Abstract
The hippocampo-prefrontal (H-PFC) pathway has been linked to cognitive and emotional disturbances in several psychiatric disorders including schizophrenia. Preclinical evidence from the NMDA receptor antagonism rodent model of schizophrenia shows severe pathology selective to the H-PFC pathway. It is speculated that there is an increased excitatory drive from the hippocampus to the prefrontal cortex due to dysfunctions in the H-PFC plasticity, which may serve as the basis for the behavioral consequences observed in this rodent model. Thus, the H-PFC pathway is currently emerging as a promising therapeutic target for the negative and cognitive symptom clusters of schizophrenia. Here, we have reviewed the physiological, pharmacological and functional characteristics of the H-PFC pathway and we propose that allosteric activation of glutamatergic and cholinergic neurotransmission can serve as a plausible therapeutic approach.
Keywords: hippocampus, NMDA antagonist, plasticity, prefrontal cortex, schizophrenia
The prefrontal cortex (PFC) is considered to be the seat for several higher order cognitive functions. It is also considered as one of the key brain areas that are involved in the pathophysiology of several neurological disorders including schizophrenia. Among the diverse inputs to the PFC, afferents from the hippocampus account for a majority of its innervation. The hippocampus projects profusely to the entire PFC via a strong monosynaptic glutamatergic projection. Although the hippocampus can also influence the PFC via several indirect and polysynaptic pathways, the monosynaptic projections are hypothesized to exert a primary influence on the PFC neurons. These monosynaptic projections from the hippocampus to the PFC, which are termed as the hippocampo-prefrontal (H-PFC) pathway, has been the subject for several studies that implicate its importance in various cognitive functions. Moreover, there is growing evidence that the H-PFC pathway could serve as the common link for the cognitive and emotional dysfunctions in several psychiatric and neurological disorders [1].
One such chronic, debilitating psychiatric disorder is schizophrenia that is characterized by psychotic or positive symptoms, as well as negative symptoms (avolition, affective flattening, social withdrawal) and cognitive disturbances. Current antipsychotic drugs act by blocking dopamine (DA) and other monoamine receptors and reduce positive symptoms but have little efficacy in reducing negative symptoms and cognitive dysfunction. Negative and cognitive symptoms have a major negative impact on patient quality of life, and represent an urgent unmet clinical need [2]. Recently, preclinical and clinical evidence demonstrates, deficits in the interplay between the hippocampus and the PFC in schizophrenia. Given the role of the H-PFC pathway in executive function and emotional regulation, it is possible that the H-PFC pathway can have a major role in the negative and cognitive symptoms and thereby can serve as a promising therapeutic target for schizophrenia.
Here, we have reviewed the anatomical, physiological and functional characteristics of the rodent H-PFC pathway with a special emphasis on the chemical regulation of this pathway. We have lso reviewed the current pre-clinical evidence that indicates the role of the H-PFC pathway in the cognitive and negative symptoms of schizophrenia.
Anatomy & physiology of the rodent H-PFC pathway
The rodent PFC is deeply innervated by the hippocampus. There are several polysynaptic pathways by which the hippocampus can influence the rodent PFC, but there also exists a strong unidirectional monosynaptic projection from the hippocampal formation to the PFC including the medial division of the PFC. Anterograde tracer studies using Phaseolus vulgaris-leucoag-glutinin showed that the projections arise from restricted portions of the CA1 (excluding dorsal CA1) of the hippocampus and the subiculum, project via the fimbria-fornix and terminate ipsilaterally [3] in the medial as well as orbital PFC of rats [4]. Projections from the ventral hippocampus/ subiculum were the strongest, while significant projections from the intermediate CA1 area were also found [5,6]. Within the medial PFC, the prelimbic, infralimbic and cingulate areas receive abundant innervation from the H-PFC pathway, which terminates in all cortical layers. Interestingly, there is a marked difference in terminal distribution along the dorsoventral extent of the PFC, with more dense fibers in the ventral areas. In the dorsal areas, the innervation is primarily located in the deeper layers [4]. Besides the medial PFC, another significant projection primarily from the intermediate hippocampal formation to the lateral PFC was also delineated [7]. Interestingly, there is no direct projection from the PFC back to the hippocampus, instead the PFC is known to communicate with the hippocampus via the nucleus reuniens of the midline thalamus [8].
Projections from the H-PFC pathways are known to terminate in both inhibitory interneurons as well as excitatory pyramidal cells. Using intracellular recordings of PFC pyramidal neurons, it was shown that single pulse stimulation of the ventral hippocampus can lead to fixed latency excitatory post synaptic potentials (EPSPs), followed by a prolonged inhibitory post-synaptic potential (IPSP). The presence of fixed latency responses was indicative of the fact that the responses were of monosynaptic origin. Moreover, these responses were seen in all three main classes of the pyramidal neurons, namely, regular spiking, nonactivating bursting and inactivating-bursting neurons [9]. The presence of the prolonged IPSP also indicated that it is possible there is simultaneous engagement of both pyramidal and nonpyramidal neurons. It was later shown, using extracellular recordings from anesthetized rats that indeed fast spiking interneurons of the prelimbic cortex respond to hippocampal stimulation. The responses in the interneurons were excitatory in nature and in accordance with the monosynaptic nature of the H-PFC pathway, had consistent latencies [10]. Based on morphological characteristics, recordings were from three types of interneurons, namely, aspiny stellate, spiny stellate and bitufted neurons and all fired action potentials in response to hippocampal stimulation. Using in vitro electrophysiological preparations in which the hippocampal afferents remained intact, it was demonstrated that both pyramidal neurons and fast spiking interneurons showed EPSPs following stimulation of the hippocampal afferents [11]. Finally, using simultaneous recording of the pyramidal neurons and interneurons in vivo it was shown that the interneurons consistently fired before the pyramidal cells after hippocampal stimulation. Therefore, monosynaptic excitation of interneurons by the H-PFC pathway could be responsible for feed-forward inhibition of the pyramidal neurons [10]. However, overall the H-PFC pathway is believed to have an excitatory influence on the PFC as low-frequency stimulation of the CA1/subicular region of anesthetized rats was shown to produce a net excitatory response evident in multiunit discharge as well as field recordings in the prelimbic area of the PFC [12]. Similar projection patterns from the hippocampus have also been reported in monkeys and humans [1,13–15].
Based on ultrastructural characteristics, it has been shown that hippocampal inputs terminate primarily in the dendritic spine heads of spiny pyramidal neurons [16,17]. On the other hand, they innervate primarily the dendritic shafts of the parvalbumin immunoreactive interneurons. Interestingly, no input to either calbindin or calretinin immunoreactive cells were observed [17]. Thus, there is evidence of a unique microstructure of the H-PFC pathway in the PFC, where hippocampal terminals make a triangular circuit with GABAergic interneurons and pyramidal neurons. This triad microcircuit may be highly involved in short- and long-term plasticity observed in this pathway and can also be the seat for dysregulation of this pathway in several neurological disorders.
Plasticity of the rodent H-PFC pathway
The H-PFC pathway has been shown to undergo both short- and long-term plasticity. Short-term paired pulse facilitation was observed in this pathway in vivo in anesthetized rats both in extracellular field recordings and intracellular recordings [9,18]. A subpopulation of the excitatory neurons also showed marked paired pulse depression [9]. Paired pulse facilitation (interstimulus intervals 20–100 ms) indicating a greater degree of facilitated presynaptic release was also confirmed using in vitro whole cell recordings using PFC slices where hippocampal afferents were preserved. The facilitation was observed in both excitatory and inhibitory cells. Interestingly, the paired pulse facilitation profile in the H-PFC pathway was very different from the cortical-PFC pathway investigated after stimulating Layer I. The facilitation observed in the H-PFC pathway was found to be higher than the cortical-PFC pathway [11]. A difference in paired pulse facilitation/inhibition was also observed based on the region of the hippocampus stimulated in both rats and mice. While stimulating the intermediate hippocampus paired pulse inhibition was observed in smaller interstimulus intervals with a gradual transition to paired pulse facilitation with increasing interstimulus intervals up to 1000 ms [19–21]. In the ventral hippocampus–PFC afferents only paired pulse facilitation was observed that gradually decreased with increasing interstimulus intervals with no evidence of paired pulse inhibition. Moreover, stimulus intensity contributed strongly to short-term plasticity in the intermediate pathway [22], whereas, in the ventral pathway, it was solely dependent on interstimulus intervals [23]. It is speculated that the paired pulse facilitation and inhibition can be a direct correlate of the triangular microcircuit observed between GABAergic interneurons, pyramidal neurons and hippocampal terminals [24].
