock-out | Salles, J. 2012. J Nutr Biochem |
| 209 | TRKβ | Increased BW & adiposity | Knock-in | Byerly, MS. 2013. PLoS One |
| 210 | TRPV4 | Increased BW & adiposity | Knock-out | O’Conor, J. 2013. Ann Rheum Dis |
| 211 | TUB | Adult-onset obesity | Knock-out | Voros, G. 2004. J Thromb Haemost |
| 212 | TW | Obesity in heterozygotes | Knock-out | Kurima, K. 2011. PLoS Genet |
| 213 | TXNIP | Increased fat to muscle ratio | Knock-out | Stubdal, H. 2000. Mol Cell Biol |
| 214 | UCP1 | Late-onset obesity with HFD | Knock-out | Kontani, Y. 2005. Aging Cell |
| 215 | VDR | Obesity | Transgenic | Wong, KE. 2011. J Biol Chem |
| 216 | WDTC1 | Obesity in heterozygotes | Knock-out | Hader, T. 2003. EMBO |
| 217 | XOR | Increased BW & adiposity | Knock-out | Murakami, N. 2014. Arterioscler Thromb Vasc Biol |
| 218 | ZEB1 | Obesity | Knock-out | Saykally, JN. 2009. PLoS One |
| 219 | ZFP90 | Obesity | Transgenic | Schadt, EE. 2005. Nat Gen |
| 220 | ZNT7 | Obesity in males only | Knock-out | Huang, L. 2012. J Biol Chem |
| 221 | ZNT8 | Obesity under HFD | Knock-out | Lemaire. K, 2009. Proc Natl Acad Sci USA |
Notes.
BW, Body weight; HFD, High fat diet.
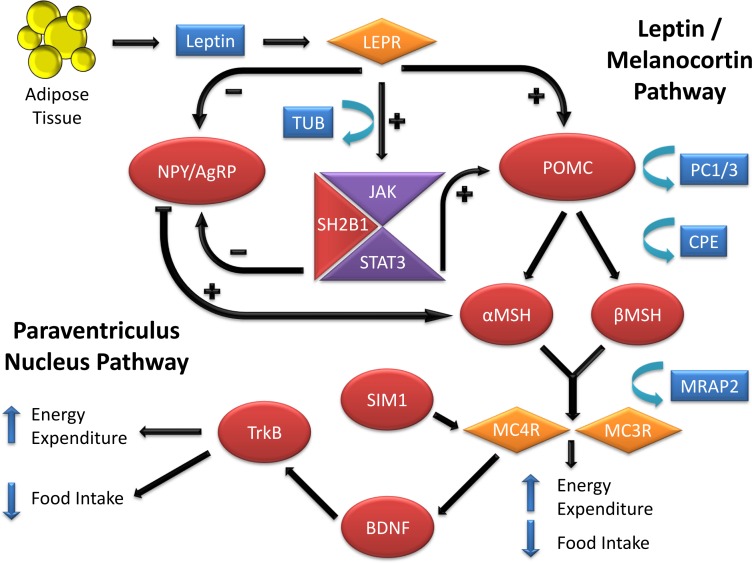
Figure 1. Genes involved in the leptin-melanocortin pathway that have been associated with monogenic obesity through their influence on food intake and energy expenditure.
Leptin secreted from adipose tissue binds to the leptin receptor in the hypothalamus. Leptin binding inhibits the neuropeptide Y/agouti-related protein (NPY/AgRP) production and stimulates pro-opiomelanocortin (POMC) production, which undergoes post-translational modifications to produce peptides such alpha and beta-melanocyte-stimulating hormone (α and βMSH) via the processing of prohormone convertase 1(PC1/3) and carboxypeptidase E (CPE) enzymes. Alpha and βMSH bind to melanocortin 3 and melanocortin 4 receptors (MC3R and MC4R) and induce their activity. Melanocortin 2 receptor accessory protein 2 (MRAP2) can reduce the responsiveness of both MC3R and MC4R to α and βMSH and result in obesity. On the other hand, Single-minded 1 (SIM1) acts as a facilitator of MC4R activity. Increase in the MC3R and MC4R activities result in a decrease in food intake and increase in energy expenditure. MC4R activity also stimulates release of Brain-derived neurotrophic factor (BDNF) which will bind to the neurotrophin receptor (TrkB) and influence food intake and energy expenditure. Aside from activation of the POMC, leptin binding to its receptor also activates the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling. This pathway, through the help of Src homology 2 B adapter protein 1 (SH2B1), results in activation of Signal transducer and activator of transcription 3 (STAT3). STAT3 will then migrate to the nucleus with the help of Tubby bipartite transcription factor (TUB) and activate its target genes related to energy homeostasis and mediate in the anorexigenic effects of leptin.
Leptin (LEP) and Leptin Receptor (LEPR)
The obese (ob) mutation was first described in 1949 by a team from Jackson Laboratory (Coleman & Hummel, 1973; Kanasaki & Koya, 2011). The ob mutation originated in non-inbred heterogeneous stock, but was subsequently transferred onto various inbred strains for further analysis (Clee & Attie, 2007; Coleman & Hummel, 1973; Coleman, 1982). This model exhibits morbid obesity associated with hyperphagia and hyperglycemia along with other neuroendocrine abnormalities (Coleman & Hummel, 1973; Coleman, 1982). Elegant parabiosis studies demonstrated that the obese gene encodes a circulating factor, while the diabetes gene encodes its receptor (Coleman, 1973). Through ground-breaking positional cloning studies, the ob mutation was characterized as a single-base deletion which results in a premature stop codon in the previously unknown leptin gene (Zhang et al., 1994). This landmark study was the first to identify this hormone and largely initiated research efforts on adipokines.
The diabetes (db) mutation was identified in 1966 in the C57BL/KsJ inbred mouse strain (Coleman & Hummel, 1967; Hummel, Dickie & Coleman, 1966). This mouse model exhibits persistent hyperphagia and obesity, resulting in hyperleptinemia, insulin resistance and increased leptin levels (Coleman & Hummel, 1967). Positional cloning and related studies identified the diabetes (db) mutation in the leptin receptor gene (Chen et al., 1996; Tartaglia et al., 1995).
After the discovery of leptin, a mutation in the leptin gene (LEP) was discovered in two severely obese cousins within a highly consanguineous family of Pakistani origin (Montague et al., 1997). The mutation was characterized as a frameshift mutation resulting in truncated transcription of leptin (Montague et al., 1997). Other reports have confirmed this initial discovery in additional homozygous patients of Pakistani, Turkish and Egyptian origin (Gibson et al., 2004; Mazen et al., 2009). In a study in Pakistan, where consanguineous marriages are preferred, 16.1% of the probands from 62 unrelated children with early onset obesity exhibited mutations in LEP. Of these probands, 9 carried a homozygous frameshift mutation. Given the high prevalence of monogenic obesity in this consanguineous population, early detection of this mutation for counselling and management of obesity could be beneficial (Saeed et al., 2012).
Similarly, shortly after its identification in mice, congenital leptin receptor (LEPR) deficiencies were found in severe obese siblings in 1998 (Clément et al., 1998). In a more recent study, 8 other patients with severe early onset obesity with homozygous or compound heterozygous mutations in LEPR were identified (Farooqi et al., 2007a; Farooqi et al., 2007b; Farooqi et al., 2007c). These patients exhibited high serum levels of leptin and loss of sensitivity of the receptor (Farooqi et al., 2007a; Farooqi et al., 2007b; Farooqi et al., 2007c).
Patients with mutations in LEP or LEPR experience rapid weight gain within the first year of life (Farooqi et al., 2002). Patients all experience hyperphagia and display aggression when food is denied (Farooqi et al., 2002). Onset of puberty is often delayed for these patients, due to hypogonadotrophic hypogonadism (Farooqi et al., 2007a; Farooqi et al., 2007b; Farooqi et al., 2007c). Leptin deficient children exhibit defective T-cell mediated immunity, explaining the high rates of infection and mortality in developing countries (Farooqi et al., 2007a; Farooqi et al., 2007b; Farooqi et al., 2007c).
The role of leptin in energy homeostasis has also been demonstrated in studies employing novel tools for genetic studies, such as whole exome sequencing. For example, whole exome sequencing was conducted in extreme obese individuals from four consanguineous families to determine the role of rare coding variants in pathogenesis of obesity (Gill et al., 2014). The study found two novel frameshift mutations (p.C186AfsX27 and p.H160LfsX9) that truncate the LEPR protein, resulting in protein products that lack the necessary binding domain for leptin signaling (Gill et al., 2014).
Considering the symptoms associated with leptin deficiency, the impact of leptin deficiency in the body is reversible via leptin treatment. A 9-year-old girl with leptin deficiency experienced reduction in weight mainly due to loss of fat, reduced energy intake, and increase in gonadotropin concentrations after treatment with recombinant human leptin for 12 months (Farooqi et al., 1999). In a different study, leptin-deficient patients in a fed state gave higher ratings to food images, but these ratings were reduced after leptin treatment (Farooqi et al., 2007a). As a result of these findings, leptin treatment has been deemed a promising therapeutic option for leptin deficient patients. It should be noted however that normal leptin levels do not preclude the presence of a deleterious mutation. A recent study described a 2-year-old boy with a deleterious leptin mutation with normal leptin levels (Wabitsch et al., 2015). This mutation had an impact on the protein function rather than expression, which questions the reliability of leptin levels as a prescreening tool for detecting leptin mutation.
TUB
Tubby bipartite transcription factor (TUB) is a member of the tubby-like proteins, which present a highly conserved C-terminus domain (Carroll, Gomez & Shapiro, 2004). TUB is a substrate for insulin receptor tyrosine kinase (IRTK) and leptin receptor Janus kinase 2 (LEPR JAK2) in the hypothalamus. TUB is translocated to the nucleus after binding to LEPR via JAK2. Inhibition of TUB expression in the hypothalamus results in increased food intake, fasting glucose levels, hepatic glucose output, decreased oxygen consumption, and reduced sensitivity of POMC to leptin (Prada et al., 2013). A mutation in the tubby gene occurred spontaneously at the Jackson Laboratory in a C57BL/6J mouse (Coleman & Eicher, 1990). These mice developed milder obesity compared to the other mutant models, hyperinsulinemia and mild hypoglycemia (Coleman & Eicher, 1990). Positional cloning of the mutated tubby gene identified a single-base change in the splice donor site that results in the incorrect retention of a single intron in the mature tub mRNA transcript (Kleyn et al., 1996).
Mutations in TUB were observed in an eleven-year-old boy from a consanguineous Caucasian family. His symptoms included deteriorating vision, obesity, and normal glucose/cholesterol/triglycerides levels but other clinical features were not observed to classify the patient as Bardet-Biedl or Alström syndrome (Borman et al., 2014). The mutation was identified as a homozygous frameshift mutation in TUB (c.1194_1195delAG, p.Arg398Serfs*9), which results in a truncated form of TUB. Homozygous loss of function of TUB is extremely rare in humans (Borman et al., 2014).
MC4R
The melanocortin-4 receptor (Mc4r) model was identified in 1997 through targeted gene disruption (Huszar et al., 1997). MC4R is a G protein couple receptor mainly expressed in the brain that is involved in both energy intake and expenditure (Gantz et al., 1993; Huszar et al., 1997). Mc4r−/− mice exhibit obesity, hyperphagia, hyperinsulinemia, hyperglycemia, and increased linear growth (Huszar et al., 1997). Comparatively, Mc4r+/− mice display milder forms of obesity, with increased weight gain in response to high-fat diet, suggesting a gene-dosage effect (Srisai et al., 2011).
The first heterozygous mutation in MC4R discovered in humans was in 1998 (Vaisse et al., 1998; Yeo et al., 1998). MC4R mutations represent the most common form of human monogenic obesity, impacting 0.2–5.6% of individuals with severe early onset obesity (Rouskas et al., 2012). Majority of these mutations are heterozygous, with homozygous mutants having a fully penetrant early-onset severe form of obesity. Not all heterozygote mutants are obese however, which is indicative of the dosage effect described previously in the mouse models (Farooqi et al., 2003; Stutzmann et al., 2008). In addition to obesity, MC4R deficient children display hyperinsulinemia and increased linear growth (Farooqi et al., 2000). Also patients experience an increase in adiposity, as well as an increase in lean mass, which is a phenotype that is not observed in other forms of monogenic obesity (Farooqi et al., 2003). Interestingly, the degree of hyperphagia in patients depends on level of receptor dysfunction, which is generally lower than that of leptin deficient patients (Farooqi et al., 2003).
MC3R
Both melanocortin receptor 3 (MC3R) and MC4R are expressed in the hypothalamus and are involved in energy homeostasis (Roselli-Rehfuss et al., 1993). Mc3r deficient mice exhibit 50–60% more adipose mass and 50% reduction in energy expenditure (Butler et al., 2000). Mc3r deficient mice are also hyperleptinaemic and male Mc3r−/− mice develop mild hyperinsulinemia (Chen et al., 2000). Mice lacking both Mc3r and Mc4r become significantly heavier than either mutation alone, suggesting that Mc3r and Mc4r have non-redundant roles in energy homeostasis (Chen et al., 2000).
While the role of MC4R in monogenic obesity is well-defined, the role of MC3R mutations in human monogenic obesity is debated (Zegers, Beckers & Hendrickx, 2013). Although mutations in the MC3R gene may not be involved in autosomal dominant form of monogenic obesity, these mutations could predispose humans to increased risk of obesity. MC3R mutations that result in defective receptors have been associated with obesity in French and Italian populations (Mencarelli et al., 2011). A non-significant two-fold enrichment in MC3R loss-of-function mutations was observed in a severe obese population from United States (Calton et al., 2009).
POMC
The pro-opiomelanocortin (Pomc) derived peptides have a variety of biological functions, such as pigmentation, adrenocortical function, and energy stores (Smith & Funder, 1988). Figure 2 is a depiction of POMC-derived peptides, including α and β-MSH. Deleting the coding region of Pomc in mouse models resulted in obesity, defective adrenal development and altered pigmentation (Yaswen et al., 1999).
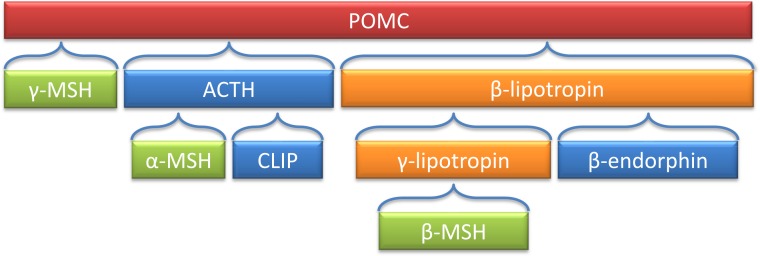
Figure 2. Processing of the POMC precursor protein.
Adrenocorticotropic hormone (ACTH) and β-lipotropin are products generated in the corticotrophic cells of the anterior pituitary under the control of corticotropin releasing hormone (CRH). Alpha-melanocyte stimulating hormone (α-MSH), corticotropin-like intermediate lobe peptide (CLIP), γ-lipotropin and β-endorphin are products generated in the intermediate lobe of the pituitary under the control of dopamine. α-, β- and γ-MSH are collectively referred to as melanotropin or intermedin.
Interest in the melanocortin pathway stemmed from the studies of agouti mice. Mouse coat color was a trait studied by the mouse model experts whose stocks founded many of the commonly studied strains (Clee & Attie, 2007). The dominant lethal “yellow” mutation (Ay) was identified in 1905 (Dickies, 1962). A non-lethal, viable, allele (Avy) occurred as a spontaneous mutation in 1960 in the C3H/HeJ strain at the Jackson Laboratory (Dickies, 1962). In addition to their yellow coat color, mutant mice exhibit adult-onset obesity, type 2 diabetes associated with insulin resistance, hyperleptinemia, higher benign tumor susceptibility and infertility (Klebig et al., 1995). The mutations in the agouti gene in carriers of the yellow alleles leads to dysregulation of its expression in multiple tissues (Bultman, Michaud & Woychik, 1992; Duhl et al., 1994; Miller et al., 1993). The agouti model displays a defect in proopiomelanocortin (POMC) signaling pathway and is desensitized to leptin signaling (Boston et al., 1997).
The first recessive mutation in POMC was discovered in 1998 (Krude et al., 1998). In addition to obesity, patients with POMC mutations displayed hypocortisolism, hair and skin hypopigmentation, neonatal hypoglycemia, seizures, cholestasis and voracious appetite (Farooqi et al., 2006; Krude et al., 1998; Krude et al., 2003).The hypopigmentation of the hair and skin is not always observed in non-European populations, and POMC mutations should still be considered in individuals with severe early onset obesity even if typical pigmentary phenotype is missing (Cirillo et al., 2012; Clément et al., 2008; Mendiratta et al., 2011). In general, POMC deficiencies are extremely rare in human population (Beales, 2010) and the position of the mutation is important, as missense mutations have been reported to directly impact the melanocortin peptide-encoding regions, whereas other missense mutations have been reported to impact the peptide-receptor binding affinity (Challis et al., 2002).
A novel mutation in the alpha-melanocyte stimulating hormone (α-MSH) gene was found in a 12-year-old girl with early onset obesity (transmitted through the father) (Dubern et al., 2008). The mutation was characterized by dramatic impairment of α-MSH (Dubern et al., 2008). The patient’s obese father had less pronounced form of obesity in comparison to the daughter, which may be due to a gene-environment interaction. This means the younger generation is more exposed to obesogenic environment, thus more likely to develop obesity (Dubern et al., 2008). Most research has been focused on α-MSH since rodent models lack beta-melanocyte stimulating hormone (β-MSH) (Bennett, 1986) but a loss of function missense mutation in β-MSH has been associated with childhood obesity. The lack of function of β-MSH reduces the amount of MSH peptide in the POMC/MC4R pathway, resulting in obesity (Biebermann et al., 2006). β-MSH mutations may result in a non fully penetrant form of monogenic obesity, as some patients with this mutation are not obese (Lee et al., 2006).
PCSK1
A mutation was discovered in 1973 in the HRS/J inbred mouse strain which homozygous mice exhibit a slower increase in body weight compared to ob/ob and db/db mice but ultimately develop severe obesity, with hyperinsulinemia and transient hyperglycemia in males (Coleman & Eicher, 1990). Coding non-synonymous mutation in the carboxypeptidase E (Cpe) gene was found to induce the fat mouse phenotype (Naggert et al., 1995). Cpe gene is involved in processing of prohormone convertase 1 (PC1) as illustrated in Fig. 1, which led scientists to study the association of this gene to obesity as well (Li et al., 2014).
PC1/3 functions as a processing enzyme of precursor proteins in the regulated secretory pathways (Creemers, Jackson & Hutton, 1998). Pc1/3 knock-out mice do not exhibit obesity, but instead show growth retardation and multiple neuroendocrine disorders (Zhu et al., 2002). An ENU mutagenesis experiment resulted in development of a mouse model with mutation on the Pc1/3 (N222D allele) that exhibits obesity (Lloyd, Bohan & Gekakis, 2006). Pc1N222D/N222D mice have lower α-MSH and display defects in POMC processing, affecting the melanocortin signaling (Lloyd, Bohan & Gekakis, 2006). Pc1N222D/N222D mice exhibit abnormal proinsulin processing, multiple endocrinological defects, hyperphagia and obesity, while heterozygous mice exhibit an intermediate phenotype for weight gain and fasting insulin processing (Lloyd, Bohan & Gekakis, 2006).
In human studies, three patients with recessive monogenic form of obesity were deficient in the pro-protein convertase subtilisin/kexin type 1 (PCSK1) gene (Farooqi et al., 2007b; Jackson et al., 2003; Jackson et al., 1997). Complete prohormone convertase 1 deficiency results in early on-set severe obesity, hyperphagia, hypoglycemia, and endocrine dysfunction (Farooqi et al., 2007a; Farooqi et al., 2007b; Farooqi et al., 2007c; Jackson et al., 2003; Jackson et al., 1997). Null mutations causing prohormone convertase 1 congenital deficiency also lead to generalized malabsorptive diarrhea and diabetes insipidus in some instances (Farooqi et al., 2007a; Farooqi et al., 2007b; Farooqi et al., 2007c; Frank et al., 2013; Jackson et al., 1997; Martín et al., 2013; Yourshaw et al., 2013). Partial loss of function heterozygous mutations in PCSK1 present a non-fully penetrant intermediate obesity phenotype (Creemers et al., 2012) However, heterozygous carriers of a null mutation show a dominantly inherited form of Mendelian obesity (Philippe et al., 2014).
SH2B1
The SH2B adaptor protein 1 (SH2B1) activates the JAK2 cytoplasmic tyrosine kinase to mediate cell signaling (Ren et al., 2005). SH2-B is a key regulator of leptin sensitivity. Sh2b1−/− mice exhibit hyperphagia, hyperlipidemia, hyperglycemia, hyperleptinemia, hyperinsulinemia and hepatic steatosis (Ren et al., 2005).
In humans, loss of function mutations in SH2B1 patients resulted in severe early onset obesity (Doche et al., 2012; Pearce et al., 2014). These patients exhibit hyperphagia, childhood onset obesity, insulin resistance, and reduced height (Doche et al., 2012). Behavioral abnormalities were also noted in patients, such as social isolation and aggression (Doche et al., 2012). The severity of the phenotype may depend on the impact of mutations on the disruption of different isoforms of SH2B1 (Pearce et al., 2014). Genomic imbalances and recurrent deletions of the SH2B1 containing region on the short arm of chromosome 16 have been associated with behavioral disorders and obesity (Bachmann-Gagescu et al., 2010). It is interesting to note that while deletion of a region on chromosome 16 that contains SH2B1 increases the risk of obesity significantly (Bochukova et al., 2010; Walters et al., 2010), reciprocal duplication of this region results in an increase in gene dosage which influences BMI in the reverse manner (leanness) (Jacquemont et al., 2011). The relevance of SH2B1 locus in human energy balance is strengthened by the identification via GWAS of common variants near SH2B1 associated with BMI variation or obesity risk (Berndt et al., 2013; Willer et al., 2009).
