Abstract
The action of hypochlorous acid (HOCl) and γ-radiation on aqueous lysosphingolipid dispersions was found to produce 2-hexadecenal (Hex). This process includes the stages of formation of nitrogen-centered radicals from the starting molecules and the subsequent fragmentation of these radicals via the rupture of C–C and O–H bonds. These findings prove the existence of a nonenzymatic pathway of sphingolipid destruction leading to the formation of Hex, which possesses a wide spectrum of biological activity. Analysis of the effect of HOCl on transplantable rat glioma C6 cells and human embryonic kidney 293 cells points to the formation of Hex. This suggests that the described mechanism of free-radical destruction of sphingolipids may be replicated on cell culture under the stress of active chlorine forms.
Keywords: free-radical destruction, sphingolipids, 2-hexadecenal, reactive oxygen species, reactive chlorine species, myeloperoxidase
Introduction
Sphingolipids represent a lipid class based on sphingosine (SPH), an aliphatic amino alcohol. It was believed for a long time that sphingolipids mainly play a structural role in biomembrane formation.1 Most of them are colocalized with cholesterol as “lipid rafts” in defined areas of the membranes and on lipoprotein surfaces and take an important part in cellular processes.2,3 In recent years, convincing evidence has been obtained indicating that sphingolipids such as ceramide, SPH, and sphingosine-1-phosphate (S1P) are signaling molecules that regulate the processes responsible for apoptosis, proliferation, aging, and inflammation.4,5 In particular, ceramide and S1P were shown to act in opposite directions, building up a “sphingolipid rheostat”, ie, a dynamic equilibrium among various sphingolipid metabolites responsible for cell proliferation and apoptosis. The variety of biological properties of sphingolipids is a serious ground for investigating these compounds, and such studies are being performed in many research laboratories worldwide.
At the same time, free-radical transformations of sphingolipids, unlike those of glycerophospholipids, represent a virtually unexplored area. This is due to the fact that sphingolipids contain mainly saturated fatty acid residues, which make them poor substrates for peroxidation.
It has been shown in our earlier studies that some lipids can undergo free-radical fragmentation in addition to oxidation. So, while studying radiation- and reactive oxygen species (ROS)-induced transformations of hydroxyl-containing glycerophospholipids and sphingolipids, we demonstrated for the first time that these compounds undergo free-radical fragmentation.6–9 These processes occur in polar moieties of the lipids and include the formation of α-hydroxyl-containing carbon-centered radicals, which undergo decomposition via the rupture of two β-bonds. In the case of sphingolipids, such as ceramide and cerebroside, such fragmentation leads to the formation of fatty acid amides and ceramides, respectively,7,8 which are signaling molecules.
A different type of fragmentation is observed upon radiolysis and photolysis of sphingolipids. In our earlier studies,10,11 the possibility of free-radical fragmentation of starting substrates that result in 2-hexadecenal (Hex) formation was demonstrated by sphingosylphosphorylcholine (SPC) radiolysis and photolysis of N-acetylated sphingolipid species.
It should be noted that there is an enzymatic pathway of Hex formation from the degradation of S1P. S1P undergoes irreversible destruction in a biochemical reaction involving S1P lyase, which catalyzes the dissociation of the carbon chain in the substrate, resulting in the formation of Hex and aminoethanol phosphate.12 S1P lyase plays an important role in the regulation of intracellular S1P levels and is also involved in various physiological and pathological processes.13,14 Hex possesses a wide spectrum of biological activity; in particular, it promotes reorganization of the cell cytoskeleton and induces apoptosis.15 It also forms adducts with DNA, which can lead to mutagenic consequences.16 The importance of Hex in the functioning of the cell dictates the necessity of investigating various ways of its formation from biomolecules.
Taking into account the findings mentioned above, investigation of free-radical transformations of sphingolipids under the action of radiation and other agents capable of generating reactive radical intermediates appears to be an important task.
In this study, new data have been obtained on free-radical destructive processes occurring in SPH, SPC, S1P, and sphingomyelin (SM) under the action of γ-radiation or HOCl-containing systems on aqueous dispersions of the starting substrates. We also studied the possibility of degradation reactions of sphingolipid on HOCl-treated cultured cells.
