ABSTRACT
Phylogenetic studies can reveal patterns of evolutionary change, including the gain or loss of elaborate courtship traits in males. Male African clawed frogs generally produce complex and rapid courtship vocalizations, whereas female calls are simple and slow. In a few species, however, male vocalizations are also simple and slow, suggesting loss of male-typical traits. Here, we explore features of the male vocal organ that could contribute to loss in two species with simple, slow male calls. In Xenopus boumbaensis, laryngeal morphology is more robust in males than in females. Larynges are larger, have a more complex cartilaginous morphology and contain more muscle fibers. Laryngeal muscle fibers are exclusively fast-twitch in males but are both fast- and slow-twitch in females. The laryngeal electromyogram, a measure of neuromuscular synaptic strength, shows greater potentiation in males than in females. Male-specific physiological features are shared with X. laevis, as well as with a species of the sister clade, Silurana tropicalis, and thus are likely ancestral. In X. borealis, certain aspects of laryngeal morphology and physiology are sexually monomorphic rather than dimorphic. In both sexes, laryngeal muscle fibers are of mixed-twitch type, which limits the production of muscle contractions at rapid intervals. Muscle activity potentiation and discrete tension transients resemble female rather than male X. boumbaensis. The de-masculinization of these laryngeal features suggests an alteration in sensitivity to the gonadal hormones that are known to control the sexual differentiation of the larynx in other Xenopus and Silurana species.
KEY WORDS: Anuran, Vocalization, Sexual dimorphism, Evolution, Larynx, Muscle
Highlighted article: How do nervous systems change evolutionarily to generate species-specific behaviors? The simplified male courtship songs of a frog, Xenopus borealis, reflect feminization of the larynx and its connection to the brain.
INTRODUCTION
Communication during courtship relies on sexually differentiated behaviors that attract mates and communicate reproductive state. In anurans, vocalizations form the primary courtship signals. African clawed frogs (Xenopus and Silurana; Fig. 1A) provide an informative model system for understanding species- and sex-specific courtship calls (Tobias et al., 2011, 2014). Across genera, the two most common vocalizations are male advertisement calls (Tobias et al., 2011), which are used to advertise receptivity and to negotiate dominance interactions, and release calls (Tobias et al., 2014), which are produced by both sexes to terminate clasps. Call types are distinguishable by parameters that include sound pulse patterns, rates and intensity modulation. Male advertisement calls are usually rapid (inter-pulse intervals 7–50 ms), temporally complex (bursts or longer trills that can have multiple phases) and intensity modulated (sound intensity progressively increases over the course of a call). Male release calls are also rapid but temporally simpler, and are not intensity modulated (see X. laevis, Fig. 1B). Female release calls, when present, are typically slower than male release calls (50–200 ms intervals), and are not temporally complex or intensity modulated.
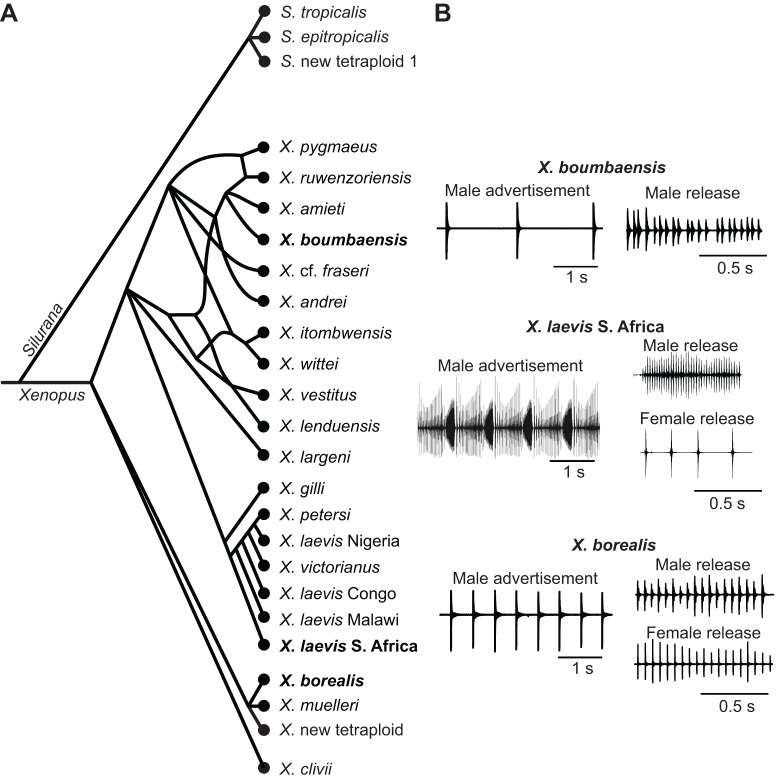
Fig. 1.
Phylogenetic relationships and vocalizations of Xenopus species used in this study. (A) The genus Xenopus includes three major species groups (Evans et al., 2004): a reticulated group that includes X. boumbaensis (bold; used in this study), a clade that includes X. laevis (bold; a well-studied model species) and a clade that includes X. borealis (bold; used in this study). The most recent common ancestor of X. borealis and X. boumbaensis is the most recent common ancestor of all Xenopus. The sub-family Xenopodinae also includes Silurana, and S. tropicalis serves as an outgroup for evolutionary comparisons. (B) Vocal sex differences vary across Xenopus species. Calls are composed of sound pulses repeated at characteristic rates. The X. boumbaensis male advertisement call (top panel; Tobias et al., 2011) is slower than the male release call (Tobias et al., 2014); females do not produce release calls. The X. borealis male advertisement call (bottom panel; Tobias et al., 2011) is also slower than release calls; male and female release call pulse rates do not differ significantly (Tobias et al., 2014). In both X. borealis and X. boumbaensis, advertisement calls lack the temporal complexity and click intensity modulation found in other species such as X. laevis S. Africa (middle panel). Xenopus laevis S. Africa female release calls are not intensity modulated and are markedly slower than both male advertisement and release calls. Timescales differ between advertisement and release calls: 1 s and 0.5 s, respectively.
In three species – X. boumbaensis, X. borealis and X. new tetraploid – the temporal structure of the male advertisement call is unusual for the genus: single sound pulses repeated at long (200–1000 ms) intervals (Tobias et al., 2011 and Yager 1992a; Fig. 1B). A parsimony analysis suggests that the ancestral advertisement call pattern was a burst (a bout of 2–14 pulses), and that both simpler and more-complex call types are derived. Phylogenetic relationships (Fig. 1B) suggest that the loss of burst-type calls in X. boumbaensis has occurred independently from the loss in X. borealis and X. new tetraploid (Tobias et al., 2011). In these three species, male release calls, although more rapid than advertisement calls, are slower than male release calls in other species (Tobias et al., 2014). Female release calls are absent from the species group that includes X. boumbaensis (Tobias et al., 2014). The extent of vocal sexual dimorphism is thus reduced in X. borealis and X. new tetraploid relative to other Xenopus species, and extreme in the species group that includes X. boumbaensis.