BDNF/NTRK2
The brain derived neurotrophic factor (BDNF) model demonstrates the numerous roles of BDNF in neural development through activation of TrkB and p75 receptors and involvement in anorexigenic activity (Noble et al., 2011). In the mature central nervous system, BDNF is expressed in various hypothalamic nuclei associated with eating behavior and obesity (Kernie, Liebl & Parada, 2000). To circumvent the problem of early mortality associated with total knock-out of the BDNF gene, conditional BDNF knockout mice were developed. Conditional knockout of BDNF in the brain via cre-loxP recombinase system resulted in mice exhibiting hyperphagia, hyperactivity and aggression as well as elevated levels of POMC (Rios et al., 2001). Since BDNF is only absent in the brain, the resulting obesity can be attributed to the lack of BDNF function therein (Rios et al., 2001). In another conditional knockout study, selective knockout of BDNF in brains of adult mice resulted in impaired hippocampal function, whereas selective knockout of BDNF in earlier stages of development resulted in more drastic phenotypes, such as hyperactivity and severe impairments in hippocampal-dependent learning (Monteggia et al., 2004).
Neurotrophin receptor (TrkB) is a member of the neurotrophin family and is known to be involved in development, maintenance and function of peripheral and central neurons and is hypothesized to play a role in mediating neuronal plasticity in the hypothalamus (Gray et al., 2006a). TrkB and its ligand BDNF are also known to be involved in the regulation of food intake and body weight (Gray et al., 2006a; Xu et al., 2003). Homozygous mutations in the gene encoding TrkB (Ntrk2) are lethal in mice, but heterozygous mutations resulting in 25% of TrkB expression display hyperphagia, increased linear growth and obesity as well as complex neurobehavioral phenotypes (Xu et al., 2003).
The neurotrophic tyrosine kinase receptor type 2 (NTRK2) gene was screened in a boy with early onset obesity, hyperphagia developmental delay, impairments in short-term memory and impaired nociception, revealing a missense mutation in NTRK2 (Yeo et al., 2004). Further analysis showed an impairment in BDNF-stimulated protein kinase phosphorylation (Yeo et al., 2004). The developmental and neurological impairments in this case is consistent with the wide spread of TrkB (encoded via NTRK2) throughout the central nervous system, where it assumes the responsibility for neuronal survival and differentiation and regulation of synaptic function (Indo et al., 1996). In another case, a girl with loss of one functional copy of BDNF presented with hyperphagia, severe obesity, cognitive impairment and hyperactivity (Gray et al., 2006b). Moreover, hyperphagia and obesity observed in a subgroup of patients with WAGR syndrome has been attributed to deletions on chromosome 11 that induce haploinsufficiency of BDNF (Han et al., 2008).
SIM1
Single-minded homolog 1 (SIM1) is a member of the helix-hoop-helix PAS family of nuclear transcription factors (Crews, 1998). Homozygous Sim1 mice die perinatally (Michaud et al., 1998), but heterozygous mutants exhibit hyperphagic obesity, increased body fat percentage (Holder et al., 2004; Michaud et al., 2001), as well as higher levels of POMC expression and resistance to α-MSH (Kublaoui et al., 2006). They are also more prone to diet-induced obesity (Holder et al., 2004) and are associated with defects in the MC4R signaling pathway (Kublaoui et al., 2006). To illustrate these signaling defects, Sim1 heterozygous mouse injected with a melanocortin agonist showed a blunted suppression of food intake, while wild-type mice exhibited a robust reduction in food intake (Kublaoui et al., 2006).
Severe early-onset obesity was observed in a girl with haploinsufficiency of SIM1, possibly acting upstream or downstream of MC4R (Holder, Butte & Zinn, 2000). Further support for the involvement of SIM1 in obesity came from studies in which patients displayed Prader-Willie like phenotypes due to heterozygous mutations in SIM1 (Bonnefond et al., 2013). In another study, heterozygous mutations in SIM1 were associated with severe obesity accompanied by a neurobehavioral phenotype for a majority of them (Ramachandrappa et al., 2013). Deletions on chromosome 6q16, including SIM1 region, has been similarly associated with obesity and Prader-Willi like phenotype (Bonaglia et al., 2008). SIM1 is expressed in kidneys and central nervous system and plays an essential role in formation of PVN of the hypothalamus (Michaud et al., 2000). This could be a mechanism in which SIM1 plays a role in energy homeostasis, as PVN neurons express MC4R which inhibits food intake (Harris et al., 2001).
Polygenic obesity mouse models and candidate gene studies in human
Given the success in identifying mutations causing severe monogenic obesity from mouse models, in parallel with the development of methods for linkage analysis, other mouse models have been developed for genetic studies of polygenic obesity. For example, the New Zealand Obese Mouse (NZO) characterizes a combination of hyperphagia, reduced energy expenditure and insufficient physical activity (Herberg & Coleman, 1977). The Kuo Kondo Mouse displays hyperphagia, hyperinsulinemia, insulin resistance (Igel et al., 1998) which precedes onset of obesity (Ikeda, 1994). Later modifications of this model led to development of KKAy from transferring the Ay gene, which is now used for obesity and diabetes research and testing of experimental therapies (Okazaki et al., 2002). The Tsumura Suzuki Obese Diabetes Mouse (TSOD) models polygenic obesity with diabetes (hyperglycemia and hyperinsulinemia) (Suzuki et al., 1999). The M16 mouse was developed to characterize the phenotypic consequences of long-term selective breeding for rapid weight gain (Allan, Eisen & Pomp, 2004). The M16 is an outbred model of early onset polygenic obesity and is characterized by hyperphagia, hyperinsulinemia, and hyperleptinemia (Allan, Eisen & Pomp, 2004). Lastly, the BSB mouse models are a backcross progeny obtained by crossing C57BL/6J x Mus spretus F1 females with C57BL/6J males to model polygenic obesity (Fisler et al., 1993; Warden et al., 1993). BSB mice range from 1% to 50% body fat with an increase in both intra-abdominal and subcutaneous fat (Fisler et al., 1993). Obesity in BSB model is associated with hyperinsulinemia, hyperglycemia, and hyperlipidemia (Fisler et al., 1993).
Studies of polygenic mouse models have involved the analysis of numerous inbred strains using multiple experimental designs, and dozens of loci have been mapped across all mouse chromosomes (Pomp, 2007; Rankinen et al., 2006). These QTLs affect body weight, body fat, high fat diet-induced weight gain, the severity of obesity, and more specific traits such as food intake, energy expenditure and exercise habits (Fawcett et al., 2008). Only a few studies revealed QTLs in regions that had been previously identified in monogenic studies. For example, a study using QTL mapping in the BSB mouse model identified a locus that is very proximal to the LEP gene, which had been previously identified via positional cloning (Warden et al., 1995; Warden et al., 1993; Zhang et al., 1994). To identify the causative variation, each locus identified in a chromosomal region is isolated in a congenic strain, essentially converting it a monogenic study where interactions with other loci are held constant. This facilitates the analysis of the locus under study.
However, positional cloning of genes underlying obesity QTLs has proven to be a difficult task with a limited success rate (Wuschke et al., 2007). Several factors have contributed to this, including the time and cost required to generate and phenotype sufficient congenic and sub-congenic strains to localize the QTL to a region where a single candidate can be identified. Another challenge has been that many of the QTLs that were originally mapped appear to have resulted from the combined effects of multiple nearby QTLs (Buchner et al., 2012; Laplante et al., 2012; Mollah & Ishikawa, 2011; Prevorsek et al., 2010; Shao et al., 2008; Yazbek et al., 2011). Thus, when isolating the loci in progressively smaller congenic strains, the individual effect sizes (i.e., the phenotypic difference between congenic genotypes) can diminish and could even seemingly disappear if they are within a strain that also harbors a locus acting in the opposite direction. Similar to the case in human polygenic obesity, adiposity in mice seems largely controlled by multiple loci having modest effects. Finally, between any pair of strains, there are haplotype blocks where the strains have numerous genetic differences, both coding and non-coding, that could contribute to a QTL.
Despite the relatively low success rate of positional cloning in identifying polygenic obesity genes, few success stories of employing this approach in mouse models are described below.
Cntnap2 and Tag1
Pioneering the use of chromosome substitution strains for positional cloning in mice, the Nadeau laboratory has recently identified two genes associated with diet-induced obesity (Buchner et al., 2012). A mutation in the Cntnap2 gene which is required for proper potassium channel localization at neuronal nodes of Ranvier was identified through congenic analysis. Depending on the genetic background of the mouse model under investigation, this mutation either protected or predisposed mice to diet-induced obesity (Buchner et al., 2012). Using a candidate gene approach based on this finding, the group also assessed its known interacting protein, Tag1, in knockout mice and found that this gene also affects obesity by protecting mice against diet-induced obesity (Buchner et al., 2012). These studies have provided further evidence linking neuronal function with the regulation of body weight. Copy number variation in Cntnap2 has recently been identified in a child with syndromic obesity (Vuillaume et al., 2014).
Deptor
The Fob3a locus was identified in studies of the Fat and Lean strains generated by long-term selection for these traits (Stylianou et al., 2004). Recently, through congenic analysis, genetic variation in Deptor has been identified as a strong obesity candidate gene at the Fob3a locus (Laplante et al., 2012). This gene was previously known for its roles in mammalian target of rapamycin (mTor) signaling, but its role in obesity development was unknown. Through the subsequent generation of transgenic mice, Deptor overexpression was associated with increased adipogenesis (Laplante et al., 2012).
Other obesity candidate genes identified through congenic analysis
Studies of the Nob3 QTL have led to the identification of a microdeletion that eliminates expression of Ifi202b (Vogel et al., 2012). The authors showed that this altered the expression of several genes including 11 β-Hsd1. 11 β-Hsd1 encodes the cortisone reductase and is a relevant candidate gene for energy balance. Another gene recently identified from mouse positional cloning studies is Bhlhe40, which affects muscle fatty acid oxidation (Takeshita et al., 2012).
Mouse models have also been helpful in elucidating genes that play a role in polygenic obesity risk/protection in humans. For example, candidate gene studies of MC4R common genetic variants revealed that the gain-of-function mutation of the variants lower the risk of obesity (Geller et al., 2004; Stutzmann et al., 2007). Additionally, the study of BDNF as a candidate gene led to the association of a coding non-synonymous variant (Val66Met) with BMI variation in healthy adults (Gunstad et al., 2006), an association confirmed later on by hypothesis-free GWAS for BMI (Thorleifsson et al., 2009; Willer et al., 2009). Similarly, two non-synonymous variants on PCSK1 were consistently associated with childhood and adult severe obesity in a study of 13,659 participants of European ancestry, making PCSK1 a candidate gene for polygenic obesity (Benzinou et al., 2008).
From Human Obesity to Mouse Models: The Back and Forth
Mouse models have been invaluable in dissecting the genetic origin of human monogenic and polygenic obesity. The reverse approach also holds true as gene discoveries in humans have pioneered new mouse models for obesity. This section will review how genuine genetic discoveries in humans using high throughput, agnostic approaches such as positional cloning, GWAS or WES have inspired new experiments in mice to investigate the function of the genes.
Positional cloning
BBS
Bardet-Biedl Syndrome (BBS) is a rare recessive developmental disorder, and people with heterozygous mutations in BBS gene are more prone to obesity (Croft et al., 1995; Sheffield, 2010). Positional cloning, homozygosity mapping, candidate gene and whole exome sequencing approaches have led to the discovery of 18 genes that are linked to BBS so far (Scheidecker et al., 2014). Follow-up studies in animal models revealed the association of BBS genes with cilia function, and in intracellular and intraflagellar trafficking (Sheffield, 2010). More specifically, mice homozygous for a single BBS mutation lack spermatozoa flagella (Sheffield, 2010). Further analysis of BBS resulted in development of BBS knockout mice (Bbs2, Bbs4 and Bbs6) that developed hyperphagia, reduced energy expenditure and increased circulating leptin levels, which suggests that leptin deficiency is not the mechanism of obesity in BBS (Rahmouni et al., 2008). This was further confirmed by administration of leptin, which failed to change body weight or food intake, indicative of leptin resistance in BBS mice (Rahmouni et al., 2008). On the other hand, expression of Pomc in BBS knock-out mice were significantly lower than that of controls, while Agrp and Npy levels were comparable to controls, pointing to the idea that Pomc is the main player in obesity in BBS mice (Rahmouni et al., 2008). This phenomenon is compatible with the role of BBS in cilia function, as abrogating cilia in POMC neurons increases food intake and causes obesity in mice (Davenport et al., 2007).
TBC1D1
A major predisposing locus for obesity was identified at 4p.15-14, affecting more females than males (Arya et al., 2004; Meyre et al., 2008; Stone et al., 2002). Positional cloning of this 4p15-14 linkage peak led to the identification of a coding non-synonymous variant (R125W) in the TBC1 domain family member 1 (TBC1D1) gene associated with female familial obesity in populations from Utah and France (Meyre et al., 2008; Stone et al., 2006). TBC1D1 is a Rab-GTPase activating protein and is closely related to insulin signaling protein AS160. It is predominantly expressed in skeletal muscle, as it is involved in regulation of lipid utilization in skeletal muscles (An et al., 2010; Chadt et al., 2008; Sano et al., 2003). These discoveries in humans were followed-up by animal work. Studies of the Nob1 QTL identified a mutation in the Tbc1d1 gene that protects against obesity (Chadt et al., 2008). Tbc1d1 knockout mice were shown to exhibit reduction in body weight, impaired glucose utilization, and increased lipid oxidation in skeletal muscles (Dokas et al., 2013). Further analysis of the deleterious effects of the human R125W mutation has been confirmed by in vivo overexpression of wild-type and mutant TBC1D1 proteins in mouse tibialis anterior muscles (An et al., 2010). In this study, the R125W mutation impaired insulin-stimulated glucose transport, but did not impair contraction-stimulated glucose transport. Experiments conducted on phosphorylation sites of other TBC1D1 mutations had opposing effects, as these mutations impaired contraction-stimulated glucose transport but did not impact insulin-stimulated glucose transport (An et al., 2010). Overall, impairment of muscle glucose transport could lead to increased fat accumulation in adipose tissue and result in subsequent obesity (An et al., 2010).
ENPP1
A positional cloning experiment led to identification of ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) as a possible contributor to obesity and type 2 diabetes in humans. A significant linkage for childhood obesity was detected on chromosome 6q22.23 in French pedigrees (Meyre et al., 2004). By using overlapping published linkage studies on obesity (Atwood et al., 2002), insulin secretion (Abney, Ober & McPeek, 2002; Duggirala et al., 2001) and type 2 diabetes (Demenais et al., 2003; Ehm et al., 2000; Ghosh et al., 2000; Xiang et al., 2004), the ENPP1 gene was picked for further analysis. ENPP1 directly inhibits insulin-induced conformational changes of the insulin receptor, and affects its activation and signaling (Maddux & Goldfin, 2000; Maddux et al., 1995). A risk haplotype of ENPP1 was associated with childhood obesity, glucose intolerance and type 2 diabetes (Meyre et al., 2005). Subsequent development of Enpp1 knockout mice highlighted a phenotype of more efficient adipocyte maturation in mesenchymal embryonal cells compared to wild-type (Liang et al., 2007). Transgenic mice overexpressing human ENPP1 in muscle and liver tissue exhibited elevation in glucose and insulin levels compared to wild-type, conveying that ENPP1 plays a role in insulin resistance and hyperglycemia (Dong et al., 2005; Maddux et al., 2006).
Genome-wide association studies
GWAS in obesity field have identified 119 independent loci associated with BMI and obesity status (Choquet & Meyre, 2011; Locke et al., 2015). Table 2 provides a summary of these identified genes and associated SNPs. Interestingly, GWAS showed that almost all genes involved in Mendelian forms of obesity in mice and humans (LEPR, POMC, MC4R, BDNF, PCSK1, TUB, NTRK2, SH2B1) display common variants associated with BMI and polygenic obesity as well (Dickson et al., 2010). Genetic animal models were developed prior to GWAS discoveries for these genes whose functions were well-established. However, most of the genes located in or near GWAS signals were of unknown function. This prompted the scientific community to develop new genetic mouse models for some of these genes that we describe below.
Table 2. List of genes and SNPs associated with body mass index (BMI) or binary obesity from genome-wide association studies (GWAS).
