Abstract
Background
Evolution and improvements in microsurgical techniques and tools have paved the way for super-microsurgical anastomoses with vessel diameters often approaching below 0.8 mm in the clinical realm and even smaller (0.2–0.3 mm) in murine models. Several imaging and monitoring devices have been introduced for post-operative monitoring but intra-operative guidance, assessment and predictability have remained limited to binocular optical microscope and surgeon’s experience. We present a high-resolution real time 3D imaging modality for intra-operative evaluation of luminal narrowing, thrombus formation and flow alterations.
Methods
An imaging modality that provides immediate, in-depth high resolution 3D structure view and flow information of the anastomosed site called phase resolved Doppler optical coherence tomography (PRDOCT) was developed. 22 mouse femoral artery anastomoses and 17 mouse venous anastomoses were performed and evaluated with PRDOCT. Flow status, vessel inner lumen 3D structure, and early thrombus detection were analyzed based on PRDOCT imaging results. Initial PRDOCT based predictions were correlated with actual long term surgical outcomes. Eventually four cases of mouse orthotopic limb transplantation were carried out and PRDOCT predicted long term patency were confirmed by actual results.
Results
PRDOCT was able to provide high-resolution 3D visualization of the vessel flow status and vessel inner lumen. The assessments based on PRDOCT visualization shows a 92% sensitivity and 90% specificity for arterial anastomoses and 90% sensitivity and 86% specificity for venous anastomoses.
Conclusions
PRDOCT is an effective evaluation tool for microvascular anastomosis. It can predict the long term vessel patency with high sensitivity and specificity.
Introduction
Vascular anastomosis is a procedure commonly performed by a broad range of surgeons. For reconstructive, vascular and transplant surgeries, it is an integral part. Since the introduction of the triangulation anastomosis method by Carrel, there have been developments of many advanced techniques such as the vascular coupling devices [1], thermo-reversible poloxamers [2] and non-suture-cuff techniques [3]. Microvascular anastomosis for vessels with an outer diameter smaller than 1.0 mm is extremely challenging and thus effective evaluation of the vessel patency is of great importance. Problems such as hematoma, luminal narrowing, and thrombus formation hinder the long term success of the procedure. Re-intervention is sometimes necessary for free flap salvage. However, the effectiveness of re-intervention is inversely related to the time elapsed between suspicion of vascular compromise and re-exploration necessitating early intervention.
There has been limited advancement in the field of medical imaging that can predict technical failures and luminal thrombosis while anastomosing extremely small vessels. There is an immense demand for devices that can help predict the surgical outcome objectively in conjunction with surgeons' subjective evaluation based on accumulated experience over the years. Such devices can also aid in training and evaluation of trainees. Ideally postoperative imaging should be objective, direct, noninvasive, reliable, easy and cost-effective [4].
While visual assessment and vital monitoring is most widely used method for post-anastomosis surgical monitoring, it is time-consuming and often unreliable due to various factors, such as ambient temperature, vasospasm, and positioning [5]. Different methods were proposed to provide objective evaluation of the blood perfusion status, such as indirect measurement through surface temperature, transcutaneous oxygen and laser Doppler flowmeters [4] and direct method using implantable venous Doppler monitoring [5] and transit-time flow monitoring [6]. Transit-time flow monitoring is an effective intraoperative measurement that provides objective evaluation of the anastomosis patency and flow status. Pulsatility index and flow volume rate can be measured real-time. It can supplement clinical evaluation or handheld Doppler analysis to diagnose a microvascular complication intraoperatively and thus alter the surgical plan. However, it is not an imaging method and quality of the anastomosis cannot be revealed directly at the junction site or immediately distal to anastomosis. Study of the effect of transit-time flow monitoring for vessels less than 1 mm or even smaller is also unknown. Therefore a non-invasive imaging modality that allows surgeons to visualize the surgical site immediately after the surgery would be invaluable. This would allow a salvage procedure to be performed immediately if a failure is doomed based on intraoperative assessment.
Traditional diagnostic imaging modalities such as computed tomography (CT) angiograms, magnetic resonance (MR) angiograms, and ultrasound Doppler, due to relatively low spatial resolution, slow imaging speed and poor temporal resolution prohibit applications in real-time intraoperative microsurgical imaging [7–8]. Optical coherence tomography (OCT) is a well-established, non-invasive optical imaging technology that can provide high-speed, high-resolution, three-dimensional images of biological samples. In recent years, OCT has been found to be highly effective in microsurgical intraoperative imaging and guidance [9]. Phase resolved Doppler OCT (PRDOCT) can provide simultaneous tissue structure and blood flow imaging [10]. Our lab recently introduced and demonstrated the capability of real-time PRDOCT system to microvascular anastomosis for suturing guidance and postoperative evaluation [8]. Based on our pilot studies, we tested this modality in technically challenging mouse femoral artery anastomosis with diameters ranging less than 0.3 mm.
In this study, we expanded our earlier work and evaluated PRDOCT for its capability as a prognostic tool in microvascular anastomosis success or failure. 22 murine femoral artery anastomoses and 17 murine venous anastomoses were performed and evaluated with PRDOCT. Flow status, vessel inner lumen 3D structure, and early thrombus morphology were analyzed to generate a prognosis, and this was correlated with the actual long term surgical outcome. Subsequently, we utilized these findings to analyze the lumen patency and flow alterations in a mouse orthotopic limb transplantation model (n=4).
