Abstract
The hippocampus plays an important role in memory, mood and spatial navigation. In the dentate gyrus of the adult hippocampus, in the subgranular zone (SGZ), new cells are generated that differentiate and mature into new neurons. Cisplatin, a highly effective antineoplastic drug with nephrotoxic and ototoxic side effects, induces apoptosis and suppresses neurogenesis in the hippocampus leading to memory impairment. Previous studies have shown that the antioxidant D-methionine protects against cisplatin-induced ototoxicity and nephrotoxicity suggesting that it might also prevent neurogenesis from being suppressed by cisplatin treatment. To test this hypothesis, rats were treated with cisplatin, D-methionine, cisplatin plus D-methionine, or saline (controls). Seven days after treatment, the rats were sacrificed and hippocampal sections immunolabeled for doublecortin (DCX) to identify neuronal precursor cells and maturing neurons in the SGZ. Cisplatin significantly reduced the number of DCX labeled cells (~80%) relative to controls. In contrast, DCX cell counts in rats treated with D-methionine prior to cisplatin were similar to controls. D-methionine treatment alone did not affect the number of DCX cells. These results indicate that D-methionine prevents the dramatic cisplatin-induced decrease of neurogenesis.
Keywords: Neurogenesis, Hippocampus, Neuronal precursor cells, Doublecortin, D-methionine, Cisplatin
Introduction
Hippocampal neurogenesis (Altman and Chorover 1963; Eriksson et al. 1998; Gage 2000) is believed to play an important role in long term memory (Snyder et al. 2005), whereas neuronal procursor cells in the subgranular zone (SGZ) in the dentate gyrus give rise to new neurons which migrate to the granular cell layer. Treatment with chemotherapeutic agents causes impairment in cognitive functions, memory and attention, a phenomenon commonly known as “chemo-brain" or "chemo-fog” (Staat and Segatore 2005). Cisplatin is one of the most commonly used platinum-based chemotherapeutic drugs and has been shown to damage the central nervous system and negatively affect the rate of neurogenesis (Dietrich et al. 2006; Duffner 2006). Importantly, cisplatin was found to be more toxic to the neuronal precursor cells and oligodendrocytes than it was to tumor cells.
One mechanism by which cisplatin causes damage is by oxidative stress and depletion of antioxidant enzymes (Chen et al. 2007). Thus, treatment with antioxidants may help rebalance endogenous antioxidants to normal levels and prevent tissue damage. Antioxidants have been shown to protect the inner ear against cisplatin-induced ototoxicity by reducing oxidative stress (Vogt 1995; Campbell et al. 2003; Rybak and Whitworth 2005). The antioxidant D-methionine, the dextro-isomer of the amino acid L-methionine was found to protect normal tissues, but not tumor cells against radiation and cisplatin induced toxicity (Vuyyuri et al. 2008). D-methionine, which protects against cisplatin-induced weight loss, also provided significant protection against cisplatin-induced damage in the inner ear (Campbell et al. 1996).
In this study, we tested the hypothesis that D-methionine would prevent cisplatin from suppressing hippocampal neurogenesis. Doublecortin (DCX), a microtubule-associated protein, is expressed in hippocampal neuronal precursor cells and maturing neurons (Brown et al. 2003; Rao and Shetty 2004; Couillard-Despres et al. 2005) and therefore used as a marker of neurogenesis in the hippocampus.
Methods
Fourteen adult male Sprague-Dawley rats (SASCO, Charles River Laboratories, International, Inc., Wilmington, MA, USA), three months of age, were used for this study. All animal procedures were carried out in accordance with the ethical standards in the NIH guidelines for use and care of laboratory animals and approved by the University of Buffalo Institutional Animal Care and Use Committee (protocol HER05080Y).