Both hippocampus and the PFC have been shown to play critical role in learning and memory in rodents. Recently, interactions between the PFC and hippocampus via the H-PFC pathway have been shown to be an important mechanism underlying learning and memory processes in the rodent brain. Classically, one of the key neurological basis underlying learning and memory has been synaptic plasticity including long-term potentiation (LTP) and long-term depression (LTD). Justifying its role in learning and memory, the H-PFC pathway also shows significant long-term synaptic plasticity. Long-term plasticity was first demonstrated in early 90s where, tetanic stimulation of the CA1/ subicular region in anesthetized rats induced an immediate, stable and persistent LTP of the prelimbic field potentials [18]. Using the same tetanic stimulation, similar LTP was induced in this pathway, in freely moving rats and shown to last for up to 3 days after induction [12]. High-frequency stimulation or low-frequency paired pulse stimulations, however, failed to induce LTD in freely moving rats and anesthetized rats [12,25]. However, a two-pulse burst low-frequency train could depotentiate a previously established LTP for more than 2 h after induction [25]. Long-term depression (LTD) in freely moving rats can be achieved using a stimulus train instead, where 900 stimulus trains (5 pulses at 250 Hz) applied at 1 Hz to the ventral hippocampus can lead to LTD which can be reversed by a subsequent 12 stimulus train (50 pulses at 250 Hz) at 0.1 Hz [26]. Bidirectional plasticity was also observed in the PFC slice preparations where the hippocampal afferents are preserved [11]. A theta burst pairing stimulus protocol in the hippocampal afferents produced strong LTP in the prelimbic cortex, whereas pairing action potentials and EPSPs at 3 Hz, produced strong LTD in this preparation. Evidence suggests that this LTP and LTD in the H-PFC pathway are involved in various cognitive functions in the rodents and they have been found to be disrupted in models of various neurobiological diseases. For example, extinction of fear conditioning and fear recall is dependent on potentiation of H-PFC pathway [27] and acute stress as well as chronic mild stress in rodents can lead to impaired LTP in this pathway, which in turn can be part of the underlying pathophysiology of stress [27,28]. Moreover, it was also shown that plasticity in the H-PFC pathway is ‘flexible’ or ‘compatible’, meaning LTD can be induced in the pathway after inducing LTP and vice versa [29]. This presence of bidirectional plasticity serves as evidence for the basis of several learning, memory and other cognitive tasks that involve the H-PFC pathway. Moreover, the ‘compatibility’ in the induction of LTP and LTD can explain the ability of PFC in preventing behavioral perseveration driven by previous history [29,30].
Pharmacology of the rodent H-PFC pathway
Following focal injection of retrograde tracer, D-[3H] aspartate in the PFC which enabled labeling of hippocampal neurons, it was shown that the major neurotransmitter used in this pathway is glutamate. Moreover, microiontophoretic application of CNQX, an AMPA receptor antagonist, in PFC was able to completely block hippocampus stimulus induced PFC activity in vivo [5]. Thus, the monosynaptic H-PFC pathway consists of primarily excitatory glutamatergic pyramidal neurons, where stimulation of the pathway produces AMPA receptor mediated excitation in the PFC. There is contrasting evidence for a role of NMDA receptors in the H-PFC pathway. First application of D-AP5 a selective NMDA receptor antagonist only affected the excitatory response in a few cells, indicating that AMPA receptor dependent synaptic responses were predominant in the PFC [5]. Second, while some studies show a role of NMDA receptor in the plasticity of the H-PFC pathway, others have shown that NMDA receptor independent mechanisms of plasticity also exist in this pathway. In anesthetized rats, local perfusion of D-AP5 during the tetanic stimulus of the hippocampus, but not after LTP induction was able to block LTP induction of field potentials, while not having any effect on test stimulus driven responses [31].
The dopaminergic system is also known to have significant influence on the H-PFC pathway. PFC receives majority of its dopaminergic inputs from the ventral tegmental area (VTA), targeting primarily the deeper layers of the PFC [32–34]. This mesocortical DA system is known to primarily exert an inhibitory influence in the PFC [35,36], whereas, an excitatory influence in in vitro preparations has also been observed [37]. Reportedly, there is close proximity of the DA terminals with the hippocampal terminals in deep layers of the PFC [16], making the mesocortical system an ideal candidate for modulations of the excitatory H-PFC pathway. Moreover, in vivo activation of the mesocortical DA system by stimulating VTA can block the excitatory responses induced by hippocampal stimulation in anesthetized rats [38]. Interestingly, activation of the mesocortical system at a frequency that leads to DA overflow causes a long-lasting enhancement in the magnitude of H-PFC tetanic LTP in vivo. On the other hand, depletion of DA in the PFC via electrolytic lesion of the VTA has opposing effects. Thus, there was a significant correlation between the amount of DA level in the PFC and the magnitude of LTP in the H-PFC pathway [39]. Between the two major DA receptor subtypes, D1 plays an important role in influencing NMDA receptor-dependent LTP. Reverse microdialysis of SKF81297, a D1 receptor agonist in the PFC significantly increases the magnitude of LTP in a dose dependent manner in PFC following hippocampal stimulation. Similarly, infusion of D1 antagonist SCH23390 in PFC also blocked H-PFC LTP in a dose-dependent manner. However, blocking of D2 with sulpiride did not inhibit LTP, indicating little or no role of D2 in modulation of NMDA dependent LTP in this synapse. It was also shown, that the D1 receptor engages the c-AMP-Protein Kinase A pathway to induce its effect on the NMDA receptor dependent LTP [40]. This shows that there is a definite convergence of the mesocortical DA system and the H-PFC pathway in the PFC cortical neurons.
Like the dopaminergic influence on the H-PFC, there is also significant influence of the noradrenergic and serotonergic system on glutamatergic transmission of this pathway. In anesthetized rats, simultaneous stimulation of the ipsilateral locus coeruleus (LC) with the tetanic stimulation of hippocampus led to an enhancement of LTP in the PFC for at least 40 min post induction of LTP. Conversely pharmacological inactivation of LC with lidocaine or noradrenergic depletion in PFC also significantly reduced the magnitude of LTP but did not completely block the induction of LTP in anesthetized or freely moving rats. On the same line, systemic administration of nisoxetine (a noradrenaline reuptake inhibitor) and clonidine (α2 adrenergic receptor agonist) augmented and partially blocked LTP in the H-PFC pathway in vivo [41]. There is contrasting evidence on the role of serotonin on H-PFC pathway. Acute and repeated administration of a selective serotonin reuptake inhibitor fluvoxamine was shown to increase the magnitude of PFC field responses following hippocampal stimulation after fluvoxamine treatment in anesthetized rats. Moreover, following repeated fluvoxamine administration there was hypersensitivity in the PFC responses to hippocampal stimulation intensity. Repeated administration of fluvoxamine also led to a significant augmentation of H-PFC LTP in anesthetized rats [42]. On the other hand, lesions of serotonergic neurons in the PFC via intracerebroventricular injections of 5,7-dihydroxytryptamine (5,7-DHT) also led to an augmentation of short-term paired pulse facilitation as well as LTP in the H-PFC pathway, while the basal synaptic transmission in this pathway remained unaffected [43]. It is speculated that depletion of serotonin in PFC can lead to an increased glutamate release (indicated by the increase in paired pulse facilitation) and also disinhibit 5-HT1A receptor actions on NMDA receptors leading to an increase in the magnitude of LTP after hippocampal stimulation [43]. The contrasting results regarding the role of serotonin on the H-PFC pathway is interesting. It is possible increased synaptic serotonin over longer periods of time can have different effects on the H-PFC pathway as opposed to acute serotonin neurotransmission. Also, systemic dosing of fluvoxamine can affect several brain regions including the hippocampus, whereas, intracerebroventricular injections of 5,7-DHT only has local action on PFC. Thus serotonin may modulate the H-PFC pathway in the cortex differently than it does in the hippocampus.