Materials and Methods
The animal study was conducted in accordance with the Johns Hopkins University Animal Care and Use Committee guidelines with consideration of minimum animal suffering (Approval Number: MO12M348).
OCT System Configuration
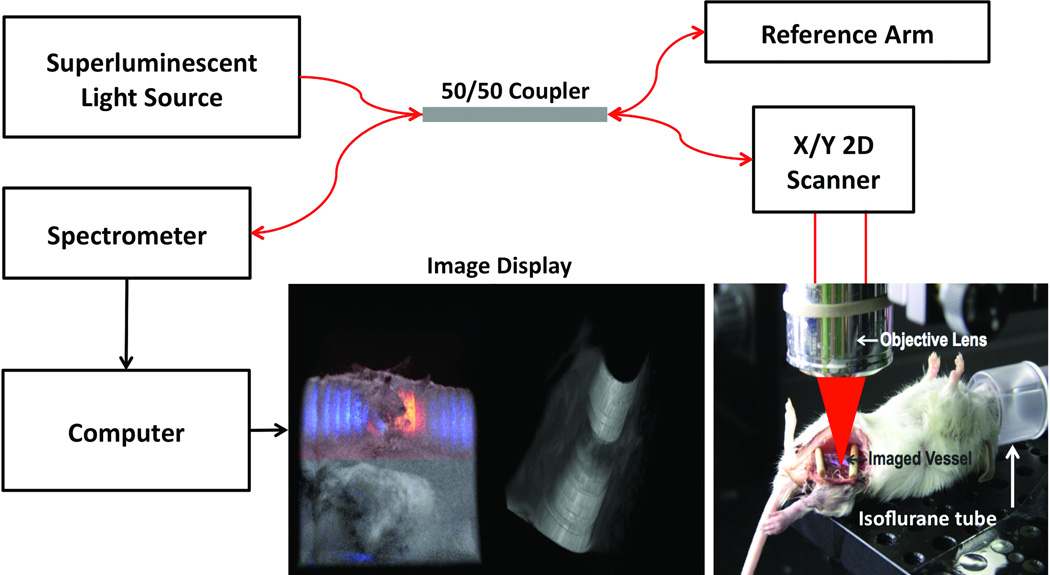
OCT system configuration and experimental set-up is shown in Figure 1. The system used a line-scan camera with 12-bit depth, 70 kHz line rate, and 2048 pixels as the spectrometer detector [11]. A superluminescent light source with an output power of 10 mW and an effective bandwidth of 105 nm centered at 845 nm was used which gave an axial resolution of 3.0 µm in air. The transversal resolution was approximately 12 µm, assuming a Gaussian beam profile. The system sensitivity is 92.5 dB with an imaging range of 1.6 mm in air. The maximum illumination of 3.7 mW on the exposed vessel was used which is in conformity with the ANSI. The system has a detectable flow range of [0.294, 14.2] mm/s for both positive and negative directions parallel to the scanning beam. Each B-mode image consisted of 1000 A-scans and each C-scan consists of 250 B-scans. Imaging frame rate was set at 70 frames per second.
Figure 1.
Illustration of system configuration for vessel imaging.
Vascular Anastomosis Procedures
A Carl Zeiss surgical microscope with maximum optical magnification of 20× was setup next to the OCT system. At the current stage, the animal needs to be slided to the adjacent OCT imaging station after the surgery. Eight to ten week old C57B6 mice were anesthetized using an isoflurane-based anesthesia system before performing femoral artery and vein end-to-end anastomoses. Room temperature was kept between 70°F and 75°F in the operation room. In our experimental model femoral artery and vein were exposed, dissected and transected. Subsequently approximating clamps (S&T, Neuhausen, Switzerland) were applied to facilitate the anastomosis. End-to-end anastomosis was performed using conventional interrupted 11-0 Nylon 4–6 sutures (S&T, Neuhausen, Switzerland). A plastic rubber sheet was placed underneath the vessel to isolate the vessel from surrounding soft tissue for clear imaging. Sutures were placed in the vessel across full thickness of the vessel wall (adventitia, media and intima) on one end and then from intima to adventitia in the other vessel end. Sutures were laid down flat and tightened. Sutures were applied circumferentially for the anastomosis to form a complete seal. Finally the clamp was removed to allow reestablishing blood flow.
