Abstract
OBJECTIVE
Fibroblast growth Factor 21 (FGF21) is a metabolic regulator of glucose and lipid metabolism. The physiological role of FGF21 is not yet fully elucidated however administration of FGF21 lowers blood glucose in diabetic animals. Moreover, increased levels of FGF21 are found in obese and diabetic rodents and humans compared to lean/non-diabetic controls.
METHODS
Adult male rhesus macaque monkeys were chronically maintained on a high-fat diet (HFD) or a standard diet (CTR). Plasma levels of FGF21, triglycerides, and cholesterol were measured and body weight was record. Glucose stimulated insulin secretion (GSIS) and glucose clearance was determined during an i.v. glucose tolerance test. Furthermore, expression of FGF21 and its receptors were determined in liver, pancreas, three white adipose tissues (WATs) and two skeletal muscles.
RESULTS
A cohort of the high-fat fed monkeys responded to the HFD with increasing body weight, plasma lipids, total cholesterol, GSIS, and decreased glucose tolerance. These monkeys were termed HFD-sensitive. Another cohort of monkeys did not become obese and maintained normal insulin sensitivity. These animals were defined as HFD-resistant. Plasma FGF21 levels were significantly increased in all HFD fed monkeys compared to the CTR group. The HFD-sensitive monkeys showed a significant increase in FGF21 mRNA expression in all examined tissues compared to CTR, while FGF21 expression in the HFD-resistant group was only increased in the liver, pancreas and the retroperitoneal WAT. In the WAT, the co-receptor β-klotho was down-regulated in the HFD-sensitive monkeys compared to the HDF-resistant group.
CONCLUSION
The current study demonstrates that HFD changes FGF21 and FGF21 receptor expression in a tissue-specific manner in rhesus monkeys; differential regulation is moreover observed between HFD-sensitive and resistant monkeys. Monkeys which maintain normal levels of the FGF21 co-receptor β-klotho in the WAT upon HFD were protected towards development of dyslipidemia and hyperglycemia.
Keywords: Diet-induced obesity, high-fat diet, rhesus macaque, FGF21-resistance, β-klotho, FGFR1
Introduction
Fibroblast growth factor 21 (FGF21) is a pleiotropic hormone-like protein and a metabolic regulator of glucose and lipid metabolism (1–5). FGF21 is a member of an atypical fibroblast growth factor (FGF) subfamily, which also includes FGF19 and FGF23. The subfamily differs from the normal FGFs by lacking the conventional FGF heparin-binding domain, enabling them to diffuse away from their tissues of origin and function as endocrine regulators (6;7). FGF21 is highly expressed in the liver, pancreas and testis (5;8;9), and to a lesser extent in skeletal muscle (10;11) and adipose tissues (12–15). FGF21 mediates its effect through the three FGF receptor (FGFR) isotypes FGFR 1c, 2c and 3c, complexed with the essential co-receptor β-klotho (16–19). FGFR1c is considered to be the most important partner for β-klotho in FGF21 signaling (20–22). Activation of FGFRs by FGF21 induces a signaling cascade with stimulation of extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt (4;5), and in cultured mouse and human adipocytes FGF21 stimulates glucose uptake (4).
In rodents, activation of the peroxisome proliferator-activated receptor α (PPARα) induces hepatic FGF21 expression in response to prolonged fasting (1;3;23). In mice fasted for 24 hours, plasma and hepatic FGF21 expression were increased (1), and in humans, 7 days of starvation also led to elevated levels of plasma FGF21 (24). Moreover, transgenic mice over expressing FGF21 display increased gluconeogenesis, fatty acid oxidation, and ketogenesis (3;25), indicating an important role in the adaption to fasting/starvation. Paradoxically, high-fat feeding also induced FGF21 in white adipose tissues (WAT) through peroxisome proliferator-activated receptor γ (PPARγ) activation (13–15;26–28). In humans high-fat feeding for five days leads to more pronounced up-regulation of plasma FGF21 (29) than 7 days of fasting.
Systemic administration of FGF21 in diabetic mice models (ob/ob, db/db, diet-induced obese) lowers blood glucose, triglycerides and total cholesterol (4;30–32). These beneficial effects have also been observed in rhesus monkeys (Macaca mulatta) treated with FGF21 (33;34). The effect of FGF21 on WAT seems to play a crucial role in the effect on the metabolic parameters as both WAT-selective β-klotho- and FGFR1 knockout mice lead to loss of metabolic effect of FGF21 (35;36). FGF21 may be a candidate for treating hyperglycemia and dyslipidemia; however several studies have shown elevated levels of FGF21 in obese and diabetic mice models (13;15;37). In obese and diabetic patients, plasma FGF21 is also higher, and furthermore correlates with several factors involved in the metabolic syndrome, e.g. non-alcoholic fatty liver disease and dyslipidemia (15;38–41). The increased endogenous levels of FGF21 indicate that obesity could be a state of FGF21 resistance. This has been shown in obese mice where increased plasma FGF21 led to a decrease in WAT and hepatic FGF21 receptors and signaling (42). However, FGF21 resistance can, despite down-regulation of FGFR1c, 2c and β-klotho, be overcome in rodents (43). Therefore, down-regulation of FGFR1c, 2c and β-klotho does not lead to overt FGF21 resistance and diet-induced obese and ob/ob mice have a better response to FGF21 treatment in terms of glucose- and lipid lowering effects than the wild type mice (43).
Extensive species similarities have been reported between human and non-human primates (NHPs) as they both show a spontaneous development of obesity and type 2 diabetes. This has made the obese/diabetic rhesus monkeys an ideal model to study the effect of anti-obesity and anti-diabetic therapies (44–46). Here, we show that, similar to humans and mice, FGF21 plasma and mRNA levels correlate positively with obesity in diet-induced obese rhesus monkeys. However, we demonstrate for the first time that in a tissue-specific manner, consumption of a high-fat diet (HFD), independent of glucose intolerance, causes rises in FGF21 and that this tissues-specific increase in FGF21 expression are observed between monkeys with and without development of glucose intolerance and dyslipidemia.
Materials and Methods
Animals
Tissues and blood samples used in this study were obtained from the Oregon National Primate Research Center (ONPRC) Obese Resource, from adult male rhesus macaque monkeys (Macaca Mulatta) that were euthanized for other studies. For this study we chose animals that had segregated into diet-sensitive, becoming obese and insulin resistant, and diet-resistant. The experimental group consisted of 15 monkeys (age 6–13; but only with an age difference of 1.5 years between each sub group). One cohort of the animals was maintained on a control diet (CTR, 14.6 % fat calorie diet, Test Diet, Richmond, IN). Another cohort of the animals was chronically maintained on a high-fat diet (HFD, 36 % fat calories, Test Diet, Richmond, IN). In addition, the HFD was supplemented with 500 ml of Kool-Aid+20% fructose three times a week. Lean and fat mass of the monkeys were determined with dexa scannings. Based on their relative change in body weight and insulin sensitivity, the high fat fed monkeys were divided into two groups: a HFD-resistant group (n = 5) that had body weights and glucose stimulated insulin secretion (GSIS) within 2 standard deviations of the CTR animals, and a HFD-sensitive group (n = 5), that had significant increased body weight and GSIS. All animal care and procedures were done according to the Institutional Animal Care and Use Committee at the ONPRC at Oregon Health & Science University. For all studies, food intake was carefully recorded every day and water was provided ad libitum. Lights were on from 7am–7pm.