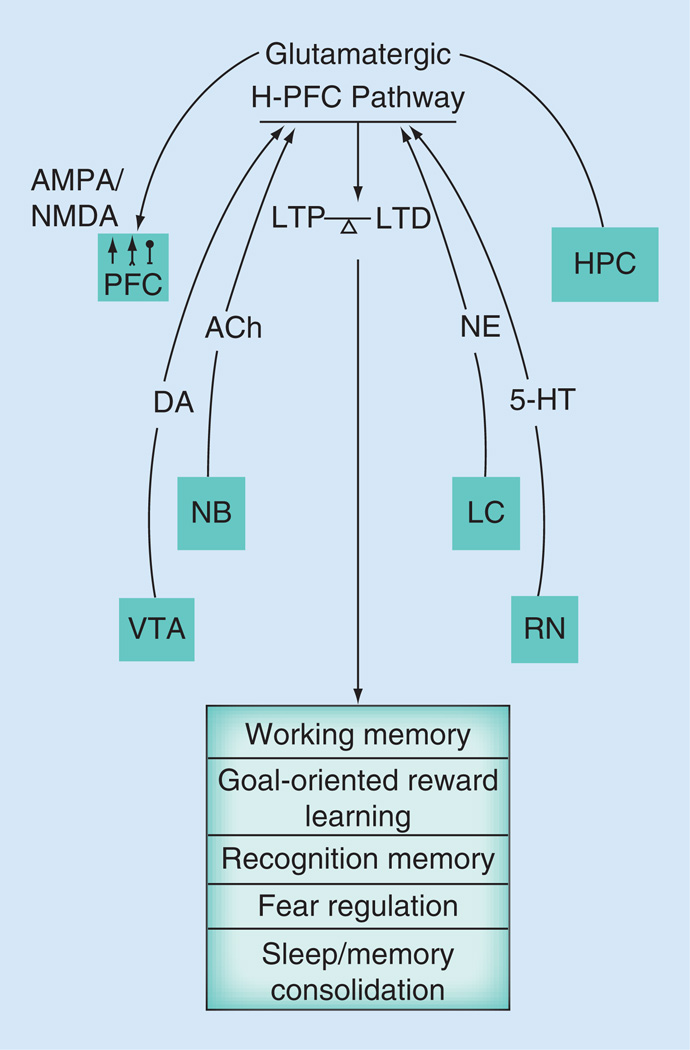
There is also a tight cholinergic regulation of the H-PFC pathway that has been reported. The PFC receives cholinergic input from the brainstem and the basal forebrain structures. This cholinergic innervation acts especially via the muscarinic cholinergic receptors to modulate both LTP and LTD in the H-PFC pathway. A nonselective muscarinic receptor agonist pilocarpine when systemically dosed before tetanic stimulation of the hippocampus was able to potentiate the NMDA dependent form of LTP in the PFC, in urethane anesthetized animals. This potentiation of LTP following pilocarpine administration was characterized by a simultaneous increase in monoaminergic transmission, with significant decrease in DA, serotonin and noradrenaline levels [44]. Muscarinic activation in PFC was also able to potentiate low-frequency hippocampal stimulation induced LTD in vivo. Thus, intracerebroventricular administration of pilocarpine in the PFC was able to potentiate a sub-threshold form of LTD without affecting basal neurotransmission of the H-PFC pathway. This form of low-frequency stimulation-induced LTD in vivo is also known to be NMDA receptor-dependent. Interestingly, blockade of NMDA receptors before the muscarinic activation blocks pilocarpine’s ability to potentiate the subthreshold form of LTD [45]. Thus, muscarinic receptors interact with the monoaminergic system as well as the glutamatergic system in the PFC to influence the H-PFC pathway. In addition, muscarinic activation alone has been shown to induce plasticity in this pathway. Using a slice preparation for PFC where hippocampal inputs are preserved [11], bath application of the cholinergic agonist carbachol can induce LTD in the H-PFC pathway in a concentration dependent manner. A subsequent increase in paired pulse facilitation was also observed during the LTD, indicating possible presynaptic mechanisms for this form of LTD. Using various pharmacological tools, it was shown that the muscarinic subtype primarily governs this LTD, possibly with a role for M2 muscarinic receptor subtype. This form of muscarinic LTD in the PFC is NMDA independent, as co-application of AP-5, an NMDA receptor antagonist failed to block the synaptic depression. However, co-application of nifedipine, voltage gated L-type calcium channel blocker and BAPTA, a postsynaptic calcium chelator block the muscarinic LTD. This indicates that postsynaptic elevation of calcium is also important for induction of this form of LTD [46]. Interestingly, this form of carbachol LTD was also observed in regular PFC slices when neurons from deeper layers of PFC were recorded intracellularly, while stimulating the superficial layers, which includes but is not exclusively comprised of projections from the hippocampus [47]. This indicates that cholinergic modulation, either in conjunction with the monoaminergic and glutamatergic neurotransmission or by itself can effectively modulate plasticity in the H-PFC pathway (Figure 1).
Figure 1. Characteristics of the hippocampo-prefrontal pathway.
The H-PFC pathway comprises glutamatergic monosynaptic projections from the HPC to the PFC. It has an excitatory influence (↑) on both pyramidal and inhibitory interneurons in PFC via both NMDA and AMPA receptor activation. There is dopaminergic, cholinergic and monoaminergic influence on the H-PFC pathway that helps to maintain a balance between long-term potentiation and long-term depression in this pathway. This balance is thought be an important factor for the normal behavioral phenotype in rodents associated with the pathway.
5-HT: Serotonin; ACh: Acetylcholine; DA: Dopamine; H-PFC: Hippocampo-prefrontal cortex; HPC: Hippocampus; LC: Locus coeruleus; NB: Nucleus basalis; NE: Norepinephrine; PFC: Prefrontal cortex; RN: Raphe nuclei; VTA: Ventral tegmental area.
Functional relevance of the H-PFC pathway in normal & pathophysiological processes
In recent years, the functional coupling between the hippocampus and the PFC has been a subject of widespread interest due to its role in cognitive and emotional processing. The functional aspect of the H-PFC pathway is elaborately reviewed by Godsil et al. [1]. In short, there is evidence that links this pathway to learning and memory, fear regulation and even sleep-governing processes. In early studies, disconnection between hippocampus and PFC was used as a method to study its role in animal behavior. Since the hippocampus and the PFC connect ipsilaterally, disconnection between the two structures was possible by pharmacological inactivation or lesions of the hippocampus and contralateral PFC. Using this technique, Floresco et al. [48] showed the role of the H-PFC pathway in working memory. Animals with H-PFC disconnections performed poorly in a delayed win-shift radial arm maze task while not affecting their normal random foraging behavior. It was further shown that the H-PFC pathway is engaged in situations with increased cognitive demand during the working memory task [49]. There is also evidence for increased synchronous activity between the PFC and hippocampus during spatial working memory tasks [50,51]. Lesions of the ventral hippocampus disrupt cognitive flexibility in animals [52]. The role of functional interaction between the two structures in goal-oriented reward learning was evident when increased coherence was observed between the two structures when animals were at the point of choice in a Y-maze while acquiring a new learning rule [53]. Synchronized activity between the two structures was important for the animal to perform accurately in such tasks indicating a possible role of the H-PFC pathway in information transfer between hippocampus and the PFC during reward learning. The H-PFC pathway has also been shown to be involved in recognition memory. While the H-PFC disconnection spared simple novel object recognition in animals, it severely impaired the animals’ ability to perform in more complex recognition tasks that involved contextual and temporal details [54]. The H-PFC pathway along with its interactions with the amygdala has been shown to be critical for regulation of fear. Plasticity studies mentioned above have shown that fear extinction led to synaptic potentiation of the H-PFC pathway [27], indicating its role in fear extinction memory. Inactivation and H-PFC disconnections studies have also shown its importance in fear recall and fear renewal [55,56]. Finally, there is evidence of functional coupling between the hippocampus and PFC during sleep. The two structures are known to communicate heavily during slow wave sleep, while this is attenuated during rapid eye movement (REM) sleep. Interestingly, PFC neurons consistently fire after the hippocampus in sleeping animals, indicating possible directional interaction between these areas. This in turn can have implications in memory consolidation [57].