Results and discussion
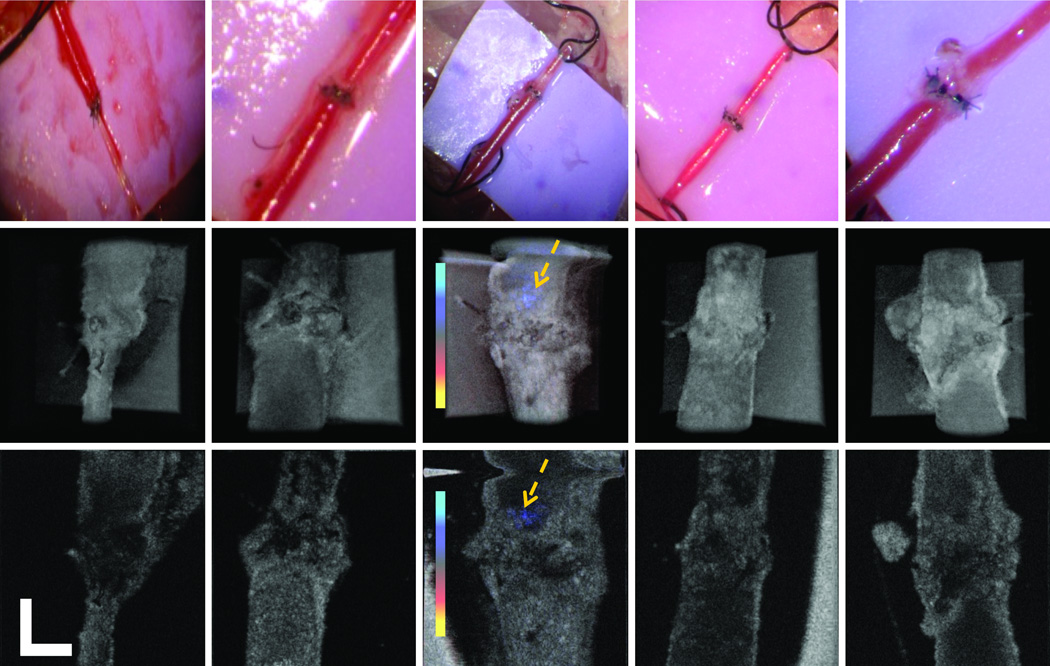
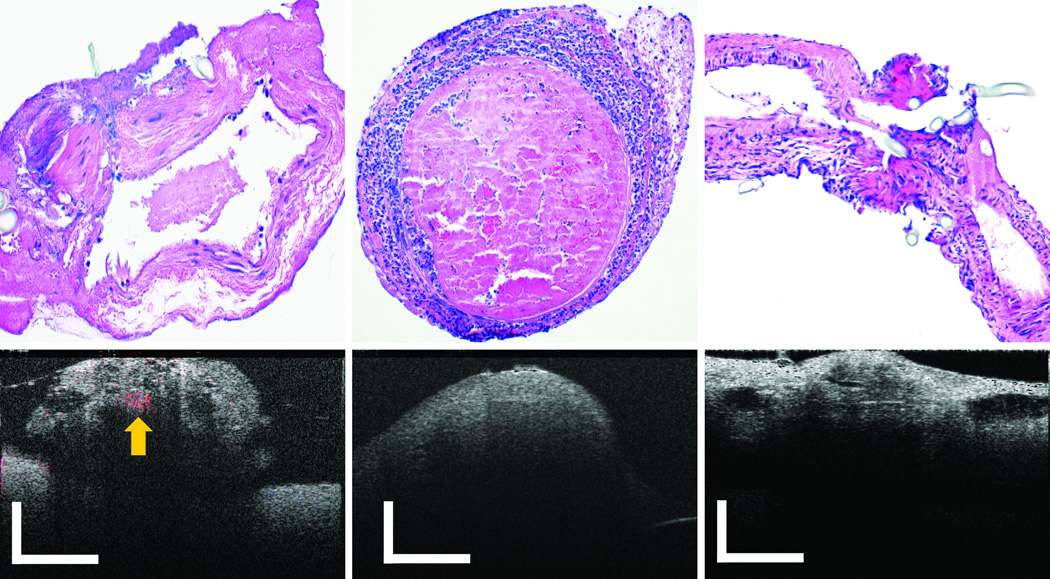
First, we intentionally introduced a series of erroneous technical steps during the surgery. PRDOCT images and camera captured surgical images were acquired to test whether PRDOCT can detect all these problems. Fig. 2(a) shows suture placed across both anterior and posterior walls of the vessel, and Fig. 2(b) shows an adventitial flap within the lumen. From Fig. 2(a), it is very easy to identify problems from both the camera and PRDOCT images. An adventitial flap can potentially induce thrombus formation by activating the coagulation cascade and is usually very difficult to detect with optical microscope. However, the PRDOCT images demonstrated the diminished flow eventually leading to complete occlusion, which could not be appreciated on still images from an early microscopic video output. Fig. 2(c)–(e) demonstrates the consequence of severe lumen narrowing at the anastomotic site. Optimal flow restoration is not easily discernable in such a scenario with the surgical microscope image. From the camera image, we can see that all distal part of the vessel, bottom part of the camera image, was filled with blood although there is no blood flow. However, PRDOCT clearly demonstrate surgical failure. Fig. 2(c) shows one example of severe lumen narrowing with barely noticeable blood flow. The blue flow region was pointed out by yellow arrows on the images. Fig. 2(d)–(e) show two more cases of complete vessel lumen occlusion. Gold standard H&E histology analysis of the problematic vessel was also performed to confirm the PRDOCT imaging results. Figure 3 is showing three representative histology results compared to OCT images(a) partial thrombosis at the junction site; (b) heavy full thrombosis and (c) suture placed across both anterior and posterior walls (note the slides was cut along the long axis of blood vessel, lumen was closed). All these results confirmed PRDOCT evaluation.
Figure 2.