At the day of euthanasia, the animals were sedated with ketamine HCL (20mg/kg, i.m.) and then deeply anesthetized with sodium pentobarbital (30mg/kg, i.v.). For measurement of plasma FGF21, glucose- and insulin levels as well as triglycerides, total cholesterol, LDL and HDL, blood was collected from the abdominal aorta in EDTA tubes and kept on ice until centrifugation within 0.5 h. Plasma triglyceride, cholesterol and HDL levels were measured by Rhein Consulting Laboratories (Portland, OR). Liver, pancreas, three white adipose tissues (WATs; subcutaneous, retroperitoneal and mesenteric), and two skeletal muscles (soleus and gastrocnemius) were removed, frozen in liquid nitrogen and stored at −80 °C for later determination of mRNA expression levels.
Glucose tolerance test
For glucose tolerance test (GTT), which was conducted pre-necropsy, the monkeys were fasted overnight with free access to drinking water. Animals were sedated with Telazol (3 mg/kg) and a baseline blood sample was collected from the lateral saphenous vein. Subsequent, a bolus of 50 % sterile dextrose (0.6 g/kg) was administered i.v. in the saphenous vein. Timed collections of blood were taken (1, 3, 5 10, 15, 20, 40 and 60 minutes), blood glucose was measured immediately in whole blood with a glucometer (Onetouch Ultra Blood Glucose Monitor, LifeScan, Milpitas, CA, USA) and plasma was assayed for insulin by the ONPRC/Oregon Health and Science University (OHSU) Endocrine Services Laboratory using an Immunolite 2000.
FGF21 ELISA
FGF21 plasma levels were measured using a commercial FGF21-specific human ELISA kit (BioVendor, Prague, Czech Republic). Plasma samples were diluted 1:1 and analyzed according to manufacturer’s instructions. The sensitivity was 7.0 pg/ml and both the intra- and interassay variability was 4.0%. We only had plasma samples from an n of 3 in each group for these measurements.
Quantitative real-time PCR analysis
Total RNA was extracted from liver, pancreas, WAT, and skeletal muscles using Trizol (Invitrogen) and RNeasy mini kit (Qiagen) according to manufacturer’s instructions. cDNA was synthesized using iScript reverse transcription kit (BioRad). Quantitative real-time PCR was performed on an ABI 7900 Sequence Detection System (Applied Biosystems) using a LNA probe based system from Roche. Primers were designed using Primer3 software (47) (Supplementary table 1). All samples were run in triplicates and expression was calculated using the ΔΔCT method. Samples were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ribosomal protein L13a (RPL13A) expression. We were only able to obtain an n of 3–4 of the tissues from each group.
Statistical analysis