| Gene | SNPs | Chrom. | Phenotype | Reference |
|---|---|---|---|---|
| ADCY9 | rs2531995 | 16 | Obesity | Berndt, SI. 2013. Nat Genet |
| AGBL4 | rs657452 | 1 | BMI | Locke, AE. 2015. Nature |
| ASB4 | rs6465468 | 7 | BMI | Locke, AE. 2015. Nature |
| BDNF | rs6265, rs4923461,rs10767664, rs2030323, rs988712 | 11 | BMI, Obesity, Overweight | Thorleifsson, G. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Jiao, H. 2011. BMC Med Genomics, Okada, Y. 2012. Nat Genet, Wen, W. 2012. Nat Genet 2012 |
| BRE | rs116612809 | 2 | BMI | Gong, J. 2013. Am J Hum Genet |
| C9orf93 | rs4740619 | 9 | BMI | Locke, AE. 2015. Nature |
| CADM1 | rs12286929 | 11 | BMI | Locke, AE. 2015. Nature |
| CADM2 | rs13078807 | 3 | BMI, Overweight | Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet |
| CALCR | rs9641123 | 7 | BMI | Locke, AE. 2015. Nature |
| CBLN1 | rs2080454 | 16 | BMI | Locke, AE. 2015. Nature |
| CDKAL1 | rs2206734, rs9356744 | 6 | BMI | Okada, Y. 2012. Nat Genet, Wen, W. 2012. Nat Genet 2012 |
| CLIP1 | rs11057405 | 12 | BMI | Locke, AE. 2015. Nature |
| CREB1, KLF7 | rs17203016 | 2 | BMI | Locke, AE. 2015. Nature |
| EHBP1 | rs11688816 | 2 | BMI | Locke, AE. 2015. Nature |
| ELAVL4 | rs11583200 | 1 | BMI | Locke, AE. 2015. Nature |
| EPB41L4B, C9orf4 | rs6477694 | 9 | BMI | Locke, AE. 2015. Nature |
| ERBB4 | rs7599312 | 2 | BMI | Locke, AE. 2015. Nature |
| ETS2 | rs2836754 | 21 | BMI | Locke, AE. 2015. Nature |
| ETV5 | rs7647305, rs9816226 | 3 | BMI, Obesity, Overweight | Thorleifsson, G. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet |
| FAIM2 | rs7138803, rs7132908 | 12 | BMI, Obesity, Overweight | Thorleifsson, G. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Paternoster, L. 2011. PLoS One, Bradfield, JP. 2012. Nat Genet, Berndt, SI. 2013. Nat Genet |
| FANCL | rs887912 | 2 | BMI, Obesity, Overweight | Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet |
| FANCL, FLJ30838 | rs12617233 | 2 | BMI | Guo, Y. 2012. Hum Mol Genet |
| FHIT | rs2365389 | 3 | BMI | Locke, AE. 2015. Nature |
| FIGN | rs1460676 | 2 | BMI | Locke, AE. 2015. Nature |
| FLJ35779 | rs2112347 | 5 | BMI, Obesity, Overweight | Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet |
| FOXO3, HSS00296402 | rs9400239 | 6 | BMI | Locke, AE. 2015. Nature |
| FTO | rs9939609, rs9930506, rs1121980, rs1421085, rs8050136, rs1558902, rs17817449, rs12149832, rs9940128, rs62033400, rs1421085, rs1121980, rs9936385, rs9941349, rs3751812, rs1558902, rs17817449 | 16 | BMI, Obesity, Childhood Obesity | Dina, C. 2007. Nat Genet, Hinney, A. PLoS One. 2007, Frayling, TM. 2007. Science, Scuteri, A. 2007. PLoS Genet, Loos, RJ. 2008. Nat Genet, Meyre, D. 2009. Nat Genet, Thorleifsson, G. 2009. Nat Genet, Willer, CJ. 2009. Nat Genet, Cho, YS. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Scherag, A. 2010. PLoS Genet, Paternoster, L. 2011. PLoS One, Wang, K. 2011. PLoS One, Bradfield, JP. 2012. Nat Genet, Wen, W. 2012. Nat Genet, Okada, Y. 2012. Nat Genet, Guo, Y. 2012. Hum Mol Genet, Graff, M. 2013. Hum Mol Genet, Pei, YF. 2013. Hum Mol Genet, Berndt, SI. 2013. Nat Genet, Wheeler, E. 2013. Nat Genet |
| GBE1 | rs3849570 | 3 | BMI | Locke, AE. 2015. Nature |
| GDF15, PGPEP1 | rs17724992 | 19 | BMI | Locke, AE. 2015. Nature |
| GIPR | rs2287019, rs11671664 | 19 | BMI | Speliotes, EK. 2010. Nat Genet, Wen, W. 2012. Nat Genet, Okada, Y. 2012. Nat Genet |
| GNAT2 | rs17024258 | 1 | Obesity | Berndt, SI. 2013. Nat Genet |
| GNPDA2 | rs10938397, rs13130484, rs348495 | 4 | BMI, Obesity, Overweight | Willer, CJ. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Graff, M. 2013. Hum Mol Genet, Berndt, SI. 2013. Nat Genet |
| GP2 | rs12597579 | 16 | BMI | Wen, W. 2012. Nat Genet 2012 |
| GPRC5BB | rs12444979 | 16 | BMI, Obesity, Overweight | Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet |
| GRID1 | rs7899106 | 10 | BMI | Locke, AE. 2015. Nature |
| GRP | rs7243357 | 18 | BMI | Locke, AE. 2015. Nature |
| GRP120 | rs116454156 | 10 | Obesity | Ichimura, A. 2012. Nature |
| HHIP | rs11727676 | 4 | BMI | Locke, AE. 2015. Nature |
| HIF1AN | rs17094222 | 10 | BMI | Locke, AE. 2015. Nature |
| HIP1, PMS2L3, PMS2P5, WBSCR16 | rs1167827 | 7 | BMI | Locke, AE. 2015. Nature |
| HMGA1 | rs206936 | 6 | BMI | Speliotes, EK. 2010. Nat Genet |
| HNF4G | rs4735692 | 8 | Obesity | Berndt, SI. 2013. Nat Genet |
| HOXB5 | rs9299 | 17 | Childhood Obesity | Bradfield, JP. 2012. Nat Genet |
| HS6ST3 | rs7989336 | 13 | Obesity | Berndt, SI. 2013. Nat Genet |
| HSD17B12 | rs2176598 | 11 | BMI | Locke, AE. 2015. Nature |
| IFNGR1, OLIG3 | rs13201877 | 6 | BMI | Locke, AE. 2015. Nature |
| KAT8, ZNF646, VKORC1, ZNF668, STX1B, FBXL19 | rs9925964 | 16 | BMI | Locke, AE. 2015. Nature |
| KCNK3 | rs11126666 | 2 | BMI | Locke, AE. 2015. Nature |
| KCNMA1 | rs2116830 | 101 | Obesity | Jiao, H. 2011. BMC Med Genomics |
| KCTD15 | rs11084753, rs29941 | 19 | BMI | Willer, CJ. 2009. Nat Genet, Thorleifsson, G. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet |
| KLF9 | rs11142387 | 9 | BMI | Okada, Y. 2012. Nat Genet |
| LEPR | rs11208659 | 1 | Childhood Obesity | Wheeler, E. 2013. Nat Genet |
| LMX1B | rs10733682 | 9 | BMI | Locke, AE. 2015. Nature |
| LOC100287559, BBS4 | rs7164727 | 15 | BMI | Locke, AE. 2015. Nature |
| LOC284260, RIT2 | rs7239883 | 18 | BMI | Locke, AE. 2015. Nature |
| LOC285762 | rs9374842 | 6 | BMI | Locke, AE. 2015. Nature |
| LPIN2 | rs643507 | 18 | Obesity (Asthmatic patients) | Melen, E. 2013. Clin Exp Allergy |
| LRP1B | rs2890652 | 2 | BMI | Speliotes, EK. 2010. Nat Genet |
| LRRN6C | rs10968576 | 9 | BMI, Obesity | Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet |
| MAF | rs1424233 | 16 | Obesity | Meyre, D. 2009. Nat Genet |
| MAP2K5 | rs2241423, rs4776970, rs997295 | 15 | BMI, Obesity, Overweight | Speliotes, EK. 2010. Nat Genet, Wen, W. 2012. Nat Genet 2012, Guo, Y. 2012. Hum Mol Genet, Berndt, SI. 2013. Nat Genet |
| MAPK3, KCTD13, INO80E, TAOK2, YPEL3, DOC2A, FAM57B | rs4787491 | 16 | BMI | Locke, AE. 2015. Nature |
| MC4R | rs17782313, rs571312, rs12970134, rs2331841, rs6567160, rs8089364, rs7234864, rs723486, rs7227255, rs2229616, rs17782313, rs17700144,rs663129, rs571312, rs476828 | 18 | BMI, Obesity, Overweight | Loos, RJ. 2008. Nat Genet, Thorleifsson, G. 2009. Nat Genet, Meyre, D. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Scherag, A. 2010. PLoS Gene, Paternoster, L. 2011. PLoS One, Guo, Y. 2012. Hum Mol Genet, Okada, Y. 2012. Nat Genet, Wen, W. 2012. Nat Genet, Bradfield, JP. 2012. Nat Genet, Berndt, SI. 2013. Nat Genet, Wheeler, E. 2013. Nat Genet, Graff, M. Hum Mol Genet. 2013, Pei, YF. Hum Mol Genet. 2013 |
| MIR548A2 | rs1441264 | 13 | BMI | Locke, AE. 2015. Nature |
| MIR548X2, PCDH9 | rs9540493 | 13 | BMI | Locke, AE. 2015. Nature |
| MRPS33P4 | rs13041126 | 20 | Obesity | Berndt, SI. 2013. Nat Genet |
| MTCH2 | rs10838738, rs3817334 | 11 | BMI, Obesity, Overweight | Willer, CJ. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet |
| MTIF3 | rs4771122 | 13 | Speliotes, EK. 2010. Nat Genet | |
| NAV1 | rs2820292 | 1 | BMI | Locke, AE. 2015. Nature |
| NEGR1 | rs2815752, rs2568958, rs1993709 | 1 | BMI, Obesity, Overweight | Willer, CJ. 2009. Nat Genet, Thorleifsson, G. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet, Wheeler, E. 2013. Nat Genet |
| NLRC3 | rs758747 | 16 | BMI | Locke, AE. 2015. Nature |
| NPC1 | rs1805081 | 18 | Obesity | Meyre, D. 2009. Nat Genet |
| NRXN3 | rs10150332 | 14 | BMI, Obesity | Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet |
| NT5C2, CYP17A1, SFXN2 | rs11191560 | 10 | BMI | Locke, AE. 2015. Nature |
| NTRK2 | rs1211166 | 9 | BMI | Guo, Y. 2012. Hum Mol Genet |
| NUP54, SCARB2 | rs17001654 | 4 | BMI | Locke, AE. 2015. Nature |
| OLFM4 | rs9568856, rs9568867 | 13 | Obesity | Bradfield, JP. 2012. Nat Genet, Berndt, SI. 2013. Nat Genet |
| PACS1 | rs564343 | 11 | Childhood Obesity | Wheeler, E. 2013. Nat Genet |
| PARK2 | rs13191362 | 6 | BMI | Locke, AE. 2015. Nature |
| PCSK1 | rs261967, rs6232, rs6234, rs6235 | 5 | BMI, Obesity | Benzinou, M. 2008. Nat Genet, Wen, W. 2012. Nat Genet |
| PLCD4, CYP27A1, USP37, TTLL4, STK36, ZNF142,RQCD1 | rs492400 | 2 | BMI | Locke, AE. 2015. Nature |
| PMS2L11 | rs2245368 | 7 | BMI | Locke, AE. 2015. Nature |
| POMC | rs713586, rs6545814, rs1561288, rs6752378, rs10182181 | 2 | BMI, Obesity, Overweight | Speliotes, EK. 2010. Nat Genet, Wen, W. 2012. Nat Genet 2012, Bradfield, JP. 2012. Nat Genet, Berndt, SI. 2013. Nat Genet, Graff, M. 2013. Hum Mol Genet |
| PRKCH | rs1957894 | 14 | Childhood Obesity | Wheeler, E. 2013. Nat Genet |
| PRKD1 | rs11847697, rs12885454 | 14 | BMI | Speliotes, EK. 2010. Nat Genet, Locke, AE. 2015. Nature |
| PTBP2 | rs1555543 | 1 | BMI | Speliotes, EK. 2010. Nat Genet |
| QPCTL | rs2287019 | 19 | Obesity, Overweight | Berndt, SI. 2013. Nat Genet |
| RABEP1 | rs1000940 | 17 | BMI | Locke, AE. 2015. Nature |
| RALYL | rs2033732 | 8 | BMI | Locke, AE. 2015. Nature |
| RARB | rs6804842 | 3 | BMI | Locke, AE. 2015. Nature |
| RASA2 | rs16851483 | 3 | BMI | Locke, AE. 2015. Nature |
| RMST | rs11109072 | 12 | Childhood Obesity | Wheeler, E. 2013. Nat Genet |
| RPL27A | rs11042023 | 11 | Obesity | Berndt, SI. 2013. Nat Genet |
| RPTOR | rs7503807 | 17 | Overweight | Berndt, SI. 2013. Nat Genet |
| SBK1, APOBR | rs2650492 | 16 | BMI | Locke, AE. 2015. Nature |
| SCG3, DMXL2 | rs3736485 | 15 | BMI | Locke, AE. 2015. Nature |
| SDCCAG8 | rs12145833 | 1 | Childhood Obesity | Scherag, A. 2010. PLoS Gene |
| SEC16B | rs10913469, rs543874, rs574367, rs516636, rs591120 | 1 | BMI, Obesity, Overweight | Thorleifsson, G. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Bradfield, JP. 2012. Nat Genet, Berndt, SI. 2013. Nat Genet, Graff, M. 2013. Hum Mol Genet, Wen, W. 2012. Nat Genet, Okada,Y. 2012. Nat Genet |
| SH2B1 | rs7498665, rs4788102, rs7359397, rs4788099 | 16 | BMI, Obesity, Overweight | Willer, CJ. 2009. Nat Genet, Thorleifsson, G. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Guo, Y. 2012. Hum Mol Genet, Berndt, SI. 2013. Nat Genet |
| SLC39A8 | rs13107325 | 4 | BMI | Speliotes, EK. 2010. Nat Genet |
| SMG6, N29617 | rs9914578 | 17 | BMI | Locke, AE. 2015. Nature |
| STXBP6 | rs10132280 | 14 | BMI | Locke, AE. 2015. Nature |
| TAL1 | rs977747 | 1 | BMI | Locke, AE. 2015. Nature |
| TCF7L2 | rs7903146 | 10 | BMI | Locke, AE. 2015. Nature |
| TDRG1, LRFN2 | rs2033529 | 6 | BMI | Locke, AE. 2015. Nature |
| TFAP2B | rs987237, rs734597, rs2272903 | 6 | BMI, Obesity, Overweight | Speliotes, EK. 2010. Nat Genet, Paternoster, L. 2011. PLoS One, Guo, Y. 2012. Hum Mol Genet, Berndt, SI. 2013. Nat Genet |
| TLR4 | rs1928295 | 9 | BMI | Locke, AE. 2015. Nature |
| TMEM160 | rs3810291, rs3810291 | 19 | BMI, Obesity | Speliotes, EK. 2010. Nat Genet, Berndt, SI. 2013. Nat Genet |
| TMEM18 | rs6548238, rs7561317, rs2867125, rs12463617, rs4854344 | 2 | BMI, Obesity, Overweight | Willer, CJ. 2009. Nat Genet, Thorleifsson, G. 2009. Nat Genet, Speliotes, EK. 2010. Nat Genet, Guo, Y. 2012. Hum Mol Genet, Bradfield, JP. 2012. Nat Genet, Berndt, SI. 2013. Nat Genet, Wheeler, E. 2013. Nat Genet, Graff, M. 2013. Hum Mol Genet |
| TNKS | rs17150703 | 8 | Childhood Obesity | Scherag, A. 2010. PLoS Gene |
| TNNI3K | rs1514175, rs12142020, rs1040070, rs1514174 | 1 | BMI, Obesity | Speliotes, EK. 2010. Nat Genet, Bradfield, JP. 2012. Nat Genet, Graff, M. 2013. Hum Mol Genet, Berndt, SI. 2013. Nat Genet |
| TOMM40, APOE, APOC1 | rs2075650 | 19 | BMI | Guo, Y. 2012. Hum Mol Genet |
| TUB | rs4929949 | 11 | BMI | Speliotes, EK. 2010. Nat Genet |
| UBE2E3 | rs1528435 | 2 | BMI | Locke, AE. 2015. Nature |
| ZBTB10 | rs16907751 | 8 | BMI | Locke, AE. 2015. Nature |
| ZNF608 | rs48361333 | 5 | BMI | Speliotes, EK. 2010. Nat Genet |
| ZZZ3 | rs17381664 | 1 | Obesity | Berndt, SI. 2013. Nat Genet |
FTO
The fat mass and obesity associated gene (FTO) is the first gene that has been convincingly associated with obesity using GWAS. The role of FTO as an important contributor to polygenic obesity was confirmed in GWAS for type 2 diabetes, BMI, early onset obesity, and incidentally in a population stratification approach (Dina et al., 2007; Frayling et al., 2007; Hinney et al., 2007; Scuteri et al., 2007). Initial GWAS on FTO identified FTO as an unknown gene in an unknown pathway (Frayling et al., 2007). The exact molecular mechanism of how FTO might contribute to obesity is still under investigation, but high level of expression of FTO in the hypothalamus is suggestive of its role in food intake (Fredriksson et al., 2008). This required further studies of FTO in knockout and overexpression animal models to understand the mechanism in which FTO influences obesity (Choquet & Meyre, 2011).
Fto knockout mice exhibit high perinatal lethality, significant reduction in body length, fat mass and lean mass, indicative of the role of Fto in energy homeostasis (Church et al., 2009; Fischer et al., 2009; McMurray et al., 2013). Deletion of Fto in the hypothalamus via adeno-associated viral vectors encoding Cre recombinase resulted in small reduction in food intake and decreased weight gain with no effect on energy expenditure (McMurray et al., 2013). Overexpression of Fto in mice results in increased food intake, increase in body and fat mass (Church et al., 2010).
Despite the metabolic phenotypes found in FTO overexpression/inactivation rodent models and the location of SNPs associated with human obesity in the intron 1 region of FTO, the implication of other neighboring genes in obesity is not excluded. As an illustration, the obesity-associated FTO sequence directly interacts with the promoters of IRX3 as well as FTO in the human, mouse and zebrafish genomes (Smemo et al., 2014). Expression QTL studies in human brains revealed that obesity associated SNPs in FTO are associated with expression of IRX3, but not FTO itself (Smemo et al., 2014). Irx3 knockout mice show reduction in body weight and increase in basal metabolic rate, indicative of a direct link between Irx3 and body composition (Smemo et al., 2014). The rs8050136 SNP located in the first intron of FTO modulates the binding for CUX1 P110 and P200 isoforms which in turn regulate the expression of FTO and of the nearby ciliary gene RPGRIPL1 (Stratigopoulos et al., 2014; Stratigopoulos et al., 2011). Homozygous mutants of Rpgripl1 are lethal, but Rpgrip1l +/− mice are leptin resistant, hyperphagic and obese (Stratigopoulos et al., 2014). Overall, these animal experiments suggest that the genes IRX3 and RPGRIPL1 may mediate at least in part the association of SNPs in FTO with obesity.
NPC1
Besides FTO, genome wide association studies revealed that two non-synonymous variants in high linkage disequilibrium (H215R/I858V) in the Niemann-Pick C1 (NPC1) gene were associated with extreme obesity in European adults (Meyre et al., 2009). Subsequent mouse models of partial inactivation of Npc1 evidenced significant weight gain in mice fed a high-fat diet, indicating a possible gene-diet interaction (Jelinek et al., 2010). More recently, SNPs at the Npc1 locus have been associated with differences of body fat (%) in response to high-fat high-sucrose diet in a GWAS performed in mice (Parks et al., 2013).
ETV5
Ets variant 5 (ETV5) is a transcription factor that can act either as an activator or repressor of transcription of genes involved in cell proliferation, differentiation, apoptosis and cell–cell or cell–matrix interaction (Sementchenko & Watson, 2000; Sharrocks, 2001). Two GWAS in populations of European ancestry identified SNPs near ETV5 as associated with BMI and obesity (Berndt et al., 2013; Speliotes et al., 2010; Thorleifsson et al., 2009). This association triggered further research on ETV5 in animal models. Etv5 knockout mice exhibit lean bodies, resistance to diet-induced obesity and severe glucose intolerance due to impaired insulin exocytosis and hypoinsulinaemia (Gutierrez-Aguilar et al., 2014).
NEGR1
GWAS identified SNPs near the neuronal growth regulator 1 (NEGR1) gene associated with BMI variation and obesity (Berndt et al., 2013; Thorleifsson et al., 2009; Wheeler et al., 2013; Willer et al., 2009). NEGR1 is expressed in the brain and participates in the neurite outgrowth in the developing brain (Marg et al., 1999). With the use of ENU mutagenesis, mice carrying a loss of function of Negr1 displayed reduced food intake and physical activity, unchanged energy expenditure and reduction in overall body mass (Lee et al., 2012).
Next generation sequencing (NGS)
SDCCAG8
Nephronophthisis-related ciliopathies (NPHP-RCs) are developmental problems that impact kidneys and are associated with renal degeneration, intellectual disability and obesity. Exome sequencing identified 12 truncating mutations on serologically defined colon cancer antigen 8 (SDCCAG8) gene, showing that a loss of function of SDCCAG8 is causal for human retinal-renal ciliopathy (Otto et al., 2010). The candidacy of SDCCAG8 gene was strengthened by the identification of common variants associated with childhood obesity through a GWAS in German and French populations (Scherag et al., 2010). To better understand the function of SDCCAG8, a gene-trap mouse line (Sdccag8gt/gt) was subsequently developed (Airik et al., 2014). The Sdccag8gt/gt mice exhibited the human phenotype of NPHP-RCs and revealed that retinal degeneration associated with the disorder exhibits early and leads to progressive loss of vision, whereas the renal degeneration occur later due to DNA damage from signaling activity (Airik et al., 2014).
The Waltz Between Mouse and Human Genetic Studies
Recent attempts in understanding genetics of obesity utilizing both human and animal genetic approaches are discussed below.
Linkage study
Arrestin domain-containing 3 protein (ARRDC3) is a regulator of cell receptor signaling, and also plays a role in metabolism (Luan et al., 2009). Genome wide linkage for human obesity identified a linkage peak on chromosome 5, and positional cloning identified ARRDC3 associated with higher BMI in males but not in females (Patwari et al., 2011). Higher ARRDC3 expression is associated with visceral adipose tissue and obesity in males. Animal models such as the Arrdc3 deficient mice have validated the role of ARRDC3 in metabolism by being resistant to obesity in a dosage dependent manner (both genders, but with greater impact on males than females) (Patwari et al., 2011).
Candidate gene approach
G-protein coupled receptor 120 (GPR120) is a receptor for unsaturated long chain fatty acids, and plays a role in adipogenesis, regulation of appetite and food preference (Hirasawa et al., 2005). Gpr120 deficient mice fed a high fat diet exhibit obesity, glucose intolerance, fatty liver, decreased adipocyte differentiation and lipogenesis (Ichimura et al., 2012), but no difference in body weight between Gpr120 deficient and wild type mice was observed when both groups were fed a normal diet (Ichimura et al., 2012). When assessed in humans, GPR120 was expressed in adipose tissue, with obese individuals having a higher expression in both subcutaneous and omental adipose tissue (1.8 fold increase) (Ichimura et al., 2012). In order to study the contribution of GPR120 to human obesity, the four GPR120 exons were sequenced in 312 non-consanguineous extremely obese French children and adults. Exon sequencing revealed a deleterious non-synonymous variant (p.R270H) of minor allele frequency (MAF) of 3% that inhibits GPR120 signaling activity and increases the risk of obesity by 62% in 6,942 obese individuals and 7,654 control subjects from Europe (Ichimura et al., 2012). Thus, GPR120 plays a role in sensing dietary fat, and is important in energy balance.
Melanocortin 2 receptor accessory protein 2 (MRAP2) is a homologue of MRAP, expressed in the brain and adrenal gland (Chan et al., 2009). MRAP2 can interact with all melanocortin receptors, which results in MC2R surface expression and signaling. MRAP2 can also reduce the responsiveness of MC1R, MC3R, MC4R and MC5R to α-MSH (Chan et al., 2009). Mouse models deficient in Mrap2 exhibit obesity (Asai et al., 2013). Selective knockout of Mrap2 in neurons expressing Sim1 also exhibit obesity, similar to global knockout of Mrap2, consistent with the idea that Sim1 expressing neurons are key regulators of energy balance (Asai et al., 2013). Four rare heterozygous mutations of MRAP2 have been identified in obese humans (Asai et al., 2013).
Whole exome sequencing
Kinase suppressor of Ras 2 (KSR2) is a scaffolding protein involved in multiple signaling pathways through kinase cascades (Dougherty et al., 2010; Pearce et al., 2013) that are linked to regulation of food intake, body fat content and glucose homeostasis (Revelli et al., 2011). By using a whole-exome sequencing strategy, KSR2 loss-of-function mutations were identified in humans and were associated with hyperphagia, early-onset obesity, low heart rate, reduced basal metabolic rate and severe insulin resistance (Pearce et al., 2013). Ksr 2−/− mice display obesity, high insulin levels, and impaired glucose tolerance (Pearce et al., 2013). Obesity persisted in Ksr 2−/− mice despite being fed the same amount of diet as Ksr2+/+ littermates (Pearce et al., 2013).
Perspectives
To conclude this review, we provide helpful suggestions to accelerate the identification of obesity predisposing genes as well as their functional interrogation in mouse and human.
The use of approaches integrating multiple types of data (system biology/functional genomics) could boost the identification of genes predisposing to obesity (Yang, Jiang & He, 2009). Such studies could benefit from the use of mice to access tissues that are not readily available in humans or to perform deep phenotyping that is too expensive in humans. The co-mapping of gene expression levels (eQTLs), protein levels (pQTLs) and even metabolites (mQTLs) to a location in the genome associated with a disease may generate novel hypotheses for direct testing in humans and mice (Davis et al., 2012; Mackay, Stone & Ayroles, 2009; Yang et al., 2009). As an illustration, a study combining positional cloning and high-throughput transcriptome approaches identified two novel candidate genes driving adiposity in mice (Akr1b8 and Rgs2) that deserve further investigation in humans (Derry et al., 2010).
Stringent P-value thresholds are classically used in GWAS (P < 5 × 10−8) to adjust the experiment for the many hypotheses tested (Dudbridge & Gusnanto, 2008). As a result, many true associations that do not reach stringent P-value thresholds are missed by conventional GWAS (Stahl et al., 2012). Similar issues are now experienced in next generation sequencing (NGS) studies (Do, Kathiresan & Abecasis, 2012). Identifying these true positive associations is challenging and so far has been addressed by the ever expanding meta-analyses (Stahl et al., 2012). Another way to ‘separate the wheat from the chaff’ could include the utilization of hypothesis-driven GWAS and next generation sequencing (NGS) approaches as opposed to hypothesis-free strategies (Li & Meyre, 2013). This method is beneficial in its decreased number of statistical tests and less stringent significance thresholds for the hypotheses being tested (Li & Meyre, 2013). Narrowing down the hypothesis to a specific linkage region or molecular pathway for example, could lead to identification of association signals previously missed by conventional GWAS (Li & Meyre, 2013). These high-throughput hypothesis-driven experiments would greatly benefit from the inclusion of data collected in mouse models of obesity (Li & Meyre, 2013).
It is also important to emphasize on the value of expression studies in future experiments. Expression studies in mice can add valuable knowledge of the expression and regulation of genes under diverse environmental exposures (Yoganathan, Karunakaran & Clee, 2012) especially when studying the expression of a gene in a certain tissue is difficult to obtain in human studies. In a recent study, the expression of a subset of GWAS obesity candidate genes was observed to be different in the hypothalamus and/or adipose tissue of fed vs. fasted animals (Yoganathan, Karunakaran & Clee, 2012). These experiments are helpful in moving from GWAS association signals to relevant candidate genes for obesity (Yoganathan, Karunakaran & Clee, 2012).
Improving on methodology and techniques used in animal studies could also provide better insight in upcoming genetics studies. Employing more recent tools in genome editing such as CRISPR/Cas9 could allow for more precise targeted mutagenesis (Zhang, Wen & Guo, 2014). This method depends on small RNA for sequence-specific cleavage (Jinek et al., 2012) for DNA targeting which is relatively cheap and easy to produce (Zhang, Wen & Guo, 2014). It involves a non-specific Cas9 nuclease and a set of programmable sequence-specific CRIPS RNA (crRNA) which can guide Cas9 to cleave the DNA and generate double-strand breaks at target sites (Zhang, Wen & Guo, 2014). CRISP/Cas9 is able to simultaneously allow for genomic modifications at multiple independent sites (Cong et al., 2013), but it can also induce non desired insertion deletions (Lin et al., 2014).