Phylogenetic surveys across taxa reveal that the loss of sexual dimorphisms is frequent and can be due either to the loss of male-typical features or to the gain of masculine traits in females (reviewed by Wiens, 2001). In X. borealis, for example, reduced behavioral sex differences appear due to a loss of male-typical call features in males, rather than to their gain in females. What are the proximate mechanisms that underlie loss of dimorphic traits? Behavioral sex differences can be produced by the central nervous system and/or shaped by behavioral effectors; modifications to either (or both) could underlie loss. When sex-specific behavioral traits are lost independently by several species, the underlying proximate mechanisms may be shared or distinct. These mechanisms can be investigated directly in Xenopus using two robust reduced preparations: the isolated brain that generates fictive vocal patterns (Rhodes et al., 2007); and the isolated larynx that produces sound pulses in response to nerve stimulation (Tobias and Kelley, 1987). These ex vivo preparations have been useful in separating central (hindbrain) and peripheral (vocal motor neurons and muscle) contributions to sex differences in vocal behaviors (reviewed by Zornik and Kelley, 2011).
List of abbreviations
- ATPase
adenosine triphosphatase
- EMG
electromyogram
- ISI
inter-stimulus interval
- LM
laryngeal myosin heavy chain isoform
- PI
EMG potentiation index
- %tt
percent transient tension
We used these preparations to determine the physiological mechanisms producing the independently evolved, simple and slow advertisement calls of male X. borealis and X. boumbaensis (Leininger and Kelley, 2013). These phylogenetically distant species use distinct central and peripheral mechanisms to generate a similar, derived advertisement call structure. The isolated male X. boumbaensis brain produces doublet pulses of nerve activity, which the laryngeal synapse converts into single muscle contractions and sounds via potentiation. By contrast, the male X. borealis brain produces single pulses of nerve activity, which the laryngeal synapse converts into single muscle contractions and sound pulses without requiring potentiation. Thus, species differences in both central and peripheral structures contribute to an ultimately similar behavioral output.
Physiological features of the larynx also constrain call parameters between the sexes. Laryngeal muscle fiber types differ in males and females, in at least two species (S. tropicalis and X. laevis): male laryngeal muscle comprises entirely fast-twitch fibers, while female laryngeal muscle fibers are a mixture of fast- and slow-twitch types (Baur et al., 2008; Sassoon et al., 1987). In X. laevis, this sex difference in muscle fiber types allows the male (but not female) larynx to produce rapid muscle contractions and sound pulse trains (Tobias et al., 1991). Muscle electromyogram (EMG) potentiation, which is much greater in males, reflects progressive recruitment of laryngeal fibers and produces intensity-modulated trains. Muscle EMG potentiation in male X. boumbaensis plays a different functional role: it enables the production of single sound pulses in response to doublets of nerve activity (Leininger and Kelley, 2013).
Here, we examine the neuromuscular mechanisms responsible for vocal sex differences in two species with simple and slow advertisement calls: X. boumbaensis and X. borealis. In X. borealis, we focused on the neuromuscular basis for the sexually monomorphic pulse rates of the release call; this comparison is not possible in X. boumbaensis as females do not produce release calls. Given the similarities in advertisement calls of X. boumbaensis and X. borealis males, we also asked whether the laryngeal physiology underlying call parameters is shared across these species. We used the isolated larynx preparation across sexes and species to compare laryngeal morphology and muscle fiber types, as well as laryngeal EMGs and muscle contraction recordings.
RESULTS
Laryngeal sexual dimorphism in X. borealis and X. boumbaensis
The Xenopus larynx consists of a hyaline cartilage structure flanked by paired bipennate muscles that insert onto sound-producing arytenoid discs via a tendon (Fig. 2A). The larynx connects with the buccal cavity via the glottis and with the lungs via the trachea; the hyaline cartilage structure creates interior, air-filled chambers (Fig. 2B). Sounds are produced underwater, independently of respiration, by movement of the discs (Yager, 1992b).
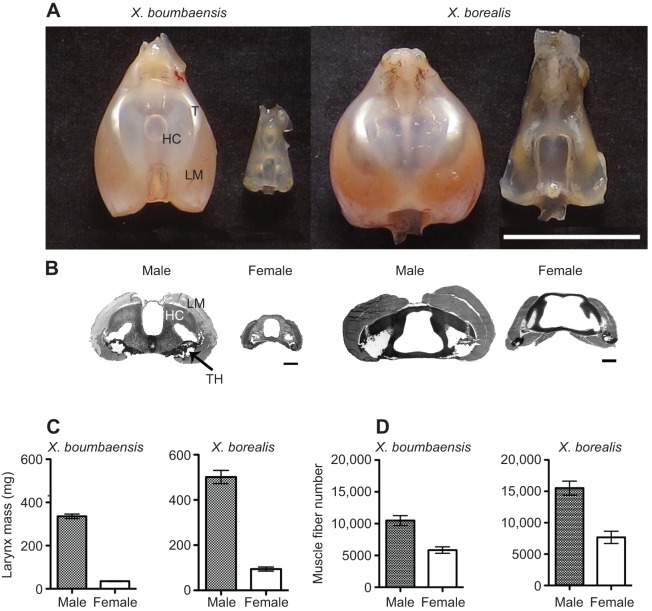
Fig. 2.
Sexual dimorphism in laryngeal size and number of muscle fibers in X. boumbaensis and X. borealis. (A) Dorsal view of X. boumbaensis (left) and X. borealis (right) larynges. A tendon (T) connects laryngeal muscle (LM) to sound-producing discs within the hyaline cartilage (HC). Scale bar: 1 cm. (B) Cross-sections of X. boumbaensis (left) and X. borealis (right) larynges at the anterior-posterior level with the largest cross-sectional muscle area. Muscle fiber numbers were determined from the region lateral to the thyohyal cartilage (TH). Scale bars: 1 mm. (C) Male larynges are significantly heavier than female larynges in both species, but the extent of dimorphism is larger in X. boumbaensis (10-fold difference in mass) than in X. borealis (5-fold difference in mass). (D) Males of both species have significantly more muscle fibers than females (X. borealis, 2×; X. boumbaensis, 1.7×). Graphs show mean±s.e.m.; N=4 for each sex.
In both X. boumbaensis and X. borealis, the larynx is sexually dimorphic in size, in morphology and in muscle fiber number (Fig. 2). Male bipennate muscles are markedly larger than female muscles (Fig. 2A,B), but sex differences are more extreme in X. boumbaensis than in X. borealis. Xenopus boumbaensis male larynges weigh about 10 times more than female larynges (Fig. 2C: 335.6±10.5 versus 35.2±1.4 mg; Mann–Whitney U-test, P=0.0043). Male X. borealis larynges (501.4±29.3 mg) weigh about five times more than female larynges (92.3±8.8 mg; Mann–Whitney U-test, P=0.0159; Fig. 2C). The X. boumbaensis larynx accounts for 8.7% and 0.3% of the total body mass of males and females, respectively. The comparable values in X. borealis are 2.4% and 0.4% for males and females, respectively.