Data are presented as mean ± SEM. Statistical comparison among the groups was made using one-way ANOVA followed by Tukey’s multiple comparison test were appropriate. The correlation studies were evaluated by regression analysis. Data were analyzed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA), and the results were considered statistical significant for p < 0.05.
Results
The effect of HFD on body weight, plasma lipids, glucose and insulin levels
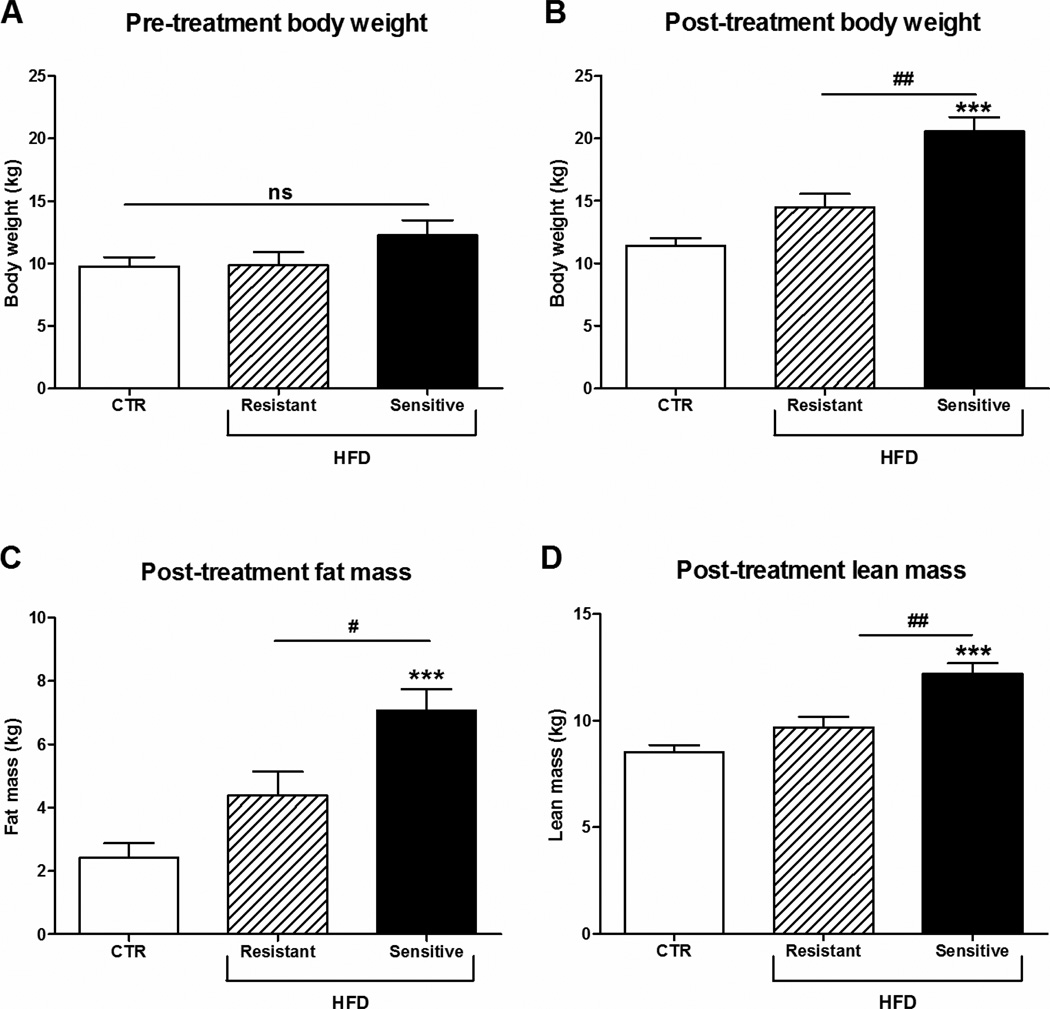
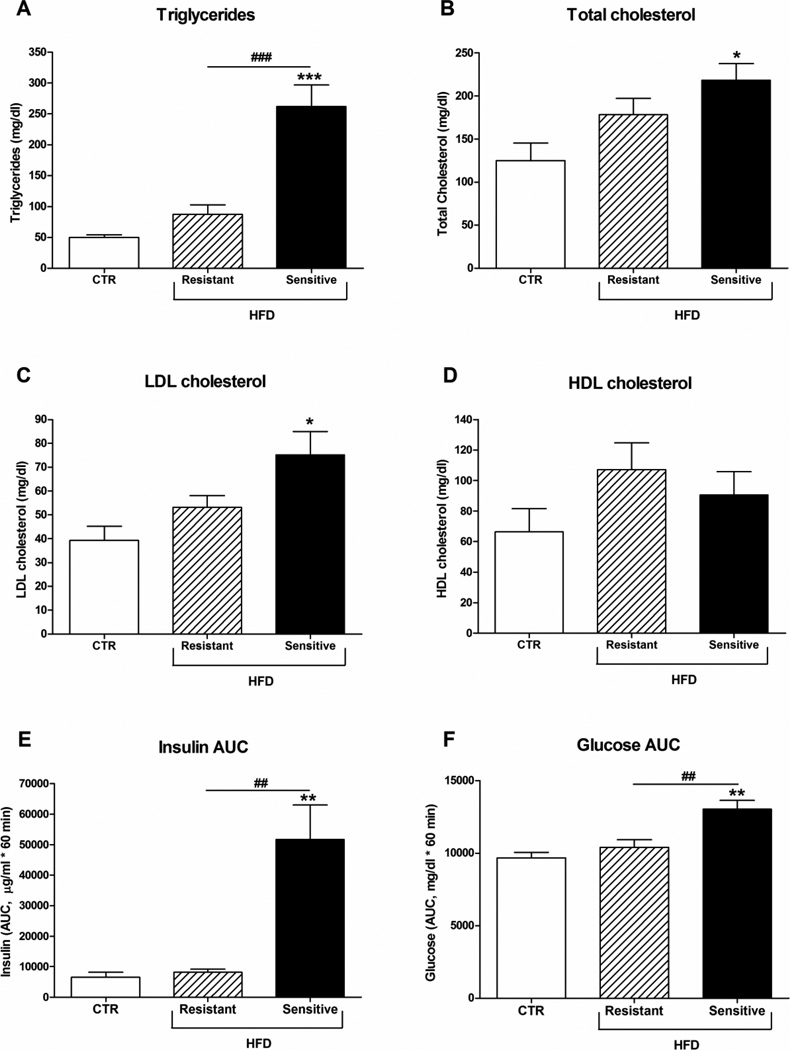
The aim of this study was to investigate whether HFD has an effect on FGF21 levels in plasma and in metabolic relevant tissues in rhesus monkeys. Similar to observations in humans, monkeys chronically maintained on the HFD have a broad distribution of weight gain and insulin sensitivity. For this study we have grouped cohorts of animals based on their relative changes in body weight and insulin sensitivity. HFD-resistant animals were defined as animals chronically maintained on HFD that have body weights and GSIS within 2 standard deviations of the controls. Based on these criteria, while a group HFD-resistant animals tended to have increased body weight and adiposity (Fig. 1), and lipids (Fig. 2A–D), none of these changes were significantly different from control fed animals (CTR). In contrast, the HFD-sensitive monkeys had significant increased body weight, fat and lean mass (Fig. 1), lipids (Fig. 2A–C), GSIS (Fig. 2E) as well as decreased glucose clearance during an i.v. GTT (Fig. 2F).
Figure 1. Effect of HFD on body weight, fat and lean mass in rhesus monkeys.
Pre-treatment (A) body weight, post-treatment (B) body weight, (C) fat and (D) lean body mass of male rhesus monkeys on control diet or a high-fat diet to which they were either resistant or sensitive, n = 5/group. Data are means ± SEM; ***p < 0.0001 versus control fed rhesus monkeys; #P < 0.05, ##P < 0.01; ns: non-significant.
Figure 2. Lipid levels and GTT in rhesus monkeys on a high-fat diet.
Male rhesus monkeys on control diet or a high-fat diet to which they were either resistant or sensitive, n = 5/group. (A) Triglyceride, (B) cholesterol, (C) LDL and (D) HDL levels. (E) Insulin and (F) glucose area under the curve (AUC) after an i.v. GTT. Data are means ± SEM; *p < 0.05, **p < 0.01, ***p < 0.0001 versus control fed rhesus monkeys; ##P < 0.01, ###P < 0.0001.
The effect of HFD on FGF21
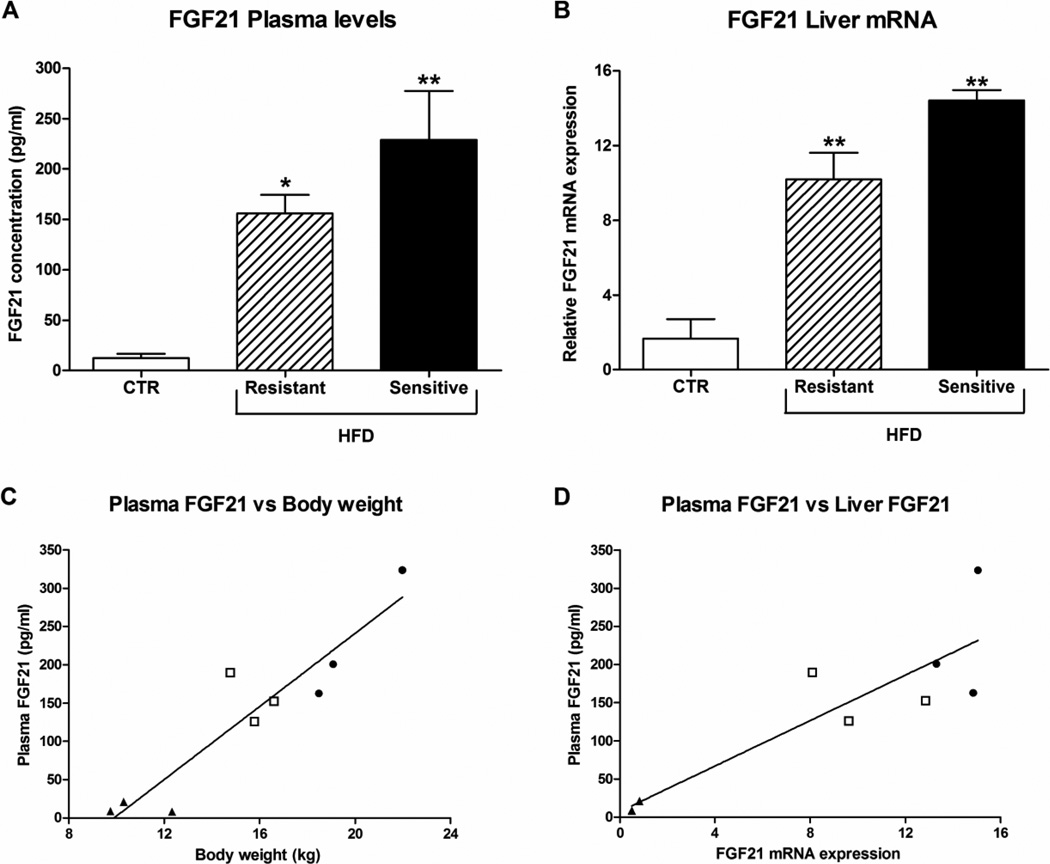
Plasma FGF21 levels were significantly increased ~12 and 18 fold in the HFD-resistant and sensitive group, respectively, compared to the CTR monkeys (Fig. 3A). The HFD-resistant monkeys tended to weigh slightly more than the CTR group, even though this was not significant, and FGF21 plasma levels correlated positively with body weight (R2 = 0.9, P < 0.0005, Fig. 3C).