In light of improvement in animal models, utilizing tissue or time specific knockouts, knock-in or transgenic mice could help in better understanding the function of genes that were previously associated with obesity. For example, mice with global PPAR γ inactivation showed reduced adipose tissue and mild glucose intolerance (Koutnikova et al., 2003). In comparison, fat-specific PPAR γ knockout animals showed complete lipoatrophy, impaired adipokine secretion, profound insulin resistance and hyperglycemia, abnormal bone, mammary gland and skin metabolism (Wang et al., 2013). Although not frequently used in the obesity genetics field, time-specific knockouts are helpful in understanding gene expression at different developmental stages (Loebel et al., 2014). For instance, complete post-natal inactivation of BDNF in mice was associated with hyperphagic obesity, whereas pre-natal inactivation of the same gene was lethal (Rios et al., 2001).
Understanding the importance of gene-gene interactions in development of obesity is another key area of investigation. Studying gene-gene interactions in humans are experimentally demanding because they require large sample sizes to detect significant interactions, and are statistically challenging due to multiple testing issues (Hu, Wang & Wang, 2014). Therefore model organisms are an important tool for studying gene-gene interactions (MacKay, 2014). Genetic studies in mice may also facilitate the discovery of gene-gene interactions or loci whose effects are only evident in the context of specific alleles at other loci. This approach is based on the hypothesis that a QTL for a trait in mouse that maps to a homologous location for the same trait in humans, is most likely caused by the same gene (Leduc et al., 2011). The BSB mouse model is an example of mouse model used to study epistatic effects on obesity QTLs (Yi et al., 2004). Mapping and identification of gene × gene interactions in mice could be examined in humans, again, since the homologous regions of mouse and human chromosome regions are well-defined (Chiu et al., 2006). Double-knockout mouse models, where two genes are inactivated, can reveal valuable information about gene-gene interactions (Chiu et al., 2006). Double-knockout mice have not been commonly studied in obesity field, so we present an example from the diabetes field. Irs1−/− have mild glucose intolerance and Irs3−/− have no detectable phenotype, but Irs1−/−/Irs3−/− are hyperglycemic and display severe lipoatrophy (Terauchi et al., 2003), indicative of their interaction in developing the type 2 diabetes phenotype. Complex mouse models showing evidence of epistasis can be tested in human genetic epidemiology studies. Although the number of known gene-gene interactions in human obesity is relatively small, we hope that increased sample size in epidemiological studies could help elucidate more of these interactions. Double knock out or transgenic mouse models are an attractive model to confirm the interactions identified in humans.
Aside from the importance of gene-gene interactions, recognizing the importance of gene-environment interactions in developing obesity is crucial (Andreasen & Andersen, 2009). For instance, physical activity could offset the aggregated genetic risk of multiple obesity loci (Ahmad et al., 2013; Li et al., 2010). Gene-environment studies can help in targeting populations that may respond well or poorly to a specific lifestyle or therapeutic interventions (Ahmad et al., 2013). Establishing large case-control studies in population-based cohorts, precise phenotyping of quantitative trait studies with precise measurements of lifestyle exposure, and target testing of interaction in existing lifestyle trials will provide the best understanding of gene-environment interactions (Franks et al., 2007).
To effectively demonstrate how genetic variations at a specific locus modify the effect of an environmental stimuli on a metabolic trait requires a combination of environmental modifications on animal models and human etiological trials (Franks & Roth, 2008). Different environmental modifications can be tested in homozygous or heterozygous knock out or knock in models and they can eventually be studied in inbred strains with naturally occurring allelic variations. Testing the gene-environment interactions in genes with prior evidence of their role in interacting with an environmental stimuli in mouse models can improve the probability that an observed effect is true (Franks & Roth, 2008), which may help to better control for the high likelihood of false positives in gene-environment interactions in human studies. Studying gene-environment interactions in knock-out animal models may be used as a first step to prioritize genes for gene environment interaction studies in human populations. For instance, Gipr knockout mice are protected against obesity and disturbance to their glucose homeostasis under a high-fat diet (Sonestedt et al., 2012). Human studies of GIPR common gene variants found a significant interaction between the rs10423928 SNP and a high-fat/low carbohydrate diet on risk of type 2 diabetes (Sonestedt et al., 2012).
Lastly, genes involved in diet and immune response have been preferential targets in positive selection during mammalian evolution, highlighting the importance of nutrient availability and pathogens as powerful driving forces of evolution (Kosiol & Vinar, 2008). Evolutionary assessment of mutations associated with highly penetrant from of obesity through mammal evolution (including rodents) may be complementary to in vitro and in vivo characterization studies in evaluating their functional relevance (Stäubert et al., 2007). This approach may be less relevant to common variants of obesity with modest effect. For instance, more than 90% of 70 missense mutations in MC4R identified in obese patients were located at amino acid positions that are highly conserved during 450 million years of MC4R evolution in vertebrates (Stäubert et al., 2007).
Conclusions
We have reviewed the synthesis between mouse and human genetics in the field of obesity. We describe the approaches and techniques that are available for mouse and human geneticists, and provide a striking illustration of the synergy between these approaches that led to successful obesity causing gene identifications these last decades. We list innovative approaches to not only ensure a higher yield of novel obesity genes, but also a deeper understanding of their function. Figure 4 is an illustration that summarizes the discussions in this review paper. Integrative mouse human strategies have the potential to lead to the identification of more genes responsible for common Mendelian forms of obesity, as well as gene × gene and gene × environment interactions. This may help to unravel the missing heritability of obesity. We believe that an exhaustive understanding of obesity genetics will help to identify novel drug targets and to design more efficient and personalized obesity prevention and management programs that, with the support of populations and stakeholders, will ultimately curb the obesity epidemic (Agurs-Collins et al., 2008).
Figure 4. Graphical representation of the main concepts of the review.
Summary of the major concepts in methodology of mouse include natural, chemical and insertional mutagenesis, as well as inbreeding and genetic manipulation techniques. In humans, linkage analysis, homozygous mapping, candidate gene studies, genome-wide association studies (GWAS) and whole exome/genome studies (WES/WGS) are discussed. These methodologies have led to genetic studies in monogenic and polygenic obesity, where mouse models paved the way for genetic discoveries in humans. The reverse concept also holds true, as genetic discoveries in humans led to development of new mouse models. In recent attempts of genetic discovery, an integrative approach of animal and human studies have promoted new gene discoveries as well as functional analysis. Current studies are utilizing innovative approaches such as use of omics, hypothesis driven GWAS, expression studies, improved genetic manipulation techniques, gene × gene or gene x environment analysis as well as evolutionary analysis to improve our understanding of the genetic architecture of obesity.
Acknowledgments
The authors would like to thank Dr. S Robiou du Pont and Dr. A Al Abadi for critically reading and commenting on the manuscript.
Funding Statement
Funding was provided by Canada Research Chairs and Michael Smith Foundation for Health Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
David Meyre is an Academic Editor for PeerJ.
Author Contributions
Fereshteh T. Yazdi and David Meyre conceived and designed the experiments, performed the experiments, wrote the paper, prepared figures and/or tables.
Susanne M. Clee performed the experiments, wrote the paper, reviewed drafts of the paper.
References
- Abney, Ober & McPeek (2002).Abney M, Ober C, McPeek MS. Quantitative-trait homozygosity and association mapping and empirical genomewide significance in large, complex pedigrees: fasting serum-insulin level in the Hutterites. American Journal of Human Genetics. 2002;70(4):920–934. doi: 10.1086/339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Arozena et al. (2008).Acevedo-Arozena A, Wells S, Potter P, Kelly M, Cox RD, Brown SDM. ENU mutagenesis, a way forward to understand gene function. Annual Review of Genomics and Human Genetics. 2008;9(1):49–69. doi: 10.1146/annurev.genom.9.081307.164224. [DOI] [PubMed] [Google Scholar]
- Agurs-Collins et al. (2008).Agurs-Collins T, Khoury MJ, Simon-Morton D, Olster DH, Harris JR, Milner JA. Public health genomics: translating obesity genomics research into population health benefits. Obesity (Silver Spring, Md.) 2008;16 (Suppl 3):S85–S94. doi: 10.1038/oby.2008.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad et al. (2013).Ahmad S, Rukh G, Varga TV, Ali A, Kurbasic A, Shungin D, Ericson U, Koivula RW, Chu AY, Rose LM, Ganna A, Qi Q, Stanáková A, Sandholt CH, Elks CE, Curhan G, Jensen MK, Tamimi RM, Allin KH, Jørgensen T, Brage S, Langenberg C, Aadahl M, Grarup N, Linneberg A, Paré G, Magnusson PKE, Pedersen NL, Boehnke M, Hamsten A, Mohlke KL, Pasquale LT, Pedersen O, Scott RA, Ridker PM, Ingelsson E, Laakso M, Hansen T, Qi L, Wareham NJ, Chasman DI, Hallmans G, Hu FB, Renström F, Orho-Melander M, Franks PW. Gene × physical activity interactions in obesity: combined analysis of 111,421 individuals of European ancestry. PLoS Genetics. 2013;9(7):e856. doi: 10.1371/journal.pgen.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, Varga & Franks (2013).Ahmad S, Varga T, Franks P. Gene × environment interactions in obesity: the state of the evidence. Human Heredity. 2013;75:106–115. doi: 10.1159/000351070. [DOI] [PubMed] [Google Scholar]
- Airik et al. (2014).Airik R, Slaats GG, Guo Z, Weiss A-C, Khan N, Ghosh A, Hurd TW, Bekker-Jensen S, Schrøder JM, Elledge SJ, Andersen JS, Kispert A, Castelli M, Boletta A, Giles RH, Hildebrandt F. Renal-retinal ciliopathy gene Sdccag8 regulates DNA damage response signaling. Journal of the American Society of Nephrology. 2014;25:1–11. doi: 10.1681/ASN.2013050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan, Eisen & Pomp (2004).Allan MF, Eisen EJ, Pomp D. The M16 mouse: an outbred animal model of early onset polygenic obesity and diabesity. Obesity Research. 2004;12(9):1397–1407. doi: 10.1038/oby.2004.176. [DOI] [PubMed] [Google Scholar]
- An et al. (2010).An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, Goodyear LJ. Glucose transport in mouse skeletal muscle. Diabetes. 2010;59:1358–1366. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen & Andersen (2009).Andreasen CH, Andersen G. Gene-environment interactions and obesity–further aspects of genomewide association studies. Nutrition (Burbank, Los Angeles County, Calif.) 2009;25(10):998–1003. doi: 10.1016/j.nut.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Arya et al. (2004).Arya R, Duggirala R, Jenkinson CP, Almasy L, Blangero J, O’Connell P, Stern MP. Evidence of a novel quantitative-trait locus for obesity on chromosome 4p in Mexican Americans. American Journal of Human Genetics. 2004;74(2):272–282. doi: 10.1086/381717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai et al. (2013).Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, Ho C, Novoselova TV, Garg S, Ridderstrale M, Marcus C, Hirschhorn JN, Keogh JM, O’Rahilly S, Chan LF, Clark AJ, Farooqi IS, Majzoub JA. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341(6143):275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood et al. (2002).Atwood LD, Heard-Costa NL, Cupples LA, Jaquish CE, Wilson PWF, D’Agostino RB. Genomewide linkage analysis of body mass index across 28 years of the Framingham Heart Study. American Journal of Human Genetics. 2002;71(5):1044–1050. doi: 10.1086/343822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala et al. (2010).Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Disease Models and Mechanisms. 2010;3(9–10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylor et al. (2011).Aylor DL, Valdar W, Foulds-Mathes W, Buus RJ, Verdugo RA, Baric RS, Ferris MT, Frelinger JA, Heise M, Frieman MB, Gralinski LE, Bell TA, Didion JD, Hua K, Nehrenberg DL, Powell CL, Steigerwalt J, Xie Y, Kelada SNP, Collins FS, Yang IV, Schwartz DA, Branstetter LA, Chesler EJ, Miller DR, Spence J, Liu EY, McMillan L, Sarkar A, Wang J, Wang W, Zhang Q, Broman KW, Korstanje R, Durrant C, Mott R, Iraqi FA, Pomp D, Threadgill D, Pardo-Manuel de Villena F, Churchill GA. Genetic analysis of complex traits in the emerging Collaborative Cross. Genome Research. 2011;21(8):1213–1222. doi: 10.1101/gr.111310.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann-Gagescu et al. (2010).Bachmann-Gagescu R, Mefford HC, Cowan C, Glew GM, Hing AV, Wallace S, Bader PI, Hamati A, Reitnauer PJ, Smith R, Stockton DW, Muhle H, Helbig I, Eichler EE, Ballif BC, Rosenfeld J, Tsuchiya KD. Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genetics in Medicine. 2010;12(10):641–647. doi: 10.1097/GIM.0b013e3181ef4286. [DOI] [PubMed] [Google Scholar]
- Bahreini et al. (2013).Bahreini N, Noor MI, Koon PB, Talib RA, Lubis SH, Dashti MG, Salehi-Abargouei A, Esmaillzadeh A. Weight status among Iranian adolescents: comparison of four different criteria. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences. 2013;18(8):641–646. [PMC free article] [PubMed] [Google Scholar]
- Beales (2010).Beales P. Genes and obesity. New York: Academic Press; 2010. Obesity in single gene disorder; pp. 125–147. [Google Scholar]
- Belizário et al. (2012).Belizário JE, Akamini P, Wolf P, Strauss B, Xavier-Neto J. New routes for transgenesis of the mouse. Journal of Applied Genetics. 2012;53(3):295–315. doi: 10.1007/s13353-012-0096-y. [DOI] [PubMed] [Google Scholar]
- Bennett (1986).Bennett HP. Biosynthetic fate of the amino-terminal fragment of pro-opiomelanocortin within the intermediate lobe of the mouse pituitary. Peptides. 1986;7(4):615–622. doi: 10.1016/0196-9781(86)90036-7. [DOI] [PubMed] [Google Scholar]
- Benzinou et al. (2008).Benzinou M, Creemers JWM, Choquet H, Lobbens S, Dina C, Durand E, Guerardel A, Boutin P, Jouret B, Heude B, Balkau B, Tichet J, Marre M, Potoczna N, Horber F, Le Stunff C, Czernichow S, Sandbaek A, Lauritzen T, Borch-Johnsen K, Andersen G, Kiess W, Körner A, Kovacs P, Jacobson P, Carlsson LMS, Walley AJ, Jørgensen T, Hansen T, Pedersen O, Meyre D, Froguel P. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nature Genetics. 2008;40(8):943–945. doi: 10.1038/ng.177. [DOI] [PubMed] [Google Scholar]
- Berndt et al. (2013).Berndt SI, Gustafsson S, Mägi R, Ganna A, Wheeler E, Feitosa MF, Justice AE, Monda KL, Croteau-Chonka DC, Day FR, Esko T, Fall T, Ferreira T, Gentilini D, Jackson AU, Luan J, Randall JC, Vedantam S, Willer CJ, Winkler TW, Wood AR, Workalemahu T, Hu Y-J, Lee SH, Liang L, Lin D-Y, Min JL, Neale BM, Thorleifsson G, Yang J, Albrecht E, Amin N, Bragg-Gresham JL, Cadby G, den Heijer M, Eklund N, Fischer K, Goel A, Hottenga J-J, Huffman JE, Jarick I, Johansson Å, Johnson T, Kanoni S, Kleber ME, König IR, Kristiansson K, Kutalik Z, Lamina C, Lecoeur C, Li G, Mangino M, McArdle WL, Medina-Gomez C, Müller-Nurasyid M, Ngwa JS, Nolte IM, Paternoster L, Pechlivanis S, Perola M, Peters MJ, Preuss M, Rose LM, Shi J, Shungin D, Smith AV, Strawbridge RJ, Surakka I, Teumer A, Trip MD, Tyrer J, Van Vliet-Ostaptchouk JV, Vandenput L, Waite LL, Zhao JH, Absher D, Asselbergs FW, Atalay M, Attwood AP, Balmforth AJ, Basart H, Beilby J, Bonnycastle LL, Brambilla P, Bruinenberg M, Campbell H, Chasman DI, Chines PS, Collins FS, Connell JM, Cookson WO, de Faire U, de Vegt F, Dei M, Dimitriou M, Edkins S, Estrada K, Evans DM, Farrall M, Ferrario MM, Ferrières J, Franke L, Frau F, Gejman PV, Grallert H, Grönberg H, Gudnason V, Hall AS, Hall P, Hartikainen A-L, Hayward C, Heard-Costa NL, Heath AC, Hebebrand J, Homuth G, Hu FB, Hunt SE, Hyppönen E, Iribarren C, Jacobs KB, Jansson J-O, Jula A, Kähönen M, Kathiresan S, Kee F, Khaw K-T, Kivimäki M, Koenig W, Kraja AT, Kumari M, Kuulasmaa K, Kuusisto J, Laitinen JH, Lakka TA, Langenberg C, Launer LJ, Lind L, Lindström J, Liu J, Liuzzi A, Lokki M-L, Lorentzon M, Madden PA, Magnusson PK, Manunta P, Marek D, März W, Leach IM, McKnight B, Medland SE, Mihailov E, Milani L, Montgomery GW, Mooser V, Mühleisen TW, Munroe PB, Musk AW, Narisu N, Navis G, Nicholson G, Nohr EA, Ong KK, Oostra BA, Palmer CNA, Palotie A, Peden JF, Pedersen N, Peters A, Polasek O, Pouta A, Pramstaller PP, Prokopenko I, Pütter C, Radhakrishnan A, Raitakari O, Rendon A, Rivadeneira F, Rudan I, Saaristo TE, Sambrook JG, Sanders AR, Sanna S, Saramies J, Schipf S, Schreiber S, Schunkert H, Shin S-Y, Signorini S, Sinisalo J, Skrobek B, Soranzo N, Stanáková A, Stark K, Stephens JC, Stirrups K, Stolk RP, Stumvoll M, Swift AJ, Theodoraki EV, Thorand B, Tregouet D-A, Tremoli E, Van der Klauw MM, van Meurs JBJ, Vermeulen SH, Viikari J, Virtamo J, Vitart V, Waeber G, Wang Z, Widén E, Wild SH, Willemsen G, Winkelmann BR, Witteman JCM, Wolffenbuttel BHR, Wong A, Wright AF, Zillikens MC, Amouyel P, Boehm BO, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Cupples LA, Cusi D, Dedoussis GV, Erdmann J, Eriksson JG, Franks PW, Froguel P, Gieger C, Gyllensten U, Hamsten A, Harris TB, Hengstenberg C, Hicks AA, Hingorani A, Hinney A, Hofman A, Hovingh KG, Hveem K, Illig T, Jarvelin M-R, Jöckel K-H, Keinanen-Kiukaanniemi SM, Kiemeney LA, Kuh D, Laakso M, Lehtimäki T, Levinson DF, Martin NG, Metspalu A, Morris AD, Nieminen MS, Njølstad I, Ohlsson C, Oldehinkel AJ, Ouwehand WH, Palmer LJ, Penninx B, Power C, Province MA, Psaty BM, Qi L, Rauramaa R, Ridker PM, Ripatti S, Salomaa V, Samani NJ, Snieder H, Sørensen TIA, Spector TD, Stefansson K, Tönjes A, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Vollenweider P, Wallaschofski H, Wareham NJ, Watkins H, Wichmann H-E, Wilson JF, Abecasis GR, Assimes TL, Barroso I, Boehnke M, Borecki IB, Deloukas P, Fox CS, Frayling T, Groop LC, Haritunian T, Heid IM, Hunter D, Kaplan RC, Karpe F, Moffatt MF, Mohlke KL, O’Connell JR, Pawitan Y, Schadt EE, Schlessinger D, Steinthorsdottir V, Strachan DP, Thorsteinsdottir U, van Duijn CM, Visscher PM, Di Blasio AM, Hirschhorn JN, Lindgren CM, Morris AP, Meyre D, Scherag A, McCarthy MI, Speliotes EK, North KE, Loos RJF, Ingelsson E. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nature Genetics. 2013;45(5):501–512. doi: 10.1038/ng.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessesen (2008).Bessesen DH. Update on obesity. The Journal of Clinical Endocrinology and Metabolism. 2008;93(6):2027–2034. doi: 10.1210/jc.2008-0520. [DOI] [PubMed] [Google Scholar]
- Biebermann et al. (2006).Biebermann H, Castañeda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, Hebebrand J, Hinney A, Tschöp MH, Grüters A, Krude H. A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metabolism. 2006;3(2):141–146. doi: 10.1016/j.cmet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Boch (2011).Boch J. News and views TALEs of genome targeting A mucosal gateway for vaccines. Nature Biotechnology. 2011;29(2):135–136. doi: 10.1038/nbt0211-135. [DOI] [PubMed] [Google Scholar]
- Bochukova et al. (2010).Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, Saeed S, Hamilton-Shield J, Clayton-Smith J, O’Rahilly S, Hurles ME, Farooqi IS. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463(7281):666–670. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaglia et al. (2008).Bonaglia MC, Ciccone R, Gimelli G, Gimelli S, Marelli S, Verheij J, Giorda R, Grasso R, Borgatti R, Pagone F, Rodrìguez L, Martinez-Frias M-L, van Ravenswaaij C, Zuffardi O. Detailed phenotype-genotype study in five patients with chromosome 6q16 deletion: narrowing the critical region for Prader-Willi-like phenotype. European Journal of Human Genetics. 2008;16(12):1443–1449. doi: 10.1038/ejhg.2008.119. [DOI] [PubMed] [Google Scholar]
- Bonnefond et al. (2013).Bonnefond A, Raimondo A, Stutzmann F, Ghoussaini M, Ramachandrappa S, Bersten DC, Durand E, Vatin V, Balkau B, Lantieri O, Raverdy V, Pattou F, Van Hul W, Van Gaal L, Peet DJ, Weill J, Miller JL, Horber F, Goldstone AP, Driscoll DJ, Bruning JB, Meyre D, Whitelaw ML, Froguel P. Brief report loss-of-function mutations in SIM1 contribute to obesity Prader-Willi—like features. Journal of Clinical Investigation. 2013;123(7):3037–3041. doi: 10.1172/JCI68035DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman et al. (2014).Borman AD, Pearce LR, Mackay DS, Nagel-Wolfrum K, Davidson AE, Henderson R, Garg S, Waseem NH, Webster AR, Plagnol V, Wolfrum U, Farooqi IS, Moore AT. A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Human Mutation. 2014;35(3):289–293. doi: 10.1002/humu.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston et al. (1997).Boston BA, Blaydon KM, Varnerin J, Cone RD. Independent and additive effects of central POMC and leptin pathways on murine obesity. Science. 1997;278(5343):1641–1644. doi: 10.1126/science.278.5343.1641. [DOI] [PubMed] [Google Scholar]