Materials and Methods
Materials
SPC, d-sphingosine (SPH) synthetic, S1P, bovine brain SM, (Z11)-hexadecenal, myeloperoxidase (MPO) from human leukocytes, 2-diphenylacetyl-1,3-indandion-1-hydrazone (DAIH), sodium hypochlorite solution, Dulbecco’s modified Eagle’s medium (DMEM), and antibiotics G-418 and gentamicin were purchased from Sigma-Aldrich Chemie GmbH. Fetal bovine serum was obtained from HyClone. The transplanted strain of rat glioma C6 cells and human embryonic kidney 293 cells (HEK 293) were obtained from the collection of cultures at the Institute of Cytology, Russian Academy of Sciences (St. Petersburg). All other chemicals were of the highest purity available from commercial sources. (2E)-Hexadecenal was synthesized in four steps from the n-tetradecanol, as described elsewhere.17 The structure and purity of the product were characterized by 1H-NMR spectroscopy and gas chromatography-mass spectrometry (GC-MS).
Cell cultures
Cells were propagated in DMEM containing 10% fetal bovine serum, G-418 (150 μg/mL), and gentamicin sulfate (100 μg/mL) for 72 hours at 37°C under an atmosphere of 5% CO2. Cells were removed from the culture by treatment with trypsin (0.25%). After centrifugation, cells were resuspended in phosphate-buffered saline (PBS; pH 7.4) and washed twice with PBS.
Preparation of sphingolipid dispersions
Lipid dispersions were prepared by dispersing thin lipid films in phosphate buffer.18 To do this, the solvent was removed on a rotary evaporator from solutions of the respective lipid in chloroform/methanol mixture (2:1, v/v) and the obtained film samples were kept under vacuum for at least one hour to complete the solvent removal. To the lipid film thus obtained, an appropriate amount of 50 mM PBS was added. Sodium dodecyl sulfate (SDS) detergent was used to obtain SPH dispersions, which were stable at ambient temperatures. The mixture was shaken for 15 minutes on a Vortex mixer at 50°C. The obtained lipid dispersions were sonicated for three minutes on an ultrasound unit (Bandelin Sonorex, 35 kHz, 60/12V). Double-distilled water was used in all experiments. Deaerated dispersions of the starting lipids were used in this study to exclude possible concurrent oxidation reactions of the substrates and radiolysis products. This was done by bubbling argon (99.9%) through the substrate dispersions for 60 minutes. The dispersions were then transferred to glass ampoules that had been purged with argon. Argon was bubbled through the dispersions for 30 minutes more, and then the ampoules were sealed.
Irradiation of samples
The prepared deaerated aqueous 10 mM dispersions of SPH (50 mM PBS, pH 7.4, 1 mM SDS) and SM (50 mM PBS, pH 7.4) were irradiated on a γ-ray unit equipped with 60Co source. The employed dose rate was 0.39 ± 0.03 Gy/s, determined through Fricke dosimetry.19 The absorbed dose range for the sphingolipids dispersions was 0.70–3.51 kGy. Radiation-chemical yields of formation for the respective products were calculated from the data on radiolysis product accumulation as a function of the dose absorbed. The mean values for radiation-chemical yields were calculated from the results obtained in three independent experiments.
Modification of sphingolipids by HOCl reagent and MPO/H2O2/Cl− system
The HOCl-mediated modification of 5 mM SPH (50 mM PBS, pH 5, 1 mM SDS), 2 mM SPC (50 mM PBS, pH 5), 5 mM S1P (50 mM PBS, pH 7.4, 1 mM SDS), and SM (50 mM PBS, pH 7.4) dispersions was performed with freshly prepared HOCl solutions, ranging from 1.0 to 7.5 mM, at room temperature. At this pH, HOCl and OCl− were present in approximately equimolar concentrations (pKa 7.53), but the solution is hereafter referred to as HOCl. The concentrations of HOCl to be added were calculated by a standard procedure,20 from absorbance values measured at 292 nm (ε292(OCl−) = 350 M−1cm−1) for NaOCl/NaOH solutions (100 mM NaOH, pH 12). Deaerated aqueous dispersions of SPH (50 mM PBS, pH 4, 1 mM SDS) and SPC (50 mM PBS, pH 4) containing 140 mM of NaCl were incubated with the MPO enzyme at 37°C for 60 minutes. The reaction was initiated by adding H2O2 at concentrations in the range 0.3–1.2 mM. The final concentration of sphingolipids in the system thus obtained was 5 mM, and that of MPO was 1.5 U/mL.