Figure 3. Effect of high-fat diet on plasma and hepatic FGF21.
FGF21 (A) plasma levels and (B) liver mRNA expression in male rhesus monkeys on control diet or a high-fat diet to which they were either resistant or sensitive, n = 3–4/group. Data are means ± SEM; *p < 0.05, **p < 0.01 versus control fed rhesus macaques. Relationship between FGF21 plasma levels and (C) body weight (R2 = 0.9, P < 0.0005, n = 9), and (D) FGF21 liver mRNA (R2 = 0.8, P < 0.002, n = 9) in rhesus monkeys was determined with linear regression analysis. ▲CTR monkeys, □ HFD-resistant monkeys, ● HFD-sensitive monkeys.
The increase in plasma FGF21 could arise from various tissues, and therefore tissues where high FGF21 expression has been observed (8) were analyzed for FGF21 expression. In the liver, FGF21 mRNA expression was increased in both the HFD-resistant and sensitive group compared to the CTR monkeys. Moreover, a strong correlation was detected between plasma FGF21 and hepatic mRNA expression of FGF21 (R2 = 0.8, P < 0.002, Fig. 3D).
To determine whether down-regulation of FGF21 receptors was found in response to increased plasma FGF21, expression of the signaling receptor FGFR1, 2, 3 and the co-receptor β-klotho were determined. In all the tissues studied, no significant difference was observed in mRNA levels of FGFR2 and 3 between the three groups (data not shown). Nor in the liver were any differences found in FGFR1 and β-klotho expression between the three groups (data not shown).
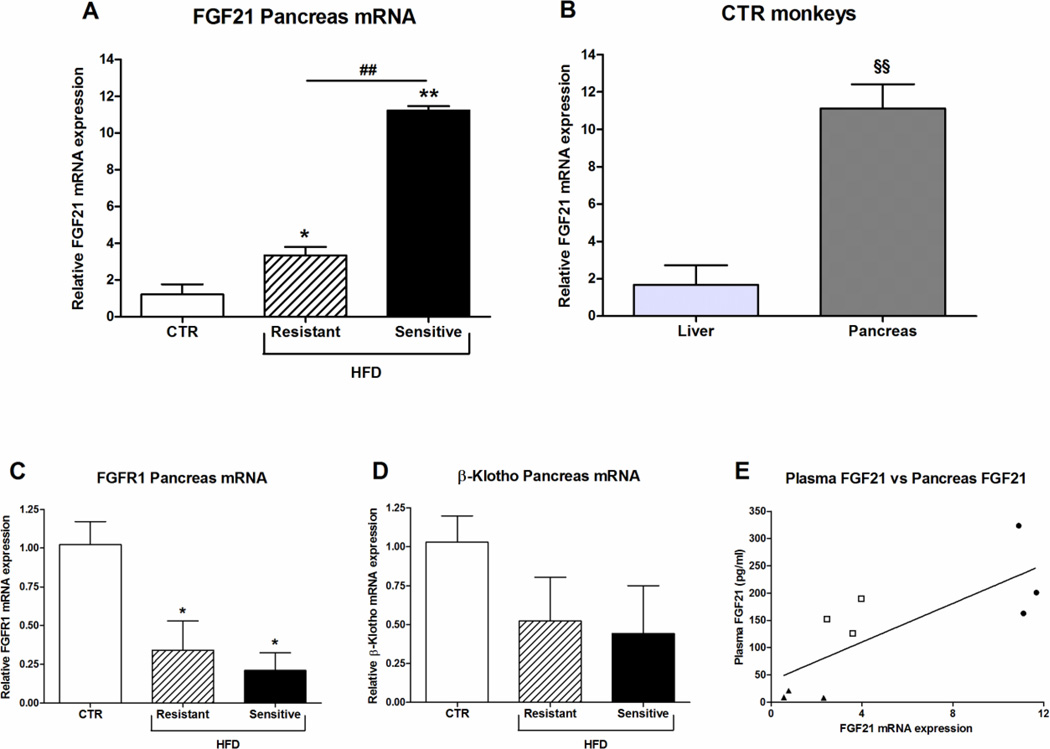
FGF21 mRNA levels were also studied in pancreas, where significant up-regulated levels were observed in both the HFD-resistant and sensitive monkeys compared to the CTR group; however, the FGF21 mRNA was significantly elevated in HFD-sensitive compared to the resistant group (p < 0.01) (Fig. 4A). As a point of reference, FGF21 mRNA levels in the CTR monkeys were approximately 6 fold higher in the pancreas compared to the liver (Fig. 4 B), and indicate that pancreas might be a major contributor of plasma FGF21. In the pancreas FGFR1 mRNA expression was significant down-regulated in the HFD-resistant and sensitive monkeys compared to the CTR group (Fig. 4C), however the mRNA expression of β-klotho was only slightly and not significantly decreased (Fig. 4D). Similar to hepatic FGF21 mRNA expression, pancreatic FGF21 mRNA expression also correlated positively with plasma FGF21 levels (R2 = 0.6, P < 0.015, Fig. 4E).
Figure 4. Effect of high-fat diet on FGF21, FGFR1 and β-Klotho mRNA expression in pancreas.
