Abstract
Objective
G1D is commonly associated with electrographic spike-wave and - less-noticeably – with absence seizures. The G1D syndrome has long been attributed to energy (i.e., ATP-synthetic) failure, as have experimental, toxic-rodent epilepsies to impaired brain metabolism and tricarboxylic acid (TCA) cycle intermediate depletion. Indeed, a (seldom-acknowledged) function of glucose and other substrates is the generation of brain TCAs via carbon-donor reactions collectively named anaplerosis. However, TCAs are preserved in murine G1D. This renders inferences about energy failure premature and suggests a different hypothesis, also grounded on our findings, that consumption of alternate TCA precursors is stimulated, potentially detracting from other functions. Second, common ketogenic diets can ameliorate G1D seizures, but lead to a therapeutically-counterintuitive reduction in blood glucose available to the brain, and they can prove ineffective in 1/3 of cases. While developing G1D treatments, all of this motivated us to: a) uphold (rather than attenuate) the residual brain glucose flux that all G1D patients possess; and b) stimulate the TCA cycle, including anaplerosis. Therefore, we tested the medium-chain triglyceride triheptanoin, a widely-used medical food supplement that can fulfill both of these metabolic roles. The rationale is that ketone bodies derived from ketogenic diets are not anaplerotic, in contrast with triheptanoin metabolites, as we have shown in the G1D mouse brain.
Design
We supplemented the regular diet of a case series of G1D patients with food-grade triheptanoin. First we confirmed that, despite their frequent electroencephalographic (EEG) presence as spike-waves, most seizures are rarely visible, such that perceptions by patients or others are inadequate for treatment evaluation. Thus, we used EEG, quantitative neuropsychological, blood analytical, and MRI cerebral metabolic rate measurements as main outcomes.
Setting
Academic and unsponsored.
Patients
Fourteen G1D children and adults not receiving a ketogenic diet.
Results
Eleven patients tolerated triheptanoin without significant adverse effects. Spike-waves decreased robustly in all patients who manifested them. Additionally, neuropsychological performance and cerebral metabolic rate increased in most patients.
Conclusions
Triheptanoin can favorably influence cardinal aspects of neural function in G1D. Additionally, our outcome measures offer a framework for the evaluation of therapies for G1D and other encephalopathies associated with impaired intermediary metabolism.
Keywords: Glucose transporter, GLUT1, G1D, triheptanoin, C7 oil, metabolism, anaplerosis, MRI, EEG
INTRODUCTION
Glucose is the principal cerebral metabolic substrate under normal circumstances. Specifically, glucose provides both energy (ATP) to neural cells and carbon for neurotransmitter production – which constitutes only an example of the numerous biosynthetic reactions that the brain carries out - via glycolysis and the tricarboxylic acid (TCA) cycle 1. But glucose metabolism also fulfills other, less often-acknowledged cerebral biosynthetic requirements, which are no less-important from a clinical standpoint when they remain unmet 2, such as anaplerosis (i.e., the replacement of the net carbon that is normally lost from the TCA pool via diffusion away from the TCA cycle). Thus, a myriad of cerebral energetic and carbon fluxes, many of which are still poorly understood quantitatively 3, stem from brain glucose availability. This is, in turn, contingent upon the activity of the facilitative membrane glucose transporter type I (GLUT1), which permits glucose to cross the blood-brain barrier and, subsequently, transit into - and perhaps through - astrocytes.
Human GLUT1 deficiency (G1D) due to mutation of the gene SLC2A1 is associated with partial loss-of-function (i.e., GLUT1 activity is never abolished) and results in decreased brain glucose accumulation 4. G1D manifests as an encephalopathy frequently (but not exclusively) characterized by medication-refractory infantile-onset seizures 5, diminished encephalic mass, intellectual disability, and complex (i.e., multiform) motor disturbances (spasticity, ataxia, chorea, dystonia and combinations thereof) 6-9. This is often accompanied by reduced cerebrospinal fluid (CSF) glucose concentration, even though CSF glucose abundance primarily reflects secretion by the choroid plexus, and exceeds the concentration typical of the brain interstitial space 10. Notably, a series of important neural function defects in G1D are independent of blood-brain barrier GLUT1 dysfunction and can be ameliorated by increasing brain tissue glucose concentration, as we have shown in the G1D mouse 11-13. We noted that these findings might bear relevance to human G1D 14, thus inviting treatment development based on the enhancement of brain glucose influx and/or the supply of an effective substitute substrate.
In this regard, the ketogenic diet, which is used for the treatment of G1D solely on the basis of biochemical assumptions and empirical observations and has not been the subject of a controlled clinical trial in G1D 15, ameliorates some movement disorders and epilepsy in 2/3 of patients (JMP, unpublished, and 16, 17). Unfortunately, neurological deficits, particularly those centered on other aspects of movement coordination such as ataxia and dysarthria, and cognition tend to persist under the diet, with a significant fraction of patients experiencing recurrent or incompletely treated abnormalities 18, whereas the remainder 1/3 of incompletely-treated or diet-unresponsive patients face an even more problematic clinical course. The diet, given with the therapeutic intent of stimulating brain metabolism in G1D, may act as an anticonvulsant through several potential mechanisms 19, 20, including the production of acetyl-coenzyme A that can stimulate the neural TCA cycle 21, 22, but its mode of action has not been investigated in G1D. Importantly, in addition to insufficiently alleviating all incapacitating features of G1D 18, ketogenic diets are not universally tolerable 23-27, such that few (<10%) adults with G1D treated with a ketogenic diet remain compliant with it or achieve employment (JMP, unpublished observations). Yet, most therapeutic efforts have been limited to early diagnosis and initiation of a ketogenic diet 16, 28.
In contrast with this form of therapy, we have shown that nutrients with additional potential to refill TCA cycle intermediates via anaplerosis such as triheptanoin (an edible triglyceride of the 7-carbon fatty acid heptanoate that can yield three molecules of heptanoate and other anaplerotic metabolites in the liver 29), offer therapeutic benefit in diseases where metabolic precursor depletion or overutilization is suspected or identified 2, 30. Dietary triheptanoin gives rise (after hepatic metabolism) to plasma heptanoate and to 5-carbon ketone bodies (β-ketopentanoate and β-hydroxypentanoate) 29, all of which can penetrate 31 and be readily metabolized by the brain (Figure 1), as we have shown in rodents, including G1D mice 32, 33. Neoglucogenesis can also be observed after heptanoate infusion, and this may act synergistically with the other heptanoate metabolites in brain glucose-deficient states such as G1D 33. Heptanoate metabolism exerts anticonvulsant effects in an unrelated animal model of toxic epilepsy and refills depleted brain TCA cycle intermediates 34. Therefore, the rationale for the present work combines potential biochemical benefits 2, 30, experience with other neurometabolic disorders that we have treated with triheptanoin 29, 35, 36, and our ex vivo G1D rodent model evidence of the metabolic effects of triheptanoin on the brain 37, all in the context of limited therapeutic alternatives.
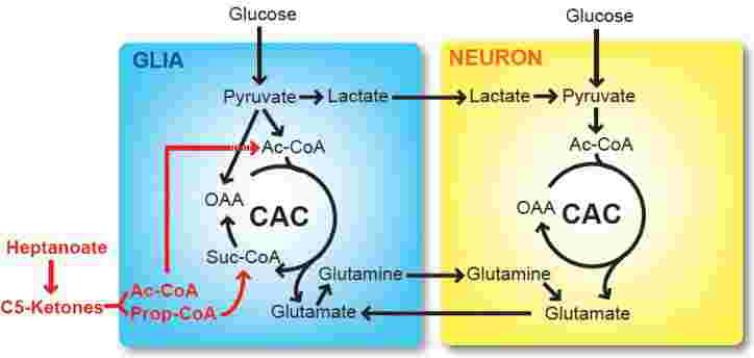
Figure 1. Metabolism of glucose, C7-derived heptanoate and 5-carbon (C5) ketones in the brain.
Glial metabolism is distinct from neuronal metabolism. Glucose can access both glia (via GLUT1) and neurons (via GLUT3), fueling the TCA cycle (CAC). In glia, pyruvate is converted into oxaloacetate (OAA) via carboxylation, donating net carbon to the TCA cycle (anaplerosis). This reaction can be impaired in G1D. Like glucose, the C7 derivative heptanoate and related metabolites (i.e., the 5-carbon ketones beta-ketopentanoate and beta-hydroxypentanoate) also generate acetyl-coenzyme A (Ac-CoA) but, unlike the 4-carbon ketone bodies beta-hydroxybutyrate and acetoacetate, they can also be incorporated into succinyl-coenzyme A (Suc-CoA) via propionyl-CoA (Prop-CoA) formation, supplying net, anaplerotic carbon to the cycle. In addition to 5-carbon (C5) ketones, the 4-carbon ketone bodies beta-hydroxybutyrate and acetoacetate are also metabolites of C7.