- Brown et al. (2010).Brown CY, Sadlon T, Gargett T, Melville E, Zhang R, Drabsch Y, Ling M, Strathdee CA, Gonda TJ, Barry SC. Robust reversible gene knockdown using a sing lentiviral short hairpin RNA vector. Human Gene Therapy. 2010;21:1005–1017. doi: 10.1089/hum.2009.107. [DOI] [PubMed] [Google Scholar]
- Buchner et al. (2012).Buchner DA, Geisinger JM, Glazebrook PA, Morgan MG, Nadeau JH. The juxtaparanodal proteins CNTNAP2 and TAG1 regulate dietinduced obesity. Mammalian Genome. 2012;23(0):431–442. doi: 10.1007/s00335-012-9400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman, Michaud & Woychik (1992).Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71(7):1195–1204. doi: 10.1016/S0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- Butler et al. (2000).Butler AA, Kesteson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the Melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141(9):3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Butler & Kozak (2010).Butler A, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59(2):323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton et al. (2009).Calton MA, Ersoy BA, Zhang S, Kane JP, Malloy MJ, Pullinger CR, Bromberg Y, Pennacchio LA, Dent R, McPherson R, Ahituv N, Vaisse C. Association of functionally significant Melanocortin-4 but not Melanocortin-3 receptor mutations with severe adult obesity in a large North American case-control study. Human Molecular Genetics. 2009;18(6):1140–1147. doi: 10.1093/hmg/ddn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, Gomez & Shapiro (2004).Carroll K, Gomez C, Shapiro L. Tubby proteins: the plot thickens. Nature Reviews. Molecular Cell Biology. 2004;5(1):55–63. doi: 10.1038/nrm1278. [DOI] [PubMed] [Google Scholar]
- Casola (2010).Casola S. Mouse models for miRNA expression: the ROSA26 locus. Methods in Molecular Biology. 2010;667:145–163. doi: 10.1007/978-1-60761-811-9_10. [DOI] [PubMed] [Google Scholar]
- Chadt et al. (2008).Chadt A, Leicht K, Deshmukh A, Jiang LQ, Scherneck S, Bernhardt U, Dreja T, Vogel H, Schmolz K, Kluge R, Zierath JR, Hultschig C, Hoeben RC, Schürmann A, Joost H-G, Al-Hasani H. Tbc1d1 mutation in lean mouse strain confers leanness protects from diet-induced obesity. Nature Genetics. 2008;40(11):1354–1359. doi: 10.1038/ng.244. [DOI] [PubMed] [Google Scholar]
- Challis et al. (2002).Challis BG, Pritchard LE, Creemers JWM, Delplanque J, Keogh JM, Luan J, Wareham NJ, Yeo GSH, Bhattacharyya S, Froguel P, White A, Sadaf Farooqi I, O’Rahilly S. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Human Molecular Genetics. 2002;11(17):1997–2004. doi: 10.1093/hmg/11.17.1997. [DOI] [PubMed] [Google Scholar]
- Chan et al. (2009).Chan LF, Webb TR, Chung T-T, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertova M, Elphick MR, Cheetham ME, Metherell LA, Clark AJL. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6146–6151. doi: 10.1073/pnas.0809918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (1996).Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, More KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84(3):491–495. doi: 10.1016/S0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2000).Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, Euan MacIntyre D, Howard Y, Chen LHT, Van der Ploeg Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nature Genetics. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Chiu et al. (2006).Chiu S, Diament A, Fisler J, Warden C. Nutritional genomics: discovering the path to personalized nutrition. New York: John Wiley & Sons; 2006. Gene–gene epistasis and gene-environment interactions influence diabetes and obesity; pp. 135–151. [Google Scholar]
- Choquet & Meyre (2011).Choquet H, Meyre D. Genetics of obesity: what have we learned? Current Genomics. 2011;12(3):169–179. doi: 10.2174/138920211795677895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church et al. (2009).Church C, Lee S, Bagg EAL, McTaggart JS, Deacon R, Gerken T, Lee A, Moir L, Mecinov J, Quwailid MM, Schofield CJ, Ashcroft FM, Cox RD. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genetics. 2009;5(8):e856. doi: 10.1371/journal.pgen.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church et al. (2010).Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, Wells S, Brüning JC, Nolan PM, Ashcroft FM, Cox RD. Overexpression of Fto leads to increased food intake results in obesity. Nature Genetics. 2010;42(12):1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo et al. (2012).Cirillo G, Marini R, Ito S, Wakamatsu K, Scianguetta S, Bizzarri C, Romano A, Grandone A, Perrone L, Cappa M, Miraglia del Giudice E. Lack of red hair phenotype in a North-African obese child homozygous for a novel POMC null mutation: nonsense-mediated decay RNA evaluation hair pigment chemical analysis. The British Journal of Dermatology. 2012;167(6):1393–1395. doi: 10.1111/j.1365-2133.2012.11060. [DOI] [PubMed] [Google Scholar]
- Clee & Attie (2007).Clee SM, Attie AD. The genetic landscape of type 2 diabetes in mice. Endocrine Reviews. 2007;28(1):48–83. doi: 10.1210/er.2006-0035. [DOI] [PubMed] [Google Scholar]
- Clément et al. (2008).Clément K, Dubern B, Mencarelli M, Czernichow P, Ito S, Wakamatsu K, Barsh GS, Vaisse C, Leger J. Unexpected endocrine features normal pigmentation in a young adult patient carrying a novel homozygous mutation in the POMC gene. The Journal of Clinical Endocrinology and Metabolism. 2008;93(12):4955–4962. doi: 10.1210/jc.2008-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément et al. (1998).Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte J-M, Basdevant A, Bougnéres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Coleman (1973).Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9(4):294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- Coleman (1982).Coleman DL. Diabetes-obesity syndromes in mice. Diabetes. 1982;31:1–6. doi: 10.2337/diab.31.1.S1. [DOI] [PubMed] [Google Scholar]
- Coleman & Eicher (1990).Coleman DL, Eicher EM. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. The Journal of Heredity. 1990;81(6):424–427. doi: 10.1093/oxfordjournals.jhered.a111019. [DOI] [PubMed] [Google Scholar]
- Coleman & Hummel (1967).Coleman DL, Hummel KP. Studies with the mutation, diabetes, in the mouse. Diabetologia. 1967;3(2):238–248. doi: 10.1007/BF01222201. [DOI] [PubMed] [Google Scholar]
- Coleman & Hummel (1973).Coleman DL, Hummel K. The influence of genetic background on the expression of the obese. Diabetologia. 1973;9:287–293. doi: 10.1007/BF01221856. [DOI] [PubMed] [Google Scholar]
- Cong et al. (2013).Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier et al. (2007).Couturier C, Sarkis C, Seron K, Belouzard S, Chen P, Lenain A, Corset L, Dam J, Vauthier V, Dubart A, Mallet J, Froguel P, Rouille Y, Jockers R. Silencing of OB-RGRP in mouse hypothalamic arcuate nucleus increases leptin receptor signaling and prevents diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19476–19481. doi: 10.1073/pnas.0706671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox & Church (2011).Cox RD, Church CD. Mouse models and the interpretation of human GWAS in type 2 diabetes and obesity. Disease Models and Mechanisms. 2011;4(2):155–164. doi: 10.1242/dmm.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers et al. (2012).Creemers JWM, Choquet H, Stijnen P, Vatin V, Pigeyre M, Beckers S, Meulemans S, Than ME, Yengo L, Tauber M, Balkau B, Elliott P, Jarvelin M-R, Van Hul W, Van Gaal L, Horber F, Pattou F, Froguel P, Meyre D. Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity. Diabetes. 2012;61(2):383–390. doi: 10.2337/db11-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers, Jackson & Hutton (1998).Creemers JW, Jackson RS, Hutton JC. Molecular and cellular regulation of prohormone processing. Seminars in Cell and Developmental Biology. 1998;9(1):3–10. doi: 10.1006/scdb.1997.0195. [DOI] [PubMed] [Google Scholar]
- Crews (1998).Crews ST. Control of cell lineage-specific development and transcription by bHLH-PAS proteins. Genes and Development. 1998;12(5):607–620. doi: 10.1101/gad.12.5.607. [DOI] [PubMed] [Google Scholar]
- Croft et al. (1995).Croft JB, Morrell D, Chase CL, Swift M. Obesity in heterozygous carriers of the gene for the Bardet-Biedl syndrome. American Journal of Medical Genetics. 1995;55(1):12–15. doi: 10.1002/ajmg.1320550105. [DOI] [PubMed] [Google Scholar]
- Davenport et al. (2007).Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity slow-onset cystic kidney disease. Current Biology. 2007;17(18):1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey & MacLean (2006).Davey RA, MacLean HE. Current and future approaches using genetically modified mice in endocrine research. American Journal of Physiology. Endocrinology and Metabolism. 2006;291:E429–E438. doi: 10.1152/ajpendo.00124.2006. [DOI] [PubMed] [Google Scholar]
- Davis et al. (2012).Davis RC, van Nas A, Castellani LW, Zhao Y, Zhou Z, Wen P, Yu S, Qi H, Rosales M, Schadt EE, Broman KW, Peterfy M, Lusis AJ. Systems genetics of susceptibility to obesity-induced diabetes in mice. Physiological Genomics. 2012;44(1):1–13. doi: 10.1152/physiolgenomics.00003.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenais et al. (2003).Demenais F, Kanninen T, Lindgren CM, Wiltshire S, Gaget S, Dandrieux C, Almgren P, Sjogren M, Hattersley A, Dina C, Tuomi T, McCarthy MI, Froguel P, Groop LC. A meta-analysis of four European genome screens (GIFT Consortium) shows evidence for a novel region on chromosome 17p11.2-q22 linked to type 2 diabetes. Human Molecular Genetics. 2003;12(15):1865–1873. doi: 10.1093/hmg/ddg195. [DOI] [PubMed] [Google Scholar]
- Derry et al. (2010).Derry JMJ, Zhong H, Molony C, MacNeil D, Guhathakurta D, Zhang B, Mudgett J, Small K, El Fertak L, Guimond A, Selloum M, Zhao W, Champy MF, Monassier L, Vogt T, Cully D, Kasarskis A, Schadt EE. Identification of genes and networks driving cardiovascular and metabolic phenotypes in a mouse F2 intercross. PLoS ONE. 2010;5(12):e856. doi: 10.1371/journal.pone.0014319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza et al. (2006).De Souza AT, Dai X, Spencer AG, Reppen T, Menzie A, Roesch PL, He Y, Caguyong MJ, Bloomer S, Herweijer H, Wolff JA, Hagstrom JE, Lewis DL, Linsley PS, Ulrich RG. Transcriptional phenotypic comparisons of Ppara knockout siRNA knockdown mice. Nucleic Acids Research. 2006;34(16):4486–4494. doi: 10.1093/nar/gkl609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diament, Fisler & Warden (2003).Diament AL, Fisler JS, Warden CH. Studies of natural allele effects in mice can be used to identify genes causing common human obesity. Obesity Reviews. 2003;4(4):249–255. doi: 10.1046/j.1467-789X.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- Dickies (1962).Dickies MM. A new viable yellow mutation in the house mouse. The Journal of Heredity. 1962;53:84–86. doi: 10.1093/oxfordjournals.jhered.a107129. [DOI] [PubMed] [Google Scholar]
- Dickson et al. (2010).Dickson SP, Wang K, Krantz I, Hakonarson H, Goldstein DB. Rare variants create synthetic genome-wide associations. PLoS Biology. 2010;8(1):e856. doi: 10.1371/journal.pbio.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina et al. (2007).Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, Carlsson LMS, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougnères P, Kovacs P, Marre M, Balkau B, Cauchi S, Chèvre J-C, Froguel P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nature Genetics. 2007;39(6):724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- Dittgen et al. (2004).Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, Helmchen F, Denk W, Brecht M, Osten P. Lentivirus-based genetic manipulations of cortical neurons their optical electrophysiological monitoring in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(52):18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do, Kathiresan & Abecasis (2012).Do R, Kathiresan S, Abecasis GR. Exome sequencing and complex disease: practical aspects of rare variant association studies. Human Molecular Genetics. 2012;21(R1):R1–R9. doi: 10.1093/hmg/dds387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doche et al. (2012).Doche ME, Bochukova EG, Su H, Pearce LR, Keogh JM, Henning E, Cline JM, Saeed S, Dale A, Cheetham T, Barroso I, Argetsinger LS, O’Rahilly S, Rui L, Carter-Su C, Farooqi IS. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. The Journal of Clinical Invesitigation. 2012;122(12):4732–4736. doi: 10.1172/JCI62696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokas et al. (2013).Dokas J, Chadt A, Nolden T, Himmelbauer H, Zierath JR, Joost H-G, Al-Hasani H. Conventional knockout of Tbc1d1 in mice impairs insulin- and AICAR-stimulated glucose uptake in skeletal muscle. Endocrinology. 2013;154(10):3502–3514. doi: 10.1210/en.2012-2147. [DOI] [PubMed] [Google Scholar]
- Dong et al. (2005).Dong H, Maddux BA, Altomonte J, Meseck M, Accili D, Terkeltaub R, Johnson K, Youngren JF, Goldfine ID. Increased hepatic levels of the insulin receptor inhibitor, PC-1/NPP1, induce insulin resistance and glucose intolerance. Diabetes. 2005;54(2):367–372. doi: 10.2337/diabetes.54.2.367. [DOI] [PubMed] [Google Scholar]
- Dougherty et al. (2010).Dougherty MK, Ritt DA, Zhou M, Specht S, Monson DM, Veenstra TD, Morrison DK. KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Molecular and Cellular Biology. 2010;34(6):652–662. doi: 10.1016/j.molcel.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubern et al. (2008).Dubern B, Lubrano-Berthelier C, Mencarelli M, Ersoy B, Frelut M-L, Bouglé D, Costes B, Simon C, Tounian P, Vaisse C, Clement K. Mutational analysis of the pro-opiomelanocortin gene in French obese children led to the identification of a novel deleterious heterozygous mutation located in the alpha-melanocyte stimulating hormone domain. Pediatric Research. 2008;63(2):211–216. doi: 10.1203/PDR.0b013e31815ed62b. [DOI] [PubMed] [Google Scholar]
- Dudbridge & Gusnanto (2008).Dudbridge F, Gusnanto A. Estimation of significance thresholds for genomewide association scans. Genetic Epidemiology. 2008;32(3):227–234. doi: 10.1002/gepi.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggirala et al. (2001).Duggirala R, Blangero J, Almasy L, Arya R, Dyer TD, Williams KL, Leach RJ, O’Connell P, Stern MP. A major locus for fasting insulin concentrations insulin resistance on chromosome 6q with strong pleiotropic effects on obesity-related phenotypes in nondiabetic Mexican Americans. American Journal of Human Genetics. 2001;68(5):1149–1164. doi: 10.1086/320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhl et al. (1994).Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nature Genetics. 1994;8:59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- Efthimiou et al. (2007).Efthimiou M, Andrianopoulos C, Stephanou G, Demopoulos NA, Nikolaropoulos SS. Aneugenic potential of the nitrogen mustard analogues melphalan, chlorambucil and p-N,N-bis(2-chloroethyl)aminophenylacetic acid in cell cultures in vitro. Mutation Research. 2007;617(1–2):125–137. doi: 10.1016/j.mrfmmm.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Ehm et al. (2000).Ehm MG, Karnoub MC, Sakul H, Gottschalk K, Holt DC, Weber JL, Vaske D, Briley D, Briley L, Kopf J, McMillen P, Nguyen Q, Reisman M, Lai EH, Joslyn G, Shepherd NS, Bell C, Wagner MJ, Burns DK. Genomewide search for type 2 diabetes susceptibility genes in four American populations. American Journal of Human Genetics. 2000;66(6):1871–1881. doi: 10.1086/302950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir et al. (2001).Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Ellacott et al. (2010).Ellacott KLJ, Morton GJ, Woods SC, Tso P, Schwartz MW. Assessment of feeding behavior in laboratory mice. Cell Metabolism. 2010;12(1):10–17. doi: 10.1016/j.cmet.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, Hollopeter & Palmiter (1996).Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274(5293):1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- Erikson, Clegg & Palmiter (1996).Erikson J, Clegg K, Palmiter R. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Farooqi et al. (2007a).Farooqi IS, Bullmore E, Keogh J, Gillard J, Rahilly SO, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007a;317(5843):25–27. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi et al. (2006).Farooqi IS, Drop S, Clements A, Keogh JM, Biernacka J, Lowenbein S, Challis BG, O’Rahilly S. Heterozygosity for a POMC-null mutation increased obesity risk in humans. Diabetes. 2006;55(9):2549–2553. doi: 10.2337/db06-0214. [DOI] [PubMed] [Google Scholar]
- Farooqi et al. (1999).Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. New England Journal of Medicine. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Farooqi et al. (2003).Farooqi IS, Keogh JM, Yeo GSH, Lank EJ, Cheetham T. Clinical Spectrum of Obesity and Mutations in the Melanocortin 4 Receptor Gene. New England Journal of Medicine. 2003;348(12):1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- Farooqi et al. (2002).Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O’Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. Journal of Clinical Investigation. 2002;110(8):1093–1103. doi: 10.1172/JCI0215693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi & O’Rahilly (2008).Farooqi IS, O’Rahilly S. Mutations in ligands and receptors of the leptin–melanocortin pathway that lead to obesity. Nature Reviews Endocrinology. 2008;4:569–577. doi: 10.1038/ncpendmet0966. [DOI] [PubMed] [Google Scholar]
- Farooqi et al. (2007b).Farooqi IS, Volders K, Stanhope R, Heuschkel R, White A, Lank E, Keogh J, O’Rahilly S, Creemers JWM. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. The Journal of Clinical Endocrinology Metabolism. 2007b;92(9):3369–3373. doi: 10.1210/jc.2007-0687. [DOI] [PubMed] [Google Scholar]
- Farooqi et al. (2007c).Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O’Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. The New England Journal of Medicine. 2007c;356(3):237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi et al. (2000).Farooqi IS, Yeo GSH, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O’Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. The Journal of Clinical Investigation. 2000;106(2):271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett et al. (2008).Fawcett GL, Roseman CC, Jarvis JP, Wang B, Wolf JB, Cheverud JM. Genetic architecture of adiposity and organ weight using combined generation QTL analysis. Obesity. 2008;16(8):1861–1868. doi: 10.1038/oby.2008.300. [DOI] [PubMed] [Google Scholar]
- Fischer et al. (2009).Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, Rüther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458(7240):894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- Fisler et al. (1993).Fisler JS, Warden CH, Pace MJ, Lusis AJ. BSB: a new mouse model of multigenic obesity. Obesity Research. 1993;1(4):271–280. doi: 10.1002/j.1550-8528.1993.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Flint & Eskin (2013).Flint J, Eskin E. Genome wide association studies in mice. Nature Reviews Genetics. 2013;13(11):807–817. doi: 10.1038/nrg3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank et al. (2013).Frank GR, Fox J, Candela N, Jovanovic Z, Bochukova E, Levine J, Fox J, Candela N, Jovanovic Z, Bochukova E, Levine J, Papenhausen PR, O’Rahilly S, Farooqi IS. Severe obesity and diabetes insipidus in a patient with PCSK1 deficiency. Molecular Genetics and Metabolism. 2013;110(1–2):191–194. doi: 10.1016/j.ymgme.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks et al. (2007).Franks PW, Mesa J-L, Harding AH, Wareham NJ. Gene-lifestyle interaction on risk of type 2 diabetes. Nutrition, Metabolism, and Cardiovascular Diseases? 2007;17(2):104–124. doi: 10.1016/j.numecd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Franks & Roth (2008).Franks PW, Roth SM. Physical activity and genetic factors in complex metabolic disease. New York: Nova Publishers; 2008. Interaction between physical activity and genetic factors in complex metabolic disease; pp. 155–173. [Google Scholar]
- Frayling et al. (2007).Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JRB, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch A-M, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin M-R, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJF, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CNA, Doney ASF, Morris AD, Smith GD, The Wellcome Trust Case Control Consortium. Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson et al. (2008).Fredriksson R, Hägglund M, Olszewski PK, Stephansson O, Jacobsson JA, Olszewska AM, Levine AS, Lindblom J, Schiöth HB. The obesity gene FTO is of ancient origin up-regulated during food deprivation expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008;149(5):2062–2071. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- Freedman et al. (2005).Freedman BD, Lee E-J, Park Y, Jameson JL. A dominant negative peroxisome proliferator-activated receptor-gamma knock-in mouse exhibits features of the metabolic syndrome. The Journal of Biological Chemistry. 2005;280(17):17118–17125. doi: 10.1074/jbc.M407539200. [DOI] [PubMed] [Google Scholar]
- Gantz et al. (1993).Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning expression gene localization of a fourth melanocortin receptor. The Journal of Biological Chemistry. 1993;268(20):15174–15179. [PubMed] [Google Scholar]
- Geller et al. (2004).Geller F, Reichwald K, Dempfle A, Illig T, Vollmert C, Herpertz S, Siffert W, Platzer M, Hess C, Gudermann T. Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. American Journal of Human Genetics. 2004;74(3):572–581. doi: 10.1086/382490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh et al. (2000).Ghosh S, Watanabe RM, Valle TT, Hauser ER, Magnuson VL, Langefeld CD, Ally DS, Mohlke KL, Silander K, Kohtamäki K, Chines P, Balow J, Jr, Birznieks G, Chang J, Eldridge W, Erdos MR, Karanjawala ZE, Knapp JI, Kudelko K, Martin C, Morales-Mena A, Musick A, Musick T, Pfahl C, Porter R, Rayman JB. The Finland-United States investigation of non-insulin-dependent diabetes mellitus genetics (FUSION) study. I. An autosomal genome scan for genes that predispose to type 2 diabetes. American Journal of Human Genetics. 2000;67(5):1174–1185. [PMC free article] [PubMed] [Google Scholar]
