Abstract
Purpose of review
Siglec-8 and Siglec-F are single pass transmembrane inhibitory receptors found on the surface of human and mouse eosinophils, respectively, but very little is known about their physiologic glycan ligands. This article reviews the latest knowledge on this topic and outlines the strategies being used to further define the production and glycobiochemical nature of these molecules in the lung.
Recent findings
Both Siglec-8 and Siglec-F recognize the same glycan structure, namely 6′-sulfated sialyl Lewis X, as determined using glycan array technologies. Studies have identified α2,3-linked sialylated glycoprotein structures localized to mouse airway epithelium in tissue sections, where their constitutive expression requires the specific sialyltransferase St3gal3. Expression of these ligands in lung is enhanced during allergic inflammation and by cytokines such as IL-13, and is maintained in primary air–liquid interface cultures of mouse lung epithelium. Further characterization suggests that they are high molecular weight sialylated proteins, putatively mucins. By combining analytic glycomics, glycoproteomic mapping, and further in-vitro eosinophil experimentation including the ability of candidate structures to enhance eosinophil apoptosis, a finely detailed appreciation of the structural requirements for productive Siglec-8 and Siglec-F engagement should soon emerge.
Summary
An enhanced understanding of Siglec-F, Siglec-8, and their ligands should improve our understanding of endogenous lung pathways limiting the survival of eosinophils within the airway in diseases such as asthma. Knowledge of this biology may also result in novel opportunities for drug development involving glycans and glycomimetics that selectively bind to Siglec-8 and induce eosinophil death.
Keywords: 6′-sulfated sialyl Lewis X, apoptosis, glycan ligands, Siglec-8, Siglec-F
INTRODUCTION
The eosinophil was discovered almost 140 years ago [1], and although it is considered to adversely contribute to many disorders, the exact roles and contributions of this cell in diseases including asthma are still being determined [2,3■■]. Siglec-F and Siglec-8 are functional paralog inhibitory receptors on mouse and human eosinophils, respectively, that were discovered more than 10 years ago [4–6]. Because engagement of Siglec-F or Siglec-8 induces eosinophil apoptosis [5–7], pharmacologic exploitation of the Siglec-8 pathway may prove to be a novel therapy in eosinophilic diseases such as bronchial asthma.
Although both Siglecs are known to preferentially recognize the sialoside ligand 6′-sulfo sialyl Lewis X (6′-su-sLex) [4], the exact composition of their natural tissue ligands are still unknown. Here, we will review the recent findings regarding the natural tissue ligands for Siglec-F and Siglec-8, ongoing glycobiological and biochemical approaches for their characterization, and future clinical use of such ligands or ligand mimetics as therapy.
NATURAL TISSUE LIGANDS FOR SIGLEC-F AND SIGLEC-8 IN THE AIRWAY
As both Siglec-F and Siglec-8 are functional paralog inhibitory receptors on eosinophils, their natural tissue ligands are expected to function as key regulators of eosinophilic airway inflammation by engagement of infiltrating inflammatory eosinophils, resulting in their apoptosis and removal.