The anti-cancer drug cisplatin (12 mg/kg; Sigma-Aldrich, Inc., St. Louis, MO, USA) was administered intraperitonally under isoflurane anesthesia (4% induction, 1.5%–2% maintenance; Webster Veterinary Supply Inc., Sterling, MA, USA) at a concentration of 1 mg/ml in 0.9% sterile saline and at a speed of 0.15 ml/minute. This dose, consistent with our previous studies (Manohar et al., 2014), resulted in strong, reliable effects on the neuronal precursor population in rats and was within the range of cisplatin doses typically used in cytotoxicity studies (Cloven et al., 2000;Campbell et al., 2003; Maimaitiyiming et al., 2013). D-methionine (Sigma-Aldrich) (300 mg/kg) diluted in 0.9% saline at a concentration 30 mg/ml was administered intraperitonally. Three sham controls received 0.9% saline instead of cisplatin. Three rats received only cisplatin. Another three rats received D-methionine 30 minutes prior to treatment with cisplatin. Injection of D-methionine prior to cisplatin treatment ensured that it was already taken up before cisplatin was applied. Finally, three rats were treated only with D-methionine. All 12 rats were sacrificed at day 7 post-treatment. For prescreening, two further rats were treated with cisplatin alone and sacrificed 2 or 21 days later. All rats were hydrated with 0.9% saline (once with 5 ml s.c. on the day of the cisplatin injection and twice a day with 10 ml/kg from post-treatment day 1 to 7). Among the rats treated with only cisplatin, 50% survived, while all the rats in the other groups survived (sham controls, cisplatin and D-methionine, D-methionine).
On day of sacrifice, rats received a lethal dose of sodium pentobarbital and were transcardially perfused with 0.1 M phosphate buffered saline (PBS, 0.1 M, pH 7.4; Sigma-Aldrich) for 5 minutes to remove red blood cells, and then with 10% phosphate buffered formalin (Fischer Scientific, Pittsburgh, PA, USA; with a formaldehyde concentration of 4%) for fixation at room temperature for 15 minutes. Brains were taken out of the skulls and post-fixated in formalin for one more week. Brain tissue containing the left or right hippocampus was immersed in 30 % sucrose (Sigma-Aldrich) in 0.1 M PBS pH 7.4 (Sigma-Aldrich) overnight at 4 °C for cryoprotection. The following day the brain tissue was cut on a cryostat into 30 μm thick sections at −30 °C. Tissue sections were collected in 0.1 M PBS, pH 7.4 and washed for 30 minutes at room temperature. Between every incubation step described below, sections were washed in PBS, pH 7.4 for 15 minutes and the buffer was changed three times. For deactivation of endogenous peroxidase, sections were treated with 0.05% H2O2 (Fischer Scientific) in distilled water for 30 minutes. Next, the sections were pre-treated with blocking solution containing 10% normal horse serum (Vector Laboratories, Burlingame, CA,USA) and 0.05% Triton X-100 (Fischer Scientific) in 0.1 M PBS, pH 7.4 ("PBS-Triton") for 30 minutes. Incubation with the primary antibody against doublecortin (DCX; polyclonal; made in goat; sc-8066; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 2 μg/ml) in PBS-Triton with 1.0% normal horse serum lasted for 2 hours at room temperature. The specificity of this polyclonal DCX-antibody was previously shown by Brown et al. (2003) with a western blot study ruling out cross-reactions. Subsequent incubation with biotinylated secondary antibody (anti-goat IgG, Vector Laboratories; 5 μl/ml), in PBS-Triton with 1.5% normal horse serum, was done for 60 minutes at room temperature. For the ABC reaction, sections were treated with avidin and biotin (Elite-ABC kit, Vector Laboratories; 20 μl of reagent A and reagent B respectively/ml) in PBS-Triton for 60 minutes at room temperature. After washing in PBS, sections were washed in 0.1 M Tris Buffer (Trizma-base, Sigma Aldrich), pH 7.2, for 15 minutes. Staining reaction using 0.05% DAB (3’3’diaminobenzidine tetrahydrochlorite) with 0.3% nickel ammonium sulfate and 0.0015% H2O2 in Tris-buffer (Trizma-base, Sigma-Aldrich) was performed which resulted in a dark brown color. Subsequently, sections were washed in Tris-buffer once then in PBS at least three times, mounted on gelatin coated slides (SuperFrost plus; Fisher Scientific) and dried overnight. The sections were dehydrated in increasing alcohol, cleared in xylene and sealed using DPX (Fisher Scientific).
To determine antibody specificity on fixed tissue sections, preadsorption procedure was performed. The immunizing peptide (sc-8066 P, Santa Cruz, 1:100) was incubated with a 1:500 dilution of the DCX antibody in blocking solution (1,5% NHS, 1% BSA in PBS-Triton) for 30 minutes at room temperature. Hippocampus sections were treated with the standard immunostaining procedure described above, just with the preadsorped DCX antibody instead.