Male X. boumbaensis larynges have ∼1.7 times the number of female muscle fibers (10,488±777 versus 5845±507 fibers; N=4 for each sex; Mann–Whitney U-test, P=0.0286; Fig. 2D). The size of individual muscle fibers, however, is very small in female X. boumbaensis (Fig. 3A), accentuating sex differences in overall muscle mass. Although muscle fiber number in male X. boumbaensis is 1.7 times that in females (Fig. 2D), muscle fiber size is 10 times larger (Fig. 3E). Thus, the sex difference in laryngeal muscle size is due primarily to the size of individual muscle fibers, and secondarily to the number of muscle fibers. Xenopus borealis male larynges (15,528±1095 fibers) have approximately twice as many muscle fibers as female larynges (7371±751; N=4 for each sex; Mann–Whitney U-test, P=0.0286; Fig. 2D). As in X. boumbaensis, the sex difference in laryngeal muscle size of X. borealis is due both to the size and to the number of individual muscle fibers.
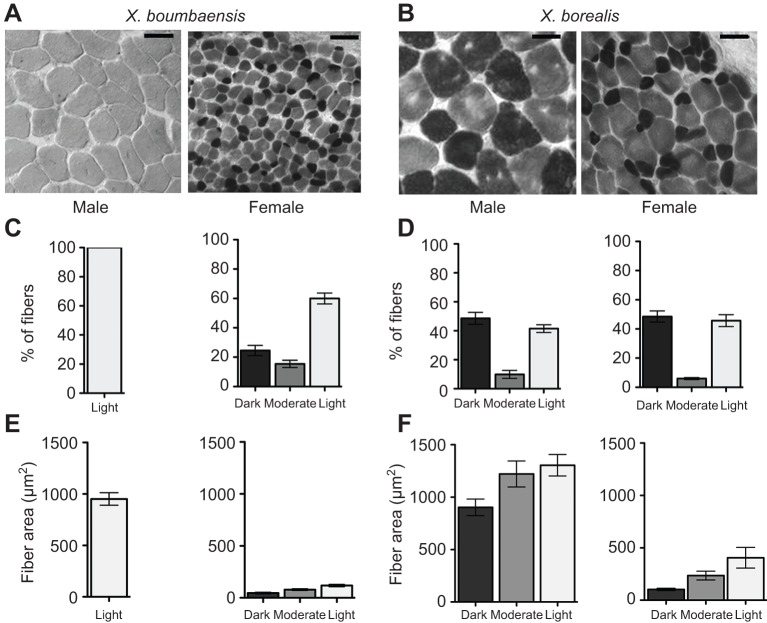
Fig. 3.
Laryngeal muscle fiber type differs by sex in X. boumbaensis but not in X. borealis. (A) ATPase histochemistry of laryngeal muscle following acidic preincubation (pH=4.6) reveals uniformly light-staining fibers in male X. boumbaensis (left), but a mixture of light, moderate and dark staining fibers in female X. boumbaensis (right). (B) Under the same conditions, both male and female X. borealis laryngeal muscle displays a mixture of staining intensities. Scale bars: 25 μm. (C) Xenopus boumbaensis male laryngeal muscle (left) is composed exclusively of light staining (fast-twitch) muscle fibers, while female laryngeal muscle contains both moderate and dark staining (slow-twitch) muscle fibers. (D) Xenopus borealis male and female laryngeal muscle contains similar proportions of light (fast-twitch), moderate and dark (slow-twitch) staining muscle fibers. (E,F) In both X. boumbaensis (E) and X. borealis (F), male muscle fiber areas are larger than female areas. For X. borealis, ATPase staining characteristics are associated with muscle fiber diameter: darkly staining fibers are smaller than more lightly staining fibers in both sexes.
Laryngeal muscle fiber type is sexually dimorphic in X. boumbaensis but not in X. borealis
The speed of muscle contraction and relaxation reflects the composition of fast- and slow-twitch fibers that express different myosin heavy chain isoforms (Lännergren, 1978). These isoforms differ in their ATPase activity and can be reliably distinguished histochemically. After ATPase histochemistry with an acidic preincubation (Sassoon et al., 1987; Baur et al., 2008), fast-twitch fibers are lightly staining and slow-twitch fibers stain more darkly. In South African X. laevis (X. laevis S. Africa), male laryngeal muscle is composed entirely of light-staining (fast-twitch) muscle fibers, while female laryngeal muscle is composed of a mixture of light-, moderate- and dark-staining (slow-twitch) muscle fibers. In females, the average size of fast-twitch muscle fibers (based on fiber cross-sectional area) is greater than that of slow-twitch fibers (Sassoon et al., 1987). We used ATPase histochemistry to assay the complement of muscle fiber types, and their cross-sectional areas, across species and sexes.
In X. boumbaensis, male laryngeal fibers were uniformly (100%) lightly staining, while female fibers were a mixture of lightly (60%), moderately (15%) and darkly (25%) staining (Fig. 3A,C). By contrast, X. borealis muscle was made up of lightly, moderately and darkly staining fibers in both sexes (Fig. 3B), and the proportions of fiber types were similar across the sexes (Fig. 3D).
In X. boumbaensis males, laryngeal muscle fibers were uniform in size [951±106 μm2 (±s.d.)] and staining intensity (Fig. 3E). In females, muscle fibers were smaller than in males; staining intensity varied with size (H=7.20, P=0.0273; Kruskal–Wallis test; N=3). Darkly staining fibers (46±7 μm2) were significantly smaller than lightly staining fibers (118±11 μm2; rank-sum difference=−6.0; P<0.05; Dunn's multiple comparisons test), while moderately staining fibers were intermediate sized (78±8 μm2) and not significantly different in size from other staining types (rank-sum difference=−3.0; P>0.05; Dunn's multiple comparisons test; Fig. 3E).
In X. borealis, male but not female fiber size also varied with staining intensity (H=8.769; P=0.0125; Kruskal–Wallis test; N=4). Darkly staining fibers (102.7±11.5 μm2) were significantly smaller than lightly staining fibers (405.5±98.9 μm2; Dunn's multiple comparisons test; rank-sum difference=−7.500; P<0.01). Moderately staining fibers (235.3±41.9 μm2) were intermediate in size but not significantly different from lightly or darkly staining fibers (Dunn's multiple comparisons; rank-sum difference=−4.500; P>0.05; Fig. 3F). In males, however, all types of fibers are larger than in females (Fig. 3B,F). In females, muscle fiber size did not vary with staining intensity: darkly staining (902.9±79.22 μm2), moderately staining (1222±124.3 μm2) or lightly staining (1305±102.7 μm2) fibers did not differ significantly in size (Kruskal–Wallis test; H=5.115; P=0.0775; N=4).
These results suggest that muscle contraction properties of male and female muscle should differ more dramatically in X. boumbaensis than in X. borealis. We tested this prediction in the ex vivo larynx preparation (Tobias and Kelley, 1987) by recording EMGs and tension transients produced by muscle contractions in response to nerve stimulation. The very small size and fragility of the female X. boumbaensis larynx only allowed for EMG recordings.