To characterize these unknown ligands, glycan array and immunohistochemical approaches using Siglec-F and Siglec-8 Ig fusion proteins (a fusion protein containing N-terminal domains of the Siglec with the human IgG1 Fc fragment) were the first to identify potential tissue distribution of these ligands [5,8■■,9–11]. Glycan array studies identified 6′-u-sLex (Fig. 1) as a unique, specific shared ligand for both Siglec-8 and Siglec-F, but it was immunohistochemical studies with these Ig fusion proteins that revealed the presence of ligands in the airway. Knowing that Siglec-F and Siglec-8 had the preference to recognize α2,3-linked sialic acid [10], the natural tissue ligands were expected to contain α2,3-linked sialic acid, and Siglec binding was expected to be eliminated by sialidase treatment of the tissue that would remove all sialic acid residues (Table 1). Indeed, studies found selective Siglec-F-Fc staining of mouse lung epithelium and goblet cells using Siglec-F-Fc as an immunohistochemical tool and confirmed that the staining was eliminated by mutating the arginine in the Siglec-F-Fc glycan-binding domain, or by pretreating the lung tissue with Maackia amurensis lectin (recognizing α2,3-linked sialic acid), sialidase, periodate, or proteinase K [5,8■■,12], suggesting that lung airway cells express α2,3-linked sialic acid-containing glycoprotein ligands for Siglec-F. Similar sialidase-sensitive, Siglec-F-binding molecules were detected in preliminary studies [13■–15■] using material derived from mouse primary tracheal epithelial cells (mTECs) and culture supernatants. Furthermore, preincubation of mouse lung tissue sections or mTEC with a novel anti-6′-su-sLex IgY antibody blocked Siglec-F-Fc binding [13■,15■]. As both Siglec-F and Siglec-8 bind to 6′-su-sLex [4], these preliminary study results also support the existence of natural Siglec-F ligands containing 6′-su-sLex or a related glycan in airway epithelial cells.
FIGURE 1.
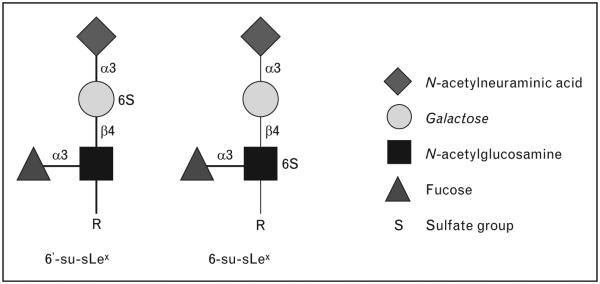
Structures of 6′-su-sLex and 6-su-sLex displayed with their various determinants using the suggested diagrammatic nomenclature (see legend) as outlined in the Essentials of Glycobiology textbook, second edition (http://www.ncbi.nlm.nih.gov/books/NBK1908/). N-acetylneuraminic acid ¼ sialic acid; R ¼ the substituent to which the glycan is attached, for example, protein or lipid; Greek symbols with numbers represent the chemical linkages between each determinant.
Table 1.
Summary of currently available information on natural tissue ligands for Siglec-8 and Siglec-F
| Question | Human Siglec-8 ligand | Mouse Siglec-F ligand |
|---|---|---|
| Where in the airway is it expressed? | Submucosal glands | Airway epithelial cells and some parenchymal cells |
| Is the ligand sialidase sensitive? | Yes | Yes |
| Does the ligand contain α2,3-linked sialic acid? | Unknown | Yes |
| Is it blocked by IgY antibody to 6′-su-sLe×? | No | Yes |
| Is ligand expression enhanced by any stimulus? | Unknown | Allergen sensitization and challenge, IL-4 and IL-13 stimulation |
Less is known about the mechanisms regulating Siglec-F ligand expression. The staining intensity for lung epithelial Siglec-F-Fc-binding material was increased following allergic lung inflammation in mice and sheep [5,9,12]. Interestingly, some peri-bronchial cells, glands, and mononuclear leucocytes seem to express Siglec-F-Fc-binding molecules under the conditions of allergic airway inflammation, but it is not known whether these ligands differ from those detected on the epithelium, nor is it clear exactly which nonepithelial cells express this ligand [5,12]. Increases in Siglec-F-Fc binding to airway epithelium were also observed after IL-4 or IL-13 stimulation, but not other cytokines such as TNF-α, in vivo and in vitro [9,14■,15■]. One knockout mouse study has helped to further delineate the enzymes responsible for Siglec-F ligand synthesis. In mouse lungs, there are several sialyltransferases which can add terminal α2,3-linked sialic acid to glycans. Lungs from one particular strain of sialyltransferase-deficient mice, St3gal3 null mice, but not St3gal2 null mice, lost virtually all Siglec-F-Fc staining [8■■], implicating the St3gal3 sialyltransferase in the generation of Siglec-F ligands. A preliminary in-vivo study [16■■] from our laboratory using St3gal3 heterozygous knockout mice finds more severe allergic eosinophilic lung inflammation than that of wildtype mice after ovalbumin sensitization and challenge. These results suggest that this Siglec-F-Fc-binding molecule is an α2,3-linked sialic acid-containing glycoprotein that increases under allergic inflammatory conditions and, when absent or reduced, results in enhanced eosinophilic inflammation.