(A) FGF21 mRNA expression in pancreas from male rhesus monkeys on control diet or a high-fat diet to which they were either resistant or sensitive, n = 3/group. (B) Relative comparison of FGF21 mRNA levels in liver and pancreas from control-fed (CTR) monkeys, n = 3–4/group. (C) FGFR1 and (D) β-Klotho mRNA expression in pancreas from male rhesus monkeys on control diet or a high-fat diet to which they were either resistant or sensitive, n = 3/group. Data are means ± SEM; *p < 0.05, **p < 0.01 versus control fed rhesus monkeys; ##P < 0.01; §§p < 0.001 (E) Relationship between FGF21 plasma levels and FGF21 liver mRNA (R2 = 0.6, P < 0.015, n = 9) in rhesus monkeys was determined with linear regression analysis. ▲CTR monkeys, □ HFD-resistant monkeys, ● HFD-sensitive monkeys.
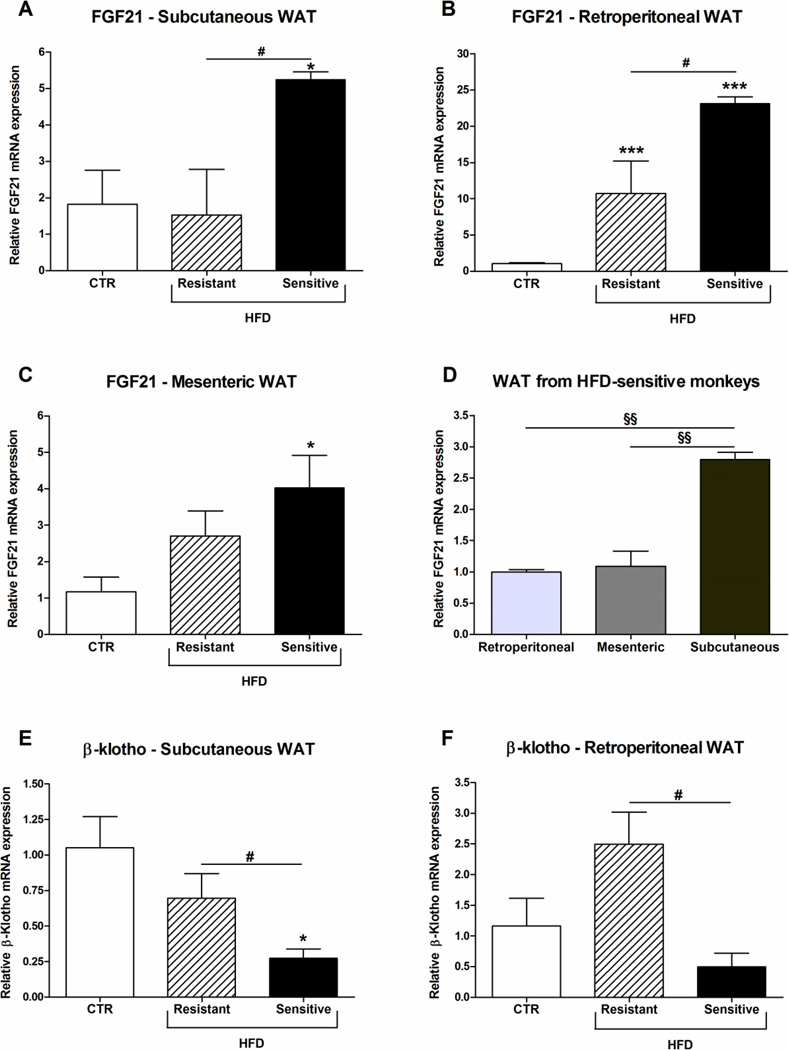
In contrast to other the tissues investigated, FGF21 mRNA expression in the subcutaneous WAT was specifically up-regulated only in the HFD-sensitive animals (Fig. 5A). In the retroperitoneal WAT FGF21 mRNA levels in both the HFD-resistant and sensitive group were increased, and furthermore a significant increase in FGF21 mRNA was observed in the HFD-sensitive compared to the HFD-resistant monkeys (Fig. 5B). In the mesenteric WAT a significant increase in FGF21 mRNA expression was observed in the HFD-sensitive monkeys compared to the CTR group (Fig. 5C). Furthermore, a comparison between the three WATs showed that the subcutaneous adipose tissue contained the highest expression of FGF21 mRNA levels (Fig. 5D). Measurement of FGFR1 expression in the three WATs showed no significant difference between the three groups (data not shown). However, β-klotho mRNA expression was significant down-regulated in the HFD-sensitive monkeys compared to both the CTR and the HFD-resistant groups in the subcutaneous WAT (Fig. 5E). In the retroperitoneal WAT, β-klotho mRNA was decreased in the HFD-sensitive compared to the HFD-resistant group, but only a tendency to a decrease is observed in the HFD-sensitive compared to the CTR monkeys (Fig. 5F).
Figure 5. Effect of high-fat diet on FGF21 and β-Klotho mRNA expression in adipose tissues.
FGF21 mRNA expression in (A) subcutaneous, (B) retroperitoneal and (C) mesenteric white adipose tissue (WAT) from male rhesus monkeys on control diet or a high-fat diet to which they were either resistant or sensitive. (D) Comparison of FGF21 mRNA expression in retroperitoneal, mesenteric and subcutaneous WAT from HFD-sensitive monkeys. Data are means ± SEM and have been normalized to the retroperitoneal tissue, §§p < 0.001. β-Klotho mRNA expression in (A) subcutaneous and (B) retroperitoneal WAT. Data are means ± SEM; *p < 0.05, ***p < 0.0001 versus control fed rhesus macaques; #P < 0.05; n = 3–4/group.
In soleus and gastrocnemius skeletal muscle we found that FGF21 mRNA expression was significant up-regulated specifically in the HFD-sensitive monkeys compared to both the CTR and the HFD-resistant group (Supplementary Fig. 1A and B). Moreover, no difference was observed between FGF21 mRNA levels in the CTR and HFD-resistant group. FGFR1 and β-klotho mRNA expression were very low in both the soleus and gastrocnemius muscle and showed no significant difference between the three groups (data not shown).
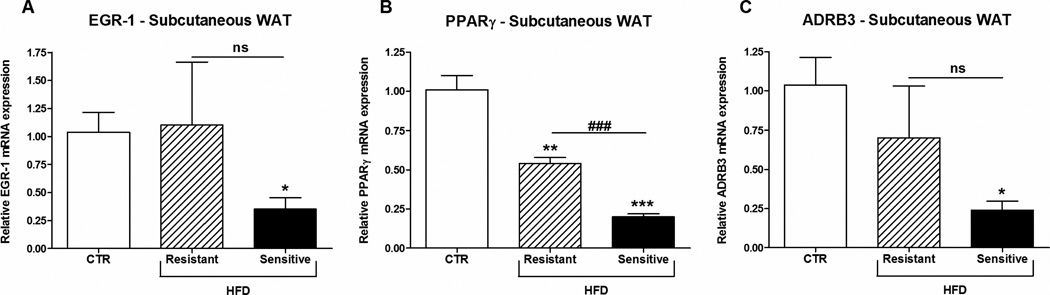
To examine if the decrease in β-klotho in subcutaneous WAT of the HFD-sensitive monkeys has any downstream effect on FGF21 signaling early growth response-1 (EGR-1) was measured. As shown in figure 6A EGR-1 was significant decreased in HFD-sensitive monkeys compared to the CTR group. Furthermore, PPARγ (Fig. 6B), involved in insulin sensitivity, as well as adrenoreceptor beta 3 (ADRB3) (Fig. 6C) were significant decreased in the HFD-sensitive monkeys. Expression of UCP1 in subcutaneous WAT was undetectable in the monkeys independent of the diet (data not shown).