Cell treatment
Sodium hypochlorite solution was diluted to working concentrations in 0.9% NaCl and used immediately. The samples containing 1.6–3 × 106 cells/mL were treated with freshly prepared NaOCl solutions, whereas the control cells were treated in similar manner with the addition of 0.9% NaCl. The final concentration of HOCl in the system thus obtained varied from 10 μM to 1 mM, and that of cells was 2.6 × 106 cells/mL. The treatment of cells with NaOCl solution was performed at room temperature under aerobic conditions and reaction time of 30 seconds while stirring.
Extraction of (2E)-hexadecenal from cells
To separate the aldehyde from the biological samples, we used a modified “Bligh & Dyer” extraction procedure.21 Hex was extracted with a chloroform/methanol/0.9% NaCl mixture (1:1:0.7, v/v), the extraction procedure was repeated twice. The resulting organic phases were combined and evaporated under vacuum. The dried samples were redissolved with 150 μL of methanol for further analysis. The extraction degree of Hex from 0.5 mL of cells was evaluated using the internal standard method, and totaled 84%.
GC-MS analysis of 2-hexadecenal formed in sphingolipid dispersions
The Hex content of irradiated and HOCl-treated sphingolipid dispersions were determined using GC-MS on a Shimadzu GCMS-QP2010 instrument equipped with an Equity-5 capillary column (30 m, 0.25 mm I.D., 0.25 μm film thickness) in the electron impact ionization mode at 70 eV. The carrier gas, helium, was applied at a flow rate of 1.0 mL/min. The column oven temperature was increased from 60°C to 280°C at the rate of 5°C/min. The oven temperature was held at 280°C for an additional four minutes. The temperature of both the molecular separator and the ion source was set at 250°C. The sample volume injected was 1 μL with a split ratio of 10:1. Quantitative determination of Hex was performed by a conventional chromatographic method using (Z11)-hexadecenal, an isomer of Hex, as an internal standard. The limit of quantitation for the compound was calculated as the compound concentration in the starting solution, for which the signal-to-noise ratio of 10 was obtained. Reproducibility throughout the whole concentration range of all compounds being analyzed was assessed using model samples. The relative standard deviation for the chromatographic methods of determination did not exceed 2%.
ESI-MS analysis of reaction products
The products formed from the reaction of sphingolipids with the HOCl-containing systems were injected into the electrospray ionization interface (ESI) in the direct infusion mode. ESI-MS experiments were carried out on quadrupole mass spectrometer (LCMS 2020, Shimadzu). Nitrogen was used as the drying and nebulizing gas at a flow rate of 15.0 and 1.5 L/min, respectively. The desolvation line temperature was 250°C, and the detector voltage was maintained at 1.2 kV.
HPLC/fluorescence analysis of 2-hexadecenal formed in cells
For sensitive quantitative analysis of Hex in cells, we applied the method based on HPLC (Shimadzu LC-10ADVP) equipped with a fluorescence detector (RF-10AXL), using the derivatization reagent 2-diphenylacetyl-1,3-indandion-1-hydrazone (DAIH). It has been shown that HPLC/fluorescence detection is a sensitive method to quantify aldehydes in biological samples.22 The chromatographic control and data processing were performed with CLASS-VP 5.0 Shimadzu software. The derivatization reaction with DAIH was carried out with the extracted compounds in the lipid fraction. Hex was identified by comparing peak’s retention time with the corresponding synthesized (2E)-hexadecenal. We used (11Z)-hexadecenal as the internal standard for the HPLC/fluorescence measurement to quantify the Hex content. HPLC separation was done in the reversed-phase mode using an acetonitrile/water (95:5 v/v) mixture at a flow rate of 1.5 mL/min on a column (25 cm, 4 mm I.D.) packed with chemically bonded octadecyl silica (Nucleosil 120–5 C18, Macherey-Nagel). The chromatogram was monitored by fluorescence detection with excitation at 425 nm and emission at 525 nm. Sample solution volumes of 5 μL were injected.
Statistical analysis
The difference between mean values was analyzed by Student’s t-test and was considered statistically significant at P < 0.05. All the data are presented as mean ± SD. The values were calculated from the results obtained in at least three independent experiments. Data averaging and error estimation were performed using the least squares method (software: OriginPro 8.5, OriginLab). The SD values for HPLC and GC-MS determination did not exceed 0.44%.