Even less is known about natural tissue ligands for Siglec-8 in human airway. Unlike Siglec-F-Fc, Siglec-8-Fc does not appear to bind well to human lung epithelium in tissue sections. Instead, it prefers to bind airway glands and binding was poorly blocked by anti-6′-su-sLex IgY [13■]. Using Western blotting, Siglec-8-Fc recognized an approximately 500 kDa sialidase-sensitive band from supernatants of human bronchial explants [13■]. Although sialidase-sensitive high molecular weight (≈460–500 kDa) material was also recognized by Siglec-F-Fc from mTEC lysates, a lower molecular weight band (≈225 kDa) was detected only in the mTEC lysate using Siglec-F-Fc [13■]. On the basis of these early results from ongoing studies, lung ligands for Siglec-F and Siglec-8 may be different in their location of expression and perhaps in their glycan composition as well, but appear to find their way into the airway lumen where they may be capable of binding to eosinophils and induce their apoptosis (Fig. 2).
FIGURE 2.
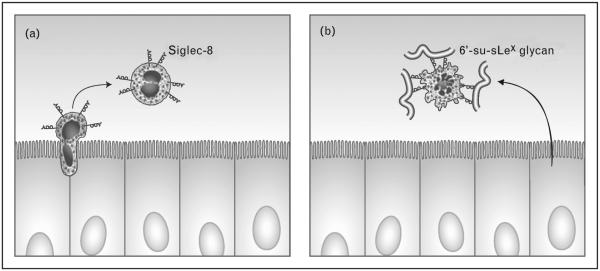
Hypothetical paradigm for control of eosinophilic lung inflammation via endogenous glycans. Eosinophils (expressing Siglec-8) are preferentially recruited into the airways during many forms of asthma and other eosinophilic lung diseases (panel a). Once in the airways, selective binding of a high molecular weight glycan ligand, putatively a mucin expressing 6′-su-sLex, results in their apoptosis (panel b). Artwork provided by Jacqueline Schaffer.
Because mouse lungs lack submucosal glands except in the trachea [17], one possible reason to explain the discordance between mouse and human Siglec-F and Siglec-8 high molecular weight ligands could be that they are similar secreted glycoproteins such as mucins. Interestingly, previous study [18] reported that MUC2 has ligand activity for Siglec-3. It is known that there are at least 12 different MUC mRNA species detected in samples from the human lower airway [19■]. For example, goblet cells typically express MUC5AC mRNA and glycoprotein, while glandular mucosal cells express MUC5B, MUC8, and MUC19 mRNA and glycoprotein [20]. As MUC5B mRNA in human fetal tissue is typically expressed only in mucosal cells of the submucosal gland and not in goblet cells just the same as for Siglec-8 ligand expression [13■,21], perhaps MUC5B might contribute some role in the Siglec-8 and Siglec-F pathways as a natural tissue ligand. To detect these unknown ligand molecules, work is underway to purify and fully biochemically characterize glycoprotein ligands for Siglec-F and Siglec-8 (http://lidpeg.jhmi.edu/).