Sex differences in laryngeal muscle EMG potentiation and transient tension
The production of sounds by the larynx requires pulling apart the arytenoid discs, which must then come together before the next sound can be produced (Yager, 1992b). If laryngeal muscle does not fully relax between contractions, the discs remain apart (Tobias and Kelley, 1987). The ability of laryngeal muscle to contract and then relax completely is quantified as the percent of transient tension recorded from the tendon inserting onto the arytenoid discs and reflects the relative proportions of fast- and slow-twitch muscle fibers (Tobias et al., 1991). Percent transient tension (%tt: calculated by dividing the height of the peak of the tension transient with respect to the following trough, by the height of the peak of the transient from baseline) was recorded at different rates of nerve stimulation.
The complement of laryngeal muscle fibers constrains how rapidly sound pulses can be produced; an entirely fast-twitch muscle can contract and relax more rapidly than one with slow-twitch fibers. To determine the range of nerve stimulation rates over which the larynx can produce the discrete muscle contractions necessary for sound, we stimulated the laryngeal nerves and measured the %tt produced by muscle contractions and the electromyogram (EMG). As EMG potentiation can also be sexually dimorphic (Tobias et al., 1991), we asked whether this sex difference is present in X. borealis and X. boumbaensis. We used a potentiation index (PI: height of the largest EMG divided by the height of the first EMG) to determine changes in the EMG size over the nerve stimulus train.
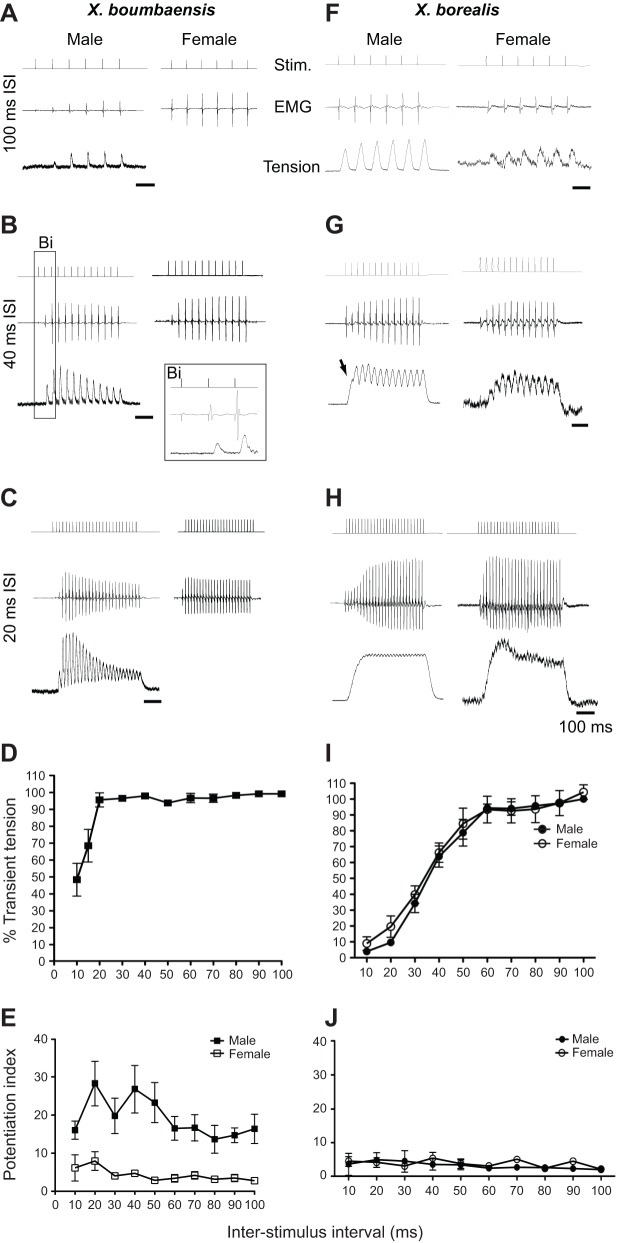
In X. boumbaensis male larynges, the first stimulus in a train typically elicited a very small EMG and no tension transient, whereas subsequent stimuli in the train elicited robust EMGs and tension transients (Fig. 4A–C; shown enlarged in Fig. 4Bi). Laryngeal muscle achieved 100% transient tension at ISIs as short as 20 ms, but 15 and 10 ms ISIs yielded only partial transients (Fig. 4D). Transients and EMGs typically reached maximum values at the beginning of the stimulus train and at short ISIs (less than 50 ms), and then decayed towards the end of the train (Fig. 4B,C). By contrast, X. boumbaensis female EMGs are robust, even in response to the first stimulus in the train, and do not potentiate as markedly as male EMGs (Fig. 4A–C, compare right with left column). Across ISIs, the female X. boumbaensis PI is markedly lower than the male PI (Fig. 4E). As noted above, we could not record robust tension transients from X. boumbaensis female larynges.
Fig. 4.
Sex differences in laryngeal physiology in X. boumbaensis but not in X. borealis. (A–H) Stimulus trains (Stim.) delivered to the laryngeal nerve ex vivo elicit a muscle electromyogram (EMG) and subsequent tension transient (tension) recorded from the tendon connecting muscle to sound-producing discs. (A–C) As inter-stimulus interval (ISI) decreases, X. boumbaensis male larynges continue to produce a potentiating EMG and full tension transients. (Bi) Very small EMG and lack of muscle tension following the first stimulus. Xenopus boumbaensis female larynges are too fragile to allow tension recordings. (D) Male X. boumbaensis larynges support robust tension transients down to 20 ms ISIs; partial transients occur at shorter ISIs. (E) Male X. boumbaensis laryngeal EMGs exhibit greater potentiation than those in females. (F–H) In X. borealis, as ISIs shorten, male and female larynges lose the ability to produce full tension transients. (G) At 40 ms ISI, the first tension transient does not return to baseline before the next transient. Unlike X. boumbaensis, X. borealis larynges of both sexes produce robust EMG and tension transients in response to each stimulus. (I) In X. borealis, both sexes lose the ability to produce robust tension transients at ISIs under 50 ms. (J) The EMG potentiation index does not differ in the sexes; values are low and resemble those of X. boumbaensis females rather than males.
In both male and female X. borealis, stimulating the laryngeal nerve at ISIs from 60–100 ms produced discrete tension transients without a maintained component (Fig. 4F,I). In both sexes, ISIs less than 60 ms resulted in partial maintained tension with superimposed transients (Fig. 4G). In contrast to X. boumbaensis, laryngeal muscle of both sexes in X. borealis displayed maintained tension at 20 ms ISI (Fig. 4H,I). In general, EMGs potentiated very little over a stimulus train. Inter-stimulus interval accounts for 94.13% of the total variation in percent transient tension (repeated measures two-way ANOVA: P<0.0001); the effect of sex (0.16%; P=0.4353) and the interaction effect (0.28%; P=0.5008) were not significant. A small proportion of variation (2.46%) is attributable to between subject differences (P<0.0001). Bonferroni post-tests revealed no significant sex differences at any inter-stimulus interval (P>0.05 for all comparisons; Fig. 4J). In male X. borealis larynges, percent transient tension did not differ significantly between 60 ms and 100 ms ISI (Fig. 4I). However, percent transient tension did decrease significantly between 50 and 10 ms ISIs (P<0.0001; Bonferroni's multiple comparisons), except for two comparisons (40 versus 50 ms and 10 versus 20 ms). In female larynges, percent transient tension did not differ significantly between 60 and 100 ms ISI, but decreased significantly between 50 and 10 ms ISI (except for 10 versus 20 ms ISIs; Fig. 4I). We conclude that, for both sexes, the X. borealis larynx does not support discrete tension transients at rapid (<50 ms) ISIs.