Results
Radiation-induced fragmentation of sphingolipids
Radiation-induced transformations of aqueous dispersions of SPH and SM were investigated. Sphingolipid dispersions were purged with argon to exclude radiation-induced concurrent oxidation reactions. Radiolysis of deaerated sphingolipid dispersions was used to establish the mechanisms of their free-radical transformations, which proceed without oxygen.
Using GC-MS (see Methods), we detected Hex among radiolysis products of SPH. Mass spectrum of this compound is shown in Figure 1.
Figure 1.
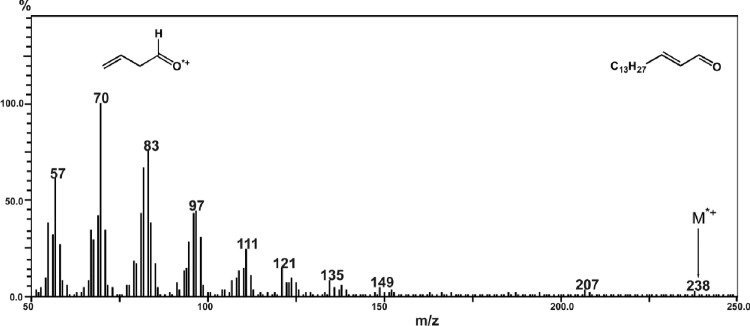
Mass spectrum of Hex formed in γ-irradiated sphingolipid dispersions.
In our previous study,10 the possibility of γ-induced Hex formation in aqueous dispersions of SPC was shown.
The radiation-chemical yields of Hex calculated from the data on its formation in 10 mM aqueous dispersions of SPH amounted to 0.14 ± 0.02 molecule/100 eV. In the case of SM, no Hex was detected among radiolysis products.
HOCl-induced fragmentation of sphingolipids
We have studied the possibility of realization of the sphingolipid destruction exposed to HOCl, which can be generated in many cells in the course of MPO-dependent reactions occurring in a living organism being in normal and in a number of pathological conditions.23–25
We examined the compositions of product mixtures formed on the addition of HOCl solutions (pH 5) to SPH, SPC, S1P, and SM dispersions. The reaction of HOCl with the sphingolipids was shown to yield chlorinated derivatives, which were identified by ESI-MS (see Methods). For example, in the mass spectrum of products formed in aqueous SPH dispersions treated with HOCl solutions (Fig. 2), mono- and dichlorinated SPH derivatives can be identified in positive mode by the molecular ion peaks [M + Na]+ at m/z 356 and 390, respectively, as well as by their characteristic fragmentation patterns.
Figure 2.
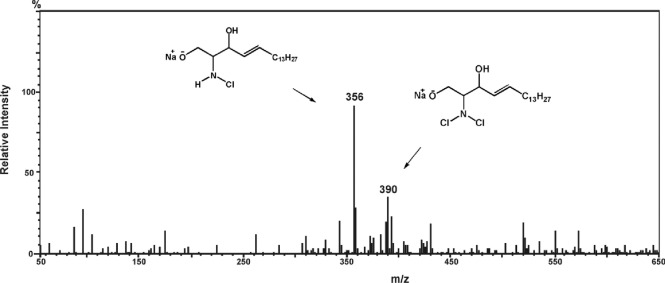
Mass spectrum of mono- and dichloramine derivatives of SPH formed in HOCl-treated deaerated aqueous SPH dispersions.
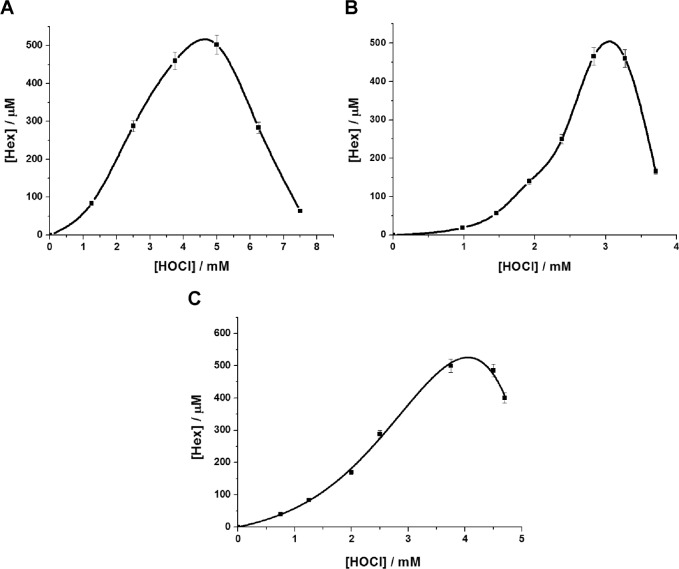
Moreover, the introduction of HOCl into SPH, S1P, and SPC dispersions resulted in the formation of Hex in amounts varying with the reagent concentration, as shown in Figure 3.