GLYCOMIC AND GLYCOBIOLGICAL APPROACHES FOR IDENTIFYING SIGLEC-F AND SIGLEC-8 LIGANDS
The glycan array developed by the Consortium for Functional Glycomics (CFG) (http://www.functio nalglycomics.org) has proven to be an extremely valuable resource for characterizing the binding specificities of many carbohydrate-binding proteins (CBPs) expressed by inflammatory cells [11,22]. More broadly, array screening has become a centerpiece of contemporary glycobiology research, allowing rapid and sensitive screening for new binding specificities. This tool has also opened up the glycobiology field to nonglycobiologists who may not have otherwise pursued glycan-based functions in their experimental systems. As the CFG glycan array has grown from a presentation of 172 glycans to now over 600 structures, new details regarding fine structural specificities have been revealed for many CBPs. As described previously, Bochner et al. [11] took advantage of this resource to identify candidate ligands for Siglec-8 using the 172 glycan version. Only one glycan on this array was recognized, NeuAcα2–3(6-O-Su)Galβ1–4(Fucα1–3)GlcNAcβ1, called 6′-su-sLex (Fig. 1). The selectivity of the binding was very high, such that, for example, loss of sulfate ester (sLex) or alteration in the position of the sulfate ester to the 6-position of GlcNAc (6-su-sLex) (Fig. 1) eliminated Siglec-8-Fc binding. These findings were validated by surface plasmon resonance (SPR), which gave an apparent Kd for binding of dimeric Siglec-8-Fc to 6′-su-sLex of 2–2.5 μM. Subsequently, glycan array screening for the candidate ligands for Siglec-F-Fc demonstrated that the mouse paralog also recognizes 6′-su-sLex [4]. Subsequently, it was shown that the α1–3 fucose of 6′-su-sLex is dispensable for Siglec-F and Siglec-8 binding, which was recently confirmed by using the latest 610 glycan version of the CFG glycan (Kiwamoto et al., unpublished observation).
Although the diversity of glycans on the current CFG array is remarkably broad and highly representative of major glycan epitopes, new structures continue to be identified in biological materials and subtle postsynthetic modifications of already known structures can induce tremendous changes in glycan binding. Therefore, while glycan array technology provides powerful leads, subsequent searches for endogenous glycan ligands and for the protein (or lipid) carriers that present these glycans must be undertaken using biological tissues. The arsenal of tools available for purification and characterization of endogenous glycans and glycoconjugates has seen rapid growth in sensitivity and utility over the last 10 years. These advances are most notable in the application of various mass spectrometric approaches for characterizing glycan structures and for mapping specific glycan structures to precise glycosylation sites on glycoprotein backbones.
On the basis of the neuraminidase-sensitive detection of high molecular weight proteins by Western blot using Siglec-F-Fc described above, we have undertaken the glycomic and glycoproteomic analysis of these endogenous candidate ligands. We immunoprecipitated an approximately 500-kDa protein from mTEC lysates using Siglec-F-Fc. The precipitated proteins were resolved by SDS-PAGE and subjected to proteomic analysis by LC-MS/MS following in-gel trypsin digestion. We identified a mucin protein from the mTEC lysate and from immunoprecipitations from mTEC media, lung tissue lysate, and mouse bronchoalveolar lavage fluid, but not from neuraminidase-treated control samples. Cross-immunoprecipitations with antimucin antibodies verified the identity of the endogenous candidate ligand. Using recently developed and optimized methods for directly releasing N-linked and O-linked glycans from glycoproteins resolved in SDS-PAGE gels, it will be possible to characterize the diversity of glycan structures presented by the identified mucin. Detecting 6′-su-sLex on the glycoproteins immunoprecipitated by Siglec-F-Fc will strongly support a function for secreted mucins as endogenous Siglec-F ligands. However, the mucin domains of mucin proteins are characterized by dense O-linked glycosylation [23]. Thus, these molecules have the capacity to present lower affinity glycans in high density, complicating the definition of a best ligand. Fortunately, specific chemical and enzymatic approaches can be brought to bear in order to assess the relative contribution of various glycan structural elements to Siglec-F interactions. Ultimately, it will be essential to return to the biology of Siglec-8 on eosinophils (and mast cells) in order to distinguish the candidate ligands of greatest relevance for the pathology of diseases such as asthma. Once purified from relevant biological sources, the apoptotic potency associated with specific modifications (sulfation, glycan branching, sialylation, fucosylation, etc.) on candidate glycoconjugate ligands can be assessed. By combining analytic glycomics, glycoproteomic mapping, and eosinophil challenge, a finely detailed appreciation of the structural requirements for productive Siglec engagement should emerge.