Cell profile counts of DCX immunopositive cells were done at 400× magnification using an Axisoscope microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA). While profile counts don't deliver absolute numbers without careful correction, they are sufficient and reliable for detecting relative differences between groups, particularly if the effect size is large as in the current study (Guillery and Herrup, 1997). In our study, there were none or only minimal changes of DCX cell body size after treatment as compared to controls. DCX positive cell profiles were counted in a total of nine sections per rat distributed along the caudal-rostral axis of the hippocampus (three random sections from the rostral end where the SGZ has a horizontal orientation, another three from the middle region where the SGZ has a diagonal orientation and extends ventrally, and finally three sections from the most caudal region where most of the SGZ is oriented vertically from dorsal to ventral). Criteria for counting DCX-positive cells in the hippocampus have been described previously (Kraus et al. 2010), specifically the presence of the nucleus or cell morphology (shape and size) with its cytoplasm homogeneously stained and clearly darker than its surroundings. Number of cells per millimeter was calculated by dividing DCX profile counts by the length of the SGZ (Kraus et al., 2010), in order to correct for length differences due to cutting angle. Cell numbers/mm were then normalized to controls and presented as mean ± standard error of mean (SEM). The results were analyzed with Graphpad Prism (Version 5.01; GraphPad Software Inc., La Jolla, CA, USA) and statistical comparisons were done by the One-way ANOVA and Tukey's Multiple Comparison Test. Significance level was set to P ≤ 0.05. Grayscale images were taken using a digital camera (SPOT Insight; Diagnostic Instruments, Inc., Sterling Heights, MI, USA) and processed with imaging software (SPOT Software, version 4.6). Figures were assembled using Adobe Photoshop 5.5 (Adobe Systems, Inc., San Jose, CA, USA).
Results
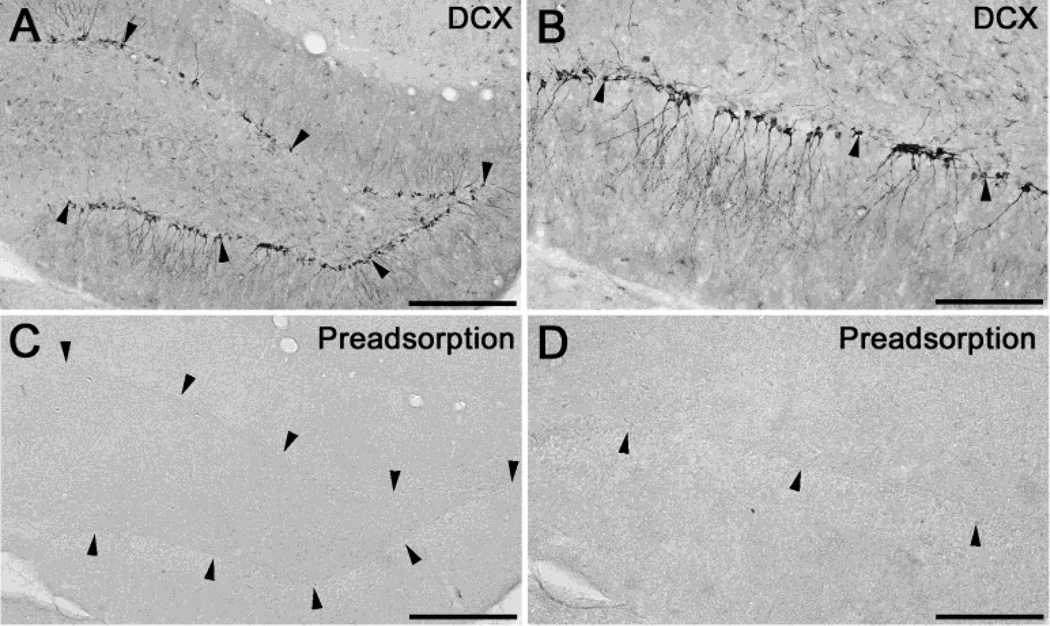
Immunostaining of the rat hippocampus visualized DCX positive cell bodies along the SGZ in the dentate gyrus (Fig. 1A, B). Preadsorption of the DCX antibody resulted in unstained sections (Fig. 1C, D), verifying DCX-antibody specificity on fixed tissue sections. Figure 2 shows the effect of cisplatin on the DCX positive neuronal precursor cells at different time points after treatment. In controls, the hippocampus shows a large number of DCX positive cells. The cell bodies were arranged in a line along the SGZ. Two days after cisplatin treatment we saw a slight reduction of the number of cell bodies as compared to the controls. Seven days after treatment, a very large reduction was observed. After 21 days the number of DCX cells had almost recovered back to the control level. Based on these observations, the protective properties of D-methionine were investigated on rats surviving for 7 days after cisplatin treatment.