This prompted us to conduct an open-label study to evaluate the impact of dietary food-grade triheptanoin on central, disease-relevant phenomena by studying a broad age-range of epileptic G1D patients exhibiting varying degrees of disease severity not receiving a ketogenic diet. To our knowledge, this is the first systematic study of a therapeutic agent in G1D. Based on the glucose-dependence of the abnormalities cited above, we reasoned that, if triheptanoin operates on mechanisms relevant to disease pathogenesis, the therapeutic outcome should be promptly noticeable (i.e., consistent with the intestinal absorption, hepatic metabolism and brain penetration of triglyceride metabolites) and sustained while the therapy is maintained. We characterized the response to triheptanoin across 4 major axes: safety and tolerability, epilepsy by EEG, cerebral metabolic rate of oxygen consumption (CMRO2) by magnetic resonance imaging (MRI), and cognitive ability by neuropsychological assessment. All G1D patients receiving triheptanoin experienced decreased spike-wave seizures, and several of them exhibited a rapid increase in cerebral metabolic rate (i.e., those with the highest frequency of spike-waves) and improved neuropsychological performance, suggesting that triheptanoin is effective in ameliorating the brain glucose-depletion state associated with G1D encephalopathy. The results establish that triheptanoin is endowed with sufficient potential to favorably and significantly impact outcome measures directly relevant to G1D and encourage further trials, including G1D patients receiving a ketogenic diet, young infants, patients with non-convulsive forms of G1D and genetically-unrelated encephalopathies associated with decreased glucose metabolism or abundance, as noted in several neurodegenerative diseases.
METHODS
This study was conducted under the IRB approval of UT Southwestern Medical Center and was referenced under FDA IND 59303 and ClinicalTrials.gov identifier NCT02018315. Informed consent was obtained from each participant over the age of 18 years, whereas informed consent was obtained from one parent of all younger participants. Assent was also obtained from children between 10 and 18 years. Travel and lodging costs up to a total of $500 per family were reimbursed for each visit.
Participants
A summary of patient age, SLC2A1 G1D causative mutation and other parameters is given in Table 1. Study participants were recruited a) from the Rare Brain Disorders Program at UT Southwestern Medical Center and Children’s Medical Center Dallas, including existing patients and those responding to our official website announcement and b) via public announcement by the Glut1 Deficiency Syndrome Foundation. Patients enrolled included male or female gender; age 1 month to 28 years of age and English or Spanish speakers. Subjects were consecutively enrolled from all eligible patients who contacted us. There was no consideration given to geographic location (including the U.S and Canada), disease severity nor to any selection factors other than those stated below. Exclusion criteria included the current use of a ketogenic diet. Participants with metal implants incompatible with exposure to a research magnetic field or unable to tolerate MRI due to anxiety were excluded from MRI procedures only. Participants who were not previously genetically verified for G1D received genotyping via the NIH Office of Rare Diseases Research Collaboration, Education and Test Translation (CETT) program for G1D at UT Southwestern and Children’s Medical Center Dallas. Because about 15% of G1D patients do not harbor identifiable pathogenic mutations in the GLUT1 gene 38, one subject, who exhibited clinical symptoms, decreased CSF glucose and a characteristic brain FDG (18fluorodeoxyglucose)-PET scan 4, 39 indicative of G1D, was enrolled despite normal clinically available DNA sequencing of SLC2A1 and standard chromosomal microarray analysis. No medications were changed immediately prior to or during the study.
TABLE 1.
Demographic, clinical and analytical characteristics of G1D subjects and duration of follow-up on triheptanoin nutritional supplementation. Fasting blood glucose before and after triheptanoin ingestion and fasting beta-hydroxybutyric levels illustrate that triheptanoin did not significantly increase glucose and that patients were not significantly ketotic under their regular diet. Subject GDO1O was diagnosed with G1D as described in the text via clinical features, a characteristic cerebral 18fluorodeoxy-glucose PET, a CSF glucose of 39 mg/dl and a CSF lactate of 1.2 mM.
| G1D subject |
Age at seizure onset (months) |
SLC2A1 G1D mutation |
Age at enrolment (years) |
Fasting blood glucose before triheptanoin (mg/dl) |
Fasting blood beta- hydroxybutyric acid before triheptanoin (mM) |
Blood glucose after triheptanoin |
Total duration of triheptanoin use (months) |
|---|---|---|---|---|---|---|---|
| GD001 | 9 | Missense R218H |
10 | 86 | 0.1 | 84 | 3 |
| GD002 | 8 | Nonsense Y449X |
22 | 92 | 0.1 | 73 | 2 |
| GD003 | 20 | Missense P485L |
15 | 82 | 1.3 | 92 | 3 |
| GD004 | 11 | Missense R333W |
2 | 86 | 0.2 | 89 | 21 |
| GD005 | 14 | Exon III-IV deletion |
9 | 88 | 0.1 | 84 | 20 |
| GD006 | 5 | Intron I splice-site mutation |
11 | 97 | 0.1 | 76 | 20 |
| GD007 | 9 | Missense R126H |
28 | 90 | 0.2 | 83 | 19 |
| GD008 | 11 | Complete hemizygous deletion |
15 | 94 | 0.1 | 80 | 2 |
| GD009 | 9 | Missense R333W |
16 | 73 | 0.1 | 87 | 19 |
| GD010 | 30 | None detected |
4 | 74 | 0.1 | 89 | 19 |
| GD011 | 6 | Intron VII splice-site mutation |
21 | 87 | 0.3 | 81 | 5 |
| GD012 | 1 | Exon X frameshift mutation |
11 | 97 | 0.3 | 84 | 18 |
| GD013 | 17 | Deletion of L169 |
18 | 90 | 0.2 | 73 | 18 |
| GD014 | 3 | Exon II frameshift mutation |
12 | 86 | 0.1 | 92 | 16 |
Dietary supplement
Triheptanoin is a naturally-occurring fat 40 that is readily synthesized from castor bean oil for use in the human food industry as an additive to dairy products or as an emollient in cosmetics 41. In the U.S., food-grade triheptanoin first received orphan designation for the treatment of fatty-acid oxidation disorders after work performed under our IND 59303 (sponsor-investigator CRR) and, more recently (2012), it achieved an equivalent legal status in the European Union for the treatment of very-long-chain 3-hydroxyacyl-CoA-dehydrogenase (VLCAD) deficiency (designation EU/3/12/1081).
G1D participants received open-label, adjunctive supplementation with food-grade triheptanoin, a medium chain triglyceride of heptanoic acid that is colorless and odorless. Triheptanoin was manufactured in oil form by SASOL Germany GmbH (Witten, Germany) and permitted, per the manufacturer (Product Information documentation version 4.02; revision date 1/16/2008), for applications in the food industry as a food additive (butter marker-fat) and commercialized as Spezialöl® 107 (CAS /EINECS numbers: 620-67-7 / 210-647-2; minimum 95% triheptanoate and 99% C7 fatty acid as determined by gas chromatography by the manufacturer). The triheptanoin oil used in this study was purchased from the SASOL North American distributor.
Procedures
The experimental procedures are illustrated in Figure 2. Each patient underwent a pre-treatment medical history, complete physical and neurological exams, general analytical laboratory evaluation (following overnight fasting), EEG (electroencephalogram), and neuropsychological evaluation. Participants who consented to imaging also underwent a 30 minute MRI examination of the total rate of oxygen consumption by the brain (i.e., the cerebral metabolic rate CMRO2).