POSSIBLE CLINICAL IMPLICATIONS REGARDING SIGLEC-8 AND ITS LIGANDS
There is only one published study examining the genetic association between sequence variants in Siglec-8 and the diagnosis of asthma. A significant association with asthma was observed for various single-nucleotide polymorphisms among African American, Japanese, and Brazilian populations [24]. As genetic linkage studies of asthma and related phenotypes have previously implicated chromosome 19q13.33–q13.41, where many Siglec genes including Siglec-8 are located [25,26], Siglec-8 might play a role in the pathogenesis of asthma.
Eosinophils obtained by bronchoalveolar lavage from asthmatic patients have similar levels of surface Siglec-8 when compared to their blood counterparts (unpublished observations), so it appears that Siglec-8 is not shed during eosinophil extravasation into the lung. However, these primed cells are particularly susceptible to undergoing apoptosis with Siglec-8 engagement, as is also seen following cytokine priming in vitro [6,27]. This suggests that activated eosinophils at the sites of inflammation may be particularly susceptible to Siglec-8-directed therapies. A separate study [28■] found increased levels of a soluble form of Siglec-8 in a small number of human serum samples from donors with hypereosinophilic syndrome (HES) or lymphocytic HES (LHES) patients, but not in other conditions associated with eosinophilia or in normal serum, so perhaps soluble Siglec-8 levels might prove useful as a biomarker in certain eosinophilic diseases.
Several animal studies using anti-Siglec-F antibody demonstrated benefits. For example, administration of anti-Siglec-F reduced the number of eosinophils in blood and bronchoalveolar lavage after ovalbumin sensitization and challenge [29]. This treatment reduced markers of airway remodeling, in particular subepithelial fibrosis [29]. Anti-Siglec-F reduced mouse intestinal and esophageal eosinophilic inflammation and disease activity [30,31■■]. In mouse models of HES and eosinophilic leukemia, anti-Siglec-F antibody administration also showed efficacy [7]. Although the function of alveolar macrophage Siglec-F is still unknown [32], this may not be relevant to human lung inflammation because human lung macrophages do not express Siglec-8.
CONCLUSION
Much biological research focuses on how responses are initiated, often with less emphasis on how responses are terminated. Yet, there are numerous examples of cellular processes that are specifically designed to dampen or eliminate reactions. Indeed, there are many such ‘inhibitory receptors’ on the surface of cells, including Siglecs, and they presumably function in this capacity. The work reviewed here sought to answer two important key questions: ‘What are the glycan ligands for Siglec-F and Siglec-8?’ and ‘Where do these ligands exist, and in what form, in vivo?’ Recent and ongoing broadly collaborative work combining cell biology, biochemistry, animal modeling, glycoproteomics, and glycobiological approaches, supported by the National Institutes of Health (http://pegnac.ucsd.edu and http:// www.functionalglycomics.org), has resulted in great progress toward answering these and other questions related to the roles played by glycans and their lectin counter receptors in a wide range of biological responses. Data generated to date indicate a negative regulatory role via Siglec-F during eosinophilic airway inflammation and suggest that natural sialoside ligands from epithelial cells and in the airways play a key role in downregulating eosinophilic airway inflammation (Fig. 2). Whether airway epithelial dysfunction and structural changes that are part of ‘airway remodeling’ might induce dysfunction of the Siglec-F and Siglec-8 ligand pathways remains to be determined. Further studies across many areas of expertise will be needed to fully elucidate the role of natural tissue ligands for Siglec-F and Siglec-8 in disease control and pathophysiology.