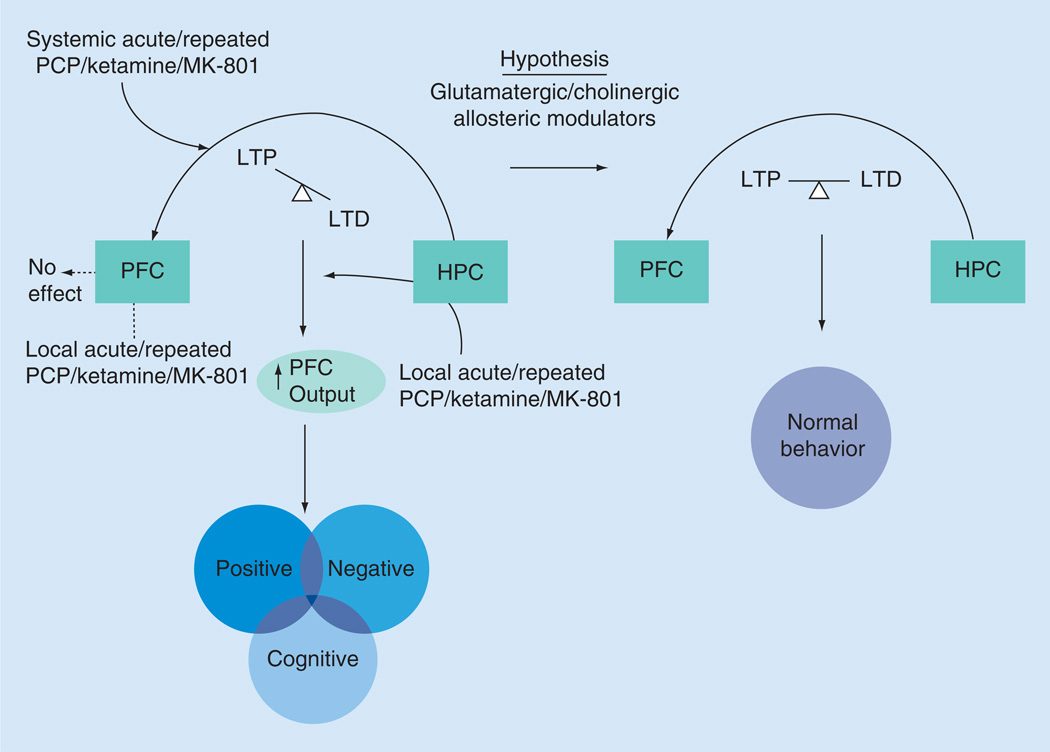
In addition to the evidence for behavioral contribution of the H-PFC pathway, there is growing evidence that several psychiatric disorders including schizophrenia can heavily affect the interaction between the two structures. Several lines of evidence suggest that plasticity in the H-PFC pathway is highly compromised in acute and chronic stress models in animals [27–28,58] directly implicating a role of the H-PFC pathway in stress regulation. There is also strong clinical evidence of involvement of the H-PFC pathway in major depression [59,60] and post traumatic stress disorders [61]. Patients with schizophrenia also show aberrant functional coupling between the hippocampus and the PFC at resting state [62], as well as during working memory tasks [63,64]. The role of the H-PFC pathway in schizophrenia is also evident in preclinical rodent models of the disease. Neurodevelopmental models of schizophrenia readily show a decreased theta-coherence between the hippocampus and the PFC [65]. NMDA receptor antagonism is an effective pharmacological model of schizophrenia that mimics positive, negative and cognitive symptoms of the disorder [66]. Acute or repeated administration of the NMDA receptor antagonists phencyclidine (PCP), MK-801 or ketamine induce a behavioral syndrome that includes several symptom clusters of the disease [2]. One advantage of this model has been its faithful representation of the negative and cognitive symptoms of schizophrenia [66,67], a current unmet clinical need for the disorder. Thus, administration of NMDA receptor antagonists in rodents produces a myriad of cognitive dysfunctions including deficits in novel object recognition [68–72], reversal learning [73–78], attentional set shifting [79,80] and attention [81–83]. Significant deficits in social interaction [84,85] and anhedonia [86,87] of rodents have also been shown, thus representing the negative symptom cluster of schizophrenia. The presence of the negative and cognitive symptom profiles in this animal model provides a unique opportunity to tease apart the pathophysiology related to these symptoms. Interestingly, multiple behavioral deficits observed in the animal model are directly or indirectly related to the functionality of the PFC. Indeed, the function of PFC is heavily compromised after treatment with NMDA receptor antagonists. Acute systemic administration of PCP or MK-801 led to a tonic excitation of PFC neurons in anesthetized as well as freely moving rats [88–91]. This increase in PFC excitability was also observed after repeated administration of PCP in rats [92]. It is possible that the glutamatergic H-PFC pathway can be a primary source for the NMDA receptor antagonist induced dysfunction of the PFC. Evidence supporting this hypothesis comes from studies that show that PCP can lead to disinhibition of the hippocampus [93]. Other studies have shown that local application of PCP or MK-801 in the rodent PFC fails to mimic the tonic excitation of PFC neurons observed after systemic administration [91,94]. Local application of PCP even failed to elicit any detectable increase in excitatory synaptic currents of PFC neurons [95]. On the other hand, local application of PCP or MK-801 in the ventral hippocampus led to enhanced excitation of the PFC [94]. Moreover, only hippocampal neurons projecting to the PFC were disinhibited and excited and not the ones that were unconnected to the PFC [94]. Also, acute MK-801 treatment has been shown to cause a long lasting increase in PFC responses evoked by ventral hippocampus. This potentiation of PFC responses shared a common mechanism involved with high-frequency stimulation induced LTP generation in the H-PFC pathway as it occluded the generation of such LTP in this pathway. This aberrant plasticity occurred in conjunction with cognitive deficits in the animals and also decayed in parallel within 24 h after the MK-801 treatment [96,97]. A recent study also showed that following repeated administration of MK-801 in rodents there is a shift from LTD to LTP in the H-PFC pathway. However, no changes were observed in the amygdala to PFC pathway, indicating a central role of the H-PFC pathway in this animal model. In addition, a normal inhibitory regulation of the amygdala-PFC pathway by the H-PFC pathway was also disrupted following MK-801 administration [98]. All of these studies directly support the hypothesis that H-PFC pathway functionality and plasticity can be directly linked to the pathophysiology observed in PFC following NMDA receptor antagonism. Moreover, the striking similarity of the functional impact of the H-PFC pathway and the behavioral deficits observed in the NMDA receptor antagonist model of schizophrenia makes it a promising target for the negative and cognitive symptoms of schizophrenia (Figure 2).
Figure 2. Pathophysiology of the hippocampo-prefrontal pathway after NMDA receptor antagonism.
Systemic administration of both acute and repeated treatment or local application in the HPC of NMDA receptor antagonists leads to an excessive excitatory drive in the PFC (increased PFC output), which is lacking when they are locally infused in the PFC. This heavily implicates that NMDA receptor antagonists affect the H-PFC pathway and tilts the LTP–LTD balance in this pathway toward potentiation. This loss in LTP–LTD balance can be responsible for the increased PFC output and the behavioral phenotype observed in a NMDA receptor antagonist rodent model of schizophrenia. We hypothesize that glutamatergic and cholinergic allosteric modulators with supreme subtype selectivity will be ideal candidates to act on this pathway and restore the LTP–LTD balance and thereby rescue the behavioral abnormalities in this rodent model.