Surgical failures detected by PRDOCT, camera image (top); top-view volumetric rendering (middle) and selective en-face slice (bottom): (a) suture crosses both posterior and anterior walls; (b) adventitial folding; (c) heavy thrombosis with little blood blow, yellow arrow points out the flowing signal; (d) and (e) complete occlusion with no blood blow case 1 and 2. (scale bar: 250 µm; Doppler color bar red to blue: −14.2 to 14.2 mm/s).
Figure 3.
Representative histology analysis results: (a) partial thrombosis at the junction site (b) heavy full thrombosis (c) suture placed across both anterior and posterior walls (scale bar: 250 µm, Doppler flow signal was labeled by yellow arrow).
While PRDOCT can detect severe surgical failures intraoperatively, the capability of PRDOCT to predict long-term successful surgical outcome is equally important. The difficulty of clinical assessment goes up significantly when there is partial thrombus developing along the surgical site as there is still blood flowing inside the vessel providing positive clinical assessment feedback. Fig.4 illustrates three typical or commonly encountered posteroperative blood flowing imaging results. Fig. 4(a), result of Mouse 32 Artery, shows a healthy artery blood flowing status with no thrombus. Fig. 4(b) demonstrates a healthy blood flow status with resolvable thrombus at the junction site while Fig. 4(c), result of Mouse 10 artery, represents flow alteration with non-resolvable thrombus, which fully occluded on day 1 after surgery. White dashed line outlines the vessel intima and yellow dashed circles outline the thrombus area. Stenosis and turbulence flow at the junction site can be visualized. However, the camera images of all three vessels look indistinguishable from each other.
Figure 4.
Immediate postoperative blood flow (a) no thrombosis, (b) partial resolvable thrombosis, and (c) heavy non-resolvable thrombosis. (Top: en face slice. Bottom: camera images. Inner lumen marked with white dashed line. Yellow dashed circles marked the thrombosis area.)
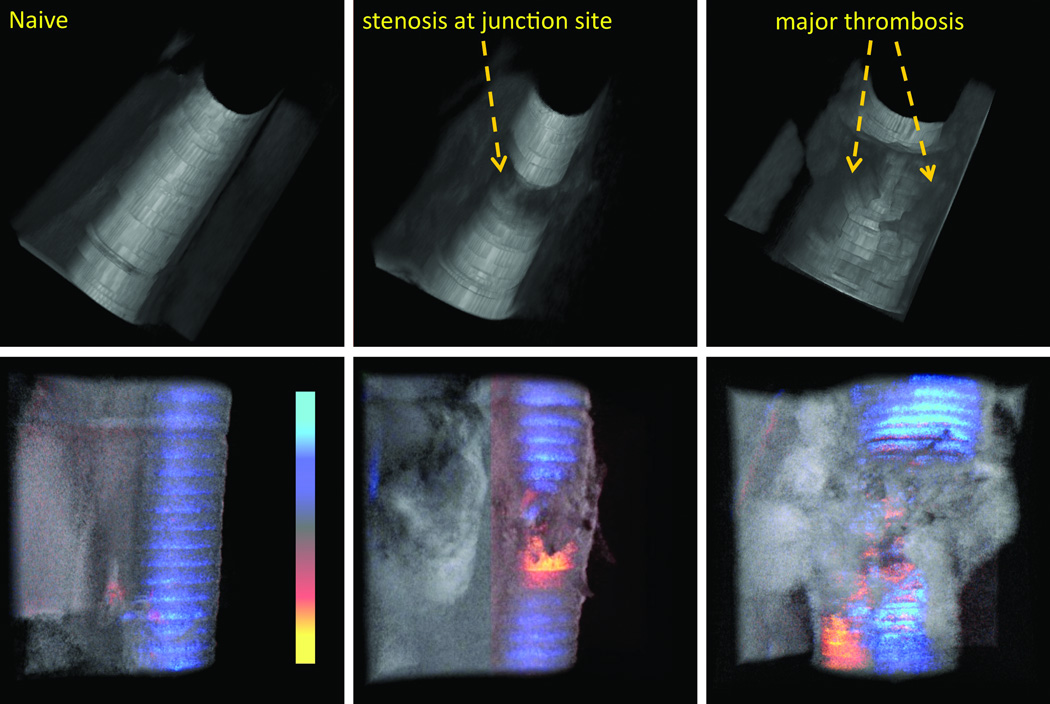
By manually segmenting the image of inner blood flow area, the flow tunnel can be reconstructed as shown in the top row of Figure 5. Further image processing and segmentation technique will be developed to realize real-time flow tunnel reconstruction. First column is the results of a naïve artery imaging. Second and third columns correspond to results shown in Fig.4 (a) and (c) respectively. The naïve blood vessel shows a smooth lumen tunnel (Top of Fig. 5(a)), while the stenosis at the junction site of the vessel after surgery is obvious in top of Fig.5 (b). We can see that the there is hardly any thrombus formation away from the junction. Top of Fig. 5(c) shows a large thrombus volume inside the vessel lumen. The vessel was occluded in its entirety. Corresponding volumetric structure and flow were shown below the tunnel rendering.
Figure 5.
3D volume rendering of the vessel inner lumen tunnel (top) and top view blood vessel structure and Doppler flow overlaid (bottom): (a) naïve (b) vessel with no thrombosis detected and (c) vessel with heavy non-resolvable thrombosis.