KEY POINTS.
Siglec-8 in humans and Siglec-F in mice, both preferentially expressed on eosinophils, recognize the identical sialoside, namely 6′-sulfo-sialyl Lewis X, but not closely related structures such as 6-sulfo-sialyl Lewis X or sialyl Lewis X.
Using a Siglec-F-Fc fusion protein as a probe in histology and Western blotting, it has been shown that lung epithelium is a major source of endogenous ligands for Siglec-F.
Expression and secretion of these endogenous glycoprotein lung ligands are enhanced under Th2-type inflammatory conditions.
Preliminary characterization suggests that these ligands are mucins.
Sialoside ligands for eosinophil Siglecs may exist in the airway at least in part to serve as a mechanism for inducing apoptosis in order to help eliminate eosinophils that have accumulated in the airway during Th2-type inflammation.
Acknowledgements
This work was supported by the grants AI72265 (to B.S.B.) and HL107151 (to B.S.B. and M.T.) from the National Institutes of Health. Dr Bochner also received support as a Cosner Scholar in Translational Research from Johns Hopkins University. The authors thank Jacqueline Schaffer for her outstanding artwork.
Footnotes
Conflicts of interest
Dr Bochner is a co-inventor on existing and pending Siglec-8-related patents. Dr Bochner may be entitled to a share of royalties received by Johns Hopkins University on the potential sales of such products. Dr Bochner is also a cofounder of, and owns stock in, Allakos, Inc., which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. Dr Bochner has current or recent consulting or scientific advisory board arrangements with Sanofi-Aventis, Merck, TEVA, and Allakos, and holds stock options in Glycomimetics, Inc. The other authors have no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 123–124).
- 1.Ehrlich P. Beiträge zur Kenntnis der granulierten Bindegewebszellen und der eosinophilen Leucocyten. Arch Anat Physiol. 1879;13:166–182. [Google Scholar]
- 2.Bochner BS, Gleich GJ. What targeting eosinophils has taught us about their role in diseases. J Allergy Clin Immunol. 2010;126:16–25. doi: 10.1016/j.jaci.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochner BS, Book W, Busse WW, et al. Workshop report from the National Institutes of Health Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD) J Allergy Clin Immunol. 2012;130:587–596. doi: 10.1016/j.jaci.2012.07.024. Summary of unmet needs in human eosinophil-associated disease research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Angata T, Cho JY, et al. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann N, McBride ML, Yamada Y, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo JP, Brummet ME, Myers AC, et al. Characterization of expression of glycan ligands for Siglec-F in normal mouse lungs. Am J Respir Cell Mol Biol. 2011;44:238–243. doi: 10.1165/rcmb.2010-0007OC. The first study to provide glycobiochemical characterization of Siglec-F lung ligands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho JY, Song DJ, Pham A, et al. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angata T, Hingorani R, Varki NM, Varki A. Cloning and characterization of a novel mouse Siglec, mSiglec-F: differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. J Biol Chem. 2001;276:45128–45136. doi: 10.1074/jbc.M108573200. [DOI] [PubMed] [Google Scholar]
- 11.Bochner BS, Alvarez RA, Mehta P, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 12.Patnode ML, Abraham WM, Crocker PR, Rosen SD. Goblet cells express carbohydrate ligands for Siglec-8 and Siglec-F [abstract] Glycobiology. 2009;19:1309. [Google Scholar]
- 13.Kiwamoto T, Na HJ, Brummet ME, et al. Similarities and differences between lung ligands for mouse Siglec-F and human Siglec-8. J Allergy Clin Immun. 2012;129:AB49. [abstract]. Data suggesting that the location and other characteristics of these mouse and human ligands may not be identical. [Google Scholar]