HPC: Hippocampus; LTD: Long-term depression; LTP: Long-term potentiation; PCP: Phencyclidine; PFC: Prefrontal cortex.
Conclusion & future perspective
In summary, the H-PFC pathway provides a strong excitatory drive in the PFC and is subject to bidirectional short- and long-term plasticity. It is under tight chemical regulation from the glutamatergic, monoaminergic and cholinergic neurotransmission. There is also a direct correlation in the physiology and plasticity of this pathway to its functional relevance. It by means of its bidirectional plasticity is strongly involved in working memory, goal oriented learning, fear regulation and sleep (Figure 1). Thus, much is now known about the characteristics and functional relevance of the H-PFC pathway but a number of unanswered questions remain. For example, PFC has high expression of metabotropic glutamate receptors (mGluR) including the subtypes mGlu2, mGluR3 and mGluR5, each of which is being investigated as a potential target for treatment of schizophrenia and other disorders that include disruptions in PFC function. Surprisingly, little is known regarding the role of mGluRs in regulating the glutamatergic projections from the hippocampus. mGluR5, being a strong signaling partner of NMDA receptors [99–102] has the potential to exert a strong influence on the H-PFC pathway. Also, the group II mGluRs may regulate transmission at this excitatory synapse. Similarly, although it is known that muscarinic receptors are involved in long-term plasticity in this pathway, important information regarding the muscarinic subtype that governs such mechanisms is either missing or less convincing. Information regarding receptor subtypes is important from a drug discovery perspective as identifying subtype specific targets will enable reducing the risk of adverse effects. One of the challenges in the past has been the lack pharmacological tools to address such questions. Moreover, the long range of the H-PFC pathway, makes it a difficult target to study, where researchers have to rely on extremely difficult in vivo techniques to characterize this pathway. However, recently with the advent of several important pharmacological tools, such as selective mGlu5, mGlu2/3, M1 and M4 modulators [103–108] as well as optogenetic techniques to reliably and selectively study long-range projections, makes it an exciting time to revisit the H-PFC pathway.
The importance of the H-PFC pathway in schizophrenia has also been apparent. From clinical evidence in schizophrenic patients to strong preclinical indications, the H-PFC pathway is slowly emerging as one of the therapeutic targets for the negative and cognitive symptoms of schizophrenia. The pharmacological rodent model that relies on NMDA receptor antagonism provides reliable face, construct and predictive validity for schizophrenia. Although not devoid of drawbacks it is still considered as a fantastic tool to study therapeutic efficacy in the different symptom clusters of schizophrenia [2,66,109]. The existence of such a model makes it a relatively easier task to further tease apart the role of the H-PFC pathway in the negative and cognitive symptom clusters. The increased excitatory drive of the PFC from the hippocampus and a shift in balance in H-PFC plasticity in favor of LTP provide strong evidence for the involvement of this pathway in NMDA receptor antagonism model of schizophrenia. Given the varied and tight pharmacological regulation of the plasticity in this pathway, there are several avenues by which the disrupted balance between LTP and LTD can be restored. For example, developing agents that modify cholinergic and glutamatergic neurotransmission in the PFC can help to reverse the pathophysiology in this pathway following NMDA receptor antagonism. Indeed, it has been shown that applying LY379268 a group II mGluR agonist can normalize PCP induced excessive release of glutamate [110]. However, developing direct agonists or antagonists as potential drug candidates has its disadvantages due to its adverse effect liabilities. Also, they severely lack subtype selectivity and thus it is impossible to tease apart the role of each subtype. Instead, allosteric modulators are one class of compounds that can serve as effective candidates for such a purpose. Allosteric modulators are compounds that do not bind to the orthosteric ligand binding site of the receptors, but instead bind and act on other sites to either potentiate activation of the receptors by its natural ligand (positive allosteric modulator or PAMs) or negatively modulate the receptor activation following binding of the natural ligand (negative allosteric modulators or NAMs) [111]. Such modulators have the potential to be extremely subtype selective due to less conservation of allosteric sites between subtypes as compared with orthosteric sites. The subtype selectivity combined with a lack of direct activation of receptors makes them favorable drug candidates over direct agonists and antagonists [112]. Thus, positive allosteric modulation of mGluR5 may serve in potentially promoting NMDA dependent LTD and thereby restoring the balance between LTP and LTD. In line with this hypothesis, there are already emerging reports that mGluR5 PAMs can reverse cognitive deficits in object recognition following ketamine administration [68]. Similarly, allosteric activation of muscarinic receptors can promote LTD in PFC and restore normal excitatory drive in the H-PFC pathway. Supporting the hypothesis, it is shown that BQCA, an M1 PAM was able to enhance muscarinic LTD in the PFC [47]. Whether, such allosteric modulators of the cholinergic neurotransmission can be potentially promising candidates in ameliorating the H-PFC dysfunctions following NMDA receptor hypofunction, remains to be seen (Figure 2).
Thus, in conclusion the H-PFC pathway has immense functional relevance that is evident even in rodents. Moreover, preclinical evidence from the NMDA receptor antagonism model heavily implicates its role in the negative and cognitive symptoms clusters of schizophrenia. Therefore, designing and optimizing drugs and compounds that show efficacy in modulating the H-PFC pathway will be a promising approach to counter the negative and cognitive symptom cluster of schizophrenia.
EXECUTIVE SUMMARY.
Anatomy & physiology of the rodent hippocampo-prefrontal (H-PFC) pathway
The intermediate and ventral hippocampus projects monosynaptically to the medial as well as orbital prefrontal cortex (PFC). The projections span all layers of the PFC and innervate both excitatory and inhibitory neurons.
Plasticity of the rodent H-PFC pathway
The H-PFC pathway shows both short- and long-term plasticity. Paired pulse facilitation and depression as well as LTP and LTD are observed in this pathway. Long-term plasticity in this pathway is bidirectional as well as compatible and is relevant for several functions served by this pathway.
Pharmacology of the rodent H-PFC pathway
The monosynaptic connections of the H-PFC pathway are primarily glutamatergic in nature, and synaptic responses are mediated primarily by AMPA receptors with modulation from the N-methyl-d-aspartate (NMDA) receptors. There is presence of both NMDA receptor-dependent and NMDA receptor-independent forms of plasticity in this pathway. Both these forms of plasticity are under tight modulation from dopaminergic, serotonergic, noradrenergic as well as cholinergic neurotransmission.
Functional relevance of the H-PFC pathway in normal & pathophysiological processes
The H-PFC pathway has been shown to be important for cognitive performance and emotional regulation in rodents. It has been shown to be important for working memory, reversal learning, goal-oriented reward learning, fear regulation and sleep. Preclinical evidence from NMDA receptor antagonism models show that there is a shift in balance between LTP and LTD in favor of LTP in the H-PFC pathway. This can underlie the increased excitatory drive observed in PFC and subsequent behavioral abnormalities following NMDA receptor antagonist administration.
Conclusion & future perspective
The evidence from the NMDA receptor antagonism model of schizophrenia suggests that the H-PFC pathway can be heavily implicated in negative and cognitive symptoms of schizophrenia and thus can be a promising therapeutic target. We propose that modulating glutamatergic and cholinergic neurotransmission, potentially by allosteric modulators can serve as a promising therapeutic approach.
Acknowledgments
Financial
PJ Conn receives research funding and salary support from Bristol-Meyers Squibb and AstraZeneca. The works of the authors listed here are supported by grants from NIMHS and NINDS.