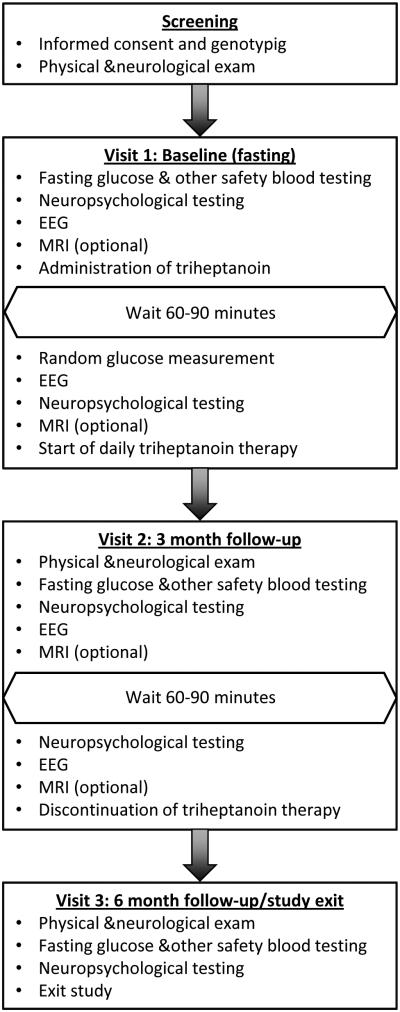
Figure 2. Flow diagram of study visits and procedures.
Each subject participated in 3 visits: Screening/baseline (initiation of triheptanoin supplementation), 3 month follow-up (discontinuation of triheptanoin supplementation), and 6 month follow up (study exit).
All participants underwent EEG recording. The EEG was acquired by placing an MRI-compatible electrode cap containing 32 active electrodes except where noted below. An additional electrode was placed directly on the skin of the chest. All preparation equipment coming into contact with skin was discarded after a single use, and electrodes and caps were disinfected after use following standardized UT Southwestern Medical Center guidelines.
Baseline (resting) EEG recordings after overnight (8 hr or greater) fasting (except for water and medications) were obtained for 30-45 minutes. During this time, baseline neuropsychological testing was completed. Parents and patients (when sufficiently mature) were asked to note all visible seizures and their observations were contrasted with EEG. After a sufficiently informative EEG baseline (i.e., defined as a tracing containing a steady rate of spike-wave discharges, Figure 3) had been established based on online and off-line analyses, participants consumed a single dose of triheptanoin oil over 1 to 3 min, in amounts ranging from 15-60 cc, according to body weight (0.75-1 g/kg). Participants remained EEG-monitored for at least an additional 90 min after consuming the oil. Post-triheptanoin neuropsychological testing was repeated approximately 90-120 minutes after oil ingestion. Blood samples were collected from each participant before and at the conclusion of the EEG. A seizure rate, defined as the total duration of EEG recorded spike-wave seizures divided by the total duration of the EEG recording, was calculated for baseline period (before triheptanoin oil consumption) and post-triheptanoin period (from 5 min after triheptanoin oil consumption to the conclusion of EEG recording).
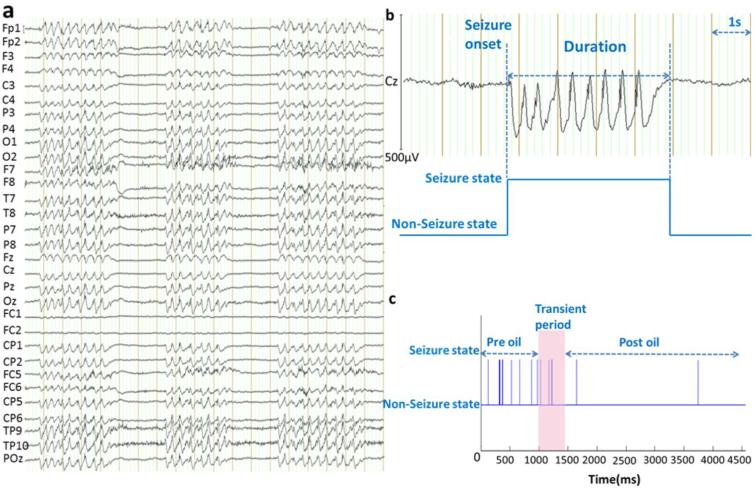
Figure 3. EEG-captured spike-wave seizures in G1D patients.
(a) An example segment of EEG recording containing three spike-wave seizure periods. Electrode position followed standard abbreviated nomenclature. Vertical bars: 1 s. (b) Illustration of the identification of seizure duration. (c) The seizure-nonseizure binarized time course of a representative subject: Spike-wave periods are represented as bar plots against EEG time course in the setting of a non-seizure baseline state. Triheptanoin (oil) administration is marked as a pink bar in (c).
Participants who consented to imaging received MRI before and after triheptanoin. The second MRI took place 60 - 90 min following triheptanoin consumption. This time interval was selected to capture peak metabolism of triheptanoin in blood as separately determined by us (CRR, unpublished observations, n=11). MRI was obtained pre- and post-oil, immediately after each session of neuropsychological testing followed by a 5 min rest period. EEG acquisition was continuous during MRI.
MRI included a routine axial T1-weighted sequence to establish that brain configuration was normal in all subjects and, more importantly, allowed for CMRO2 quantification as developed by us 42-47, with a test-retest CMRO2 reliability of less than 5% error. In brief, using phase-contrast MRI and T2-Relaxation Under Spin Tagging (TRUST) MRI measurements, the whole-brain arteriovenous oxygenation gradient was determined and used to calculate brain oxygen extraction with the aid of full brain volumetric measurements excluding cerebrospinal fluid. Scan acquisition time was 5 min per MRI. No sedation or stimulation was administered.
Neuropsychological testing consisted of the Peabody Picture Vocabulary Test, Fourth Edition (PPVT-4) and the Expressive Vocabulary Test, second edition (EVT-2). These two tests were chosen for their ease of administration, relatively short administration time, and availability of parallel forms, allowing for quick re-administration without the concern of practice effects (Dunn, L.M. & Dunn, D.M. (2007). Peabody picture vocabulary test manual (4th ed.). Minneapolis, MN: Pearson Assessments. Williams, K.T. (2007). Expressive vocabulary test manual (2nd ed.). Minneapolis, MN: Pearson Assessments. Strauss, E., Sherman, E., & Spreen, O. (2006). A compendium of neuropsychological tests: Administration, norms, and commentary (3rd ed.). New York: Oxford University Press). Administration of the tests took approximately 30-45 minutes per testing session.
3-month triheptanoin consumption
To investigate long-term impact on outcome measures, triheptanoin oil was then given for 3 months, at approximately 1 g/kg of body weight per day, divided into 4 doses per day, given 30 to 60 min before a routine meal. This dose represents ~30% of the total dietary fat. Participants followed additional dietary modifications with the goal of maintaining total daily fat intake and body weight constant. A reduction in simple carbohydrate consumption was also instituted with the intent of lowering glycemic index so as to minimize insulin release, interference with ketogenesis or excessive weight gain. Daily caloric and protein intake remained unmodified.
Long-term follow-up
Participants were evaluated again at 3 months and 6 months for follow-up testing and post-treatment advice as necessary. Triheptanoin was discontinued at the 3 month visit. Follow-up visits included complete physical and neurological exams, general analytical laboratory analyses, and neuropsychological testing. Additional CMRO2 determination or EEG was performed as described below for select participants.
Longer-term (indefinite) consumption of triheptanoin
All participants who reported clinical benefit and requested to continue triheptanoin beyond the 3 month visit were removed from the initial research protocol. In order to continue triheptanoin for an indefinite period, patients voluntarily submitted a signed letter requesting continuation and then were enrolled in a second IRB-approved protocol, agreeing to provide semi-annual blood tests and general physical examination reports. Contact was maintained with each of these participants via telephone or secure, HIPAA-compliant email communications as needed.
Statistical analyses
The primary outcome of our study was a reduction in electrographic seizures as measured by seizure rate (total time spent seizing divided by total EEG record time) and increased neurocognitive performance as measured by the PPVT and EVT. Secondary outcomes included CMRO2. Descriptive statistics are provided for demographic and clinical measures. Wilcoxon matched pairs signed rank sum or Student’s paired t-tests were performed on EEG and CMRO2 data before and after triheptanoin consumption at baseline, and baseline and 3-month measures of EEG, neuropsychological measures and blood analyses (cholesterol, HDL, LDL, triglycerides, glucose levels, and beta hydroxybutyrate). IBM© SPSS V20 was used to analyze these data using a two-sided p-value (unless otherwise noted).
RESULTS
Patient characteristics
Fourteen patients diagnosed with G1D were enrolled (Table 1). The median age at enrollment was 13.5 years of age (range: 2-28), and 42% (6/14) of the patients were female. Most patients identified themselves as Caucasian (N=12; 1 patient was African American and 1 patient was Caucasian and Asian) and non-Hispanic (N=13). The median age of onset of symptoms was 8.5 months old (range: 0-30 months, n=13), and the median age at diagnosis was 7.3 years (range: 2-12 years old, n=11). Patients were enrolled from across the U.S. and Canada.