The X. borealis larynx produces muscle contractions and sound pulses in response to each stimulus in a train (Leininger and Kelley, 2013). Complete muscle relaxation following contraction is necessary to produce pulse trains; substantial maintained tension prevents the sound-producing discs from returning to their original positions before the next nerve impulse (Tobias and Kelley, 1987). Because the X. borealis release call (40–50 ms ICI) is slightly faster than the stimulation rates at which we observed partial maintained muscle tension (60 ms), we asked whether isolated X. borealis male larynges (N=6) could produce pulse trains over this crucial range of ISIs (Fig. 5). We stimulated isolated larynges with progressively decreasing ISIs (90–30 ms) and noted at what point the larynx switched from producing robust pulse trains to just one or two pulses at the start of a stimulus train (indicating muscle relaxation for the next pulse). After finding this interval for each larynx, we lengthened the ISI once again to confirm that the larynx was still capable of producing robust trains of sound pulses. All of the larynges produced robust pulse trains in response to nerve stimulus trains with ISIs of 70–90 ms. By 47±7 ms ISI (range=37–60 ms ISI) robust trains were no longer produced. We conclude that the X. borealis larynx cannot produce pulse trains appreciably more rapid than those that make up the release call.
Fig. 5.
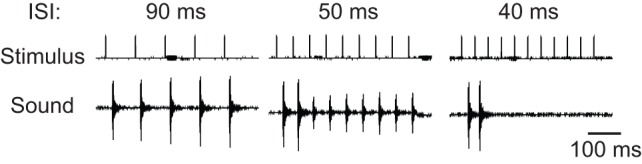
Sound production in the X. borealis male larynx in response to bilateral laryngeal nerve stimulation. The loss of robust tension transients at ISIs close to release call rates is accompanied by a progressive loss of sound production ability (N=6 larynges). Three example traces from one larynx are shown. At 90 ms ISI (left), the larynx is capable of producing robust sound pulses following each stimulus. At 50 ms ISI (middle), the larynx produces two robust sound pulses, followed by additional sound pulses at a reduced intensity. At 40 ms ISI (right), sound pulses do not follow all nerve pulses; the larynx produces only two sound pulses at the beginning of the stimulus train and then falls silent.
In X. borealis, the mean EMG PI ranged from 2.0±0.2 at 100 ms ISI to 3.9±1.0 at 10 ms ISI (Fig. 4J). At some ISIs (70 and 90 ms), the PI was greater in female than in male larynges. Overall, the female PI appeared more variable over the range of inter-stimulus intervals, whereas the male PI decreased gradually as the ISI increased. We conclude that EMG potentiation is not a sexually dimorphic feature of X. borealis larynges. Xenopus borealis EMG potentiation more closely resembles that of X. boumbaensis (this study; compare Fig. 4E with Fig. 4J) and X. laevis S. Africa (Tobias and Kelley, 1987) females than males. Thus for both %tt and potentiation, the physiological properties of the male X. borealis larynx resemble those of females in other species.
DISCUSSION
X. borealis has lost certain male-specific laryngeal characters
We examined sex and species differences in the vocal organ of X. borealis and X. boumbaensis. These species have converged on similar male advertisement and release calls but have diverged in vocal sex differences. In many Xenopus species, male vocalizations are intensity modulated and rapid, whereas female vocalizations lack intensity modulation and are slower. One exception is X. borealis, which lacks a sex difference in release call rate (Tobias et al., 2014; Yager, 1992a). Xenopus boumbaensis shows a different, more extreme vocal sex difference because female release calls are absent (Tobias et al., 2014). Here, we show that morphological features of the larynx – overall mass, and number and size of muscle fibers – are sexually dimorphic in both species, and in the same direction (larger in males). For X. boumbaensis, laryngeal muscle fiber type and the extent of EMG potentiation are also sexually dimorphic. However, the X. borealis larynx is not sexually dimorphic in fiber type, %tt and EMG potentiation. These features are more typical of female than male larynges in X. laevis S. Africa (Sassoon et al., 1987; Tobias and Kelley, 1987), S. tropicalis (Baur et al., 2008) and, as shown here, X. boumbaensis. We propose that, as for call rates and patterns, the lack of sex differences in the X. borealis larynx arises from a loss in males, rather than from a gain in females.
The subfamily Xenopodinae includes two genera: Xenopus and Silurana (Evans et al., 2004) (see Fig. 1A). Silurana is a monophyletic, sister genus that serves as an outgroup for comparisons with the three Xenopus species groups. S. tropicalis and X. laevis both exhibit laryngeal sex differences: male larynges are morphologically complex, larger than female larynges, and contain larger and more muscle fibers (Baur et al., 2008; Sassoon and Kelley, 1986). All of these features are also found in X. boumbaensis and X. borealis (Yager, 1992b) (see Fig. 1A). As these sex differences are also shared with S. tropicalis, we propose that they are ancestral.
Rapid, intensity-modulated calls require an entirely fast-twitch complement of muscle fibers, and a weak and facilitating neuromuscular synapse responsible for EMG potentiation (Ruel et al., 1998; Tobias et al., 1998). Given the widespread rapidity and intensity modulation of male advertisement calls in Xenopus and Silurana (Tobias et al., 2011), an entirely fast-twitch complement of laryngeal muscle fibers and a facilitating synapse may also represent the male ancestral state. We propose that the X. borealis larynx has lost select male-specific characters from this ancestral state.
Laryngeal muscle fiber size, number and type: evolutionary and developmental hypotheses
The proximate mechanisms for producing elaborate, male-specific behaviors in vertebrates include the action of steroid hormones on behavioral neuroeffectors during development and in adulthood (Johnson and Wade, 2010; Kelley, 1988; Luine et al., 1980; Remage-Healey and Bass, 2006). As sexually dimorphic laryngeal features are under the developmental control of gonadal hormones in both X. laevis and S. tropicalis (Zornik and Kelley, 2011), we propose that specific, hormonally mediated developmental processes have been lost in X. borealis, resulting in reduced sexual dimorphisms. In X. laevis, gonadal androgens masculinize overall laryngeal size, and muscle fiber size, number and twitch type from initial values shared by the sexes (Zornik and Kelley, 2011; Yang and Kelley, 2009).
Because X. borealis retains a sexually dimorphic larynx and muscle fiber sizes, we hypothesize that androgens can still masculinize these features. In X. laevis, laryngeal muscle fiber numbers in adult males exceed those of females due to the addition of a greater number of muscle fibers produced by androgen-mediated proliferation of myoblasts (Marin et al., 1990; Sassoon and Kelley, 1986). The greater number of laryngeal muscle fibers in male X. borealis observed here suggests that this underlying developmental program has been retained.