Fig. 1.
DCX antibody specificity. The dentate gyrus of the hippocampus show DCX immunopositive cell bodies in the SGZ (a, b). When the DCX-antibody was preadsorped with the immunizing peptide (sc-8066 P), sections remained unstained. Arrowheads indicate the SGZ. Scale bars: 200 μm (a, c); 100 μm (b, d).
Fig. 2.
Hippocampal neuronal precursor cells in the dentate gyrus immunolabeled for DCX in a control rat (a) and rats treated with cisplatin (Cis) surviving for 2 days (b), 7 days (c) and 21 days (d): Seven days after cisplatin treatment the number of cell bodies (arrow heads) was considerably reduced. Scale bar: 50μm
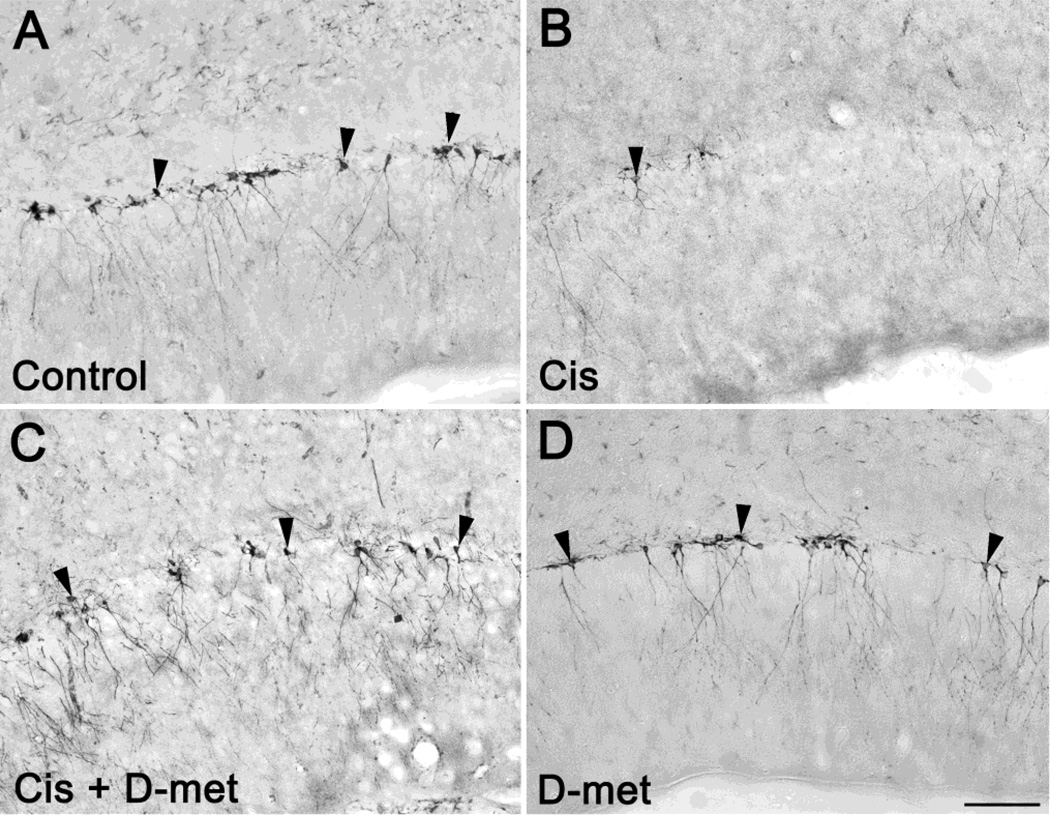
Figure 3 shows DCX positive neuronal precursor cells from controls (saline treatment) and experimental rats treated with cisplatin, cisplatin plus D-methionine, or only D-methionine 7 days after treatment. Rats treated with cisplatin plus D-methionine had a greater number of DCX positive cells when compared to the rats treated with only cisplatin. The number of DCX positive cells was comparable to that of controls as well as to rats treated with only D-methionine. The number of DCX labeled cells in rats treated with D-methionine was similar to the controls.