Of the 14 patients enrolled in the trial, 5 participated in imaging. These patients were older than 15 years. The 9 patients who elected not to participate did so for specific reasons (inability to remain immobile due to movement disorder or anxiety, n=5; immaturity, n=2; metal implants preventing exposure to research MRI, n=1; personal choice, n=1).
Adverse events
No patients experienced serious or unexpected adverse events. One patient discontinued triheptanoin after three weeks due to gastric discomfort. Another patient experienced significant (10%) weight gain at 2 months that did not lead to discontinuation. This patient did not follow the recommended dietary and nutritional advice by decreasing extra sources of fat and simple sugars. Additionally, two patients experienced diarrhea and/or digestive discomfort within days of treatment initiation, but these symptoms resolved by reducing the dose of triheptanoin by one-half and gradually increasing the amount to the target levels over several days.
Correlation between EEG and absences
All 14 patients were observed by their primary caretaker while undergoing EEG. Mature patients and their caretakers were asked to report all absence events that were noticeable to them. Patients and caretakers had no access to the EEG tracing and were visually supervised in a separate room through a window by the EEG recording operator. The EEG operator noted the correlation between reported absence or any other potential seizure events and the EEG tracing. 100% of subjects underreported EEG spike-wave seizures. For 7 subjects, over 50% of spike-wave seizures lasting over 3 sec were not noticeable by the observer as absences or other abnormal behavior. For 4 subjects, no absence or other abnormal manifestation was reported, despite the occurrence of abundant spike-wave seizures lasting over 3 sec.
Immediate response to therapy
EEG
EEG recordings were analyzed as described in our previous work 48-50 with the exception that only the clinically-relevant aspects of the EEG (i.e., those that are part of a standard EEG report) were analyzed. Of the 14 patients enrolled, 2 patients were urgently initiated on triheptanoin in the inpatient setting due to unmanageable epilepsy (more frequent than hourly absence seizures) and thus baseline research EEG recordings were not performed as described above and were thus not included in the quantitative EEG analysis. However, these patients were EEG-monitored just as triheptanoin was initiated and its administration was followed by the termination of absences and a decrease in EEG spike-waves within 2 hr after ingestion. Of the 12 remaining patients, one patient exhibited no EEG or observable absence seizures at baseline for the duration of the EEG recording and was also excluded from the EEG data analysis. Of note, the overall self-reported or parental-reported absence seizure rate was below 20% of all electrographic spike-wave seizures, regardless of EEG seizure frequency or duration.
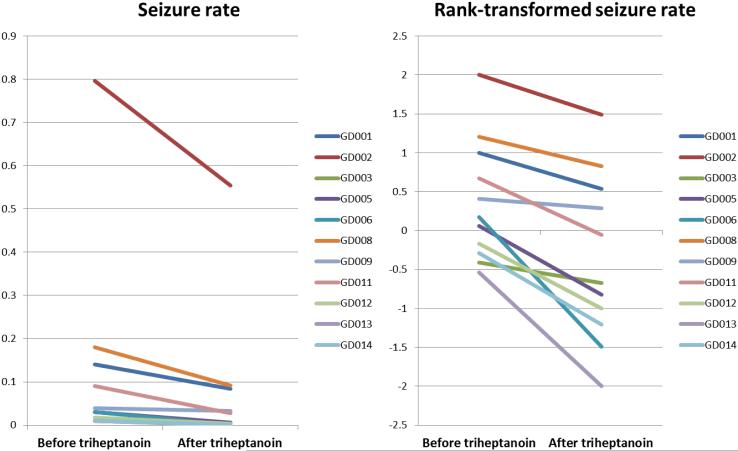
Electrographic spike-wave seizures were precisely captured by EEG recording (Figure 3). There were no other types of EEG seizures in any of the patients. The seizure rate was calculated both before and after oil administration for each subject by dividing the total duration of recorded spike-wave seizures by the total duration of the EEG recording (Table 2 and Figure 3). As shown in Figure 3b, the duration of each spike-wave segment was delimited by the onset of the first spike and the return to the baseline rhythm immediately after the last wave. In order to meet the assumption of normal distribution of the data, a rank-transformation was performed. A paired t-test was used to compare the rates of seizures before and after oil administration at baseline. A significant decrease in seizure rate was observed in all patients (p<0.05) (Figure 4).
TABLE 2.
Seizure rates before and after acute triheptanoin ingestion. Spike-wave rate changes (expressed in %) were calculated as: (rateafter − ratebefore) / ratebefore × 1OO%. The mean ± SD of all the seizure rate changes was −62.5±29.8%, p= 0.0004.
| G1D subject | Seizure rate | Rank-transformed Seizure rate |
|||
|---|---|---|---|---|---|
| Before C7 | After C7 | Change (%) | Before C7 | After C7 | |
|
| |||||
| GD001 | 0.1404 | 0.0845 | −39.83% | 0.9982 | 0.5375 |
| GD002 | 0.7970 | 0.5545 | −30.43% | 2.0004 | 1.4895 |
| GD003 | 0.0098 | 0.0064 | −35.03% | −0.4100 | −0.6745 |
| GD005 | 0.0296 | 0.0054 | −81.64% | 0.057 | −0.8255 |
| GD006 | 0.0302 | 0.0005 | −98.24% | 0.1717 | −1.4895 |
| GD008 | 0.1800 | 0.0918 | −49.01% | 1.2074 | 0.8255 |
| GD009 | 0.0390 | 0.0333 | −14.68% | 0.41 | 0.2888 |
| GD011 | 0.0898 | 0.0271 | −69.78% | 0.6745 | −0.057 |
| GD012 | 0.0178 | 0.0035 | −80.30% | −0.1717 | −0.9982 |
| GD013 | 0.0097 | 0.0000 | −100.00% | −0.5375 | −2.0004 |
| GD014 | 0.0101 | 0.0011 | −88.82% | −0.2888 | −1.2074 |
Figure 4. Seizure rate reduction after acute triheptanoin oil consumption.
(a) Seizure rate of each subject pre- and post-triheptanoin oil consumption. (b) Rank-transformed seizure rate of each subject pre- and post-triheptanoin oil consumption.
Neuropsychological indices
Nine patients completed baseline neuropsychological testing. The first three participants did not undergo neuropsychological testing owing to logistic limitations, and two participants were urgently enrolled as inpatients as described above. Therefore, the PPVT and EVT could not be administered to them. In all, the PPVT and EVT could not be administered in 5 patients.
At the baseline visit, patients underwent testing while fasting, then again about one hour after ingesting triheptanoin. During this visit, on the PPVT, 5 out of 9 participants showed improvement on receptive vocabulary and no participants worsened. On the EVT, 4 out of 9 participants showed improvement and, again, no participants worsened. Overall, 7 out of 9 participants showed improved scores on some aspect of neuropsychological testing between the fasting baseline and testing an hour post-oil, but these changes were not significant (Figure 5).
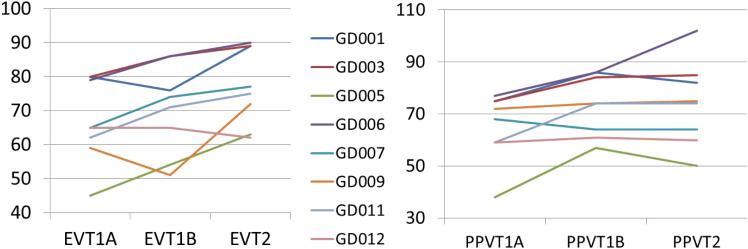
Figure 5. Neuropsychological indices in G1D patients after triheptanoin food supplementation.
Vocabulary performance improved acutely and long term with triheptanoin supplementation. The neuropsychological scores of all 8 G1D subjects before and after triheptanoin are represented. Subject designations are the same through all the figures and tables. Standardized PPVT and EVT ratings (y-axis) were obtained in the fasting state (baseline) at time 0 min (x-axis 1A suffix) and 60 min (x-axis 1B suffix) following triheptanoin ingestion, and then subsequently after 3 months of daily triheptanoin supplementation (x-axis T2 suffix). PPVT and EVT scores were below normal age ranges and increased at subsequent time points in rigorously statistically significant fashion. The 95% confidence intervals for the PPVT scaled scores at each of the three time points studies were 54.4 - 76.3; 63.6 - 82.9 and 60.4 - 87.6, respectively. PPVT scores improved significantly over time (F2,14 = 5.945, p=0.014), although there were no significant pairwise comparisons after Bonferroni adjustment for multiple comparisons. The 95% confidence intervals for the EVT scaled scores at each of the three time points studies were 56.6 - 77.2; 59.4 - 81.3 and 67.6 - 86.6, respectively. EVT scores improved significantly over time (F2,14 = 9.571, p=0.002). Pairwise comparisons yielded (pre-oil relative to follow-up) p=0.006 (Bonferroni adjustment for multiple comparisons).