X. borealis lacks a characteristically masculine complement of fast-twitch laryngeal muscle fibers. In X. laevis, androgens convert laryngeal muscle from a female-like mixed state in juveniles to the exclusively fast-twitch type of adult males (Sassoon et al., 1987). As fibers convert developmentally from slow- to fast-twitch, the male X. laevis larynx becomes progressively able to produce discrete tension transients at high rates (Tobias et al., 1991). Fast-twitch fibers express a larynx-specific myosin isoform: laryngeal myosin (LM) (Catz et al., 1992). Conversion of fibers from slow- to fast-twitch type is due to androgen-driven proliferation of a specific population of LM-expressing myoblasts, which then fuse with existing fibers (Nasipak and Kelley, 2012). S. tropicalis also possesses a myosin isoform whose expression is specific to the larynx, is regulated by androgen and is sexually differentiated in adults (Nasipak and Kelley, 2008), suggesting that this cellular mechanism is ancestral. This population may either be missing in X. borealis or be maintained but androgen insensitive.
Laryngeal EMG potentiation and synaptic strength: evolutionary and developmental hypotheses
Muscle potentiation in male X. boumbaensis enables production of single, same intensity clicks in response to doublets of nerve compound action potentials (Leininger and Kelley, 2013). We show here that EMG potentiation is marked, suggesting a weak facilitating neuromuscular synapse. The temporally simplified male advertisement call of this species has more likely evolved via modifications to the hindbrain vocal pattern generator (Leininger and Kelley, 2013), rather than by changes to the neuromuscular synapse, the properties of which appear similar to those of X. laevis. In X. borealis, EMG potentiation in both sexes resembles that produced by the strong laryngeal synapse of female X. laevis (Tobias and Kelley, 1987). Thus, X. borealis has apparently lost not only the developmental program for muscle fiber conversion but also the mechanism underlying EMG potentiation.
In X. laevis, estrogen secretion from the ovaries produces and maintains strong laryngeal synapses in females (Tobias et al., 1998). Changes in estrogen regulation could explain a lack of sex difference in EMG potentiation (and underlying synaptic strength) in X. borealis, via several possible mechanisms. For example, male X. borealis might produce enough estrogen via aromatization of gonadal androgens to strengthen their laryngeal synapse. As both this estrogen-driven mechanism and androgen-driven differentiation of fiber type appear to be absent in X. borealis, their loss could reflect change in a common element, such as a shared and required receptor co-regulator (Feng and O'Malley, 2014). Alternatively, the laryngeal synapse of both sexes may be strong and independent of endocrine control.
In summary, endocrine regulation of laryngeal morphology and physiology described for X. laevis and S. tropicalis suggest possible mechanisms for loss of specific laryngeal sex differences in X. borealis. Unresolved issues include how androgens masculinize laryngeal size and muscle fiber number, but not muscle fiber type. A highly specific form of insensitivity to sex hormones should be considered a candidate mechanism for behavioral evolution. Exploration of these endocrine-related proximate mechanisms will provide additional insights into candidates for evolutionary change.
MATERIALS AND METHODS
Animals
All animal care and experimental procedures conformed to guidelines set forth by the National Institutes of Health and were approved by Columbia University's Institutional Animal Care and Use guidelines (protocol no. AC-AAAA8203). Xenopus borealis males (N=20; mass=20.3±1.3 g) and females (N=12; mass=37.6±3.9 g), and X. boumbaensis males (N=8; mass=4.1±0.7 g) and females (N=9; mass=8.4±1.0 g) were obtained from Xenopus Express (Brooksville, FL, USA) or the Kelley lab colony (some X. boumbaensis originated from a previous colony at the University of Geneva, Switzerland). Animals were group housed in polycarbonate cages filled with 10 l of dechlorinated water, changed twice weekly. Animals were fed frog brittle (Nasco, Fort Atkinson, WI, USA) at least 3 h in advance of changing.
Behavioral recordings
Calls in Fig. 1B were recorded as described previously (Leininger and Kelley, 2013). Briefly, males were primed for calling with human chorionic gonadotropin (100 IU X. borealis; 50 IU X. boumbaensis; Sigma, St Louis, MO, USA, product CG10-10VL) 24–48 and 6 h prior to recording. Each male was placed with an unreceptive female conspecific (for advertisement calls) or with a primed male conspecific (for release calls) in a glass aquarium; females were paired with a primed male conspecific. Vocalizations were recorded with a hydrophone (High Tech, Gulfport, MI, USA; output sensitivity: 2164.5 dB at 1 V mPa21, 127 frequency sensitivity, 0.015–10 kHz) and written to disk with a Marantz CD recorder (CDR300, Mahwah, NJ, USA; 44.1 kHz sampling rate).
Laryngeal size and muscle fiber number
Frogs were deeply anesthetized with a subcutaneous injection of 1.2% MS-222 and placed on ice before dissection. Larynges were rapidly removed, weighed and cleared of connective tissue while submersed in ice-cold saline. Whole larynges were frozen in 40% OCT (Sakura Finetechnical, Torrance, CA, USA) on dry ice and sectioned transversely (20-30 µm) at −20°C using a cryostat (Hacker Instruments and Industries, Winnsboro, SC, USA). Sections were mounted on Superfrost slides (ThermoFisher Scientific, Waltham, MA, USA) and kept at −80°C until use (typically within 2 days of sectioning).
X. borealis and X. boumbaensis (N=4 of each species/sex) laryngeal sections were stained with Hematoxyolin (Harris progressive method), using Eosin as a counterstain (Humason, 1979). Slide-mounted sections were fixed in 10% formalin, stained in Harris Hemotoxylin solution (Sigma-Aldrich, St Louis, MO, USA; HHS32), washed with water, incubated in Scott solution, washed in water, counterstained with 1% Eosin (E-511; ThermoFisher Scientific, Waltham, MA, USA), dehydrated through an ethanol series, cleared with xylene and coverslipped with Cytoseal (Richard-Allen Scientific, Kalamazoo, MI, USA). Tissue was visualized and photographed using a Leica DMR microscope with a Spot RT slider camera (Diagnostic Instruments, Sterling Heights, MI, USA; Software version 4.0.4) connected to a Dell computer. Whole sections were photographed at 5× magnification and merged using Adobe Photoshop photomerge function; smaller fields of view for counting individual fibers were photographed at 20× (males) and 40× (females; fibers are smaller) magnification.
Fiber number was calculated using a method similar to that of Marin et al. (1990). Three sections were identified from the region of greatest muscle area in each larynx (X. borealis: 4.2 mm from caudal end in males, 2.6 mm from caudal end in females; X. boumbaensis: 4.0 mm from caudal end in males, 0.7 mm from caudal end in females; Fig. 2B), and the overall muscle area was measured using ImageJ (NIH, Bethesda, MD, USA). Individual muscle fibers were counted within a 0.093 mm2 field in the inner leaf of the bipennate muscle, adjacent to the thyohyal cartilage. Total fiber number for each section was determined by multiplying the number of fibers per mm2 as calculated from the smaller field by the total area of laryngeal muscle; values were averaged across the three representative sections for an individual mean. Male and female muscle fiber numbers were compared using a Mann–Whitney U-test.