Footnotes
competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur. Neuropsychopharmacol. 2013;23(10):1165–1181. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Neill JC, Harte MK, Haddad PM, Lydall ES, Dwyer DM. Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: a translational link to humans. Eur. Neuropsychopharmacol. 2014;24(5):822–835. doi: 10.1016/j.euroneuro.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Jay TM, Glowinski J, Thierry AM. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res. 1989;505(2):337–340. doi: 10.1016/0006-8993(89)91464-9. [DOI] [PubMed] [Google Scholar]
- 4. Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 1991;313(4):574–586. doi: 10.1002/cne.903130404. • Article showing anatomical characteristics of the hippocampo-prefrontal (H-PFC) pathway: the authors use an anterograde tracer technique to reveal that ventral and intermediate hippocampus projects to the medial and orbital areas of the prefrontal cortex (PFC).
- 5.Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory amino acid pathway from the hippocampus to the prefrontal cortex. Contribution of AMPA Receptors in hippocampo-prefrontal cortex transmission. Eur. J. Neurosci. 1992;4(12):1285–1295. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 6.Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res. Rev. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verwer RW, Meijer RJ, Van Uum HF, Witter MP. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus. 1997;7(4):397–402. doi: 10.1002/(SICI)1098-1063(1997)7:4<397::AID-HIPO5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res. Bull. 2007;71(6):601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Synaptic influence of hippocampus on pyramidal cells of the rat prefrontal cortex: an in vivo intracellular recording study. Cereb. Cortex. 2003;13(7):782–792. doi: 10.1093/cercor/13.7.782. • Article showing physiological responses from pyramidal cells of PFC following hippocampal stimulation: the authors perform in vivo intracellular recordings from different types of PFC pyramidal neurons to demonstrate excitatory synaptic potentials followed by inhibitory potentials.
- 10. Tierney PL, Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur. J. Neurosci. 2004;20(2):514–524. doi: 10.1111/j.1460-9568.2004.03501.x. • Article showing the physiological excitation of interneurons in the PFC following hippocampal stimulation: the authors use extracellular recordings in PFC to show that morphologically different interneurons exhibit excitatory responses following ventral hippocampus stimulation in vivo.
- 11.Parent MA, Wang L, Su J, Netoff T, Yuan LL. Identification of the hippocampal input to medial prefrontal cortex in vitro . Cereb. Cortex. 2010;20(2):393–403. doi: 10.1093/cercor/bhp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jay TM, Burette F, Laroche S. Plasticity of the hippocampal-prefrontal cortex synapses. J. Physiol. Paris. 1996;90(5–6):361–366. doi: 10.1016/s0928-4257(97)87920-x. [DOI] [PubMed] [Google Scholar]
- 13.Croxson PL, Johansen-Berg H, Behrens TE, et al. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J. Neurosci. 2005;25(39):8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 15.Shamy JL, Carpenter DM, Fong SG, et al. Alterations of white matter tracts following neurotoxic hippocampal lesions in macaque monkeys: a diffusion tensor imaging study. Hippocampus. 2010;20(8):906–910. doi: 10.1002/hipo.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J. Comp. Neurol. 1996;369(1):1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Gabbott P, Headlam A, Busby S. Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Res. 2002;946(2):314–322. doi: 10.1016/s0006-8993(02)02487-3. [DOI] [PubMed] [Google Scholar]
- 18.Laroche S, Jay TM, Thierry AM. Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci. Lett. 1990;114(2):184–190. doi: 10.1016/0304-3940(90)90069-l. [DOI] [PubMed] [Google Scholar]
- 19.Christoffersen GRJ, Petersen S, Dacosta NM. Potentiation of prelimbic field potentials during and seconds after trains of excitations in the rat hippocampo-prefrontal pathway. Neurosci. Lett. 2003;341(2):143–146. doi: 10.1016/s0304-3940(03)00193-9. [DOI] [PubMed] [Google Scholar]
- 20.Izaki Y, Takita M, Nomura M. Mouse hippocampo-prefrontal paired-pulse facilitation and long-term potentiation in vivo. Neuroreport. 2001;12(6):1191–1193. doi: 10.1097/00001756-200105080-00028. [DOI] [PubMed] [Google Scholar]
- 21.Izaki Y, Takita M, Nomura M. Local properties of CA1 region in hippocampo-prefrontal synaptic plasticity in rats. Neuroreport. 2002;13(4):469–472. doi: 10.1097/00001756-200203250-00022. [DOI] [PubMed] [Google Scholar]
- 22.Takita M, Izaki Y, Kuramochi M, Yokoi H, Ohtomi M. Synaptic plasticity dynamics in the hippocampal-prefrontal pathway in vivo . Neuroreport. 2010;21(1):68–72. doi: 10.1097/WNR.0b013e3283344949. [DOI] [PubMed] [Google Scholar]
- 23.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 24.Takita M, Fujiwara SE, Izaki Y. Functional structure of the intermediate and ventral hippocampo-prefrontal pathway in the prefrontal convergent system. J. Physiol. Paris. 2013;107(6):441–447. doi: 10.1016/j.jphysparis.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Burette F, Jay TM, Laroche S. Reversal of LTP in the hippocampal afferent fiber system to the prefrontal cortex in vivo with low-frequency patterns of stimulation that do not produce LTD. J. NeuroPhysiol. 1997;78(2):1155–1160. doi: 10.1152/jn.1997.78.2.1155. [DOI] [PubMed] [Google Scholar]
- 26.Takita M, Izaki Y, Jay TM, Kaneko H, Suzuki SS. Induction of stable long-term depression in vivo in the hippocampal-prefrontal cortex pathway. Eur. J. Neurosci. 1999;11(11):4145–4148. doi: 10.1046/j.1460-9568.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- 27. Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol. Learn Mem. 2008;89(4):560–566. doi: 10.1016/j.nlm.2007.10.005. •• Article showing the role of plasticity in H-PFC pathway in fear regulation: the authors show that fear extinction leads to synaptic potentiation in the H-PFC pathway, which may be important for extinction memory in rodents during a subsequent retention test.
- 28.Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb. Cortex. 2004;14(2):224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- 29.Izaki Y, Takita M, Akema T. Compatibility of bidirectional synaptic plasticity on hippocampo-prefrontal cortex pathway in rats. Neurosci. Lett. 2003;345(1):69–71. doi: 10.1016/s0304-3940(03)00492-0. [DOI] [PubMed] [Google Scholar]
- 30.Kolb B. Prefrontal cortex. In: Kolb B, Tees RC, editors. The Cerebral Cortex Of The Rat. Cambridge, Mass: MIT Press; 1990. pp. 437–458. [Google Scholar]
- 31.Jay TM, Burette F, Laroche S. NMDA receptor-dependent long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in the rat. Eur. J. Neurosci. 1995;7(2):247–250. doi: 10.1111/j.1460-9568.1995.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 32.Seguela P, Watkins KC, Descarries L. Ultrastructural featuRes. of dopamine axon terminals in the anteromedial and the suprarhinal cortex of adult rat. Brain Res. 1988;442(1):11–22. doi: 10.1016/0006-8993(88)91427-8. [DOI] [PubMed] [Google Scholar]
- 33.Van Eden CG, Hoorneman EM, Buijs RM, Matthijssen MA, Geffard M, Uylings HB. Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopical level. Neuroscience. 1987;22(3):849–862. doi: 10.1016/0306-4522(87)92964-2. [DOI] [PubMed] [Google Scholar]
- 34.Verney C, Alvarez C, Geffard M, Berger B. Ultrastructural double-labelling study of dopamine terminals and GABA-containing neurons in rat anteromedial cerebral cortex. Eur. J. Neurosci. 1990;2(11):960–972. doi: 10.1111/j.1460-9568.1990.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 35. Ferron A, Thierry AM, Le Douarin C, Glowinski J. Inhibitory influence of the mesocortical dopaminergic system on spontaneous activity or excitatory response induced from the thalamic mediodorsal nucleus in the rat medial prefrontal cortex. Brain Res. 1984;302(2):257–265. doi: 10.1016/0006-8993(84)90238-5. •• A comprehensive review of the behavioral implications of the H-PFC pathway.