Blood glucose
Blood glucose levels were measured 90-120 minutes after oil consumption at the baseline visit and compared to fasting levels collected earlier that morning. A Wilcoxon matched pairs signed rank sum analysis yielded a significant decrease (Fasting Glucose Median=87.00, Post-oil Glucose Median=81.00, N=5, p<0.01).
Brain metabolic rate (CMRO2)
Five patients completed imaging at baseline. The average percent change from baseline following the first administration of triheptanoin was 5.30% (SD=7.40), but this change was not significant (p=0.18) when analyzed as a group. Three participants experienced an increase in brain metabolic rate, one experienced no change, and one participant experienced a slight lowering of brain metabolic rate (Figure 5).
Response at 3 month follow-up
Neuropsychological testing
When baseline fasting PPVT and EVT scores were compared to scores at the three month follow up, immediate gains (i.e., those detected after the first triheptanoin dose) were maintained on the PPVT, i.e., all 5 patients who exhibited immediate improvement continued to manifest similar improvement at the three month follow up. Immediate gains were also maintained on the EVT, with 2 additional patients demonstrating improvement, for a total of 6 out of 9 patients showing improvement at the three month follow up. Significant improvement was observed over time; the change from pre- to post-triheptanoin was not significant, but gains made by the 3 month follow up were significant (p<0.05; Figure 5).
Laboratory tests
Blood glucose, cholesterol, HDL, LDL, triglycerides, and beta hydroxybutyrate levels were compared between baseline and the 3 month follow up using Wilcoxon matched pairs signed rank sum. All values were normal relative to standard laboratory ranges. There were no significant differences (p>0.05; not shown).
Brain metabolic rate CMRO2
Two patients participated in imaging at 5-6 months follow up under a separate IRB-approved protocol. The first of these participants, designated GD007, who had manifested no EEG seizures and no change in CMRO2 after triheptanoin ingestion, demonstrated a 17% increase in CMRO2 from fasting baseline levels. The second patient, designated GD009, manifested no significant change in CMRO2 relative to a significant, previously increased CMRO2 after triheptanoin initiation (Figure 6).
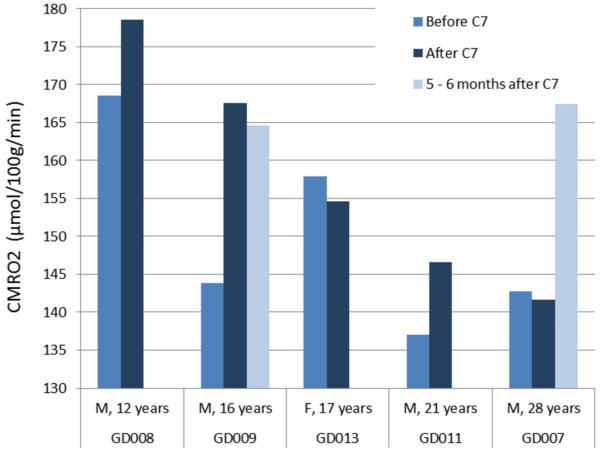
Figure 6. MRI-measured CMRO2 of each subject pre- and post- acute triheptanoin oil consumption.
In subjects GD009 and GD007, CMRO2 was measured again at 5-6 months post-triheptanoin oil consumption. The normal values for CMRO2 have been measured by us in a broad adult age-range and are gender-dependent (i.e., females exhibit higher CMRO2 than males). At ages 21 and 28 years, the normal male CMRO2 is 149 and 154 µmol/100g/min, respectively. The standard deviation none of our normal CMRO2 measurements exceeds 12.5 µmol/100g/min (HL et al, Age-related increase of resting metabolic rate in the human brain; submitted).
Continuation of the medical food supplementation regimen
Of the 14 participants, 12 completed three months of triheptanoin consumption and 11 returned for the 3-month follow up. One participant discontinued earlier than stipulated due to intolerable gastric discomfort and one discontinued due to parent-reported lack of efficacy. Of the 12 participants who completed 3 months of therapy, 1 participant declined to return for follow up due to financial hardship.
Of the 11 participants who returned for the 3-month follow up, only one elected to discontinue triheptanoin therapy at this time due to weight gain. Ten participants signed informed consent to continue treatment with triheptanoin as described above. Of the 10 participants who continued, 1 patient discontinued after 5 months of use due to lack of efficacy, although parental reporting indicated noncompliance. All other subjects continued to receive triheptanoin without adverse effects at the time of the last assessment for a total of 185 patient-months.
COMMENT
This study was motivated by two reasons. First, the pharmacological treatment of G1D seizures is generally ineffective. A potential role for several specific anticonvulsants was suggested by the spike-wave EEG pattern often identified in G1D seizures, which is typical of some absence epilepsies 51,52. Yet this approach has proven ineffective 16. In practice, anticonvulsant utilization and discontinuation in G1D remains subordinate to trial-and-error 16, 53-56.
Second, we noted that, normally, an important brain glucose-derived anaplerotic process is catalyzed by pyruvate carboxylase inside the astrocyte 57, 58, which depends on glycolysis (i.e., on pyruvate generation) to refill TCA cycle precursors and maintain byproduct output, including glutamate, glutamine and GABA 59. Neurons may carry out only a modest degree of anaplerosis via the malic enzyme 60. In contrast, common (i.e., natural) dietary fats are oxidized into only even-carbon number ketone bodies (β-hydroxybutyrate and acetoacetate) 22, which lead to acetyl-CoA generation, but cannot sustain anaplerosis because they are fully converted into water and CO2 in the TCA cycle 21, 30. Thus, any ketogenic diet in use today, especially when it results in relative hypoglycemia, represents a rudimentary – if not a restrictive - treatment for G1D when considered from this perspective: even-carbon number ketones, generated from common dietary fat or a ketogenic diet, ameliorate seizures, but are not anaplerotic, as underscored by the fact that the two key metabolic roles of glucose are (a) energy production by oxidation of acetyl-coenzyme A and (b) anaplerosis by providing pyruvate for carboxylation. Ketogenic diets can fulfill (a), but the diet’s fat cannot meet (b). In contrast, we have shown that mouse brain nuclear magnetic resonance (NMR) and mass spectrometry 32 indicate that heptanoate is anaplerotic because it can satisfy both (a) and (b) 33. Further observations made in our transgenic antisense G1D mice, which appear normal at birth but later develop frequent spontaneous seizures, decreased brain weight, spasticity, and ataxia (thus closely mimicking the most commonly recognized human G1D syndrome) also converge on the potential utilization or overutilization of alternative metabolic sources by the G1D brain: In the state of depleted brain glucose accumulation and spontaneous systemic ketosis typical of G1D mice, whole-brain TCA cycle intermediates and neurotransmitter contents are preserved, undermining the hypothesis of global brain energy failure, while suggesting that TCA refilling proceeds via enhancement of alternative metabolic fluxes such as fatty acids 13. This was illustrated for heptanoate as noted above 33.
Clinical outcomes
As indicated by the blood analytical values, there was no change in glucose levels or in lipid levels. This indicates that, despite the addition of triheptanoin to the diet, the food supplement is safe and metabolically neutral as assessed by these analyses.
EEG seizure reduction and neuropsychological results of receptive and expressive vocabulary were favorably impacted by triheptanoin. Other non-clinical data and non-systematic evidence support the clinical improvement noticed by many patients as well. The voluntary continuation rate (11 out of 14 at 3 months, 10 out of 14 at 6 months) is indicative of improvement. Several parents spontaneously reported that other caregivers (school teachers, speech and physical therapists) who were unaware of study participation noted marked improvement, both motor and cognitive. This is consistent with improvement on the vocabulary tests, which measure not only language ability, but also general intellectual function, as patients are required to maintain attention and concentration for the duration of the task. Perhaps most striking, the very young participants (age 2 and 4 years), who were significantly delayed in all aspects of intellectual and motor function, made rapid improvements in developmental milestones, meeting them at age-appropriate intervals.