- 14.Kiwamoto T, Brummet ME, Na HJ, et al. Characterization of the synthesis of the eosinophil Siglec-F glycan ligand by mouse airway epithelial cells. J Allergy Clin Immunol. 2011;127:AB204. [abstract]. Data showing that cultured mouse airway epithelial cells make Siglec-F ligands that can be a source of study in vitro. [Google Scholar]
- 15.Kiwamoto T, Na HJ, Brummet ME, Finn MG, et al. Use of Siglec-F-Fc and a novel IgY antibody recognizing 6′-sulfated-sialyl Lewis X to identify endogenous lung ligands for Siglec-F [abstract] Glycobiology. 2011;21:1484. Data demonstrating that Siglec-F lung ligands contain 6′-sulfo-sialyl Lewis X based on the use of a novel antiglycan antibody. [Google Scholar]
- 16.Kiwamoto T, Brummet ME, Hudson SA, et al. Attenuation of type 3 α2,3 sialyltransferase (St3gal3) is associated with enhancement of eosinophilic allergic airway inflammation in mice [abstract] Glycobiology. 2012;22:1541. Data suggesting that loss of an enzyme required for Siglec-F ligand sialylation results in enhanced allergic eosinophilic lung inflammation in vivo. [Google Scholar]
- 17.Wenzel S, Holgate ST. The mouse trap: it still yields few answers in asthma. Am J Respir Crit Care Med. 2006;174:1173–1176. doi: 10.1164/rccm.2609002. [DOI] [PubMed] [Google Scholar]
- 18.Ishida A, Ohta M, Toda M, et al. Mucin-induced apoptosis of monocyte-derived dendritic cells during maturation. Proteomics. 2008;8:3342–3349. doi: 10.1002/pmic.200800039. [DOI] [PubMed] [Google Scholar]
- 19.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. A comprehensive review of airway epithelium and innate immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 21.Reid CJ, Gould S, Harris A. Developmental expression of mucin genes in the human respiratory tract. Am J Respir Cell Mol Biol. 1997;17:592–598. doi: 10.1165/ajrcmb.17.5.2798. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Feinberg H, Conroy E, et al. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 23.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Ann Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 24.Gao PS, Shimizu K, Grant AV, et al. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur J Hum Genet. 2010;18:713–719. doi: 10.1038/ejhg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venanzi S, Malerba G, Galavotti R, et al. Linkage to atopy on chromosome 19 in north-eastern Italian families with allergic asthma. Clin Exp Allergy. 2001;31:1220–1224. doi: 10.1046/j.1365-2222.2001.01132.x. [DOI] [PubMed] [Google Scholar]
- 26.Ober C, Cox NJ, Abney M, et al. Genome-wide search for asthma susceptibility loci in a founder population. The Collaborative Study on the Genetics of Asthma. Hum Mol Genet. 1998;7:1393–1398. doi: 10.1093/hmg/7.9.1393. [DOI] [PubMed] [Google Scholar]
- 27.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–124. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Na HJ, Hamilton RG, Klion AD, Bochner BS. Biomarkers of eosinophil involvement in allergic and eosinophilic diseases: review of phenotypic and serum markers including a novel assay to quantify levels of soluble Siglec-8. J Immunol Methods. 2012;383:39–46. doi: 10.1016/j.jim.2012.05.017. A comprehensive review of eosinophil biomarkers in disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song DJ, Cho JY, Lee SY, et al. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song DJ, Cho JY, Miller M, et al. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol. 2009;131:157–169. doi: 10.1016/j.clim.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubinstein E, Cho JY, Rosenthal P, et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2011;53:409–416. doi: 10.1097/MPG.0b013e3182182ff8. A demonstration that an antibody to Siglec-F reduces allergic eosinophilic inflammation in the gastrointestinal tract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng YH, Mao H. Expression and preliminary functional analysis of Siglec-F on mouse macrophages. J Zhejiang Univ Sci B. 2012;13:386–394. doi: 10.1631/jzus.B1100218. [DOI] [PMC free article] [PubMed] [Google Scholar]