- Gibson et al. (2004).Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O’Rahilly S, Trussell RA. Congenital leptin deficiency due to homozygosity for the Δ133G mutation: report of another case and evaluation of response to four years of leptin therapy. Journal of Clinical Endocrinology and Metabolism. 2004;89:4821–4826. doi: 10.1210/jc.2004-0376. [DOI] [PubMed] [Google Scholar]
- Gill et al. (2014).Gill R, Cheung YH, Shen Y, Lanzano P, Mirza NM, Ten S, Maclaren NK, Motaghedi R, Han JC, Yanovski JA, Leibel RL, Chung WK. Whole-exome sequencing identifies novel LEPR mutations in individuals with severe early onset obesity. Obesity. 2014;22(2):576–584. doi: 10.1002/oby.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon & Ruddle (1981).Gordon JW, Ruddle FH. Integration and stable germ line transmission of genes injected into mouse pronuclei. Science. 1981;214(4526):1244–1246. doi: 10.1126/science.6272397. [DOI] [PubMed] [Google Scholar]
- Gray et al. (2006a).Gray J, Yeo G, Hung C, Keogh J, Clayton P, Banerjee K, McAulay A, O’Rahilly S, Farooqi IS. Functional characterization of human NTRK2 mutations identified in patients with severe early-onset obesity. International Journal of Obesity (2005) 2006a;31(2):359–364. doi: 10.1038/sj.ijo.0803390. [DOI] [PubMed] [Google Scholar]
- Gray et al. (2006b).Gray J, Yeo GSH, Cox JJ, Morton J, Adlam A-L R., Keogh JM, Yanovski JA, El Gharbawy A, Han JC, Tung YCL, Hodges JR, Raymond FL, O’Rahilly S, Farooqi IS. Hyperphagia severe obesity impaired cognitive function hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006b;55(12):3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad et al. (2006).Gunstad J, Schofield P, Paul R, Spitznagel M, Ronald C, Williams LM, Kohn M, Gordon E. BNDF Val66Met polymorphism associated with body mass index in healthy adults. Neuropsychobiology. 2006;53(3):153–156. doi: 10.1159/000093341. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Aguilar et al. (2014).Gutierrez-Aguilar R, Kim D-H, Casimir M, Dai X-Q, Pfluger PT, Park J, Haller A, Donelan E, Park J, D’Alessio D, Woods SC, MacDonald PE, Seeley RJ. The role of the transcription factor ETV5 in insulin exocytosis. Diabetologia. 2014;57(2):383–391. doi: 10.1007/s00125-013-3096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han et al. (2008).Han JC, Liu Q-R, Jones MaryP., Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. The New England Journal of Medicine. 2008;359(9):918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbers, Jahner & Jaenisch (1981).Harbers K, Jahner D, Jaenisch R. Microinjection of cloned retroviral genomes into mouse zygotes: integration and expression in the animal. Nature. 1981;293:540–544. doi: 10.1038/293540a0. [DOI] [PubMed] [Google Scholar]
- Harris et al. (2001).Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjørbæk C, Elmquist JK, Flier JS, Hollenberg AN. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. The Journal of Clinical Investigation. 2001;107(1):111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg & Coleman (1977).Herberg L, Coleman DL. Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism: Clinical and Experimental. 1977;26(1):59–99. doi: 10.1016/0026-0495(77)90128-7. [DOI] [PubMed] [Google Scholar]
- Hill (1998).Hill J. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- Hinney et al. (2007).Hinney A, Nguyen TT, Scherag A, Friedel S, Brönner G, Müller TD, Grallert H, Illig T, Wichmann H-E, Rief W, Schäfer H, Hebebrand J. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE. 2007;2(12):e856. doi: 10.1371/journal.pone.0001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa et al. (2005).Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature Medicine. 2005;11(1):90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- Hirschhorn et al. (2002).Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2002;4(2):45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Holder, Butte & Zinn (2000).Holder JL, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Human Molecular Genetics. 2000;9(1):101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- Holder et al. (2004).Holder L, Jr, Zhang L, Kublaoui BM, Dileone RJ, Bair Oz, Lee OK, Zinn CH. Sim1 gene dosage modulates the homeostatic feeding response to increased dietary fat in mice. American Journal of Physiology. Endocrinology and Metabolism. 2004;287(1):E105–E113. doi: 10.1152/ajpendo.00446.2003. [DOI] [PubMed] [Google Scholar]
- Horvat et al. (2000).Horvat S, Bünger L, Falconer VM, Mackay P, Law A, Bulfield G, Keightley PD. Mapping of obesity QTLs in a cross between mouse lines divergently selected on fat content. Mammalian Genome: Official Journal of the International Mammalian Genome Society. 2000;11(1):2–7. doi: 10.1007/s003350010002. [DOI] [PubMed] [Google Scholar]
- Hu, Wang & Wang (2014).Hu JK, Wang X, Wang P. Testing gene-gene interactions in genome wide association studies. Genetic Epidemiology. 2014;38(2):123–134. doi: 10.1002/gepi.21786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang & Kamihira (2013).Huang S, Kamihira M. Development of hybrid viral vectors for gene therapy. Biotechnology Advances. 2013;31(2):208–223. doi: 10.1016/j.biotechadv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Hummel, Dickie & Coleman (1966).Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Huszar et al. (1997).Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/S0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Ichimura et al. (2012).Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, Buzzetti R, Elliott P, Pouta A, Jarvelin M-R, Körner A, Kiess W, Pigeyre M, Caiazzo R, Van Hul W, Van Gaal L, Horber F, Balkau B, Lévy-Marchal C, Rouskas K, Kouvatsi A, Hebebrand J, Hinney A, Scherag A, Pattou F, Meyre D, Koshimizu Taka-aki, Wolowczuk I, Tsujimoto G, Froguel P. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483(7389):350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- Igel et al. (1998).Igel M, Taylor BA, Phillips SJ, Becker W, Herberg L, Joost HG. Hyperleptinemia and leptin receptor variant Asp600Asn in the obese, hyperinsulinemic KK mouse strain. Journal of Molecular Endocrinology. 1998;21(3):337–345. doi: 10.1677/jme.0.0210337. [DOI] [PubMed] [Google Scholar]
- Ikeda (1994).Ikeda H. KK Mouse. Diabetes Research and Clinical Practice. 1994;24:313–316. doi: 10.1016/0168-8227(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Indo et al. (1996).Indo Y, Tsuruta M, Hayashida Y, Karim M, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nature Genetics. 1996;13(4):485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- Jackson et al. (1997).Jackson RS, Creemers JWM, Ohagi S, Raffin-Sanson M-L, Sanders L, Montague CT, Hutton JC, O’Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nature Genetics. 1997;16:303–307. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- Jackson et al. (2003).Jackson RS, Creemers JWM, Farooqi IS, Raffin-Sanson M-L, Varro A, Dockray GJ, Holst JJ, Brubaker PL, Corvol P, Polonsky KS, Ostrega D, Becker KL, Bertagna X, Hutton JC, White A, Dattani MT, Hussain K, Middleton SJ, Nicole TM, Milla PJ, Lindley KJ, O’Rahilly S. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. The Journal of Clinical Invesitigation. 2003;112(10):1550–1560. doi: 10.1172/JCI200318784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont et al. (2011).Jacquemont S, Reymond A, Zufferey F, Harewood L, Walters RG, Kutalik Z, Martinet D, Shen Y, Valsesia A, Beckmann ND, Thorleifsson G, Belfiore M, Bouquillon S, Campion D, de Leeuw N, de Vries BBA, Esko T, Fernandez BA, Fernández-Aranda F, Fernández-Real JM, Gratacòs M, Guilmatre A, Hoyer J, Jarvelin M-R, Frank Kooy R, Kurg A, Le Caignec C, Männik K, Platt OS, Sanlaville D, Van Haelst MM, Villatoro Gomez S, Walha F, Wu Bai-lin, Yu Y, Aboura A, Addor M-C, Alembik Y, Antonarakis SE, Arveiler B, Barth M, Bednarek N, Béna F, Bergmann S, Beri M, Bernardini L, Blaumeiser B, Bonneau D, Bottani A, Boute O, Brunner HG, Cailley D, Callier P, Chiesa J, Chrast J, Coin L, Coutton C, Cuisset J-M, Cuvellier J-C, David A, de Freminville B, Delobel B, Delrue M-A, Demeer B, Descamps D, Didelot G, Dieterich K, Disciglio V, Doco-Fenzy M, Drunat S, Duban-Bedu B, Dubourg C, El-Sayed Moustafa JS, Elliott P, Faas BHW, Faivre L, Faudet A, Fellmann F, Ferrarini A, Fisher R, Flori E, Forer L, Gaillard D, Gerard M, Gieger C, Gimelli S, Gimelli G, Grabe HJ, Guichet A, Guillin O, Hartikainen A-L, Heron D, Hippolyte L, Holder M, Homuth G, Isidor B, Jaillard S, Jaros Z, Jiménez-Murcia S, Joly Helas G, Jonveaux P, Kaksonen S, Keren B, Kloss-Brandstätter A, Knoers Nine V. A. M., Koolen DA, Kroisel PM, Kronenberg F, Labalme A, Landais E, Lapi E, Layet V, Legallic S, Leheup B, Leube B, Lewis S, Lucas J, MacDermot KD, Magnusson P, Marshall C, Mathieu-Dramard M, McCarthy MI, Meitinger T, Antonietta Mencarelli M, Merla G, Moerman A, Mooser V, Morice-Picard F, Mucciolo M, Nauck M, Coumba Ndiaye N, Nordgren A, Pasquier L, Petit F, Pfundt R, Plessis G, Rajcan-Separovic E, Paolo Ramelli G, Rauch A, Ravazzolo R, Reis A, Renieri A, Richart C, Ried JS, Rieubland C, Roberts W, Roetzer KM, Rooryck C, Rossi M, Saemundsen E, Satre V, Schurmann C, Sigurdsson E, Stavropoulos DJ, Stefansson H, Tengström C, Thorsteinsdóttir U, Tinahones FJ, Touraine R, Vallée L, van Binsbergen E, Van der Aa N, Vincent-Delorme C, Visvikis-Siest S, Vollenweider P, Völzke H, Vulto-van Silfhout AT, Waeber G, Wallgren-Pettersson C, Witwicki RM, Zwolinksi S, Andrieux J, Estivill X, Gusella JF, Gustafsson O, Metspalu A, Scherer SW, Stefansson K, Blakemore AIF, Beckmann JS, Froguel P. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature. 2011;478(7367):97–102. doi: 10.1038/nature10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek et al. (2010).Jelinek D, Millward V, Birdi A, Trouard TP, Heidenreich RA, Garver WS. Npc1 haploinsufficiency promotes weight gain and metabolic features associated with insulin resistance. Human Molecular Genetics. 2010;20(2):312–321. doi: 10.1093/hmg/ddq466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek et al. (2012).Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice (2000).Justice MJ. Capitalizing on large-scale mouse mutagenesis screens. Nature Reviews. Genetics. 2000;1(2):109–115. doi: 10.1038/35038549. [DOI] [PubMed] [Google Scholar]
- Justice, Siracusa & Stewart (2011).Justice MJ, Siracusa LD, Stewart AF. Technical approaches for mouse models of human disease. Disease Models and Mechanisms. 2011;4(3):305–310. doi: 10.1242/dmm.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiyala et al. (2010).Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes. 2010;59(7):1657–1666. doi: 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki & Koya (2011).Kanasaki K, Koya D. Biology of obesity: lessons from animal models of obesity. Journal of Biomedicine and Biotechnology. 2011;2011:1–11. doi: 10.1155/2011/197636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar et al. (2002).Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, Brandon EP, Schaffer D, Verma IM, Lee K-F, Heinemann SF, Gage FH. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):2320–2325. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie, Liebl & Parada (2000).Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. The EMBO Journal. 2000;19(6):1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk, Penney & McHugh (2010).Kirk SFL, Penney TL, McHugh T-LF. Characterizing the obesogenic environment: the state of the evidence with directions for future research. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity. 2010;11(2):109–117. doi: 10.1111/j.1467-789X.2009.00611.x. [DOI] [PubMed] [Google Scholar]
- Kirk et al. (2012).Kirk SFL, Penney TL, Mchugh T-LF, Sharma AM. Effective weight management practice: a review of the lifestyle intervention evidence. International Journal of Obesity. 2012;36(2):178–185. doi: 10.1038/ijo.2011.80. [DOI] [PubMed] [Google Scholar]
- Klebig et al. (1995).Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(11):4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyn et al. (1996).Kleyn PW, Fan W, Kovats SG, Lee JJ, Pulido JC, Wu Y, Berkemeier LR, Misumi DJ, Holmgren L, Charlat O, Woolf EA, Tayber O, Brody T, Shu P, Hawkins F, Kennedy B, Baldini L, Ebeling C, Alperin GD, Deeds J, Lakey ND, Culpepper J, Chen H, Glücksmann-Kuis MA, Carlson GA, Duyk GM, Moore KJ. Identification and characterization of the mouse obesity gene tubby: a member of a novel gene family. Cell. 1996;85(2):281–290. doi: 10.1016/S0092-8674(00)81104-6. [DOI] [PubMed] [Google Scholar]
- Kootstra & Verma (2003).Kootstra NA, Verma IM. Gene therapy with viral vectors. Annual Review of Pharmacology and Toxicology. 2003;43:413–439. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- Kosiol & Vinar (2008).Kosiol C, Vinar T, da Fonseca RR, Hubisz MJ, Bustamante CD, Nielsen R, Siepel A. Patterns of positive selection in six Mammalian genomes. PLoS Genetics. 2008;4(8):e856. doi: 10.1371/journal.pgen.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutnikova et al. (2003).Koutnikova H, Cock T-A, Watanabe M, Houten SM, Champy M-F, Dierich A, Auwerx J. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14457–14462. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude et al. (1998).Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nature Genetics. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Krude et al. (2003).Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, Grüters A. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. The Journal of Clinical Endocrinology and Metabolism. 2003;88(10):4633–4640. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- Kublaoui et al. (2006).Kublaoui BM, Holder JL, Gemelli T, Zinn AR. Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Molecular Endocrinology. 2006;20(10):2483–2492. doi: 10.1210/me.2005-0483. [DOI] [PubMed] [Google Scholar]
- Kühn & Torres (2002).Kühn R, Torres R. Cre/loxp recombination system and gene targeting. In: Clarke A, editor. Methods in molecular biology. 2nd ed. Totowa: Humana Press Inc.; 2002. pp. 175–204. [DOI] [PubMed] [Google Scholar]
- Lander & Botstein (1987).Lander ES, Botstein D. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science. 1987;236(4808):1567–1570. doi: 10.1126/science.2884728. [DOI] [PubMed] [Google Scholar]
- Laplante et al. (2012).Laplante M, Horvat S, Festuccia WT, Birsoy K, Prevorsek Z, Efeyan A, Sabatini DM. DEPTOR cell-autonomously promotes adipogenesis and its expression is associated with obesity. Cell Metabolism. 2012;16(2):202–212. doi: 10.1016/j.cmet.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc et al. (2011).Leduc MS, Lyons M, Darvishi K, Walsh K, Sheehan S, Amend S, Cox A, Orho-Melander M, Kathiresan S, Paigen B, Korstanje R. The mouse QTL map helps interpret human genome-wide association studies for HDL cholesterol. Journal of Lipid Research. 2011;52(6):1139–1149. doi: 10.1194/jlr.M009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee & Cox (2011).Lee AWS, Cox RD. Use of mouse models in studying type 2 diabetes mellitus. Expert Reviews in Molecular Medicine. 2011;13(January):e856. doi: 10.1017/S1462399410001729. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2012).Lee AWS, Hengstler H, Schwald K, Berriel-Diaz M, Loreth D, Kirsch M, Kretz O, Haas CA, de Angelis MH, Herzig S, Brümmendorf T, Klingenspor M, Rathjen FG, Rozman J, Nicholson G, Cox RD, Schäfer MKE. Functional inactivation of the genome-wide association study obesity gene neuronal growth regulator 1 in mice causes a body mass phenotype. PLoS ONE. 2012;7(7):e856. doi: 10.1371/journal.pone.0041537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2006).Lee YS, Challis BG, Thompson DA, Yeo GSH, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung Chiao-Chien C., Wareham NJ, Froguel P, Millhauser GL, O’Rahilly S, Farooqi IS. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metabolism. 2006;3(2):135–140. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Lentz, Gray & Samulski (2012).Lentz TB, Gray SJ, Samulski RJ. Viral vectors for gene delivery to the central nervous system. Neurobiology of Disease. 2012;48(2):179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li & Meyre (2013).Li A, Meyre D. Challenges in reproducibility of genetic association studies: lessons learned from the obesity field. International Journal of Obesity (2005) 2013;37(4):559–567. doi: 10.1038/ijo.2012.82. [DOI] [PubMed] [Google Scholar]
- Li & Meyre (2014).Li A, Meyre D. Jumping on the train of personalized medicine: a primer for non-geneticist clinicians: Part 2. Fundamental concepts in genetic epidemiology. Current Psychiatry Reviews. 2014;10(2):101–117. doi: 10.2174/1573400510666140319235334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2014).Li P, Tiwari HK, Lin W-Y, Allison DB, Chung WK, Leibel RL, Yi N, Liu N. Genetic association analysis of 30 genes related to obesity in a European American population. International Journal of Obesity. 2014;38(5):724–729. doi: 10.1038/ijo.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2010).Li S, Zhao JH, Luan Jian’an, Ekelund U, Luben RN, Khaw K-T, Wareham NJ, Loos RJF. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Medicine. 2010;7(8):1–9. doi: 10.1371/journal.pmed.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang et al. (2007).Liang J, Fu M, Ciociola E, Chandalia M, Abate N. Role of ENPP1 on adipocyte maturation. PLoS ONE. 2007;2(9):e856. doi: 10.1371/journal.pone.0000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2014).Lin Y, Cradick TJ, Brown MT, Deshmukh H, Ranjan P, Sarode N, Wile BM, Vertino PM, Stewart FJ, Bao G. CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA guide RNA sequences. Nucleic Acids Research. 2014;42(11):7473–7485. doi: 10.1093/nar/gku402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, Bohan & Gekakis (2006).Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Human Molecular Genetics. 2006;15(11):1884–1893. doi: 10.1093/hmg/ddl111. [DOI] [PubMed] [Google Scholar]
- Locke et al. (2015).Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Hua Zhao J, Zhao W, Chen J, Fehrmann R, Hedman ÅK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Mateo Leach I, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stanáková A, Strawbridge RJ, Ju Sung Y, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Ärnlöv J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Blüher M, Böhringer S, Bonnycastle LL, Böttcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Ida Chen Y-D, Clarke R, Warwick Daw E, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Gräßler J, Grönberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga J-J, James AL, Jeff JM, Johansson Åsa, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Sin Lo K, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Müller G, Müller-Nurasyid M, Musk AW, Nagaraja R, Nöthen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Vernon Smith A, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen A-C, Tan S-T, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh H-W, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gådin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee J-Y, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van’t Hooft FM, Vinkhuyzen AAE, Westra H-J, Zheng W, Zondervan KT, Heath AC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Adrienne Cupples L, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Kees Hovingh G, Humphries SE, Hunt SC, Hyppönen E, Illig T, Jacobs KB, Jarvelin M-R, Jöckel K-H, Johansen B, Jousilahti P, Wouter Jukema J, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tönjes A, Trégouët D-A, Tremblay A, Tremoli E, Virtamo J, Vohl M-C, Völker U, Waeber G, Willemsen G, Witteman JC, Carola Zillikens M, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Eline Slagboom P, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann H-E, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebel et al. (2014).Loebel DAF, Hor ACC, Bildsoe HK, Tam PPL. Timed deletion of twist1 in the limb bud reveals age-specific impacts on autopod and zeugopod patterning. PLoS ONE. 2014;9(6):e856. doi: 10.1371/journal.pone.0098945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan et al. (2009).Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X, Li D, Jia W, Kang J, Pei G. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457(7233):1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- MacKay (2014).MacKay T. Epistasis and quantitative traits: using model organisms to study gene-gene interactions. Nature Reviews Genetics. 2014;15(1):22–33. doi: 10.1038/nrg3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, Stone & Ayroles (2009).Mackay TFC, Stone EA, Ayroles JF. The genetics of quantitative traits: challenges and prospects. Nature Reviews. Genetics. 2009;10(8):565–577. doi: 10.1038/nrg2612. [DOI] [PubMed] [Google Scholar]
- Maddux et al. (2006).Maddux BA, Chang Y, Accili D, Mcguinness OP, Youngren JF, Goldfine ID, Over IDG, et al. Overexpression of the insulin receptor inhibitor PC-1/ENPP1 induces insulin resistance and hyperglycemia. American Journal of Physiology, Endocrinology and Metabolism. 2006;290:746–749. doi: 10.1152/ajpendo.00298.2005. [DOI] [PubMed] [Google Scholar]
- Maddux & Goldfin (2000).Maddux BA, Goldfin ID. Membrane glycoprotein PC-1 inhibition of insulin receptor function occurs via direct interaction with the receptor alpha-subunit. Diabetes. 2000;49(15):13–19. doi: 10.2337/diabetes.49.1.13. [DOI] [PubMed] [Google Scholar]
- Maddux et al. (1995).Maddux BA, Sbraccia P, Kumakura S, Sasson S, Youngren J, Fisher A, Spencer S, Grupe A, Henzel W, Stewart TA, Reaven GM, Goldfine ID. Membrane glycoprotein PC-1 and insulin resistance in non-insulin dependent diabetes mellitus. Nature. 1995;373:448–451. doi: 10.1038/373448a0. [DOI] [PubMed] [Google Scholar]