Cerebral metabolic rate CMRO2
The CMRO2 is diminished in G1D (Figure 6). In contrast with the cerebral metabolic rate characteristic of normal subjects of the same age than those studied here 61, G1D patients demonstrate decreased but uniquely different CMRO2 values. This phenomenon may reflect the broad phenotypic diversity of our case series, of which the CMRO2 is but one aspect. Because G1D is a life-long genetic disorder that impacts the brain in early infancy, it is also unknown what the maximum achievable CMRO2 level is in G1D should patients experience a full restoration of glucose influx after the disorder has proven symptomatic for an extended developmental period of their lives, or even if the age-normal CMRO2 can be exceeded as the consequence of a favorable therapeutic effect. Of note, it is not plausible – nor was it attempted - to safely advance general correlations between CMRO2 and spike-wave frequency or neuropsychological indices, as this outside the scope and inferential power of this study. Therefore, only individual observations deserve remarks: Of 5 subjects who received CMRO2 determination, three demonstrated rapid (i.e., consistent with the cerebral metabolism of triheptanoin metabolites), significant increases (Figure 6, subjects GD008, GD009 and GD011). The CMRO2 of subjects GD013 and GD007 did not increase after acute triheptanoin ingestion. Subject GD013 exhibited spike-wave seizures that were completely abolished by triheptanoin (Figure 4), and subject GD007 did not exhibit spike-wave seizures neither before nor after triheptanoin. The robustness of the CMRO2 measurements prompts an explanatory framework for these single-subject observations: The simplest interpretation of these results, should they prove a general feature of G1D, is that triheptanoin metabolism may lead to increased oxygen consumption only while the brain undergoes a reduction of ictogenesis. In parallel to this contention, we hypothesize that, when ictogenesis is abolished by triheptanoin or absent at baseline, triheptanoin does exert little or no effect on CMRO2. Because the relationship between ictogenesis and whole-brain oxygen consumption is unknown (as determined by the global encephalic CMRO2 reported here), and because G1D spike-wave seizures may be caused by aberrant thalamocortical synchronization rather than global or multifocal epileptogenesis 13, further work currently in progress will aim to elucidate CMRO2 changes in regional, ictogenic G1D tissue. Also notable is the significant increase in CMRO2 for subject GD007 after 6 months of treatment. Despite the absence of epilepsy as detected by observation and by EEG, this finding is compatible with triheptanoin – induced long-term changes in the non-epileptic G1D brain.
Potential therapeutic mechanisms of triheptanoin in human G1D
Glucose supports brain metabolism and neurometabolic diseases involving dysfunctional glucose metabolism are often associated with intractable absence seizures 5. Additional molecules such as fatty acids from ketogenic diets, and their derivative ketone bodies, can partially substitute for glucose, except that: 1) normal or postprandially increased glycemia interfere with ketosis, necessitating that canonical ketogenic diets are, paradoxically for brain states associated with decreased glucose flux 62, carbohydrate-restricted; 2) mitochondrial fatty acid oxidation can compete with glucose metabolism 63; and 3) all food-derived fats and ketone bodies contain even chain carbons which are fully consumed in the tricarboxylic acid (TCA) cycle and excreted as water and CO2, thus yielding no net carbon to compensate for the natural loss of metabolites 2, 30, 64. This compensation is normally provided by anaplerosis, which, in the brain, stems principally from glucose via carbon-donor reactions 65, which are unavailable in the ketogenic diet. In contrast with ketogenic diets, the metabolism of triheptanoin generates both even-carbon and 5-carbon ketones (beta-hydroxypentanoate, beta-ketopentanoate) in the liver, and the latter are anaplerotic 30, 66, 67, potentially affording superior benefit. We have found that G1D is associated with cerebral carbon depletion in mice 13and the results of this trial support prior findings of reduced cerebral oxygen consumption (CMRO2) in man 68.
Ictogenesis, spike-wave and absence epilepsy in G1D
In this study, we attempted to follow a mechanistic approach by targeting what we believe represent primary causal events. This framework is largely based on our work illustrating that decreased glucose flux leads to cerebral metabolic deficit which, in turn, differentially impacts excitatory and inhibitory synaptic balance, and on the fact that these aberrant phenomena are poised to be relevant to disease pathogenesis based on the plausible direct relation between metabolism, synaptic function 69 and epileptogenesis 70 and, further, that they are favorably modifiable (in G1D patients and mouse brain slices) by increasing blood glucose or ketone body availability. We have observed that: 1) G1D is associated with diminished cerebral glucose flux (resulting in decreased brain acetyl-coA), 2) there is no intrinsic neuronal excitability dysfunction or gross structural abnormalities as suggested by the rapid reversibility of the human EEG and of key synaptic current abnormalities with glucose or ketone bodies, and 3) brain glucose is used for anaplerosis and stimulating this process may be of additional benefit when glucose availability is decreased. Given these observations, we reasoned that providing both a source of acetyl-coA and a source of anaplerotic carbon (via triheptanoin metabolism), results in a treatment strategy based upon disease mechanisms and one that anticonvulsants or the ketogenic diet do not target. This represents a reductionist approach, such that complementary interventions targeting secondary excitability or other abnormalities may be needed, as is the case with most neurological diseases. In line with the necessarily reductionist approach outlined above, we selected the EEG as an important outcome. However, there are many non-observable biological aspects in epilepsy and, consequently, the field continues to be driven by the most reliable and accessible indicators, which have been in use for decades (for example, EEG, quality of life and intellectual assessments). Best treatments include those that target causes, as we believe triheptanoin does. But observation need not be synonymous with causation: Absence seizures, such as those noted in G1D, are temporally well-circumscribed impairments of consciousness associated with visually-observable manifestations 71. During a typical absence, EEG recording reveals repetitive spike-waves 72. Yet, observable absences, EEG and sustained attention can be differentially impacted by drug treatment 73, 74, supporting the limited-causality clause above. However, we anticipate that further ways of assessing epilepsy will emerge – thus our focused approach here.
Implications for further trials: Is triheptanoin a drug, prodrug, or a medical food?
The IUPAC definition of a prodrug is a compound that undergoes biotransformation before exerting pharmacological effects. Prodrugs are thus canonical drug precursors containing specialized nontoxic protective groups used in a transient manner to alter or to eliminate undesirable properties in the parent molecule. In contrast, the term medical food, as defined in section 5(b) of the Orphan Drug Act (21 U.S.C. 360ee (b) (3)) identifies any food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation. In this sense, triheptanoin fulfills the requirements of a medical food.
Limitations and new questions
Although two patients discontinued triheptanoin due to gastrointestinal intolerance, this unintended effect has been observed with oil consumption and can be ameliorated by varying the administration schedule or by administering triheptanoin in solid form or in an emollient, such as sugar-free and fat-free yogurt or pudding.
The patients enrolled spanned 2 through 28 years, and this heterogeneity in ages implies heterogeneity in brain metabolism rates. The dose administered (1 g/kg), while appropriate for older adolescents and adults, did not take into account the younger ages, and therefore higher metabolism, of some of the patients enrolled in the trial. Future trials using biologically-relevant age stratification can help assess impact on outcomes over changing levels of metabolism through development.
ACKNOWLEDGMENTS
The generous support of the Glut1 Deficiency Foundation and K. Meyers family is gratefully acknowledged. Neither of them participated in the study inception, design, data interpretation or manuscript generation. JMP is supported by NIH grants NS077015, NS078059, RR002584 and RR024982. JMP and JYP are supported by the Office of Rare Diseases Research Glucose transporter type I deficiency syndrome (G1D) collaboration, education, and test translation (CETT) program for rare genetic diseases. LBG is supported by NS065640. HL is supported by NIH grants NS067015 and MH084021.