Fig. 3.
Hippocampal neuronal precursor cells in the dentate gyrus immunolabeled for DCX in controls (a) and seven days after treatment (b, c, d): cisplatin alone (Cis), cisplatin with D-methionine (Cis + D-met), and D-methionine alone (D-met). Seven days after cisplatin treatment (b) the number of cell bodies (arrow heads) was significantly reduced when compared to controls (a). When treated with D-methionine before cisplatin (c), the number of cell bodies remained similar to controls. Scale bar: 100μm
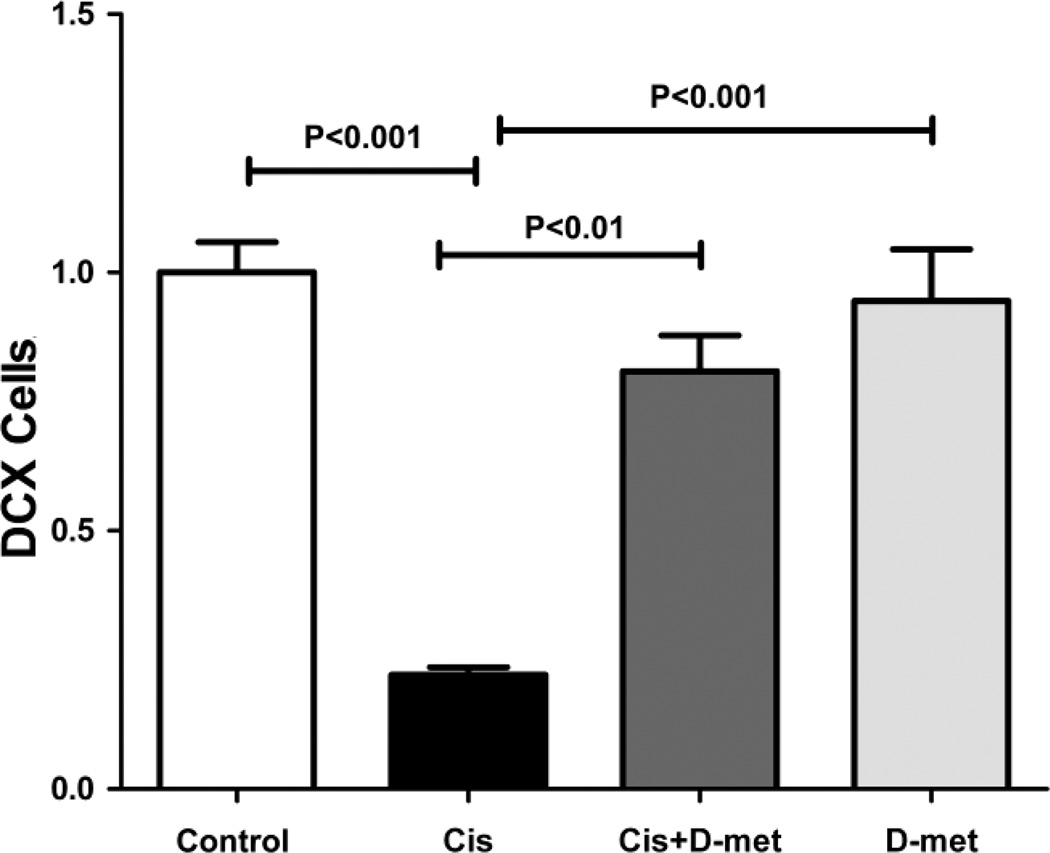
For quantification, we counted the numbers of DCX labeled cells in the control group and the three experimental groups at 7 days post-treatment (Fig. 4). The numbers of DCX labeled cells were divided by the length of the SGZ, then normalized to the average cell number in controls which was set to 1.0. The normalized average cell number 7 days after cisplatin treatment was 0.22 ± 0.015 (SEM), a reduction of almost 80% compared to controls. This reduction was highly significant (One-way ANOVA, F=27.77, df = 3, P<0.0001, Tukey post-hoc comparison, P<0.001). In contrast, when D-methionine was administered along with cisplatin the cell density was 0.8 ± 0.070 (SEM), significantly larger than in rats treated with only cisplatin (Tukey post-hoc comparison, P<0.01) while not significantly different from controls or from animals treated only with D-methionine (Tukey post-hoc analyses, P>0.05). When D-methionine was administered alone the average cell density was 0.95 ± 0.099 (SEM) and not significantly different from controls (Tukey post-hoc analysis, P>0.05).