Figure 3.
Accumulation of Hex in deaerated aqueous dispersions of sphingolipids as function of HOCl concentration added. (A) 5 mM SPH (50 mM PBS pH 5, 1 mM SDS). (B) 2 mM SPC (50 mM PBS, pH 5). (C) 5 mM S1P (50 mM PBS, pH 7.4, 1 mM SDS). Error bars indicate SD of the means (n ≥ 3).
Formation of Hex was recorded immediately after the addition of HOCl to the sphingolipid dispersions (see Methods), and its concentration increased from 0.25 to 500 μM with increase in HOCl concentrations from 0.5 to 4.5 mM for SPH (Fig. 3A), from 1 to 3 mM for SPC (Fig. 3B), and from 0.5 to 4.0 mM for S1P (Fig. 3C). Further increase of HOCl concentration resulted in an abrupt fall in the amounts of Hex being formed, most probably due to the reaction of Hex with the added reagent, since unsaturated aldehydes are known to react easily with hypochlorous acid. The addition of HOCl to SM dispersions led to formation of chlorinated derivatives of the lipid, but no Hex was detected among the final products.
Destruction of sphingolipids under the action of MPO/H2O2/chloride system
It has been found in this study that the respective chlorinated derivatives and Hex can also be formed from lysosphingolipids under the action of reactive chlorine species generated in the MPO-mediated halogenating cycle. Aqueous dispersions of SPH and SPC prepared as indicated in “Methods” were incubated for 60 minutes at 37°C with the hypochlorous acid formed from the MPO-catalyzed reaction in the presence of H2O2 at various concentrations (0.3–1.2 mM). The reaction was carried out at pH values ranging from 4 to 7.4. The sphingolipid dispersions were found to yield maximum amounts of Hex when the pH of the medium was ≤4.5, in accordance with the literature data showing that MPO acts mainly in the halogenating mode at acidic pH values, yielding maximum amounts of HOCl.26 The diagrams in Figure 4 show the concentrations of Hex formed in aqueous dispersions of SPH (A) and SPC (B) in reaction mixtures of various compositions.
Figure 4.
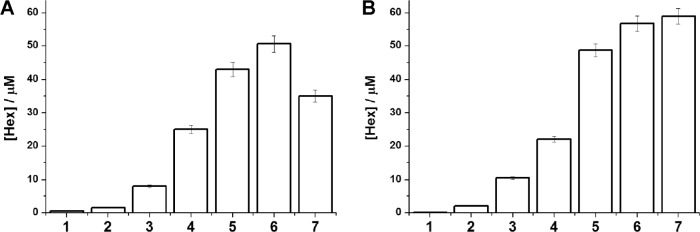
Formation of Hex in aqueous dispersions of SPH (A) and SPC (B) under the action of MPO/H2O2/Cl− system. 1—sphingolipid dispersion; 2—sphingolipid dispersion/0.3 mM H2O2; 3—sphingolipid dispersion/MPO; 4—sphingolipid dispersion/MPO/0.3 mM H2O2; 5—sphingolipid dispersion/MPO/0.6 mM H2O2; 6—sphingolipid dispersion/MPO/1.0 mM H2O2; 7—sphingolipid dispersion/MPO/1.2 mM H2O2. The reactions were conducted in 50 mM PBS, 140 mM NaCl, pH 4, T = 37°C, t = 60 minutes. The sphingolipid concentration in the system thus obtained was 5 mM, and that of MPO was 1.5 U/mL. Error bars indicate SD of the means (n ≥ 3).
Control experiments (1) were performed with deaerated aqueous dispersions of sphingolipid in the absence of MPO and H2O2 (50 mM PBS, 140 mM NaCl, pH 4). They indicated that no Hex was formed in the absence of MPO-generated reactive chlorinating species (RCS). Some quantities of Hex were detected in the MPO/Cl− systems without hydrogen peroxide added (2), probably due to the presence of certain MPO activity under such conditions. The data obtained in experiments (3)–(7) indicate that the most effective formation of Hex from SPH and SPC occurred when the initiation of the MPO-mediated reaction was performed by H2O2 at concentrations of 1.0 and 1.2 mM, respectively.