Figure 6. EGR-1, PPARγ and ADRB3 mRNA expression in subcutaneous WAT from rhesus monkeys on a high-fat diet.
Male rhesus monkeys on control diet or a high-fat diet to which they were either resistant or sensitive, n = 3/group. (A) Early growth response-1 (EGR-1), (B) peroxisome proliferator-activated receptor γ (PPARγ) and (C) adrenoreceptor beta 3 (ADRB3). Data are means ± SEM; *p < 0.05, **p < 0.01, ***p < 0.0003 versus control fed rhesus monkeys; ###P < 0.001.
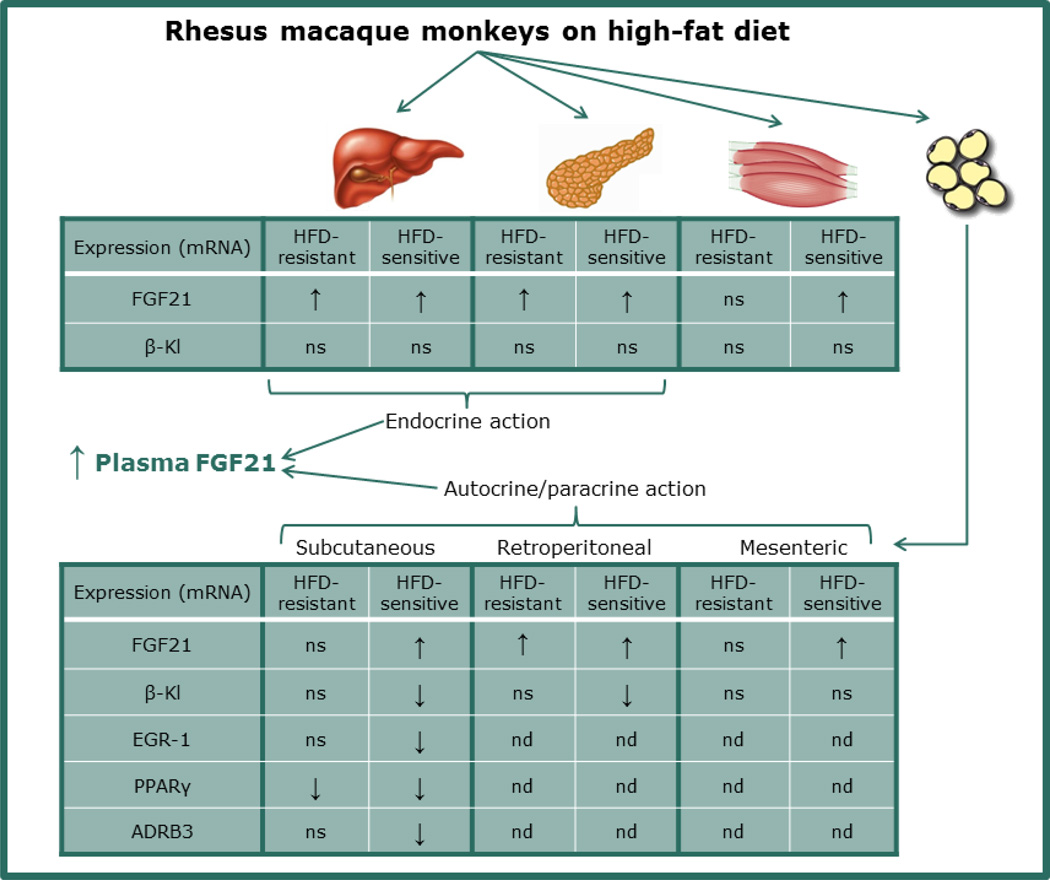
In summary, The HFD-sensitive monkeys showed a significant increase in the FGF21 mRNA expression in all examined tissues (liver, pancreas, WATs and skeletal muscles) compared to CTR, while FGF21 expression in the HFD-resistant group was only increased in the liver, pancreas and the retroperitoneal WAT (see overview in Fig. 7). FGFR1 mRNA expression is down-regulated in the pancreas in both the HFD-sensitive and resistant monkeys compared to the CTR group. β-klotho mRNA levels are reduced in the subcutaneous WAT leading to decreased EGR-1, PPARγ and ADRB3 expression in the HFD-sensitive but not the resistant monkeys.
Figure 7. Overview of the mRNA expression in rhesus macaque monkeys on high-fat diet (HFD).
FGF21 mRNA expression is elevated in liver, pancreas, skeletal muscles and white adipose tissues (WATs) in HFD-sensitive monkeys. HFD-resistant monkeys also have elevated FGF21 mRNA levels in liver and pancreas, but no significant difference was found in the WATs and skeletal muscles on FGF21 mRNA expression compared to the control fed monkeys. β-klotho (β-Kl) mRNA expression was down-regulated in the HFD-sensitive monkeys in the subcutaneous and retroperitoneal WAT, but not in any other tissue. EGR1, PPARγ and ADRB3 mRNA levels were down-regulated in subcutaneous WAT in the HFD-sensitive monkeys. HFD-resistant monkeys also have down-regulated PPARγ mRNA levels in the subcutaneous WAT compared to the control fed monkeys. FGF21 from liver and pancreas might contribute to the elevated plasma FGF21 levels found in the rhesus macaque monkeys on HFD, whereas FGF21 in the WATs might exert autocrine or paracrine actions. ns, non-significant; nd, not determined.
Discussion
In this study, we have characterized two cohorts of rhesus monkeys fed a HFD, with one group being relatively resistant to the HFD and another cohort displaying obesity, insulin resistance and glucose intolerance. These animals also display increased triglycerides and cholesterol; all of these characteristics are consistent with previously published data in human subjects (44). The reason for the observed difference in adiposity between monkeys fed the same diet is unknown, but in this study the highest FGF21 levels were observed in the HFD-sensitive monkeys. As mentioned above, studies have shown that FGF21 administration corrects dyslipidemia (4;30;31;33;34), however in this study the HFD-sensitive monkeys both had high FGF21-, cholesterol- and triglyceride levels indicating that FGF21 might be secreted as a compensatory mechanism, however a state of FGF21 resistance has been described in diet-induced obese mice (42) and despite the ability of pharmacological doses of FGF21 to overcome this (43), the endogenous level of FGF21 might not be high enough to activate the receptors. Furthermore, the strong positive correlation demonstrated between body weight and the FGF21 plasma levels, is consistent with previous studies in obese and diabetic patients (15;38–41).