Fig. 4.
Relative numbers of DCX cells in controls, cisplatin (Cis), cisplatin with D-methionine (Cis + D-met), and D-methionine (D-met) 7 days after treatment: Numbers were normalized to the control group which was set to 1.0. Rats treated with only cisplatin showed a large and significant reduction in cell density when compared to controls and to animals treated with only D-methionine. D-methionine administered 30 minutes prior to cisplatin kept the number of DCX positive cells comparable to the controls and to animals treated with only D-methionine, while it was significantly larger than in animals treated with cisplatin. There were no significant differences between controls, animals treated with cisplatin plus D-methionine, and animals treated with only D-methionine.
Discussion
Cisplatin caused a massive reduction in the number of DCX labeled cells in the dentate gyrus of the adult rat hippocampus when compared to controls one week after treatment, consistent with previous observations (Dietrich et al. 2006). This large decrease in neurogenesis would presumably impair hippocampal related functions in these rats as reported in other studies (Shabani et al. 2012) as well as in patients undergoing chemotherapy who have reported impaired cognition and memory (Kaasa et al. 1988; Troy et al. 2000). The reduced number of DCX cells in the SGZ has been linked to apoptosis-mediated cell death. Dietrich and co-workers observed a strong increase of TUNEL staining in the mouse hippocampus after treatment with cisplatin and other anti-cancer drugs, where neuronal precursor cells were far more vulnerable to cisplatin toxicity than mature neurons or neuroepithelial stem cells in vitro as well as in vivo (Dietrich et al. 2006). Consistent with these morphological observations, Manohar and co-workers (2014) observed a significant up-regulation of pro-apoptotic genes Bik, Bid, Bok, Trp53p2 and Card6 two days after cisplatin injection at same dose used in this study. An alternative explanation for reduced DCX immunoreactivity in SGZ may be down-regulation of this protein in surviving cells; however, we are unaware of any studies that have documented such a change of expression.
DCX is expressed in neuronal precursor cells as well as in maturing neurons, but is down-regulated during maturation and is absent in mature functional neurons (Brown et al. 2003; Rao and Shetty 2004; Couillard-Despres et al. 2005). Therefore, the cisplatin-induced reduction of DCX positive cells is indicative of reduced neurogenesis due to death of neuronal precursor cells or maturing neurons, to decreased proliferation of surviving precursor cells, or failure of newborn cells to differentiate and mature into neural precursors. Earlier studies have shown that cisplatin reduces the number of hippocampal cells labeled with BrdU (Dietrich et al. 2006) or the cell division marker Ki67 (Manohar et al. 2014), demonstrating a decreased rate of cell proliferation consistent with reduced neurogenesis caused by cisplatin. .
Rats treated with D-methionine plus cisplatin showed a significant increase in the number of DCX labeled cells as compared to cisplatin treated rats. This may be due to the antioxidant property of D-methionine which reduces oxidative stress by binding free radicals thereby reducing cell death (Vogt 1995). In addition, D-methionine increases the production of mitochondrial reduced glutathione (GSH) which acts as an anti-oxidant thereby reducing oxidative stress-induced apoptosis (Campbell et al. 2003; Vuyyuri et al. 2008). Alternatively, D-methionine may partially chelate cisplatin and reduce the concentration of unbound cisplatin in blood or tissues. In that case, the protective properties of D-methionine may be due to lower levels of systemic cisplatin rather than its antioxidant properties, thereby hindering the therapeutic properties of cisplatin (Ekborn et al. 2002). However, this is unlikely to be a major factor because D-methionine has been shown to protect against cisplatin toxicity without significantly lowering the anti-tumor effectiveness (Jones and Basinger 1989; Cloven et al. 2000; Campbell et al. 2003). The underlying reasons for such a selective protection of non-tumor cells may be that non-tumor cells have a different methionine metabolism than tumor cells (Vuyyuri et al. 2008). Cisplatin reduces the mitochondrial membrane potential thereby causing cell death (Maimaitiyiming et al. 2013), while D-methionine mitigates loss of mitochondrial membrane potential selectively in non-tumor cells but not in tumor cells (Campbell et al. 2003) thereby selectively protecting non-tumor cells.