ATPase histochemistry
Larynges were prepared as described above but were frozen immediately. To distinguish between muscle fiber twitch types, we followed methods of Baur et al. (2008), modified from the original method of Guth and Samaha (1970). Larynges of both sexes were always processed together to control for staining variability across reactions. For reactions using X. borealis laryngeal muscle (N=4), X. laevis larynges [previously characterized (Sassoon et al., 1987)] were included as controls to verify the staining protocol. For X. boumbaensis reactions (N=3), X. borealis male larynges were included as a control.
Briefly, slide-mounted sections with the largest muscle area were pre-incubated in acid buffer (pH 4.6) followed by ATP solution (40 min at 30°C), a wash in CaCl2, incubation in cobalt chloride, a water wash and immersion in ammonium sulfide solution; slides were then washed and coverslipped. Under these conditions, fast-twitch fibers stain lightly moderately and slow-twitch fibers stain more intensely (Sassoon et al., 1987). Tissue was photographed as described above and at least 150–200 adjacent muscle fibers were counted and cross-sectional areas measured from each larynx. Because individual sections can vary in overall absolute staining intensity, fibers were first classified by eye based on relative staining intensity (dark, moderate and light) (Sassoon et al., 1987) for species and sexes with heterogenous staining (X. boumbaensis females, X. borealis males and females). We verified these classifications by measuring light transmission (gray values) for individual muscle fibers using ImageJ (NIH). Gray values vary from section to section due to variable staining and photographic parameters. However, for each section, gray values differed according to classification. For example, within a section of X. boumbaensis female laryngeal muscle, mean gray values for light (186±13 s.d.), moderate (146±8 s.d.) and dark (116±9 s.d.) staining fibers are significantly different (ANOVA P<0.0001; Tukey post hoc tests; P<0.001 for all comparisons). Within a section of X. borealis female laryngeal muscle, mean gray values for light (124±27 s.d.), moderate (85±16 s.d.) and dark (64±16 s.d.) staining fibers are significantly different (ANOVA, P<0.0001; Tukey post hoc tests; P<0.001 for dark versus moderate, P<0.0001 for other comparisons). Within a section of X. borealis male laryngeal muscle, mean gray values for light (112±15 s.d.), moderate (90±14 s.d.) and dark (70±10 s.d.) staining fibers are significantly different (Kruskal–Wallis test, P<0.0001; Dunn's multiple comparisons test; P<0.001 for moderate versus light, P<0.0001 for other comparisons).
Isolated larynx preparation
We used the isolated larynx preparation (Tobias and Kelley, 1987) to relate physiological properties to sound production. We measured transient tension as an indication of effective muscle contraction at various rates of nerve stimulation, and EMG potentiation as an indication of laryngeal synaptic strength.
Experimental procedures are described in detail by Tobias and Kelley (1987). Briefly, larynges were isolated and pinned to a Sylgard-lined recording chamber filled with room temperature (21°C), oxygenated saline. The laryngeal nerve was stimulated with 0.5 ms square pulses (150 µA) generated by a Grass Stimulator (model S8800) and delivered to the suction electrode via stimulus isolation units (PSIU6, Grass Instruments, Warwick, RI, USA). Trains of stimuli (500 ms duration) at inter-stimulus intervals of 10 to 100 ms in increments of 10 ms were presented in a random order, separated by at least 5 s. Intervals tested were centered at 50 ms, a characteristic inter-click interval of X. borealis and X. boumbaensis release calls, the fastest call in the repertoire of both species.
Isometric tension on the tendon connecting the laryngeal muscle to the sound-producing cartilage discs was recorded with a force transducer (model FT03; Grass Technologies, Warwick, RI, USA) attached to a DC amplifier (model P16; Grass Technologies). EMG potentials were recorded with bipolar silver-wire electrodes insulated to the tip and amplified with an extracellular amplifier (A-M Systems model 1800, Carlsborg, WA, USA). All signals were digitized (10–20 kHz) and saved either with MacLab digitizer and Chart Software (v. 3.5/3.6) running on a G3 Macintosh computer, or (in most cases) with a DigiData digitizer running with pClamp software (v. 9.2; Axon Instruments/Molecular Devices, Sunnyvale, CA, USA) on a Dell computer.
Laryngeal muscle tension records and EMG potentials were analyzed in Clampfit (v. 9.2; Axon Instruments/Molecular Devices, Sunnyvale, CA, USA). Percent transient tension of laryngeal muscle tension records was calculated by dividing the height of the peak of the tension transient with respect to the following trough, by the height of the peak of the transient from baseline. Therefore, 100% transient tension represents a complete contraction and relaxation of laryngeal muscle. When possible, average percent transient tension values were taken from the middle three transients produced in response to the stimulus train. In some X. boumbaensis records, tension transients decayed over the course of stimulation (e.g. see Fig. 5C). In these cases, percent transient tension was calculated from the middle three robust tension transients. The EMG potentiation index (PI) was calculated as the height of the largest EMG in response to the first five stimuli over the height of the first EMG. In these experiments the number of stimuli varied according to the inter-stimulus interval because the train length was held constant; therefore, to compare potentiation over the same number of EMGs, we considered potentiation in response to the maximum number of stimuli produced by any stimulus train, which was five (500 ms train duration/100 ms inter-stimulus interval).
We recorded sound pulses from X. borealis larynges (N=6) to determine the minimum ISIs that were capable of eliciting robust sound pulse trains (one pulse per stimulus over the course of a train). Laryngeal dissection was as above, except that the lungs were left attached to the larynx, which increases the probability of successful sound recordings (M. L. Tobias, personal communication). The general stimulation setup is the same as described for the tension and EMG experiments. We delivered stimulus trains (200–300 ms; 90–30 ms ISI) bilaterally to the laryngeal nerve rootlets and progressively decreased the ISI until the larynx failed to produce robust pulse trains. After identifying this interval, we stimulated once more with a longer ISI to verify that the larynx was still capable of producing robust trains. Sound pulses were recorded with a hydrophone (output sensitivity – 52 dB, 0.1–6 kHz; Knowles, Itasca, IL, USA), and digitized using the DigiData. Because EMG electrodes and force transducers can hamper sound production, we confined recordings to sound for these experiments.
Statistics
Counts of laryngeal muscle fiber number were compared between sexes using a Mann–Whitney U-test (as the data did not meet the assumptions for normality). For species/sexes that displayed more than one fiber type, an ANOVA (or its non-parametric equivalent, Kruskal–Wallis) with Tukey (parametric) or Dunn's (nonparametric) post hoc tests was used to test whether muscle fiber size varied with staining intensity. In X. borealis, a two-way repeated-measures ANOVA was used to assess the effects of sex and inter-stimulus interval on percent transient tension and EMG potentiation; Bonferroni post-tests were used to examine sex differences across inter-stimulus intervals. Within a sex (X. borealis and X. boumbaensis), the effects of inter-stimulus interval on percent transient tension and EMG potentiation were assessed using a repeated-measures ANOVA with Bonferroni's multiple comparison post hoc tests. Statistics analyses used Prism v. 5.0c (GraphPad Software, La Jolla, CA, USA). Unless otherwise specified, data are expressed as mean±s.e.m.