In rodents, the highest FGF21 mRNA levels have been observed in the pancreas followed by the liver (5;8;9). In this study, an approximately 6 fold higher FGF21 mRNA expression was present in the pancreas compared to the liver of the CTR monkeys, which is again consistent with the expression pattern of FGF21 observed in mice (8). The role of FGF21 in pancreas is still not fully understood and autocrine/paracrine action of FGF21 on pancreatic cells might exist as studies have shown that FGF21 protects beta-cell function and survival and inhibits glucagon secretion (5;48). FGF21 mRNA levels in the pancreas and the liver also correlated well with the FGF21 plasma levels, i.e. FGF21 mRNA was up-regulated in both the HFD-resistant and sensitive group compared to the CTR monkeys, and therefore, both the pancreas and the liver could contribute to the increased plasma FGF21 levels. The exact role of FGF21 expressed in the various tissues is not known, but as the liver does not express FGFR1 (49) it does not seem to be a direct target of FGF21, and FGF21 expressed in the liver probably functions in an endocrine manner. The high expression of FGF21 in the pancreas also point towards an endocrine role, but FGF21 receptors are expressed on beta-cells, and thus pancreatic FGF21 might function in both an endocrine and paracrine fashion. FGF21 expression in the FGF21 responsive WAT is quite low and FGF21 from this tissue is thought to function in an autocrine manner (26;50). However, from this study it is not possible to conclude which tissues is the major contributor to plasma FGF21. In humans fed a high fat diet for 5 days, a significant increase in plasma FGF21 was observed (51), but no increased expression of FGF21 was observed in skeletal muscle or WAT pointing towards the liver and pancreas as major contributors to plasma FGF21.
No significant differences were observed in the FGF receptors in the liver; which, is in agreement with a study in diet-induced mice, were no reduction in β-klotho was observed in the liver (42). This may also indicate that the liver is not a direct target of FGF21. In the pancreas however, FGFR1 expression was significant down-regulated in the both the HFD-resistant and sensitive monkeys, and thus cannot explain the differences observed in FGF21 expression between these two groups.
In all three WATs investigated, FGF21 mRNA expression was very low compared to the pancreas and the liver. The subcutaneous WAT was found to have the highest levels of FGF21 mRNA of the three WATs studied. Importantly, in the subcutaneous WAT the FGF21 mRNA expression was only increased in the HFD-sensitive group. This observation is interesting as it has previously been observed that FGF21 expression in the subcutaneous WAT in obese humans correlated with plasma FGF21 levels (15) and high local concentrations around one of FGF21’s most important target tissues might lead to down-regulation of FGF21 receptors. Furthermore, the fact that we observed down-regulation of β-klotho in the subcutaneous WAT of the HFD-sensitive monkeys points toward a degree of FGF21 resistance. In diet-induced obese mice FGF21 resistance was more pronounced in the WAT than in the liver (42), supporting our findings. In rodents, administration of FGF21 to diet-induced obese mice lead to increased expression of UCP1 in subcutaneous WAT (52), which could potentially increase the energy expenditure and prevent obesity. However, the effect on body weight in the diabetic obese monkeys treated with FGF21 was minor, although significant. The energy expenditure has not been studied in the monkeys and a change in food intake might also have caused the weight loss (33).
Several studies have shown that FGF21 is induced by PPARγ agonists in WAT (13–15). Despite the fact that PPARγ target genes are fully activated in obese and diabetic patients, administration of PPARγ agonist commonly reduces fasting plasma insulin and improves insulin resistance (53). Moreover, a recent study showed that induction of FGF21 by the PPARγ agonist rosiglitazone in WAT, did not contribute to the circulating levels of FGF21 (13;26). This could however be explained by several factors e.g. 1) glucose is also a regulator of hepatic FGF21, and lower levels of glucose will reduce hepatic FGF21, 2) FGF21 in WAT is not the major contributor to circulating FGF21. In this study the increased expression of FGF21 in WAT does not correlate with the PPARγ expression; the opposite is actually observed but more than the expression level determines the activity of PPARγ. The decreased expression of ADRB3 could point towards a decreased responsiveness to catecholamines and might indicate impaired adaptive thermogenesis.
The expression of FGF21 mRNA determined in the skeletal muscles was very low, and the action of FGF21 in skeletal muscle is unclear as the expression of β-klotho is very limited in the skeletal muscle (10;11). Therefore the expression of FGF21 in skeletal muscles does not support an autocrine function, even though one study has shown an effect of FGF21 on glucose uptake in human skeletal muscle in vitro (54).
The regulation of FGF21 is complex and involves autocrine, paracrine and endocrine functions (see suggested overview in Fig. 7). Results obtained in this study show that up-regulation of FGF21 in diet-induced obese rhesus monkeys is in agreement with published human data (15;38;40), but as tissue expression of FGF21 and receptors is commonly not examined, this study add much more information to understanding the role of FGF21 and its receptors. Elevated FGF21 levels potentially leading to FGF21 resistance in diet-induced obese rhesus monkeys, together with published data showing that obese diabetic rhesus monkeys can be beneficially treated with FGF21 administration, indicate that FGF21 resistance likely also can be overcome in humans. Of important interest is the finding that HFD-resistant monkeys seem to have preserved FGF21 sensitivity in the WAT, as no down-regulation of β-klotho is observed. This is further supported by the observed down-regulation of β-klotho in both the subcutaneous and retroperitoneal WAT of the HFD-sensitive monkeys (see overview in Fig. 7). The findings were supported by the decrease in EGR-1 mRNA expression observed in subcutaneous WAT of HFD-sensitive monkeys. This indicates that the HFD-resistant monkeys are more FGF21-sensitive than the HFD-sensitive monkeys. In conclusion, in a tissue-specific manner, consumption of a HFD, independent of glucose intolerance, causes rises in FGF21 and this tissues-specific increase in FGF21 expression are observed between monkeys with and without development of glucose intolerance and dyslipidemia. FGF21 sensitivity in WAT might protect towards development of impaired glucose tolerance and dyslipidemia, but does not rule out that other factors might be involved as well. Thus, this study elucidates the importance of understanding the mechanisms regulating FGF21 resistance and supports a role for FGF21 as a new target in therapeutic therapy of obesity.
Supplementary Material
Acknowledgements
We thank Birgitte Wulff from Novo Nordisk A/S for helpful discussions. Eva B. Nygaard has received scholarships from the graduate research school In Vivo Pharmacology, Novo Nordisk A/S, and The Faculty of Health and Medical Sciences, University of Copenhagen. This work was partially supported by NIH grants P51 OD011092 (Kevin L. Grove) and RC4 DK090956.