- 36.Godbout R, Mantz J, Pirot S, Glowinski J, Thierry AM. Inhibitory influence of the mesocortical dopaminergic neurons on their target cells: electrophysiological and pharmacological characterization. J. Pharmacol. Exp. Ther. 1991;258(2):728–738. [PubMed] [Google Scholar]
- 37.Bernardi G, Cherubini E, Marciani MG, Mercuri N, Stanzione P. Responses of intracellularly recorded cortical neurons to the iontophoretic application of dopamine. Brain Res. 1982;245(2):267–274. doi: 10.1016/0006-8993(82)90809-5. [DOI] [PubMed] [Google Scholar]
- 38.Jay TM, Glowinski J, Thierry AM. Inhibition of hippocampo-prefrontal cortex excitatory responses by the mesocortical DA system. Neuroreport. 1995;6(14):1845–1848. doi: 10.1097/00001756-199510020-00006. [DOI] [PubMed] [Google Scholar]
- 39.Gurden H, Tassin JP, Jay TM. Integrity of the mesocortical dopaminergic system is necessary for complete expression of in vivo hippocampal-prefrontal cortex long-term potentiation. Neuroscience. 1999;94(4):1019–1027. doi: 10.1016/s0306-4522(99)00395-4. [DOI] [PubMed] [Google Scholar]
- 40.Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo . J. Neurosci. 2000;20(22):RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim EP, Tan CH, Jay TM, Dawe GS. Locus coeruleus stimulation and noradrenergic modulation of hippocampo-prefrontal cortex long-term potentiation. Int. J. Neuropsychopharmacol. 2010;13(9):1219–1231. doi: 10.1017/S1461145709991131. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi S, Matsumoto M, Otani H, et al. Changes in synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway induced by repeated treatments with fluvoxamine. Brain Res. 2002;949(1–2):131–138. doi: 10.1016/s0006-8993(02)02973-6. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi S, Matsumoto M, Togashi H, Ueno K-I, Yoshioka M. The serotonergic modulation of synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway. Neurosci. Lett. 2003;342(3):179–182. doi: 10.1016/s0304-3940(03)00293-3. [DOI] [PubMed] [Google Scholar]
- 44.Lopes Aguiar C, Romcy-Pereira RN, Escorsim Szawka R, Galvis-Alonso OY, Anselmo-Franci JA, Pereira Leite J. Muscarinic acetylcholine neurotransmission enhances the late-phase of long-term potentiation in the hippocampal-prefrontal cortex pathway of rats in vivo: a possible involvement of monoaminergic systems. Neuroscience. 2008;153(4):1309–1319. doi: 10.1016/j.neuroscience.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 45. Lopes-Aguiar C, Bueno-Junior LS, Ruggiero RN, Romcy-Pereira RN, Leite JP. NMDA receptor blockade impairs the muscarinic conversion of sub-threshold transient depression into long-lasting LTD in the hippocampus-prefrontal cortex pathway in vivo: correlation with gamma oscillations. Neuropharmacology. 2013;65:143–155. doi: 10.1016/j.neuropharm.2012.09.013. Article showing the role of muscarinic receptors in synaptic plasticity of the H-PFC pathway: the authors show that muscarinic activation by pilocarpine can potentiate in vivo long-term depression (LTD) in a NMDA-dependent manner.
- 46.Wang L, Yuan LL. Activation of M2 muscarinic receptors leads to sustained suppression of hippocampal transmission in the medial prefrontal cortex. J. Physiol. 2009;587(Pt 21):5139–5147. doi: 10.1113/jphysiol.2009.174821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caruana DA, Warburton EC, Bashir ZI. Induction of activity-dependent LTD requires muscarinic receptor activation in medial prefrontal cortex. J. Neurosci. 2011;31(50):18464–18478. doi: 10.1523/JNEUROSCI.4719-11.2011. •• Article showing M1 positive allosteric modulators can be used to enhance muscarinic LTD in the prefrontal cortex: the authors use a slice preparation to chemically induce LTD using the muscarinic agonist carbachol, which can be potentiated by the M1 PAM BQCA.
- 48. Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J. Neurosci. 1997;17(5):1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. •• Article showing the role of H-PFC pathway in working memory: in this classic study, the authors use a disconnection technique between the hippocampus and prefrontal cortex to demonstrate, for the first time, the role of the H-PFC pathway in working memory.
- 49.Goto Y, Grace AA. Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb. Cortex. 2008;18(6):1407–1414. doi: 10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujisawa S, Buzsaki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72(1):153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3(12):e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J. Neurosci. 2010;30(50):17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Benchenane K, Peyrache A, Khamassi M, et al. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. 2010;66(6):921–936. doi: 10.1016/j.neuron.2010.05.013. • Article elucidating the role of the H-PFC pathway on goal-oriented reward learning: the authors use in vivo electrophysiology in conjunction with behavior to show functional coupling between the hippocampus and prefrontal cortex during behavioral learning.
- 54.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J. Neurosci. 2011;31(29):10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J. Neurosci. 2011;31(47):17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76(4):804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61(4):587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J. Neurosci. 2007;27(11):2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goveas J, Xie C, Wu Z, et al. Neural correlates of the interactive relationship between memory deficits and depressive symptoms in nondemented elderly: resting fMRI study. Behav. Brain Res. 2011;219(2):205–212. doi: 10.1016/j.bbr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc. Natl Acad. Sci. USA. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Admon R, Lubin G, Stern O, et al. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc. Natl Acad. Sci. USA. 2009;106(33):14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y, Shu N, Liu Y, et al. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr. Res. 2008;100(1–3):120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 63.Meyer-Lindenberg AS, Olsen RK, Kohn PD, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch. Gen. Psychiatry. 2005;62(4):379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 64.Wolf RC, Vasic N, Sambataro F, et al. Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(8):1464–1473. doi: 10.1016/j.pnpbp.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 65.Dickerson DD, Restieaux AM, Bilkey DK. Clozapine administration ameliorates disrupted long-range synchrony in a neurodevelopmental animal model of schizophrenia. Schizophr. Res. 2012;135(1–3):112–115. doi: 10.1016/j.schres.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 66. Neill JC, Barnes S, Cook S, et al. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol. Ther. 2010;128(3):419–432. doi: 10.1016/j.pharmthera.2010.07.004. •• A comprehensive review on NMDA receptor antagonism model faithfully representing cognitive and negative symptoms of schizophrenia.