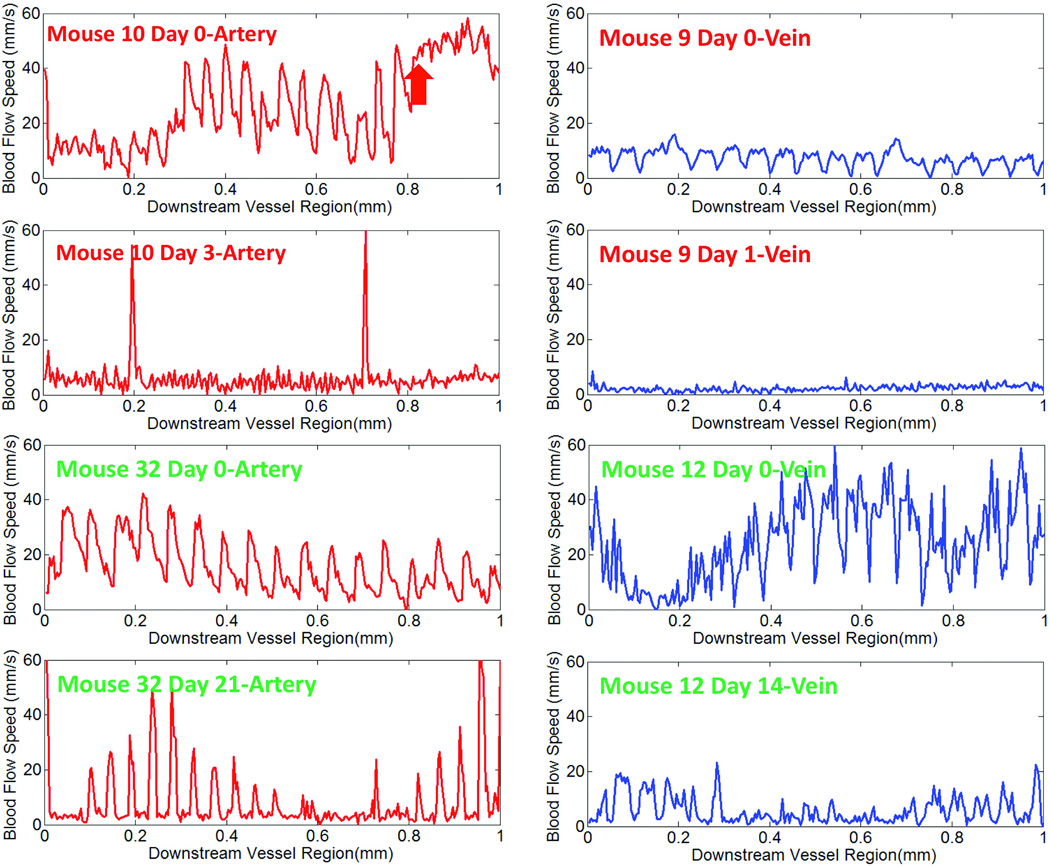
The capability of PRDOCT to provide quantitative blood flowing speed is also important. We measured the flow speed in the downstream vessel. Figure 6(a) shows artery average blood flow speed measurement of Mouse 10 and Mouse 32 at different days. Day 0 represents 5 to 10 minutes after removing vessel clamp. Figure 6(b) shows the results of vein blood flow speed measurements. The blood flow speed was calculated with a single Doppler angle deduced from the structure images and covers a range of 1 mm of the vessel. Local Doppler angle non-uniformity can potentially cause a variation of the flow speed value. The variance of Doppler angle can be addressed in the future by designing specific vessel grove with adjustable uniform Doppler angle over the scanning region. Mouse 10 artery showed strong pulsating flow speed peaks at 60 mm/s. Red arrow marks the start region of thrombus with a flat speed profile. Based on all the junction and downstream imaging and flow speed measurement, we predicted that Mouse 10 artery would be occluded later, which was confirmed by the Day 3 imaging. The flow of Mouse 10 at Day 3 showed a flat baseline speed near zero point. Two huge spikes in the flow speed were caused by the sudden motion artifact. Mouse 32 artery experiment showed long term patency. The flow speed was normal at both Day 0 and confirmed at Day 14. Similar results can be found for venous flow speed. Mouse 9 venous flow speed after the surgery was low although no major thrombus was detected. This eventually led to occlusion at day 1. Mouse 12 vein at Day 0 had optimum flow and no major thrombus was detected during day 0 imaging. Day 14 result confirmed its long term patency.
Figure 6.
Blood flow speed at downstream site of the vessel at different days for (a) artery and (b) vein (Green text has long term success while red text indicates long term failure.)
Even though Mouse 10 artery and Mouse 9 vein long term imaging demonstrated occlusion, there was some blood flow immediately after surgery. This could potentially trigger early intervention, however, further quantifiable criteria to address the time to intervene are yet to be standardized with further experiments through translational large animal models. In our semi-quantitative assessment, anastomosis was evaluated as patent if the following three criteria were met. Firstly, the vessel lumen is open and there is detectable blood flow. Secondly, the maximum thrombus cross-sectional area was less than 40% of the vessel inner lumen area, which we considered as resolvable thrombosis, Thirdly, the blood flowing speed (velocity) downstream should be above certain threshold value, which in our case was set as a relative measurement from average speed of blood flowing through a naïve vessel. For the artery experiment, 12 anastomoses remained patent beyond 7 days. PRDOCT predicted 11 out of 12 long-term successful cases. Ten arterial anastomoses were occluded at long term analysis. PRDOCT predicted 9 out of 10 surgical failures. Thus PRDOCT provided 92% sensitivity and 90% specificity in artery patency. For the vein experiment, 9 mice showed long term patency while 8 were occluded. PRDOCT provided 100% sensitivity and 86% specificity for venous patency.