Abbreviations
- FGF21
Fibroblast growth factor 21
- FGFR
Fibroblast growth factor receptor
- GSIS
Glucose stimulated insulin secretion
- HFD
high-fat diet
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Supplementary information is available at www.nature.com/ijo
References
- 1.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 6.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- 8.Fon TK, Bookout AL, Ding X, Kurosu H, John GB, Wang L, et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 2000;1492:203–206. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 10.Hojman P, Pedersen M, Nielsen AR, Krogh-Madsen R, Yfanti C, Akerstrom T, et al. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58:2797–2801. doi: 10.2337/db09-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008;582:3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol. 2009;71:369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 13.Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74:403–412. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Qiang L, Farmer SR. Identification of a Domain within Peroxisome Proliferator-Activated Receptor gamma Regulating Expression of a Group of Genes Containing Fibroblast Growth Factor 21 That Are Selectively Repressed by SIRT1 in Adipocytes. Molecular and Cellular Biology. 2008;28:188–200. doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 16.Gupte J, Yang L, Wu X, Weiszmann J, Hecht R, Lemon B, et al. The FGFR D3 Domain Determines Receptor Selectivity For Fibroblast Growth Factor 21. Journal of Molecular Biology. 2011;408:491–502. doi: 10.1016/j.jmb.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, et al. BetaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol. 2008;22:1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge H, Baribault H, Vonderfecht S, Lemon B, Weiszmann J, Gardner J, et al. Characterization of a FGF19 Variant with Altered Receptor Specificity Revealed a Central Role for FGFR1c in the Regulation of Glucose Metabolism. PLoS ONE. 2012;7:e33603. doi: 10.1371/journal.pone.0033603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C, Jin C, Li X, Wang F, McKeehan WL, Luo Y. Differential Specificity of Endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in Complex with KLB. PLoS ONE. 2012;7:e33870. doi: 10.1371/journal.pone.0033870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yie J, Wang W, Deng L, Tam LT, Stevens J, Chen MM, et al. Understanding the Physical Interactions in the FGF21/FGFR/beta-Klotho Complex: Structural Requirements and Implications in FGF21 Signaling. Chemical Biology & Drug Design. 2012:398–410. doi: 10.1111/j.1747-0285.2012.01325.x. [DOI] [PubMed] [Google Scholar]
- 23.Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, et al. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 24.Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, et al. Fibroblast Growth Factor-21 Regulates PPARgamma Activity and the Antidiabetic Actions of Thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oishi K, Konishi M, Murata Y, Itoh N. Time-imposed daily restricted feeding induces rhythmic expression of Fgf21 in white adipose tissue of mice. Biochemical and Biophysical Research Communications. 2011;412:396–400. doi: 10.1016/j.bbrc.2011.07.125. [DOI] [PubMed] [Google Scholar]
- 28.Uebanso T, Taketani Y, Yamamoto H, Amo K, Ominami H, Arai H, et al. Paradoxical Regulation of Human FGF21 by Both Fasting and Feeding Signals: Is FGF21 a Nutritional Adaptation Factor? PLoS ONE. 2011;6:e22976. doi: 10.1371/journal.pone.0022976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vienberg SG, Brons C, Nilsson E, Astrup A, Vaag A, Andersen B. Impact of short-term high-fat feeding and insulin-stimulated FGF21 levels in subjects with low birth weight and controls. Eur J Endocrinol. 2012;167:49–57. doi: 10.1530/EJE-12-0039. [DOI] [PubMed] [Google Scholar]
- 30.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast Growth Factor 21 Reverses Hepatic Steatosis, Increases Energy Expenditure, and Improves Insulin Sensitivity in Diet-Induced Obese Mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models - association with liver and adipose tissue effects. American Journal of Physiology - Endocrinology And Metabolism. 2009;297:E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 33.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 34.Véniant MM, Komorowski R, Chen P, Stanislaus S, Winters K, Hager T, et al. Long-Acting FGF21 Has Enhanced Efficacy in Diet-Induced Obese Mice and in Obese Rhesus Monkeys. Endocrinology. 2012;153:4192–4203. doi: 10.1210/en.2012-1211. [DOI] [PubMed] [Google Scholar]
- 35.Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, et al. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Molecular Metabolism. 2012 doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, et al. Beta-Klotho Is Required for Fibroblast Growth Factor 21 Effects on Growth and Metabolism. Cell Metabolism. 2012;16:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab. 2009;297:E1197–E1204. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, et al. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116:65–68. doi: 10.1055/s-2007-985148. [DOI] [PubMed] [Google Scholar]
- 39.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Bao Y, Xu A, Pan X, Lu J, Wu H, et al. Serum Fibroblast Growth Factor 21 Is Associated with Adverse Lipid Profiles and gamma-Glutamyltransferase But Not Insulin Sensitivity in Chinese Subjects. Journal of Clinical Endocrinology & Metabolism. 2009;94:2151–2156. doi: 10.1210/jc.2008-2331. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Yang G, Ning H, Yang M, Liu H, Chen W. Plasma FGF-21 levels in type 2 diabetic patients with ketosis. Diabetes Research and Clinical Practice. 2008;82:209–213. doi: 10.1016/j.diabres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hale C, Chen MM, Stanislaus S, Chinookoswong N, Hager T, Wang M, et al. Lack of Overt FGF21 Resistance in Two Mouse Models of Obesity and Insulin Resistance. Endocrinology. 2012;153:69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- 44.Hansen BC. Primates in the experimental pharmacology of obesity. In: Obesity: Pathology and therapy. 2000:461–489. [Google Scholar]
- 45.Schäfer SA, Hansen BC, Völkl A, Fahimi HD, Pill J. Biochemical and morphological effects of K-111, a peroxisome proliferator-activated receptor (PPAR)alpha activator, in non-human primates. Biochemical Pharmacology. 2004;68:239–251. doi: 10.1016/j.bcp.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Winegar DA, Brown PJ, Wilkison WO, Lewis MC, Ott RJ, Tong WQ, et al. Effects of fenofibrate on lipid parameters in obese rhesus monkeys. Journal of Lipid Research. 2001;42:1543–1551. [PubMed] [Google Scholar]
- 47.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 48.Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, et al. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795–1804. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 49.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, et al. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. J Biol Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vienberg SG, Brøns C, Nilsson E, Astrup AV, Vaag A, Andersen B. Impact of short term high fat feeding and insulin stimulated FGF21 levels in subjects with low birth weight and controls. European Journal of Endocrinology. 2012 doi: 10.1530/EJE-12-0039. [DOI] [PubMed] [Google Scholar]
- 52.Fisher fM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tontonoz P, Spiegelman BM. Fat and Beyond: The Diverse Biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 54.Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev. 2011;27:286–297. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.