- Marg et al. (1999).Marg A, Sirim P, Spaltmann F, Plagge A, Kauselmann G, Buck F, Rathjen FG, Brümmendorf T. Neurotractin, a novel neurite outgrowth-promoting Ig-like protein that interacts with CEPU-1 and LAMP. Journal of Cell Biology. 1999;145(4):865–876. doi: 10.1083/jcb.145.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín et al. (2013).Martín MG, Lindberg I, Solorzano–Vargas RS, Wang J, Avitzur Y, Bandsma R, Sokollik C, Lawrence S, Pickett LA, Chen Z, Egritas O, Dalgic B, Albornoz V, de Ridder L, Hulst J, Gok F, Aydoan A, Al–Hussaini A, Gok DE, Yourshaw M, Wu SV, Cortina G, Stanford S, Georgia S. Congenital proprotein convertase 1/3 deficiency causes malabsorptive diarrhea and other endocrinopathies in a pediatric cohort. Gastroenterology. 2013;145(1):138–148. doi: 10.1053/j.gastro.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May et al. (2000).May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, Sadelain M. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406(6791):82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- Mazen et al. (2009).Mazen I, El-Gammal M, Abdel-Hamid M, Amr K. A novel homozygous missense mutation of the leptin gene (N103K) in an obese Egyptian patient. Molecular Genetics and Metabolism. 2009;97(4):305–308. doi: 10.1016/j.ymgme.2009.04.002. [DOI] [PubMed] [Google Scholar]
- McClurg et al. (2007).McClurg P, Janes J, Wu C, Delano DL, Walker JR, Batalov S, Takahashi JS, Shimomura K, Kohsaka A, Bass J, Wiltshire T, Su AI. Genomewide association analysis in diverse inbred mice: power and population structure. Genetics. 2007;176:675–683. doi: 10.1534/genetics.106.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray et al. (2013).McMurray F, Church CD, Larder R, Nicholson G, Wells S, Teboul L, Tung YCL, Rimmington D, Bosch F, Jimenez V, Yeo GSH, O’Rahilly S, Ashcroft FM, Coll AP, Cox RD. Adult onset global loss of the fto gene alters body composition and metabolism in the mouse. PLoS Genetics. 2013;9(1):e856. doi: 10.1371/journal.pgen.1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli et al. (2011).Mencarelli M, Dubern B, Alili R, Maestrini S, Benajiba L, Tagliaferri M, Galan P, Rinaldi M, Simon C, Tounian P, Hercberg S, Liuzzi A, Di Blasio AM, Clement K. Rare melanocortin-3 receptor mutations with in vitro functional consequences are associated with human obesity. Human Molecular Genetics. 2011;20(2):392–399. doi: 10.1093/hmg/ddq472. [DOI] [PubMed] [Google Scholar]
- Mendiratta et al. (2011).Mendiratta MS, Yang Y, Balazs AE, Willis AS, Eng CM, Karaviti LP, Potocki L. Early onset obesity and adrenal insufficiency associated with a homozygous POMC mutation. International Journal of Pediatric Endocrinology. 2011;2011(1):5. doi: 10.1186/1687-9856-2011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke (2013).Menke DB. Engineering subtle targeted mutations into the mouse genome. Genesis. 2013;51(9):605–618. doi: 10.1002/dvg.22422. [DOI] [PubMed] [Google Scholar]
- Meyre et al. (2005).Meyre D, Bouatia-Naji N, Tounian A, Samson C, Lecoeur C, Vatin V, Ghoussaini M, Wachter C, Hercberg S, Charpentier G, Patsch W, Pattou F, Charles M-A, Tounian P, Clément K, Jouret B, Weill J, Maddux BA, Goldfine ID, Walley A, Boutin P, Dina C, Froguel P. Variants of ENPP1 are associated with childhood and adult obesity and increase the risk of glucose intolerance and type 2 diabetes. Nature Genetics. 2005;37(8):863–867. doi: 10.1038/ng1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre et al. (2009).Meyre D, Delplanque J, Chèvre J-C, Lecoeur C, Lobbens S, Gallina S, Durand E, Vatin V, Degraeve F, Proença C, Gaget S, Körner A, Kovacs P, Kiess W, Tichet J, Marre M, Hartikainen A-L, Horber F, Potoczna N, Hercberg S, Levy-Marchal C, Pattou F, Heude B, Tauber M, McCarthy MI, Blakemore AIF, Montpetit A, Polychronakos C, Weill J, Coin LJM, Asher J, Elliott P, Järvelin M-R, Visvikis-Siest S, Balkau B, Sladek R, Balding D, Walley A, Dina C, Froguel P. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nature Genetics. 2009;41(2):157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- Meyre et al. (2008).Meyre D, Farge M, Lecoeur C, Proenca C, Durand E, Allegaert F, Tichet J, Marre M, Balkau B, Weill J, Delplanque J, Froguel P. R125W coding variant in TBC1D1 confers risk for familial obesity contributes to linkage on chromosome 4p14 in the French population. Human Molecular Genetics. 2008;17(12):1798–1802. doi: 10.1093/hmg/ddn070. [DOI] [PubMed] [Google Scholar]
- Meyre et al. (2004).Meyre D, Lecoeur C, Delplanque J, Francke S, Vatin V, Durand E, Weill J, Dina C, Froguel P. A genome-wide scan for childhood obesity-associated traits in French families shows significant linkage on chromosome 6q22.31-q23.2. Diabetes. 2004;53(3):803–811. doi: 10.2337/diabetes.53.3.803. [DOI] [PubMed] [Google Scholar]
- Michaud et al. (2001).Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Lévy E, Mitchell GA, Himms-Hagen J, Fan CM. Sim1 haploinsufficiency causes hyperphagia obesity reduction of the paraventricular nucleus of the hypothalamus. Human Molecular Genetics. 2001;10(14):1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- Michaud et al. (2000).Michaud JL, DeRossi C, May NR, Holdener BC, Fan CM. ARNT2 acts as the dimerization partner of SIM1 for the development of the hypothalamus. Mechanisms of Development. 2000;90(2):253–261. doi: 10.1016/S0925-4773(99)00328-7. [DOI] [PubMed] [Google Scholar]
- Michaud et al. (1998).Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes and Development. 1998;12(20):3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller et al. (1993).Miller MW, Duhl DM, Vrieling H, Cordes SP, Ollmann MM, Winkes BM, Barsh GS. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes and Development. 1993;7(3):454–467. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- Misra & Khurana (2008).Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. The Journal of Clinical Endocrinology and Metabolism. 2008;93(11 Suppl 1):S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- Mollah & Ishikawa (2011).Mollah MBR, Ishikawa A. Intersubspecific subcongenic mouse strain analysis reveals closely linked QTLs with opposite effects on body weight. Mammalian Genome: Official Journal of the International Mammalian Genome Society. 2011;22(5–6):282–289. doi: 10.1007/s00335-011-9323-9. [DOI] [PubMed] [Google Scholar]
- Montague et al. (1997).Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O’Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Monteggia et al. (2004).Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(29):10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison et al. (2013).Morrison AC, Voorman A, Johnson AD, Liu X, Yu J, Li A, Muzny D, Yu F, Rice K, Zhu C, Bis J, Heiss G, O’Donnell CJ, Psaty BM, Cupples LA, Gibbs R, Boerwinkle E. Whole-genome sequence-based analysis of high-density lipoprotein cholesterol. Nature Genetics. 2013;45(8):899–901. doi: 10.1038/ng.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must et al. (1999).Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA: The Journal of the American Medical Association. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- Nadeau et al. (2000).Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nature. 2000;24:221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- Naggert et al. (1995).Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduses enzyme activity. Nature Genetics. 1995;10(2):135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- Noble et al. (2011).Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2011;300(5):1053–1069. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kane & Gehrin (1987).O’Kane C, Gehrin W. Detection in situ of genomic regulatory elements in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly & Farooqi (2008).O’Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57(11):2905–2910. doi: 10.2337/db08-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki et al. (2002).Okazaki M, Saito Y, Udaka Y, Maruyama M, Murakami H, Ota S, Kikuchi T, Oguchi K. Diabetic nephrophathy in KK and KK-Ay mice. Experimental Animals. 2002;51(2):191–196. doi: 10.1538/expanim.51.191. [DOI] [PubMed] [Google Scholar]
- Olshanky et al. (2005).Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. The New England Journal of Medicine. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Osten, Dittgen & Licznerski (2006).Osten P, Dittgen T, Licznerski P. The dynamic synapse: molecular methods in ionotropic receptor biology. New York: CRC Press; 2006. Lentivirus-based genetic manipulations in neurons in vivo; pp. 249–256. [Google Scholar]
- Otto et al. (2010).Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, van Reeuwijk J, Letteboer SJF, Sang L, Giles RH, Liu Q, Coene KLM, Estrada-Cuzcano A, Collin RWJ, McLaughlin HM, Held S, Kasanuki JM, Ramaswami G, Conte J, Lopez I, Washburn J, MacDonald J, Hu J, Yamashita Y, Maher ER, Guay-Woodford LM, Neumann HPH, Obermüller N, Koenekoop RK, Bergmann C, Bei X, Lewis RA, Katsanis N, Lopes V, Williams DS, Lyons RH, Dang CV, Brito DA, Dias MB, Zhang X, Cavalcoli JD, Nürnberg G, Nürnberg P, Pierce EA, Jackson PK, Antignac C, Saunier S, Roepman R, Dollfus H, Khanna H, Hildebrandt F. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nature Genetics. 2010;42(10):840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen (2003).Paigen K. One hundred years of mouse genetics: an intellectual history. I. The classical period (1902–1980) Genetics. 2003;163:1–7. doi: 10.1093/genetics/163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks et al. (2013).Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, Pan C, Civelek M, Rau CD, Bennett BJ, Mehrabian M, Ursell LK, He A, Castellani LW, Zinker B, Kirby M, Drake TA, Drevon CA, Knight R, Gargalovic P, Kirchgessner T, Eskin E, Lusis AJ. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metabolism. 2013;17(1):141–152. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwari et al. (2011).Patwari P, Emilsson V, Schadt EE, Chutkow WA, Lee S, Marsili A, Zhang Y, Dobrin R, Cohen DE, Larsen PR, Zavacki AM, Fong LG, Young SG, Lee RT. The arrestin domain-containing 3 protein regulates body mass and energy expenditure. Cell Metabolism. 2011;14(5):671–683. doi: 10.1016/j.cmet.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce et al. (2013).Pearce LR, Atanassova N, Banton MC, Bottomley B, Van der Klaauw AA, Revelli J-P, Hendricks A, Keogh JM, Henning E, Doree D, Jeter-Jones S, Garg S, Bochukova EG, Bounds R, Ashford S, Gayton E, Hindmarsh PC, Shield JPH, Crowne E, Barford D, Wareham NJ, UK10K consortium. O’Rahilly S, Murphy MP, Powell DR, Barroso I, Farooqi IS. KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell. 2013;155(4):765–777. doi: 10.1016/j.cell.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce et al. (2014).Pearce LR, Joe R, Doche ME, Su H-W, Keogh JM, Henning E, Argetsinger LS, Bochukova EG, Cline JM, Garg S, Saeed S, Shoelson S, O’Rahilly S, Barroso I, Rui L, Farooqi IS, Carter-Su C. Functional characterisation of obesity-associated variants involving the alpha and beta isoforms of human SH2B1. Endocrinology. 2014;155(9):3219–3226. doi: 10.1210/en.2014-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip et al. (2011).Philip VM, Sokoloff G, Ackert-Bicknell CL, Striz M, Branstetter L, Beckmann MA, Spence JS, Jackson BL, Galloway LD, Barker P, Wymore AM, Hunsicker PR, Durtschi DC, Shaw GS, Shinpock S, Manly KF, Miller DR, Donohue KD, Culiat CT, Churchill GA, Lariviere WR, Palmer AA, O’Hara BF, Voy BH, Chesler EJ. Genetic analysis in the Collaborative Cross breeding population. Genome Research. 2011;21(8):1223–1238. doi: 10.1101/gr.113886.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe et al. (2014).Philippe J, Stijnen P, Meyre D, De Graeve F, Thuillier D, Delplanque J, Gyapay G, Sand O, Creemers JW, Froguel P, Bonnefond A. A nonsense loss-of-function mutation in PCSK1 contributes to dominantly inherited human obesity. International Journal of Obesity. 2014;39:1–30. doi: 10.1038/ijo.2014.96. [DOI] [PubMed] [Google Scholar]
- Pomp (2007).Pomp D. Obesity: genomics and postgenomics. New York: CRC Press; 2007. Natural polygenic models; pp. 125–142. [Google Scholar]
- Prada et al. (2013).Prada PO, Quaresma PGF, Caricilli AM, Santos AC, Guadagnini D, Morari J, Weissmann L, Ropelle ER, Carvalheira JBC, Velloso LA, Saad MJA. Tub has a key role in insulin and leptin signaling and action in vivo in hypothalamic nuclei. Diabetes. 2013;62(1):137–148. doi: 10.2337/db11-1388. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Prevorsek et al. (2010).Prevorsek Z, Gorjanc G, Paigen B, Horvat S. Congenic and bioinformatics analyses resolved a major-effect Fob3b QTL on mouse Chr 15 into two closely linked loci. Mammalian Genome: Official Journal of the International Mammalian Genome Society. 2010;21(3–4):172–185. doi: 10.1007/s00335-010-9252-z. [DOI] [PubMed] [Google Scholar]
- Rahmouni et al. (2008).Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. The Journal of Clinical Invesitigation. 2008;118(4):1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandrappa et al. (2013).Ramachandrappa S, Raimondo A, Cali AMG, Keogh JM, Henning E, Saeed S, Thompson A, Garg S, Bochukova EG, Brage S, Trowse V, Wheeler E, Sullivan AE, Dattani M, Clayton PE, Datta V, Bruning JB, Wareham NJ, O’Rahilly S, Peet DJ, Barroso I, Whitelaw ML, Farooqi IS. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. The Journal of Clinical Invesitigation. 2013;123(7):3042–3050. doi: 10.1172/JCI68016DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankinen et al. (2006).Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Pérusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity. 2006;14(4):529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- Reed, Lawler & Tordoff (2008).Reed DR, Lawler MP, Tordoff MG. Reduced body weight is a common effect of gene knockout in mice. BMC Genetics. 2008;9(4) doi: 10.1186/1471-2156-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees & Alcolado (2005).Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabetic Medicine: A Journal of the British Diabetic Association. 2005;22(4):359–370. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- Ren et al. (2005).Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metabolism. 2005;2(2):95–104. doi: 10.1016/j.cmet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Revelli et al. (2011).Revelli J-P, Smith D, Allen J, Jeter-Jones S, Shadoan MK, Desai U, Schneider M, Van Sligtenhorst I, Kirkpatrick L, Platt KA, Suwanichkul A, Savelieva K, Gerhardt B, Mitchell J, Syrewicz J, Zambrowicz B, Hamman BD, Vogel P, Powell DR. Profound obesity secondary to hyperphagia in mice lacking kinase suppressor of ras 2. Obesity. 2011;19(5):1010–1018. doi: 10.1038/oby.2010.282. [DOI] [PubMed] [Google Scholar]
- Rios et al. (2001).Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity hyperactivity. Molecular Endocrinology. 2001;15(10):1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Rogner & Avner (2003).Rogner UC, Avner P. Congenic mice: cutting tools for complex immune disorders. Nature Reviews. Immunology. 2003;3:243–252. doi: 10.1038/nri1031. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss et al. (1993).Roselli-Rehfuss KG, Mountjoy LS, Robbins MT, Mortrud MJ, Low JB, Tatro ML, Entwistle RB, Simerly RD, Cone Identification of a receptor for gamma melanotropin other proopiomelanocortin peptides in the hypothalamus limbic system. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(19):8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal & Brown (2007).Rosenthal N, Brown S. The mouse ascending: perspectives for human-disease models. Nature Cell Biology. 2007;9(9):993–999. doi: 10.1038/ncb437. [DOI] [PubMed] [Google Scholar]
- Rouskas et al. (2012).Rouskas K, Meyre D, Stutzmann F, Paletas K, Papazoglou D, Vatin V, Marchand M, Kouvatsi A, Froguel P. Loss-of-function mutations in MC4R are very rare in the Greek severely obese adult population. Obesity. 2012;20(11):2278–2282. doi: 10.1038/oby.2012.77. [DOI] [PubMed] [Google Scholar]
- Russell et al. (1989).Russell LB, Hunsicker PR, Cacheiro NL, Bangham JW, Russell WL, Shelby MD. Chlorambucil effectively induces deletion mutations in mouse germ cells. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(10):3704–3708. doi: 10.1073/pnas.86.10.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed et al. (2012).Saeed S, Butt TA, Anwer M, Arslan M, Froguel P. High prevalence of leptin and melanocortin-4 receptor gene mutations in children with severe obesity from Pakistani consanguineous families. Molecular Genetics and Metabolism. 2012;106(1):121–126. doi: 10.1016/j.ymgme.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Sano et al. (2003).Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. The Journal of Biological Chemistry. 2003;278(17):14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- Scheidecker et al. (2014).Scheidecker S, Etard C, Pierce NW, Geoffroy V, Schaefer E, Muller J, Chennen K, Flori E, Pelletier V, Poch O, Marion V, Stoetzel C, Strahle U, Nachury MV, Dollfus H. Exome sequencing of Bardet-Biedl syndrome patient identifies a null mutation in the BBSome subunit BBIP1 (BBS18) Journal of Medical Genetics. 2014;51(2):132–136. doi: 10.1136/jmedgenet-2013-101785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherag et al. (2010).Scherag A, Dina C, Hinney A, Vatin V, Scherag S, Vogel CIG, Müller TD, Grallert H, Wichmann H-E, Balkau B, Heude B, Jarvelin M-R, Hartikainen A-L, Levy-Marchal C, Weill J, Delplanque J, Körner A, Kiess W, Kovacs P, Rayner NW, Prokopenko I, McCarthy MI, Schäfer H, Jarick I, Boeing H, Fisher E, Reinehr T, Heinrich J, Rzehak P, Berdel D, Borte M, Biebermann H, Krude H, Rosskopf D, Rimmbach C, Rief W, Fromme T, Klingenspor M, Schürmann A, Schulz N, Nöthen MM, Mühleisen TW, Erbel R, Jöckel K-H, Moebus S, Boes T, Illig T, Froguel P, Hebebrand J, Meyre D. Two new Loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and german study groups. PLoS Genetics. 2010;6(4):e856. doi: 10.1371/journal.pgen.1000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri et al. (2007).Scuteri A, Sanna S, Chen W-M, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orrú M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genetics. 2007;3(7):e856. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sementchenko & Watson (2000).Sementchenko VI, Watson DK. Ets target genes: past, present and future. Oncogene. 2000;19(55):6533–6548. doi: 10.1038/sj.onc.1204034. [DOI] [PubMed] [Google Scholar]
- Seppen et al. (2001).Seppen J, Barry SC, Harder B, Osborne WR. Lentivirus administration to rat muscle provides efficient sustained expression of erythropoietin. Blood. 2001;98(3):594–596. doi: 10.1182/blood.V98.3.594. [DOI] [PubMed] [Google Scholar]
- Shao et al. (2008).Shao H, Burrage LC, Sinasac DS, Hill AE, Ernest SR, O’Brien W, Courtland H-W, Jepsen KJ, Kirby A, Kulbokas EJ, Daly MJ, Broman KW, Lander ES, Nadeau JH. Genetic architecture of complex traits: large phenotypic effects pervasive epistasis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19910–19914. doi: 10.1073/pnas.0810388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks (2001).Sharrocks AD. The ETS-domain transcription factor family. Nature Reviews: Molecular Cell Biology. 2001;2(11):827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- Sheffield (2010).Sheffield V. The blind leading the obese: the molecular pathophysiology of a human obesity syndrome. Transactions of the American Clinical and Climatological Association. 2010;121:172–182. [PMC free article] [PubMed] [Google Scholar]
- Shendure (2011).Shendure J. Next-generation human genetics. Genome Biology. 2011;12(9):408. doi: 10.1186/gb-2011-12-9-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver (1995).Silver L. Mouse genetics: concepts and applications. New York: Oxford University Press; 1995. pp. 1–362. [Google Scholar]
- Singer et al. (2004).Singer J, Hill A, Burrage L, Olszens K, Song J, Justice M, Nadeau J, et al. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–449. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- Singleton (2011).Singleton A. Exome sequencing: a transformative technology. The Lancet Neurology. 2011;10(10):942–946. doi: 10.1016/S1474-4422(11)70196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smemo et al. (2014).Smemo S, Tena JJ, Kim K-H, Gamazon ER, Sakabe NJ, Gómez-Marín C, Aneas I, Credidio FL, Sobreira DR, Wasserman NF, Lee JH, Puviindran V, Tam D, Shen M, Son JE, Vakili NA, Sung H-K, Naranjo S, Acemel RD, Manzanares M, Nagy A, Cox NJ, Hui C-C, Gomez-Skarmeta JL, Nóbrega MA. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507(7492):371–375. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith & Funder (1988).Smith A, Funder J. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocrine Reviews. 1988;9(1):159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- Sonestedt et al. (2012).Sonestedt E, Lyssenko V, Ericson U, Gullberg B, Wirfält E, Groop L, Orho-Melander M. Genetic variation in the glucose-dependent insulinotropic polypeptide receptor modifies the association between carbohydrate and fat intake and risk of type 2 diabetes in the Malmo Diet and Cancer cohort. The Journal of Clinical Endocrinology and Metabolism. 2012;97(5):E810–E818. doi: 10.1210/jc.2011-2444. [DOI] [PubMed] [Google Scholar]