In conclusion, D-methionine may be a promising drug for reducing the central and peripheral neurotoxic effects of cisplatin treatment, without reducing the anti-tumor effectiveness of cisplatin.
Acknowledgements
This project was partially supported by National Institute of Health (NIH grants R01DC00909101; R01DC009219; R01DC011808).
Footnotes
Ethical standards
All animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Altman J, Chorover SL. Autoradiographic Investigation of the Distribution and Utilization of Intraventricularly Injected Adenine-3h, Uracil-3h and Thymidine-3h in the Brains of Cats. J Physiol. 1963;169:770–779. doi: 10.1113/jphysiol.1963.sp007295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Rybak LP, Hughes LF. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol. 2003;14:144–156. [PubMed] [Google Scholar]
- Campbell KC, Rybak LP, Meech RP, Hughes L. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102:90–98. doi: 10.1016/s0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- Cloven NG, Re A, McHale MT, Burger RA, DiSaia PJ, Rose GS, Campbell KC, Fan H. Evaluation of D-methionine as a cytoprotectant in cisplatin treatment of an animal model for ovarian cancer. Anticancer Res. 2000;20:4205–4209. [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffner PK. The long term effects of chemotherapy on the central nervous system. J Biol. 2006;5:21. doi: 10.1186/jbiol51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekborn A, Laurell G, Johnstrom P, Wallin I, Eksborg S, Ehrsson H. D-Methionine and cisplatin ototoxicity in the guinea pig: D-methionine influences cisplatin pharmacokinetics. Hear Res. 2002;165:53–61. doi: 10.1016/s0378-5955(02)00277-0. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Jones MM, Basinger MA. Thiol and thioether suppression of cis-platinum-induced nephrotoxicity in rats bearing the Walker 256 carcinosarcoma. Anticancer Res. 1989;9:1937–1942. [PubMed] [Google Scholar]
- Kaasa S, Olsnes BT, Thorud E, Host H. Reduced short-term neuropsychological performance in patients with nonsmall-cell lung cancer treated with cisplatin and etoposide. Antibiot Chemother. 1988;41:226–231. doi: 10.1159/000416209. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimaitiyiming H, Li Y, Cui W, Tong X, Norman H, Qi X, Wang S. Increasing cGMP-dependent protein kinase I activity attenuates cisplatin induced kidney injury through protection of mitochondria function. Am J Physiol Renal Physiol. 2013;305:F881–F890. doi: 10.1152/ajprenal.00192.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S, Jamesdaniel S, Salvi R. Cisplatin inhibits hippocampal cell proliferation and alters the expression of apoptotic genes. Neurotox Res. 2014;25:369–380. doi: 10.1007/s12640-013-9443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005;10:1313–1321. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- Shabani M, Nazeri M, Parsania S, Razavinasab M, Zangiabadi N, Esmaeilpour K, Abareghi F. Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology. 2012;33:1314–1321. doi: 10.1016/j.neuro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Staat K, Segatore M. The phenomenon of chemo brain. Clin J Oncol Nurs. 2005;9:713–721. doi: 10.1188/05.CJON.713-721. [DOI] [PubMed] [Google Scholar]
- Troy L, McFarland K, Littman-Power S, Kelly BJ, Walpole ET, Wyld D, Thomson D. Cisplatin-based therapy: a neurological and neuropsychological review. Psychooncology. 2000;9:29–39. doi: 10.1002/(sici)1099-1611(200001/02)9:1<29::aid-pon428>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Vogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic Biol Med. 1995;18:93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- Vuyyuri SB, Hamstra DA, Khanna D, Hamilton CA, Markwart SM, Campbell KC, Sunkara P, Ross BD, Rehemtulla A. Evaluation of D-methionine as a novel oral radiation protector for prevention of mucositis. Clin Cancer Res. 2008;14:2161–2170. doi: 10.1158/1078-0432.CCR-07-1954. [DOI] [PubMed] [Google Scholar]