Footnotes
Competing interests: None
REFERENCES
- 1.Pascual JM, Wang D, Lecumberri B, et al. GLUT1 deficiency and other glucose transporter diseases. Eur J Endocrinol. 2004 May;150(5):627–633. doi: 10.1530/eje.0.1500627. [DOI] [PubMed] [Google Scholar]
- 2.Marin-Valencia I, Roe CR, Pascual JM. Pyruvate carboxylase deficiency: mechanisms, mimics and anaplerosis. Mol Genet Metab. 2010 Sep;101(1):9–17. doi: 10.1016/j.ymgme.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Jeffrey FM, Marin-Valencia I, Good LB, et al. Modeling of brain metabolism and pyruvate compartmentation using (13)C NMR in vivo: caution required. J Cereb Blood Flow Metab. 2013 Aug;33(8):1160–1167. doi: 10.1038/jcbfm.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascual JM, Van Heertum RL, Wang D, Engelstad K, De Vivo DC. Imaging the metabolic footprint of Glut1 deficiency on the brain. Ann Neurol. 2002 Oct;52(4):458–464. doi: 10.1002/ana.10311. [DOI] [PubMed] [Google Scholar]
- 5.Pascual JM, Campistol J, Gil-Nagel A. Epilepsy in inherited metabolic disorders. Neurologist. 2008 Nov;14(6 Suppl 1):S2–S14. doi: 10.1097/01.nrl.0000340787.30542.41. [DOI] [PubMed] [Google Scholar]
- 6.Ronen GM, De Vivo DC, Harik SI. A second case of defective glucose transport at the blood-brain barrier. Emergence of a novel clinical syndrome. Ann Neurol. 1991;30:452. [Google Scholar]
- 7.De Vivo DC, Trifiletti RR, Jacobson RI, Ronen GM, Behmand RA, Harik SI. Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med. 1991 Sep 5;325(10):703–709. doi: 10.1056/NEJM199109053251006. [DOI] [PubMed] [Google Scholar]
- 8.Arsov T, Mullen SA, Rogers S, et al. Glucose transporter 1 deficiency in the idiopathic generalized epilepsies. Ann Neurol. 2012 Nov;72(5):807–815. doi: 10.1002/ana.23702. [DOI] [PubMed] [Google Scholar]
- 9.Mullen SA, Suls A, De Jonghe P, Berkovic SF, Scheffer IE. Absence epilepsies with widely variable onset are a key feature of familial GLUT1 deficiency. Neurology. 2010 Aug 3;75(5):432–440. doi: 10.1212/WNL.0b013e3181eb58b4. [DOI] [PubMed] [Google Scholar]
- 10.Lund-Andersen H. Transport of glucose from blood to brain. Physiol Rev. 1979 Apr;59(2):305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- 11.Good LB, Espinosa F, Ma Q, Heilig CW, Kavalali ET, Pascual JM. A neuronal excitability defect in a prototypic energy metabolism disorder; American Epilepsy Society annual meeting; Seattle, WA, USA. 2008. p. 364. Epilepsia 49. 364. [Google Scholar]
- 12.Good LB, Ma Q, Kavalali ET, Heilig CW, Pascual JM. Society for Neuroscience Annual Meeting. Society for Neuroscience; Chicago: 2009. Diminished synaptic quantal amplitudes in a brain energy metabolic disorder. 330.338/H327. [Google Scholar]
- 13.Marin-Valencia I, Good LB, Ma Q, et al. Glut1 deficiency (G1D): epilepsy and metabolic dysfunction in a mouse model of the most common human phenotype. Neurobiol Dis. 2012 Oct;48(1):92–101. doi: 10.1016/j.nbd.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akman CI, Engelstad K, Hinton VJ, et al. Acute hyperglycemia produces transient improvement in glucose transporter type 1 deficiency. Ann Neurol. 2010 Jan;67(1):31–40. doi: 10.1002/ana.21797. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Pascual JM, Yang H, et al. Glut-1 deficiency syndrome: clinical, genetic, and therapeutic aspects. Ann Neurol. 2005 Jan;57(1):111–118. doi: 10.1002/ana.20331. [DOI] [PubMed] [Google Scholar]
- 16.Pong AW, Geary BR, Engelstad KM, Natarajan A, Yang H, De Vivo DC. Glucose transporter type I deficiency syndrome: epilepsy phenotypes and outcomes. Epilepsia. 2012 Sep;53(9):1503–1510. doi: 10.1111/j.1528-1167.2012.03592.x. [DOI] [PubMed] [Google Scholar]
- 17.Klepper J, Scheffer H, Leiendecker B, et al. Seizure control and acceptance of the ketogenic diet in GLUT1 deficiency syndrome: a 2- to 5-year follow-up of 15 children enrolled prospectively. Neuropediatrics. 2005 Oct;36(5):302–308. doi: 10.1055/s-2005-872843. [DOI] [PubMed] [Google Scholar]
- 18.Klepper J. Glucose transporter deficiency syndrome (GLUT1DS) and the ketogenic diet. Epilepsia. 2008 Nov;49(Suppl 8):46–49. doi: 10.1111/j.1528-1167.2008.01833.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim do Y, Rho JM. The ketogenic diet and epilepsy. Curr Opin Clin Nutr Metab Care. 2008 Mar;11(2):113–120. doi: 10.1097/MCO.0b013e3282f44c06. [DOI] [PubMed] [Google Scholar]
- 20.Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013 Jan;36(1):32–40. doi: 10.1016/j.tins.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yudkoff M, Daikhin Y, Melo TM, Nissim I, Sonnewald U, Nissim I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu Rev Nutr. 2007;27:415–430. doi: 10.1146/annurev.nutr.27.061406.093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bough K. Energy metabolism as part of the anticonvulsant mechanism of the ketogenic diet. Epilepsia. 2008 Nov;49(Suppl 8):91–93. doi: 10.1111/j.1528-1167.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kielb S, Koo HP, Bloom DA, Faerber GJ. Nephrolithiasis associated with the ketogenic diet. J Urol. 2000 Aug;164(2):464–466. [PubMed] [Google Scholar]
- 24.Stewart WA, Gordon K, Camfield P. Acute pancreatitis causing death in a child on the ketogenic diet. J Child Neurol. 2001 Sep;16(9):682. doi: 10.1177/088307380101600910. [DOI] [PubMed] [Google Scholar]
- 25.Best TH, Franz DN, Gilbert DL, Nelson DP, Epstein MR. Cardiac complications in pediatric patients on the ketogenic diet. Neurology. 2000 Jun 27;54(12):2328–2330. doi: 10.1212/wnl.54.12.2328. [DOI] [PubMed] [Google Scholar]
- 26.Berry-Kravis E, Booth G, Taylor A, Valentino LA. Bruising and the ketogenic diet: evidence for diet-induced changes in platelet function. Ann Neurol. 2001 Jan;49(1):98–103. doi: 10.1002/1531-8249(200101)49:1<98::aid-ana13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Hoyt CS, Billson FA. Optic neuropathy in ketogenic diet. Br J Ophthalmol. 1979 Mar;63(3):191–194. doi: 10.1136/bjo.63.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klepper J, Leiendecker B, Bredahl R, et al. Introduction of a ketogenic diet in young infants. J Inherit Metab Dis. 2002 Oct;25(6):449–460. doi: 10.1023/a:1021238900470. [DOI] [PubMed] [Google Scholar]
- 29.Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002 Jul;110(2):259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis. 2006 Apr-Jun;29(2-3):327–331. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- 31.Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003 Jul 2;23(13):5928–5935. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin-Valencia I, Good LB, Ma Q, Jeffrey FM, Malloy CR, Pascual JM. High-resolution detection of (1)(3)C multiplets from the conscious mouse brain by ex vivo NMR spectroscopy. J Neurosci Methods. 2012 Jan 15;203(1):50–55. doi: 10.1016/j.jneumeth.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin-Valencia I, Good LB, Ma Q, Malloy CR, Pascual JM. Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain. J Cereb Blood Flow Metab. 2013 Feb;33(2):175–182. doi: 10.1038/jcbfm.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willis S, Stoll J, Sweetman L, Borges K. Anticonvulsant effects of a triheptanoin diet in two mouse chronic seizure models. Neurobiol Dis. 2010 Dec;40(3):565–572. doi: 10.1016/j.nbd.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roe CR, Yang BZ, Brunengraber H, Roe DS, Wallace M, Garritson BK. Carnitine palmitoyltransferase II deficiency: successful anaplerotic diet therapy. Neurology. 2008 Jul 22;71(4):260–264. doi: 10.1212/01.wnl.0000318283.42961.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roe CR, Bottiglieri T, Wallace M, Arning E, Martin A. Adult Polyglucosan Body Disease (APBD): Anaplerotic diet therapy (Triheptanoin) and demonstration of defective methylation pathways. Mol Genet Metab. 2010 Oct-Nov;101(2-3):246–252. doi: 10.1016/j.ymgme.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Pascual JM, Good LB, Liu P, et al. Synaptic excitation-inhibition imbalance in glucose transporter I deficiency (G1D) and first treatment of its associated human epilepsy with triheptanoin; Paper presented at: Curing the Epilepsies 2013: Pathways Forward; Bethesda, MD, USA. 2013. [Google Scholar]