- 67.Jodo E. The role of the hippocampo-prefrontal cortex system in phencyclidine-induced psychosis: a model for schizophrenia. J. Physiol. Paris. 2013;107(6):434–440. doi: 10.1016/j.jphysparis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Chan MH, Chiu PH, Sou JH, Chen HH. Attenuation of ketamine-evoked behavioral responses by mGluR5 positive modulators in mice. Psychopharmacology (Berlin) 2008;198(1):141–148. doi: 10.1007/s00213-008-1103-1. [DOI] [PubMed] [Google Scholar]
- 69.Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav. Brain Res. 2007;184(1):31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 70.Hashimoto K, Fujita Y, Shimizu E, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur. J. Pharmacol. 2005;519(1–2):114–117. doi: 10.1016/j.ejphar.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 71.King MV, Sleight AJ, Woolley ML, Topham IA, Marsden CA, Fone KC. 5-HT6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidation - an effect sensitive to NMDA receptor antagonism. Neuropharmacology. 2004;47(2):195–204. doi: 10.1016/j.neuropharm.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Sutcliffe JS, Rhaman F, Marshall KM, Neill JC. Oestradiol attenuates the cognitive deficit induced by acute phencyclidine treatment in mature female hooded-Lister rats. J. Psychopharmacol. 2008;22(8):918–922. doi: 10.1177/0269881107083839. [DOI] [PubMed] [Google Scholar]
- 73.Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J. Psychopharmacol. 2007;21(2):198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- 74.Abdul-Monim Z, Reynolds GP, Neill JC. The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. J. Psychopharmacol. 2003;17(1):57–65. doi: 10.1177/0269881103017001700. [DOI] [PubMed] [Google Scholar]
- 75.Abdul-Monim Z, Reynolds GP, Neill JC. The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav. Brain Res. 2006;169(2):263–273. doi: 10.1016/j.bbr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 76.Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav. Brain Res. 2009;204(2):396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav. Neurosci. 2005;119(22):420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- 78.Van Der Meulen JA, Bilbija L, Joosten RN, De Bruin JP, Feenstra MG. The NMDA-receptor antagonist MK-801 selectively disrupts reversal learning in rats. Neuroreport. 2003;14(17):2225–2228. doi: 10.1097/00001756-200312020-00018. [DOI] [PubMed] [Google Scholar]
- 79.Mclean SL, Beck JP, Woolley ML, Neill JC. A preliminary investigation into the effects of antipsychotics on sub-chronic phencyclidine-induced deficits in attentional set-shifting in female rats. Behav. Brain Res. 2008;189(1):152–158. doi: 10.1016/j.bbr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 80.Rodefer JS, Murphy ER, Baxter MG. PDE10A inhibition reverses subchronic PCP-induced deficits in attentional set-shifting in rats. Eur. J. Neurosci. 2005;21(4):1070–1076. doi: 10.1111/j.1460-9568.2005.03937.x. [DOI] [PubMed] [Google Scholar]
- 81.Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berlin) 2007;193(4):521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- 82.Baviera M, Invernizzi RW, Carli M. Haloperidol and clozapine have dissociable effects in a model of attentional performance deficits induced by blockade of NMDA receptors in the mPFC. Psychopharmacology (Berlin) 2008;196(2):269–280. doi: 10.1007/s00213-007-0959-9. [DOI] [PubMed] [Google Scholar]
- 83.Paine TA, Carlezon WA., Jr. Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits in rats. Neuropharmacology. 2009;56(4):788–797. doi: 10.1016/j.neuropharm.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becker A, Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Test of predictive validity. Prog. Neuropsychopharmacol. Biol Psychiatry. 2004;28(8):1267–1277. doi: 10.1016/j.pnpbp.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 85.Snigdha S, Neill JC. Improvement of phencyclidine-induced social behaviour deficits in rats: involvement of 5-HT1A receptors. Behav. Brain Res. 2008;191(1):26–31. doi: 10.1016/j.bbr.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 86.Amitai N, Semenova S, Markou A. Clozapine attenuates disruptions in response inhibition and task efficiency induced by repeated phencyclidine administration in the intracranial self-stimulation procedure. Eur. J. Pharmacol. 2009;602(1):78–84. doi: 10.1016/j.ejphar.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baird JP, Turgeon S, Wallman A, Hulick V. Behavioral processes mediating phencyclidine-induced decreases in voluntary sucrose consumption. Pharmacol. Biochem. Behav. 2008;88(3):272–279. doi: 10.1016/j.pbb.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 2007;27(43):11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc. Natl Acad. Sci. USA. 2004;101(22):8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Katayama T, Jodo E, Suzuki Y, Hoshino KY, Takeuchi S, Kayama Y. Activation of medial prefrontal cortex neurons by phencyclidine is mediated via AMPA/kainate glutamate receptors in anesthetized rats. Neuroscience. 2007;150(2):442–448. doi: 10.1016/j.neuroscience.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 91. Suzuki Y, Jodo E, Takeuchi S, Niwa S, Kayama Y. Acute administration of phencyclidine induces tonic activation of medial prefrontal cortex neurons in freely moving rats. Neuroscience. 2002;114(3):769–779. doi: 10.1016/s0306-4522(02)00298-1. •• Article showing the effect of phencyclidine (PCP) on PFC cortical neurons: the authors show that systemic administration of PCP leads to an increase in PFC neuronal excitability, but the effect was absent following local application of PCP in the PFC.
- 92.Ninan I, Jardemark KE, Wang RY. Olanzapine and clozapine but not haloperidol reverse subchronic phencyclidine-induced functional hyperactivity of N-methyl-d-aspartate receptors in pyramidal cells of the rat medial prefrontal cortex. Neuropharmacology. 2003;44(4):462–472. doi: 10.1016/s0028-3908(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 93.Greene R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus. 2001;11(5):569–577. doi: 10.1002/hipo.1072. [DOI] [PubMed] [Google Scholar]
- 94.Jodo E, Suzuki Y, Katayama T, et al. Activation of medial prefrontal cortex by phencyclidine is mediated via a hippocampo-prefrontal pathway. Cereb. Cortex. 2005;15(5):663–669. doi: 10.1093/cercor/bhh168. [DOI] [PubMed] [Google Scholar]
- 95.Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res. Brain Res. Rev. 2000;31(2–3):302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 96.Blot K, Bai J, Otani S. The effect of non-competitive NMDA receptor antagonist MK-801 on neuronal activity in rodent prefrontal cortex: an animal model for cognitive symptoms of schizophrenia. J. Physiol. Paris. 2013;107(6):448–451. doi: 10.1016/j.jphysparis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 97. Blot K, Kimura SI, Bai J, et al. Modulation of hippocampus-prefrontal cortex synaptic transmission and disruption of executive cognitive functions by MK-801. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht329. (Epub ahead of print). •• Article showing that following NMDA receptor antagonism there is enhanced long-term potentiation (LTP) in the H-PFC pathway: the authors use in vivo electrophysiology to demonstrate aberrant plasticity in this pathway following MK-801 treatment.
- 98. Thomases DR, Cass DK, Meyer JD, Caballero A, Tseng KY. Early adolescent MK-801 exposure impairs the maturation of ventral hippocampal control of basolateral amygdala drive in the adult prefrontal cortex. J. Neurosci. 2014;34(27):9059–9066. doi: 10.1523/JNEUROSCI.1395-14.2014. •• Article showing dysfunction in plasticity following repeated NMDA receptor antagonists: the authors show that following repeated MK-801 treatment there is a shift in plasticity from LTD to LTP in the H-PFC pathway, while not affecting the amygdala to PFC pathway.
- 99.Attucci S, Carla V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br. J. Pharmacol. 2001;132(4):799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ehlers MD. Synapse structure: glutamate receptors connected by the shanks. Curr. Biol. 1999;9(22):R848–R850. doi: 10.1016/s0960-9822(00)80043-3. [DOI] [PubMed] [Google Scholar]
- 101.Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci. 2001;21(16):5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl d-aspartate receptor. Curr. Drug Targets CNS Neurol. Disord. 2002;1(1):1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- 103.Bubser M, Bridges TM, Dencker D, et al. Selective activation of M muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem. Neurosci. 2014;5(10):920–942. doi: 10.1021/cn500128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dhanya RP, Sheffer DJ, Dahl R, et al. Design and synthesis of systemically active metabotropic glutamate subtype-2 and −3 (mGlu2/3) receptor positive allosteric modulators (PAMs): pharmacological characterization and assessment in a rat model of cocaine dependence. J. Med. Chem. 2014;57(10):4154–4172. doi: 10.1021/jm5000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodriguez AL, Grier MD, Jones CK, et al. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol. Pharmacol. 2010;78(6):1105–1123. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shirey JK, Brady AE, Jones PJ, et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores. impairments in reversal learning. J. Neurosci. 2009;29(45):14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shirey JK, Xiang Z, Orton D, et al. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat. Chem. Biol. 2008;4(1):42–50. doi: 10.1038/nchembio.2007.55. [DOI] [PubMed] [Google Scholar]
- 108.Wenthur CJ, Morrison RD, Daniels JS, Conn PJ, Lindsley CW. Synthesis and SAR of substituted pyrazolo[1,5-a]quinazolines as dual mGlu(2)/mGlu(3) NAMs. Bioorgan. Med. Chem. Lett. 2014;24(12):2693–2698. doi: 10.1016/j.bmcl.2014.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adell A, Jimenez-Sanchez L, Lopez-Gil X, Romon T. Is the acute NMDA receptor hypofunction a valid model of schizophrenia? Schizophr. Bull. 2012;38(1):9–14. doi: 10.1093/schbul/sbr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 111. Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8(1):41–54. doi: 10.1038/nrd2760. •• A comprehensive review on the role of allosteric modulators of G-protein-coupled receptors as a therapeutic approach for several CNS disorders including schizophrenia.
- 112.Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol. Sci. 2009;30(3):148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]