- Speakman et al. (2007).Speakman J, Hambly C, Mitchell S, Król E. Animal models of obesity. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity. 2007;8(Suppl 1):55–61. doi: 10.1111/j.1467-789X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Speliotes et al. (2010).Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan Jian’an, Mägi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segrè AV, Estrada K, Liang L, Nemesh J, Park J-H, Gustafsson S, Kilpeläinen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga J-J, Johansson Å, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JRB, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, van Wingerden S, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson J-O, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MTS, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AIF, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, Busonero F, Campbell H, Cappuccio FP, Cavalcanti-Proença C, Chen Y-DI, Chen C-M, Chines PS, Clarke R, Coin L, Connell J, Day INM, Heijer Martin den, Duan J, Ebrahim S, Elliott P, Elosua R, Eiriksdottir G, Erdos MR, Eriksson JG, Facheris MF, Felix SB, Fischer-Posovszky P, Folsom AR, Friedrich N, Freimer NB, Fu M, Gaget S, Gejman PV, Geus EJC, Gieger C, Gjesing AP, Goel A, Goyette P, Grallert H, Gräßler J, Greenawalt DM, Groves CJ, Gudnason V, Guiducci C, Hartikainen A-L, Hassanali N, Hall AS, Havulinna AS, Hayward C, Heath AC, Hengstenberg C, Hicks AA, Hinney A, Hofman A, Homuth G, Hui J, Igl W, Iribarren C, Isomaa B, Jacobs KB, Jarick I, Jewell E, John U, Jørgensen T, Jousilahti P, Jula A, Kaakinen M, Kajantie E, Kaplan LM, Kathiresan S, Kettunen J, Kinnunen L, Knowles JW, Kolcic I, König IR, Koskinen S, Kovacs P, Kuusisto J, Kraft P, Kvaløy K, Laitinen J, Lantieri O, Lanzani C, Launer LJ, Lecoeur C, Lehtimäki T, Lettre G, Liu J, Lokki M-L, Lorentzon M, Luben RN, Ludwig B, Manunta P, Marek D, Marre M, Martin NG, McArdle WL, McCarthy A, McKnight B, Meitinger T, Melander O, Meyre D, Midthjell K, Montgomery GW, Morken MA, Morris AP, Mulic R, Ngwa JS, Nelis M, Neville MJ, Nyholt DR, O’Donnell CJ, O’Rahilly S, Ong KK, Oostra B, Paré G, Parker AN, Perola M, Pichler I, Pietiläinen KH, Platou CGP, Polasek O, Pouta A, Rafelt S, Raitakari O, Rayner NW, Ridderstråle M, Rief W, Ruokonen A, Robertson NR, Rzehak P, Salomaa V, Sanders AR, Sandhu MS, Sanna S, Saramies J, Savolainen MJ, Scherag S, Schipf S, Schreiber S, Schunkert H, Silander K, Sinisalo J, Siscovick DS, Smit JH, Soranzo N, Sovio U, Stephens J, Surakka I, Swift AJ, Tammesoo M-L, Tardif J-C, Teder-Laving M, Teslovich TM, Thompson JR, Thomson B, Tönjes A, Tuomi T, van Meurs JBJ, van Ommen G-J, Vatin V, Viikari J, Visvikis-Siest S, Vitart V, Vogel CIG, Voight BF, Waite LL, Wallaschofski H, Walters GB, Widen E, Wiegand S, Wild SH, Willemsen G, Witte DR, Witteman JC, Xu J, Zhang Q, Zgaga L, Ziegler A, Zitting P, Beilby JP, Farooqi IS, Hebebrand J, Huikuri HV, James AL, Kähönen M, Levinson DF, Macciardi F, Nieminen MS, Ohlsson C, Palmer LJ, Ridker PM, Stumvoll M, Beckmann JS, Boeing H, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Collins FS, Cupples LA, Smith GD, Erdmann J, Froguel P, Grönberg H, Gyllensten U, Hall P, Hansen T, Harris TB, Hattersley AT, Hayes RB, Heinrich J, Hu FB, Hveem K, Illig T, Jarvelin M-R, Kaprio J, Karpe F, Khaw K-T, Kiemeney LA, Krude H, Laakso M, Lawlor DA, Metspalu A, Munroe PB, Ouwehand WH, Pedersen O, Penninx BW, Peters A, Pramstaller PP, Quertermous T, Reinehr T, Rissanen A, Rudan I, Samani NJ, Schwarz PEH, Shuldiner AR, Spector TD, Tuomilehto J, Uda M, Uitterlinden A, Valle TT, Wabitsch M, Waeber G, Wareham NJ, Watkins H, Wilson JF, Wright AF, Zillikens MC, Chatterjee N, McCarroll SA, Purcell S, Schadt EE, Visscher PM, Assimes TL, Borecki IB, Deloukas P, Fox CS, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, Mohlke KL, O’Connell JR, Peltonen L, Schlessinger D, Strachan DP, van Duijn CM, Wichmann H-E, Frayling TM, Thorsteinsdottir U, Abecasis GR, Barroso I, Boehnke M, Stefansson K, North KE, I McCarthy M, Hirschhorn JN, Ingelsson E, Loos RJF. Association analyses of 249,796 individuals reveal eighteen new loci associated with body mass index. Nature Genetics. 2010;42(11):937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisai et al. (2011).Srisai D, Gillum MP, Panaro BL, Zhang X-M, Kotchabhakdi N, Shulman GI, Ellacott KLJ, Cone RD. Characterization of the hyperphagic response to dietary fat in the MC4R knockout mouse. Endocrinology. 2011;152(3):890–902. doi: 10.1210/en.2010-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl et al. (2012).Stahl EA, Wegmann D, Trynka G, Gutierrez-Achury J, Do R, Voight BF, Kraft P, Chen R, Kallberg HJ, Kurreeman FAS, Kathiresan S, Wijmenga C, Gregersen PK, Alfredsson L, Siminovitch KA, Worthington J, de Bakker PIW, Raychaudhuri S, Plenge RM. Bayesian inference analyses of the polygenic architecture of rheumatoid arthritis. Nature Genetics. 2012;44(5):483–489. doi: 10.1038/ng.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford, Cohn & Cordes (2001).Stanford WL, Cohn JB, Cordes SP. Gene-trap mutagenesis: past, present and beyond. Nature Reviews. Genetics. 2001;2(10):756–768. doi: 10.1038/35093548. [DOI] [PubMed] [Google Scholar]
- Stäubert et al. (2007).Stäubert C, Tarnow P, Brumm H, Pitra C, Gudermann T, Grüters A, Schöneberg T, Biebermann H, Römpler H. Evolutionary aspects in evaluating mutations in the melanocortin 4 receptor. Endocrinology. 2007;148(10):4642–4648. doi: 10.1210/en.2007-0138. [DOI] [PubMed] [Google Scholar]
- Stone et al. (2002).Stone S, Abkevich V, Hunt SC, Gutin A, Russell DL, Neff CD, Riley R, Frech GC, Hensel CH, Jammulapati S, Potter J, Sexton D, Tran T, Gibbs D, Iliev D, Gress R, Bloomquist B, Amatruda J, Peter Rae MM, Ted Adams D, Mark Skolnick H, Shattuck D. A major predisposition locus for severe obesity at 4p15-p14. American Journal of Human Genetics. 2002;70(6):1459–1468. doi: 10.1086/340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone et al. (2006).Stone S, Abkevich V, Russell DL, Riley R, Timms K, Tran T, Trem D, Frank D, Jammulapati S, Neff CD, Iliev D, Gress R, He G, Frech GC, Adams TD, Skolnick MH, Lanchbury JS, Gutin A, Hunt SC, Shattuck D. TBC1D1 is a candidate for a severe obesity gene evidence for a gene/gene interaction in obesity predisposition. Human Molecular Genetics. 2006;15(18):2709–2720. doi: 10.1093/hmg/ddl204. [DOI] [PubMed] [Google Scholar]
- Strachan & Read (1999).Strachan T, Read A. Human molecular genetics. vol. 2. New York: Wiley-Liss; 1999. Genetic manipulation of animals. [Google Scholar]
- Stratigopoulos et al. (2011).Stratigopoulos G, Leduc CA, Cremona ML, Chung WK, Leibel RL. Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. The Journal of Biological Chemistry. 2011;286(3):2155–2170. doi: 10.1074/jbc.M110.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratigopoulos et al. (2014).Stratigopoulos G, Martin Carli JF, O’Day DR, Wang L, LeDuc CA, Lanzano P, Chung WK, Rosenbaum M, Egli D, Doherty DA, Leibel RL. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metabolism. 2014;19(5):767–779. doi: 10.1016/j.cmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann et al. (2008).Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O’Rahilly S, Farooqi IS, Froguel P, Meyre D. Prevalence of melanocortin-4 receptor deficiency in europeans their age-dependent penetrance in multigenerational pedigrees. Diabetes. 2008;57(September):2511–2518. doi: 10.2337/db08-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann et al. (2007).Stutzmann F, Vatin V, Cauchi S, Morandi A, Jouret B, Landt O, Tounian P, Levy-Marchal C, Buzzetti R, Pinelli L, Balkau B, Horber F, Bougneres P, Froguel P, Meyre D. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Human Molecular Genetics. 2007;16(15):1837–1844. doi: 10.1093/hmg/ddm132. [DOI] [PubMed] [Google Scholar]
- Stylianou et al. (2004).Stylianou IM, Christians JK, Keightley PD, Bünger L, Clinton M, Bulfield G, Horvat S. Genetic complexity of an obesity QTL (Fob3) revealed by detailed genetic mapping. Mammalian Genome: Official Journal of the International Mammalian Genome Society. 2004;15(6):472–481. doi: 10.1007/s00335-004-3039-z. [DOI] [PubMed] [Google Scholar]
- Styrkarsdottir et al. (2014).Styrkarsdottir U, Thorleifsson G, Helgadottir HT, Bomer N, Metrustry S, Bierma-Zeinstra S, Strijbosch AM, Evangelou E, Hart D, Stefansson K. Severe osteoarthritis of the hand associates with common variants within the ALDH1A2 gene and with rare variants at 1p31. Nature Genetics. 2014;46(5):498–502. doi: 10.1038/ng.2957. [DOI] [PubMed] [Google Scholar]
- Sun, Abil & Zhao (2012).Sun N, Abil Z, Zhao H. Recent advances in targeted genome engineering in mammalian systems. Biotechnology Journal. 2012;7(9):1074–1087. doi: 10.1002/biot.201200038. [DOI] [PubMed] [Google Scholar]
- Suzuki et al. (1999).Suzuki W, Iizuka S, Tabuchi M, Funo S, Yanagisawa T, Kimura M, Sato T, Endo T, Kawamura H. A new mouse model of spontaneous Diabetes derived from ddY strain. Experimental Animals. 1999;48(3):181–189. doi: 10.1538/expanim.48.181. [DOI] [PubMed] [Google Scholar]
- Svenson, Bogue & Peters (2003).Svenson K, Bogue M, Peters L. Invited review: identifying new mouse models of cardiovascular disease: a review of high-throughput screens of mutagenized and inbred strains. Journal of Applied Physiology. 2003;94:1650–1659. doi: 10.1152/japplphysiol.01029.2003. [DOI] [PubMed] [Google Scholar]
- Switzer, Mangat & Karmali (2013).Switzer NJ, Mangat HS, Karmali S. Current trends in obesity: body composition assessment, weight regulation, and emerging techniques in managing severe obesity. Journal of Interventional Gastroenterology. 2013;3(1):34–36. doi: 10.7178/jig.106. [DOI] [Google Scholar]
- Takeshita et al. (2012).Takeshita S, Suzuki T, Kitayama S, Moritani M. Bhlhe40, a potential diabetic modifier gene on Dbm1 locus , negatively controls myocyte fatty acid oxidation. Genes & Genetic Systems. 2012;87:253–264. doi: 10.1266/ggs.87.253. [DOI] [PubMed] [Google Scholar]
- Tartaglia et al. (1995).Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification expression of a leptin receptor OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Teare et al. (2005).Teare MD, Barrett JH, Road BH, Sheffield S. Genetic Epidemiology 2 Genetic linkage studies. Lancet. 2005;366(9490):1036–1044. doi: 10.1016/S0140-6736(05)67382-5. [DOI] [PubMed] [Google Scholar]
- Terauchi et al. (2003).Terauchi Y, Matsui J, Suzuki R, Kubota N, Komeda K, Aizawa S, Eto K, Kimura S, Nagai R, Tobe K, Lienhard GE, Kadowaki T. Impact of genetic background ablation of insulin receptor substrate (IRS)-3 on IRS-2 knock-out mice. The Journal of Biological Chemistry. 2003;278(16):14284–14290. doi: 10.1074/jbc.M211045200. [DOI] [PubMed] [Google Scholar]
- Thomas & Capecchi (1987).Thomas K, Capecchi M. Site-directed mutagenesis by gene targeting in mouse embroyonic stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thorleifsson et al. (2009).Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, Jonsdottir T, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Jonsson F, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Lauritzen T, Aben KK, Verbeek A, Roeleveld N, Kampman E, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Becker DM, Gulcher J, Kiemeney LA, Pedersen O, Kong A, Thorsteinsdottir U, Stefansson K. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nature Genetics. 2009;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- Toye et al. (2004).Toye AA, Moir L, Hugill A, Bentley L, Quarterman J, Mijat V, Hough T, Goldsworthy M, Haynes A, Hunter AJ, Browne M, Spurr N, Cox RD. A new mouse model of type 2 diabetes produced by N-ethyl-nitrosourea mutagenesis is the result of a missense mutation in the glucokinase gene. Diabetes. 2004;53:1577–1583. doi: 10.2337/diabetes.53.6.1577. [DOI] [PubMed] [Google Scholar]
- Tung et al. (2010).Tung Y-CL, Ayuso E, Shan X, Bosch F, O’Rahilly S, Coll AP, Yeo GSH. Hypothalamic-specific manipulation of Fto, the ortholog of the human obesity gene FTO, affects food intake in rats. PLoS ONE. 2010;5(1):e856. doi: 10.1371/journal.pone.0008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnov et al. (2010).Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nature Reviews. Genetics. 2010;11(9):636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- Vaisse et al. (1998).Vaisse C, Clement K, Guy-grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity WRN , is a 3′ → 5′ exonuclease. Nature Genetics. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- Valdar et al. (2006).Valdar W, Solberg LC, Gauguier D, Burnett S, Klenerman P, Cookson WO, Taylor MS, Rawlins J Nicholas P., Mott R, Flint J. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nature Genetics. 2006;38(8):879–887. doi: 10.1038/ng1840. [DOI] [PubMed] [Google Scholar]
- Vimaleswaran et al. (2012).Vimaleswaran KS, Tachmazidou I, Zhao JH, Hirschhorn JN, Dudbridge F, Loos RJF. Candidate genes for obesity-susceptibility show enriched association within a large genome-Wide association study for BMI. Human Molecular Genetics. 2012;21(20):4537–4542. doi: 10.1093/hmg/dds283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher et al. (2012).Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. American Journal of Human Genetics. 2012;90(1):7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel et al. (2012).Vogel H, Scherneck S, Kanzleiter T, Benz V, Kluge R, Stadion M, Kryvych S, Bluher M, Kloting N, Joost H-G, Schurmann A. Loss of function of Ifi202b by a microdeletion on chromosome 1 of C57BL/6J mice suppresses 11β-hydroxysteroid dehydrogenase type 1 expression development of obesity. Human Molecular Genetics. 2012;21(17):3845–3857. doi: 10.1093/hmg/dds213. [DOI] [PubMed] [Google Scholar]
- Vuillaume et al. (2014).Vuillaume M-L, Naudion S, Banneau G, Diene G, Cartault A, Cailley D, Bouron J, Toutain J, Bourrouillou G, Vigouroux A, Bouneau L, Nacka F, Kieffer I, Arveiler B, Knoll-Gellida A, Babin PJ, Bieth E, Jouret B, Julia S, Sarda P, Geneviève D, Faivre L, Lacombe D, Barat P, Tauber M, Delrue M-A, Rooryck C. New candidate loci identified by array-CGH in a cohort of 100 children presenting with syndromic obesity. American Journal of Medical Genetics. Part A. 2014;164A(8):1965–1975. doi: 10.1002/ajmg.a.36587. [DOI] [PubMed] [Google Scholar]
- Wabitsch et al. (2015).Wabitsch M, Funcke J-B, Lennerz B, Kuhnle-Krahl U, Lahr G, Debatin K-M, Vatter P, Gierschik P, Moepps B, Fischer-Posovszky P. Biologically inactive leptin and early-onset extreme obesity. New England Journal of Medicine. 2015;372:48–54. doi: 10.1056/NEJMoa1406653. [DOI] [PubMed] [Google Scholar]
- Walters et al. (2010).Walters RG, Jacquemont S, Valsesia A, Smith AJDE, Martinet D. A novel highly-penetrant form of obesity due to microdeletions on chromosome 16p11.2. Nature. 2010;463(7281):671–675. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2013).Wang F, Mullican SE, Dispirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-speci fi c deletion of PPAR γ. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(46):18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden et al. (1993).Warden CH, Fisler JS, Pace MJ, Svenson KL, Lusis AJ. Coincidence of genetic loci for plasma cholesterol levels and obesity in a multifactorial mouse model. The Journal of Clinical Investigation. 1993;92(2):773–779. doi: 10.1172/JCI116649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden et al. (1995).Warden CH, Fisler JS, Shoemaker SM, Wen PZ, Svenson KL, Pace MJ, Lusis AJ. Identification of four chromosomal loci determining obesity in a multifactorial mouse model. The Journal of Clinical Investigation. 1995;95(4):1545–1552. doi: 10.1172/JCI117827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler et al. (2013).Wheeler E, Huang N, Bochukova EG, Keogh JM, Lindsay S, Garg S, Henning E, Blackburn H, Loos RJF, Wareham NJ, O’Rahilly S, Hurles ME, Barroso I, Farooqi IS. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nature Genetics. 2013;45(5):513–517. doi: 10.1038/ng.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer et al. (2009).Willer CJ, Speliotes EK, Loos RJF, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw K-T, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan Jian’an, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O’Rahilly S, Purmann C, Rees MG, Ridderstråle M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YCL, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, Waeber G, Wallace C, Watanabe RM, Waterworth DM, Watkins N, Witteman JCM, Zeggini E, Zhai G, Zillikens MC, Altshuler D, Caulfield MJ, Chanock SJ, Farooqi IS, Ferrucci L, Guralnik JM, Hattersley AT, Hu FB, Jarvelin M-R, Laakso M, Mooser V, Ong KK, Ouwehand WH, Salomaa V, Samani NJ, Spector TD, Tuomi T, Tuomilehto J, Uda M, Uitterlinden AG, Wareham NJ, Deloukas P, Frayling TM, Groop LC, Hayes RB, Hunter DJ, Mohlke KL, Peltonen L, Schlessinger D, Strachan DP, Wichmann H-E, McCarthy MI, Boehnke M, Barroso I, Abecasis GR, Hirschhorn JN. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nature Genetics. 2009;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2011).World Health Organization . Obesity and overweight: factsheet. Geneva: World Health Organization; 2011. Available at http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- Wuschke et al. (2007).Wuschke S, Dahm S, Schmidt C, Joost H-G, Al-Hasani H. A meta-analysis of quantitative trait loci associated with body weight and adiposity in mice. International Journal of Obesity. 2007;31(5):829–841. doi: 10.1038/sj.ijo.0803473. [DOI] [PubMed] [Google Scholar]
- Xiang et al. (2004).Xiang K, Wang Y, Zheng T, Jia W, Li J, Chen L, Shen K, Wu S, Lin X, Zhang G, Wang C, Wang S, Lu H, Fang Q, Shi Y, Zhang R, Xu J, Weng Q. Genome-wide search for type 2 diabetes/impaired glucose homeostasis susceptibility genes in the Chinese significant linkage to chromosome 6q21-q23 and chromosome 1q21-q24. Diabetes. 2004;53(1):228–234. doi: 10.2337/diabetes.53.1.228. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2003).Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nature Neuroscience. 2003;6(7):736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura & Araki (2008).Yamamura K, Araki K. Gene trap mutagenesis in mice: new perspectives and tools in cancer research. Cancer Science. 2008;99(1):1–6. doi: 10.1111/j.1349-7006.2007.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2009).Yang X, Deignan JL, Qi H, Zhu J, Qian S, Zhong J, Torosyan G, Majid S, Falkard B, Kleinhanz RR, Karlsson J, Castellani LW, Mumick S, Wang K, Xie T, Coon M, Zhang C, Estrada-Smith D, Farber CR, Wang SS, van Nas A, Ghazalpour A, Zhang B, MacNeil DJ, Lamb JR, Dipple KM, Reitman ML, Mehrabian M, Lum PY, Schadt EE, Lusis AJ, Drake TA. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nature Genetics. 2009;41(4):415–423. doi: 10.1038/ng.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Jiang & He (2009).Yang D, Jiang Y, He F. An integrated view of the correlations between genomic and phenomic variables. Journal of Genetics and Genomics. 2009;36(11):645–651. doi: 10.1016/S1673-8527(08)60156-3. [DOI] [PubMed] [Google Scholar]
- Yaswen et al. (1999).Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nature Medicine. 1999;5(9):1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Yazbek et al. (2011).Yazbek SN, Buchner DA, Geisinger JM, Burrage LC, Spiezio SH, Zentner GE, Hsieh C-W, Scacheri PC, Croniger CM, Nadeau JH. Deep congenic analysis identifies many strong context-dependent QTLs one of which Slc35b4 regulates obesity glucose homeostasis. Genome Research. 2011;21(7):1065–1073. doi: 10.1101/gr.120741.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo et al. (1998).Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nature Genetics. 1998;20(2):111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- Yeo et al. (2004).Yeo GSH, Connie Hung C-C, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O’Rahilly S, Farooqi IS. A de novo mutation affecting human TrkB associated with severe obesity developmental delay. Nature Neuroscience. 2004;7(11):1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- Yi et al. (2004).Yi N, Diament A, Chiu S, Kim K, Allison DB, Fisler JS, Warden CH. Characterization of epistasis influencing complex spontaneous obesity in the BSB model. Genetics. 2004;167(1):399–409. doi: 10.1534/genetics.167.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin et al. (2014).Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nature Reviews. Genetics. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- Yoganathan, Karunakaran & Clee (2012).Yoganathan P, Karunakaran S, Clee Ho. Nutritional regulation of genome-wide association obesity genes in a tissue-dependent manner. Nutrition and Metabolism. 2012;9(1):1. doi: 10.1186/1743-7075-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourshaw et al. (2013).Yourshaw M, Solorzano-Vargas RS, Pickett LA, Lindberg I, Wang J, Cortina G, Pawlikowska-Haddal A, Baron H, Venick RS, Nelson SF, Martín MG. Exome sequencing finds a novel PCSK1 mutation in a child with generalized malabsorptive diarrhea and diabetes insipidus. Journal of Pediatric Gastroenterology and Nutrition. 2013;57(6):759–767. doi: 10.1097/MPG.0b013e3182a8ae6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers, Beckers & Hendrickx (2013).Zegers D, Beckers S, Hendrickx R. Prevalence of rare MC3R variants in obese cases and lean controls. Endocrine. 2013;44(2):386–390. doi: 10.1007/s12020-012-9862-1. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (1994).Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhang, Wen & Guo (2014).Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Human Molecular Genetics. 2014;15(23):1–7. doi: 10.1093/hmg/ddu125. [DOI] [PubMed] [Google Scholar]
- Zhu & Zhao (2007).Zhu M, Zhao S. Candidate gene identification approach: progress and challenges. International Journal of Biological Sciences. 2007;3(7):420–427. doi: 10.7150/ijbs.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2002).Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, Steiner DF. Disruption of PC1/3 expression in mice causes dwarfism multiple neuroendocrine peptide processing defects. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10293–10298. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou et al. (2005).Zou F, Gelfond JAL, Airey DC, Lu L, Manly KF, Williams RW, Threadgill DW. Quantitative trait locus analysis using recombinant inbred intercrosses: theoretical and empirical considerations. Genetics. 2005;170:1299–1311. doi: 10.1534/genetics.104.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]