- 38.Wang D, Pascual JM, De Vivo D. Glucose Transporter Type 1 Deficiency Syndrome. GeneReviews. 1993 2010/03/20 ed. [PubMed] [Google Scholar]
- 39.Pascual JM, Wang D, Hinton V, et al. Brain glucose supply and the syndrome of infantile neuroglycopenia. Arch Neurol. 2007 Apr;64(4):507–513. doi: 10.1001/archneur.64.4.noc60165. [DOI] [PubMed] [Google Scholar]
- 40.Addison RF, Ackman RG. Exceptional occurrence of odd-chain fatty acids in smelt (Osmerus mordax) from Jeddore Harbour, Nova Scotia. Lipids. 1970 Jun;5(6):554–557. doi: 10.1007/BF02532744. [DOI] [PubMed] [Google Scholar]
- 41.Johnson W., Jr Final report on the safety assessment of trilaurin, triarachidin, tribehenin, tricaprin, tricaprylin, trierucin, triheptanoin, triheptylundecanoin, triisononanoin, triisopalmitin, triisostearin, trilinolein, trimyristin, trioctanoin, triolein, tripalmitin, tripalmitolein, triricinolein, tristearin, triundecanoin, glyceryl triacetyl hydroxystearate, glyceryl triacetyl ricinoleate, and glyceryl stearate diacetate. Int J Toxicol. 2001;20(Suppl 4):61–94. [PubMed] [Google Scholar]
- 42.Liu P, Xu F, Lu H. Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magn Reson Med. 2013 Mar 1;69(3):675–681. doi: 10.1002/mrm.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu P, Huang H, Rollins N, et al. Quantitative assessment of global cerebral metabolic rate of oxygen (CMRO) in neonates using MRI. NMR Biomed. 2014 Jan 7; doi: 10.1002/nbm.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60(2):357–363. doi: 10.1002/mrm.21627. PMCID: PMC2587050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med. 2012 Jan;67(1):42–49. doi: 10.1002/mrm.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011 Jun;21(6):1426–1434. doi: 10.1093/cercor/bhq224. PMCID3097991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aslan S, Xu F, Wang PL, et al. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 2010 Mar;63(3):765–771. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Good LB, Sabesan S, Iasemidis LD, Tsakalis K, Treiman DM. Brain dynamical disentrainment by anti-epileptic drugs in rat and human status epilepticus. Conf Proc IEEE Eng Med Biol Soc. 2004;1:176–179. doi: 10.1109/IEMBS.2004.1403120. [DOI] [PubMed] [Google Scholar]
- 49.Sabesan S, Good LB, Tsakalis KS, Spanias A, Treiman DM, Iasemidis LD. Information flow and application to epileptogenic focus localization from intracranial EEG. IEEE Trans Neural Syst Rehabil Eng. 2009 Jun;17(3):244–253. doi: 10.1109/TNSRE.2009.2023291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang NC, Good LB, Marsh ST, Treiman DM. EEG stages predict treatment response in experimental status epilepticus. Epilepsia. 2009 Apr;50(4):949–952. doi: 10.1111/j.1528-1167.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 51.Leary LD, Wang D, Nordli DR, Jr., Engelstad K, De Vivo DC. Seizure characterization and electroencephalographic features in Glut-1 deficiency syndrome. Epilepsia. 2003 May;44(5):701–707. doi: 10.1046/j.1528-1157.2003.05302.x. [DOI] [PubMed] [Google Scholar]
- 52.Posner E. Pharmacological treatment of childhood absence epilepsy. Expert Rev Neurother. 2006 Jun;6(6):855–862. doi: 10.1586/14737175.6.6.855. [DOI] [PubMed] [Google Scholar]
- 53.Klepper J, Florcken A, Fischbarg J, Voit T. Effects of anticonvulsants on GLUT1-mediated glucose transport in GLUT1 deficiency syndrome in vitro. Eur J Pediatr. 2003 Feb;162(2):84–89. doi: 10.1007/s00431-002-1112-8. [DOI] [PubMed] [Google Scholar]
- 54.Klepper J, Fischbarg J, Vera JC, Wang D, De Vivo DC. GLUT1-deficiency: barbiturates potentiate haploinsufficiency in vitro. Pediatr Res. 1999 Dec;46(6):677–683. doi: 10.1203/00006450-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Ho YY, Yang H, Klepper J, Fischbarg J, Wang D, De Vivo DC. Glucose transporter type 1 deficiency syndrome (Glut1DS): methylxanthines potentiate GLUT1 haploinsufficiency in vitro. Pediatr Res. 2001 Aug;50(2):254–260. doi: 10.1203/00006450-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 56.Wang D, Pascual JM, De Vivo D. Glucose Transporter Type 1 Deficiency Syndrome. GeneReviews [Internet] 2002:1993. Resource available on line at http://www.ncbi.nlm.nih.gov/pubmed/20301603.
- 57.Gamberino WC, Berkich DA, Lynch CJ, Xu B, LaNoue KF. Role of pyruvate carboxylase in facilitation of synthesis of glutamate and glutamine in cultured astrocytes. J Neurochem. 1997 Dec;69(6):2312–2325. doi: 10.1046/j.1471-4159.1997.69062312.x. [DOI] [PubMed] [Google Scholar]
- 58.Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985 Mar 11;329(1-2):364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- 59.Kornberg HL. In: Essays in biochemistry. Campbell PN, Marshall RD, editors. Academic Press; London, UK: 1966. pp. 1–31. [Google Scholar]
- 60.Hassel B. Carboxylation and anaplerosis in neurons and glia. Mol Neurobiol. 2000 Aug-Dec;22(1-3):21–40. doi: 10.1385/MN:22:1-3:021. [DOI] [PubMed] [Google Scholar]
- 61.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987 Oct;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 62.Klepper J, Leiendecker B. Glut1 deficiency syndrome and novel ketogenic diets. J Child Neurol. 2013 Aug;28(8):1045–1048. doi: 10.1177/0883073813487600. [DOI] [PubMed] [Google Scholar]
- 63.Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis. 2010 Oct;33(5):469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kovac S, Abramov AY, Walker MC. Energy depletion in seizures: anaplerosis as a strategy for future therapies. Neuropharmacology. 2013 Jun;69:96–104. doi: 10.1016/j.neuropharm.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 65.Gruetter R. In vivo 13C NMR studies of compartmentalized cerebral carbohydrate metabolism. Neurochem Int. 2002 Aug-Sep;41(2-3):143–154. doi: 10.1016/s0197-0186(02)00034-7. [DOI] [PubMed] [Google Scholar]
- 66.Gu L, Zhang GF, Kombu RS, et al. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. II. Effects on lipolysis, glucose production, and liver acyl-CoA profile. Am J Physiol Endocrinol Metab. 2010 Feb;298(2):E362–371. doi: 10.1152/ajpendo.00384.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kinman RP, Kasumov T, Jobbins KA, et al. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. Am J Physiol Endocrinol Metab. 2006 Oct;291(4):E860–866. doi: 10.1152/ajpendo.00366.2005. [DOI] [PubMed] [Google Scholar]
- 68.Pascual JM, Good LB, Liu P, et al. Curing the Epilepsies 2013: Pathways Forward. Bethesda, MD: 2013. Synaptic excitation-inhibition imbalance in glucose transporter I deficiency (G1D) and first treatment of its associated human epilepsy with triheptanoin. Stroke NIoNDa. [Google Scholar]
- 69.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012 Jul;32(7):1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trevelyan AJ, Schevon CA. How inhibition influences seizure propagation. Neuropharmacology. 2013 Jun;69:45–54. doi: 10.1016/j.neuropharm.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 71.Penry JK, Dreifuss FE. Automatisms associated with the absence of petit mal epilepsy. Arch Neurol. 1969 Aug;21(2):142–149. doi: 10.1001/archneur.1969.00480140042004. [DOI] [PubMed] [Google Scholar]
- 72.Tenney JR, Glauser TA. The current state of absence epilepsy: can we have your attention? Epilepsy Curr. 2013 May;13(3):135–140. doi: 10.5698/1535-7511-13.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010 Mar 4;362(9):790–799. doi: 10.1056/NEJMoa0902014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masur D, Shinnar S, Cnaan A, et al. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology. 2013 Oct 29;81(18):1572–1580. doi: 10.1212/WNL.0b013e3182a9f3ca. [DOI] [PMC free article] [PubMed] [Google Scholar]