Acknowledgements
We gratefully acknowledge Brian Nasipak for experimental advice and Ben Evans, Martha Tobias, Irene Ballagh, Ian Hall, Charlotte Barkan and two anonymous reviewers for helpful comments on previous drafts of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
E.C.L. and D.B.K. designed the experiments, analyzed the data and interpreted the results. E.C.L. carried out the experiments, with the assistance of K.K. on fiber type and fiber size experiments.
Funding
This study was supported by Columbia University (research support for the Weintraub Chair), the National Institutes of Health [NS23684] and a Columbia Summer Undergraduate Research Fellowship (to K.K.). Deposited in PMC for release after 12 months.
References
- Baur L. A., Nasipak B. T. and Kelley D. B. (2008). Sexually differentiated, androgen-regulated, larynx-specific myosin heavy-chain isoforms in Xenopus tropicalis; comparison to Xenopus laevis. Dev. Genes Evol. 218, 371-379 10.1007/s00427-008-0223-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz D. S., Fischer L. M., Moschella M. C., Tobias M. L. and Kelley D. B. (1992). Sexually dimorphic expression of a laryngeal-specific, androgen-regulated myosin heavy chain gene during Xenopus laevis development. Dev. Biol. 154, 366-376 10.1016/0012-1606(92)90075-R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans B. J., Kelley D. B., Tinsley R. C., Melnick D. J. and Cannatella D. C. (2004). A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol. Phylogenet. Evol. 33, 197-213 10.1016/j.ympev.2004.04.018 [DOI] [PubMed] [Google Scholar]
- Feng Q. and O'Malley B. W. (2014). Nuclear receptor modulation - Role of coregulators in selective estrogen receptor modulator (SERM) actions. Steroids 90, 39-43 10.1016/j.steroids.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L. and Samaha F. J. (1970). Procedure for the histochemical demonstration of actomyosin ATPase. Exp. Neurol. 28, 365-367 10.1016/0014-4886(70)90244-X [DOI] [PubMed] [Google Scholar]
- Humason G. L. (1979). Animal Tissue Techniques. San Francisco: WH Freeman Co. [Google Scholar]
- Johnson M. A. and Wade J. (2010). Behavioural display systems across nine Anolis lizard species: sexual dimorphisms in structure and function. Proc. R. Soc. B Biol. Sci. 277, 1711-1719 10.1098/rspb.2009.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. B. (1988). Sexually dimorphic behaviors. Annu. Rev. Neurosci. 11, 225-251 10.1146/annurev.ne.11.030188.001301 [DOI] [PubMed] [Google Scholar]
- Lännergren J. (1978). The force-velocity relation of isolated twitch and slow muscle fibres of Xenopus laevis. J. Physiol. 283, 501-521 10.1113/jphysiol.1978.sp012516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger E. C. and Kelley D. B. (2013). Distinct neural and neuromuscular strategies underlie independent evolution of simplified advertisement calls. Proc. R. Soc. Lond. B Biol. Sci. 280, 20122639 10.1098/rspb.2012.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V., Nottebohm F., Harding C. and McEwen B. S. (1980). Androgen affects cholinergic enzymes in syringeal motor neurons and muscle. Brain Res. 192, 89-107 10.1016/0006-8993(80)91011-2 [DOI] [PubMed] [Google Scholar]
- Marin M. L., Tobias M. L. and Kelley D. B. (1990). Hormone-sensitive stages in the sexual differentiation of laryngeal muscle fiber number in Xenopus laevis. Development 110, 703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasipak B. T. and Kelley D. B. (2008). The genome of the diploid anuran Xenopus tropicalis contains a novel array of sarcoplasmic myosin heavy chain genes expressed in larval muscle and larynx. Dev. Genes Evol. 218, 389-397 10.1007/s00427-008-0225-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasipak B. and Kelley D. B. (2012). Developing laryngeal muscle of Xenopus laevis as a model system: androgen-driven myogenesis controls fiber type transformation. Dev. Neurobiol. 72, 664-675 10.1002/dneu.20983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L. and Bass A. H. (2006). A rapid neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 1126, 27-35 10.1016/j.brainres.2006.06.049 [DOI] [PubMed] [Google Scholar]
- Rhodes H. J., Yu H. J. and Yamaguchi A. (2007). Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator. J Neurosci 27, 1485-1497 10.1523/JNEUROSCI.4720-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel T. D., Kelley D. B. and Tobias M. L. (1998). Facilitation at the sexually differentiated laryngeal synapse of Xenopus laevis. J. Comp. Physiol. A 182, 35-42 10.1007/s003590050155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D. A. and Kelley D. B. (1986). The sexually dimorphic larynx of Xenopus laevis: development and androgen regulation. Am. J. Anat. 177, 457-472 10.1002/aja.1001770404 [DOI] [PubMed] [Google Scholar]
- Sassoon D. A., Gray G. E. and Kelley D. B. (1987). Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis. J. Neurosci. 7, 3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M. L. and Kelley D. B. (1987). Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. J. Neurosci. 7, 3191-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M. L., Marin M. L. and Kelley D. B. (1991). Development of functional sex differences in the larynx of Xenopus laevis. Dev. Biol. 147, 251-259 10.1016/S0012-1606(05)80022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M. L., Tomasson J. and Kelley D. B. (1998). Attaining and maintaining strong vocal synapses in female Xenopus laevis. J. Neurobiol. 37, 441-448 [DOI] [PubMed] [Google Scholar]
- Tobias M. L., Evans B. J. and Kelley D. B. (2011). Evolution of advertisement calls in African clawed frogs. Behaviour 148, 519-549 10.1163/000579511X569435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M. L., Korsh J. and Kelley D. B. (2014). Evolution of male and female release calls in African clawed frogs. Behaviour 151, 1313-1334 10.1163/1568539X-00003186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens J. J. (2001). Widespread loss of sexually selected traits: how the peacock lost its spots. Trends Ecol. Evol. 16, 517-523 10.1016/S0169-5347(01)02217-0 [DOI] [Google Scholar]
- Yager D. D. (1992a). Underwater acoustic communication in the African Pipid frog Xenopus borealis. Bioacoustics 4, 1-24 10.1080/09524622.1992.9753201 [DOI] [Google Scholar]
- Yager D. D. (1992b). A unique sound production mechanism in the pipid anuran Xenopus borealis. Zool. J. Linnean Soc. 104, 351-375 10.1111/j.1096-3642.1992.tb00927.x [DOI] [Google Scholar]
- Yang E. J. and Kelley D. B. (2009). Hormones and the regulation of vocal patterns in amphibians: Xenopus laevis vocalizations as a model system. In Hormones, Brain, and Behavior (ed. Pfaff D., Arnold A., Etgen A., Fahrbach S. and Rubin R.), pp. 693–707 London, UK: Academic Press. [Google Scholar]
- Zornik E. and Kelley D. B. (2011). A neuroendocrine basis for the hierarchical control of frog courtship vocalizations. Front. Neuroendocrinol. 32, 353-366 10.1016/j.yfrne.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]