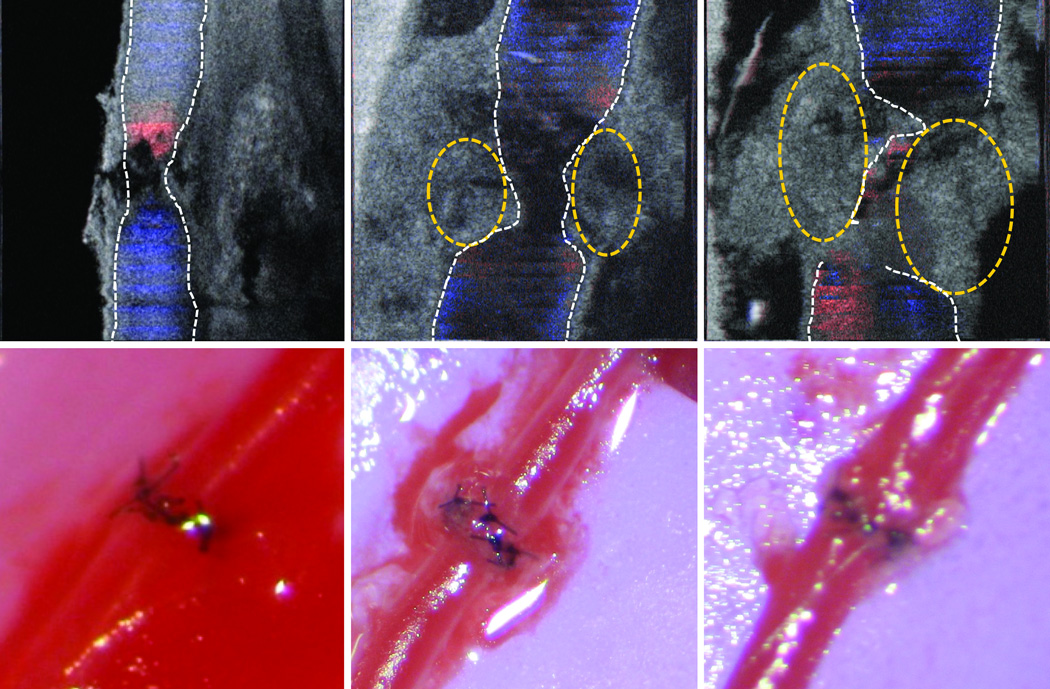
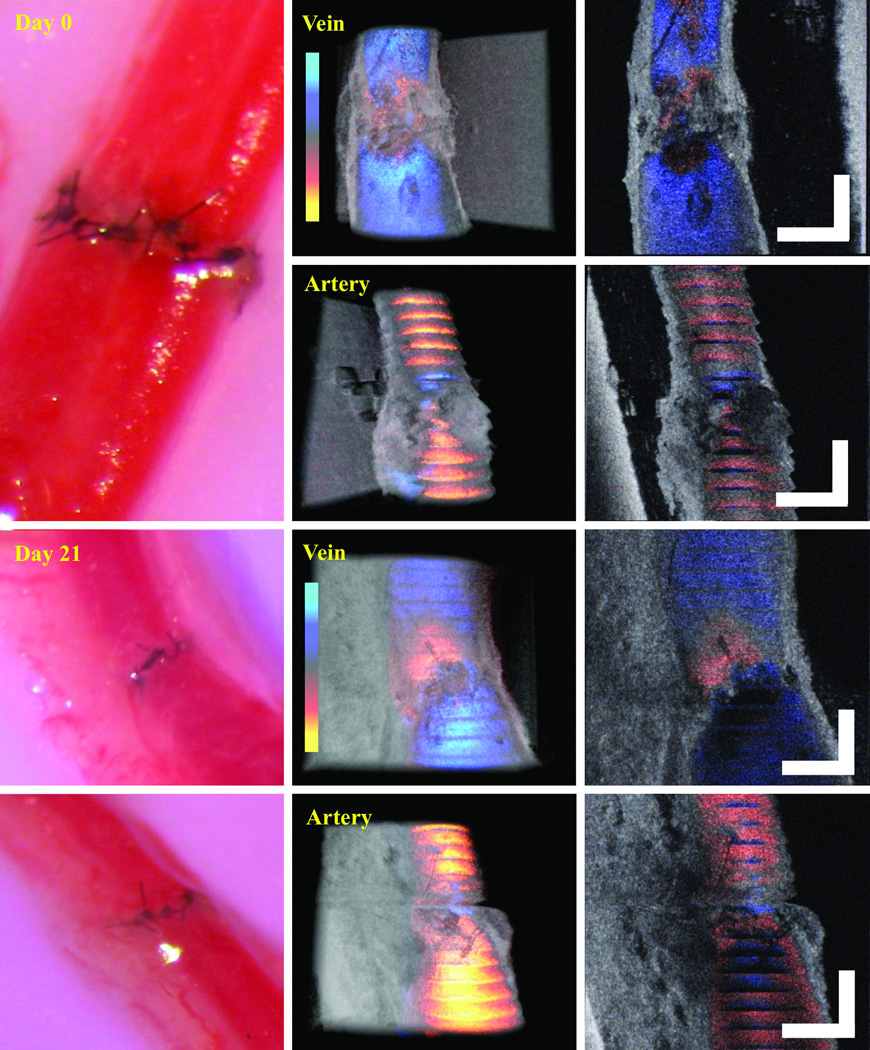
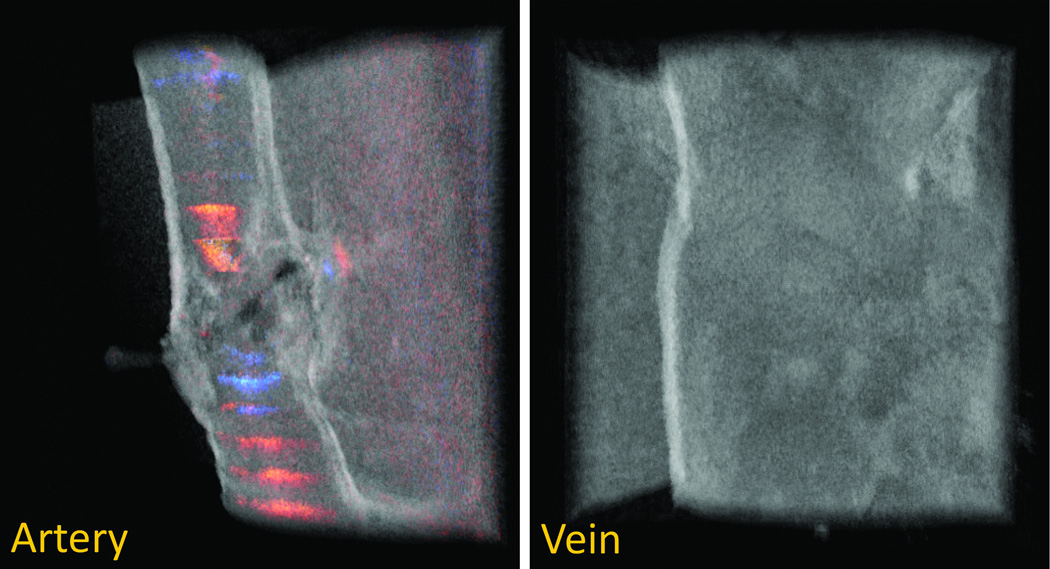
After successful evaluation of both arterial and venous anastomoses using PRDOCT, we also tested re-vascularization of mouse hind limb transplant model described by Sucher at al [3]. This translational model holds its importance for testing therapeutic and diagnostic approaches in vascularized composite allotransplantation. Briefly, hind limb is harvested from an allogeneic mouse and transplanted in an orthotopic manner. Following femur osteosynthesis, muscle inset, femoral artery and veins are anastomosed using a suture based method, note this is different from sutureless cuff based method in [3], which we think would be another interesting study using PRDOCT to systematically study the cuff based anastomosis evaluation. The extremely small vessel diameter of 0.2–0.3 mm also correlates with the clinically applicable lymphaticovenular anastomoses (LVA) that are gaining popularity in reconstructive microsurgery. We, therefore, tested the patency and flow immediately following re-vascularization of a hind limb transplant and 21 days post-operatively (n=4). Two cases passed the PRDOCT evaluation and exhibited predicted long term success. The representative images from day 0 and day 21 are depicted in Figure 7 with no evidence of luminal narrowing and minimal flow aberrancy. However two failed the PRDOCT evaluation: one is the case of thrombosis in the vein and the other is immediate thrombosis in both the artery and vein. Representative images is shown in Figure 8. This investigative tool can also potentially detect flow alterations, luminal narrowing and technical failures in LVA.
Figure 7.
Representative image set of mouse hind limb transplantation immediate after surgery (top) and 21 days after surgery (bottom) (scale bar: 250 µm). Image set consists of camera image of the surgical site (left) and top-view structure and flow overlaid volume rendering of the junction (middle) and en-face slices (right).
Figure 8.
Mouse hind limb transplantation failure representative case: artery flow restored with no vein flow restoration.
Conclusion and future work
Phase Resolved Doppler optical coherence tomography (PRDOCT) can provide valuable structural and flow information during and after microvascular anastomoses especially in the case of technically challenging extremely small vessels. It is shown that surgical failure can be detected by PRDOCT earlier than visual feedback from optical microscope. In addition surgical success and long term patency of vascular anastomoses can also be predicted based on initial structural framework and flow characteristics by PRDOCT imaging. The findings from these preliminary experiments will form the bases of future animal studies aimed at standardization of OCT imaging evaluation and its potential translation to clinical microvascular surgery as a diagnostic and intraoperative guidance tool.
One apparent limitation of OCT technology is depth of light penetration. By switching the imaging wavelength towards longer band and applying angle-compounding method through handheld probe development, we can extend the imaging depth [11]. In its current state, OCT technique holds a great potential for vessels with diameter less than 2 mm with wall thickness of less than 300 micrometer as seen corresponding to a wide variety of commonly used perforator flaps and lymphaticovenular anastomoses. This technology can also be incorporated into traditional optical microscopes with some modifications and can be applied in clinical setting as an imaging technology to provide real time intra-operative guidance and decision-making in complex and technically challenging cases of microvascular anastomoses.
Acknowledgments
This work was supported in part by NIH grants 1R01EY021540-01A1.
Footnotes
Authors' Contribution:
Imaging system fabrication: Yong Huang and Jin U Kang
Animal experiment: Dedi Tong, Shan Zhu, Lehao Wu, Qi Mao, and Zuhaib Ibrahim
Experiment design: Yong Huang, Dedi Tong, Shan Zhu, Zuhaib Ibrahim, WP Andrew Lee, Gerald Brandacher, and Jin U Kang
Conception, Analysis, Interpretation of data: Yong Huang, Dedi Tong, Shan Zhu, Lehao Wu, Qi Mao, Zuhaib Ibrahim, and Gerald Brandacher
Draft and revision of manuscript: Yong Huang, Zuhaib Ibrahim, and Gerald Brandacher
Financial Disclosure and Products
The authors have no financial interest to declare in relation to the content of this article.
Contributor Information
Yong Huang, Email: yhuang60@jhu.edu.
Dedi Tong, Email: tongdedi@gmail.com.
Shan Zhu, Email: zhushanpumc@gmail.com.
Lehao Wu, Email: lwwu@icloud.com.
Qi Mao, Email: maoqimdphd@gmail.com.
Zuhaib Ibrahim, Email: zuhaib.ibrahim@gmail.com.
WP Andrew Lee, Email: wpal@jhmi.edu.
Gerald Brandacher, Email: gbranda2@jhmi.edu.
Jin U. Kang, Email: jkang@jhu.edu.
References
- 1.Ahn CY, Shaw WW, Berns S, Markowitz BL. Clinical experience with the 3M microvascular coupling anastomotic device in 100 free-tissue transfers. Plast. Reconstr. Surg. 1994;93:1481. doi: 10.1097/00006534-199406000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Chang EI, Galvez MG, Glotzbach JP, Hamou CD, El-Ftesi S, et al. Vascular anastomosis using controlled phase transitions in poloxamer gels. Nat. Med. 2011;17:1147. doi: 10.1038/nm.2424. [DOI] [PubMed] [Google Scholar]
- 3.Sucher R, Lin CH, Zanoun R, Atsina KK, Weinstock M, et al. Mouse hind limb transplantation: a new composite tissue allotransplantation model using nonsuture supermicrosurgery. Transplantation. 2010;90:1374. doi: 10.1097/TP.0b013e3181ff4fc3. [DOI] [PubMed] [Google Scholar]
- 4.Hovius SER, Adrichem Van LNA, Mulder HD, Strik van R, Meulen van der JC. The predictive value of the laser Doppler flowmeter for postoperative microvascular monitoring. Annals of Plastic Surgery. 1993;31:307. doi: 10.1097/00000637-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Swartz WM, Izquierdo R, Miller MJ. Implantable venous Doppler microvascular monitoring: laboratory investigation and clinical results. Plast. Reconstr. Surg. 1994;93:152. doi: 10.1097/00006534-199401000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Selber JC, Garvey PB, Clemens MW, Chang EI, Zhang H, Hanasono MM. A prospective study of transit-time flow volume measurement for intraoperative evaluation and optimization of free flaps. Plast. Reconstr. Surg. 2013;131:270. doi: 10.1097/PRS.0b013e3182789c91. [DOI] [PubMed] [Google Scholar]
- 7.Boppart SA, Bouma BE, Pitris C, Tearny GJ, Southern JF, et al. Intraoperative assessment of microsurgery with three-dimensional optical coherence tomography. Radiology. 1998;208:81. doi: 10.1148/radiology.208.1.9646796. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Ibrahim Z, Tong DD, Zhu S, Mao Q, et al. Microvascular anastomosis guidance and evaluation using real-time three-dimensional Fourier-domain Doppler optical coherence tomography. J. Biomed. Opt. 2013;18:111404. doi: 10.1117/1.JBO.18.11.111404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang JU, Huang Y, Zhang K, Ibrahim Z, Cha J, Lee AWP, Brandacher G, Gehlbach P. Real-time 3-D Fourier domain optical coherence tomography video image guided microsurgeries. J. Biomed. Opt. 2012;17:081403. doi: 10.1117/1.JBO.17.8.081403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZP, Milner TE, Sriniyas S, Wang X, Malekafzali A, et al. Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography. Opt. Lett. 1997;22:1119. doi: 10.1364/ol.22.001119. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Liu X, Kang JU. Real-time 3D and 4D Fourier domain Doppler optical coherence tomography based on dual graphics processing units. Biomed. Opt. Express. 2012;3:2162. doi: 10.1364/BOE.3.002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wicks RT, Huang Y, Zhang K, Zhao MT, Tyler BM, et al. Extravascular optical coherence tomography: evaluation of carotid atherosclerosis and pravastatin therapy. Stroke. 2014;45:1123. doi: 10.1161/STROKEAHA.113.002970. [DOI] [PMC free article] [PubMed] [Google Scholar]