HOCl-induced formation of Hex in cells
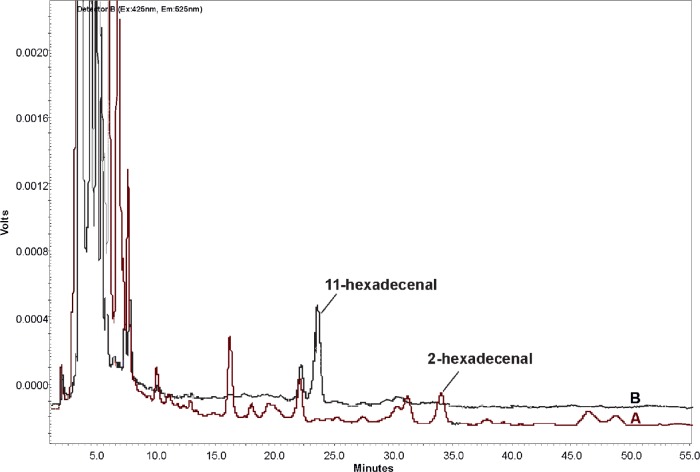
We investigated the effect of HOCl on transplanted cells of rat glioma C6 and human embryonic kidney 293 cells. We found that HOCl at concentrations from 10 μM to 1 mM induced the formation of Hex in cells. The lipid fraction analysis from HOCl-treated C6 glioma and HEK 293 cells gave a peak at 34 minutes corresponding to Hex. Figure 5A shows the HPLC chromatogram of the derivatized products of C6 glioma cells’ lipid extraction after HOCl treatment at the concentration of 1 mM.
Figure 5.
HPLC chromatograms of derivatized products of C6 glioma cells’ lipid extraction after HOCl treatment (A) and the blank cell extraction without added HOCl (B).
Meanwhile, the blank cell extraction without added HOCl was performed to discount any peaks from the cell components themselves (Fig. 5B). (11Z)-hexadecenal was used as the internal standard to quantify Hex content. Hex concentration in the 1 mM HOCl-treated C6 gliomaand HEK 293 cells was approximately 3 nmol per 106 cells.
Discussion
In this study, we investigated transformations of SPH, SPC, S1P, and SM under the action of γ-radiation and HOCl-containing systems on aqueous dispersions of the named substrates. In the cases of SPH and SPC, the formation of Hex was found to occur under such conditions. However, no trace of Hex was detected among the reaction products of SM. These facts indicate that the presence of a free amino group is a necessary condition for the γ-radiation- or HOCl-induced C–C destruction of lipids.
The possibility of radiation-induced destruction involving C–C bond cleavage in amine-containing organic compounds has been shown in our earlier studies that were performed on a series of 1,2-amino alcohols.27 The mechanism of amino alcohol destruction on radiolysis of their aqueous solutions includes the stages of formation and subsequent fragmentation of nitrogen-centered radicals according to the scheme below:
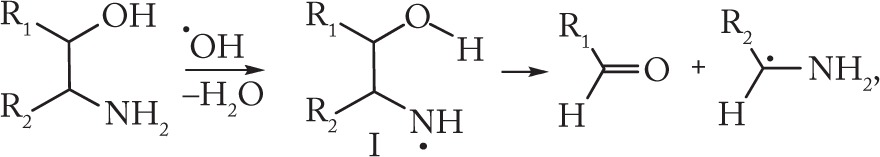 |
(1) |
To enable decomposition via cleavage of two bonds in β-position with respect to the radical-center formation of a five-membered transition state (I) has been proposed.27 The reaction (1) is strongly favored by the hydrogen bond –N⋯H–O–between vicinal hydroxyl and amino groups of α-amino alcohols. It should be noted that cysteine addition suppressed the C–C destruction of amino alcohols, which may be accounted for by the reduction of aminyl radicals by S–H groups of cysteine molecules. At the same time, oxygen only just slightly affected the yields of the C–C destruction products.27 The nitrogen-centered radicals formed from starting substrates do not interact with oxygen because they are oxidizers themselves.
Lysosphingolipids contain both proton donors and proton acceptors, such as −OH and −NH2 groups, and hence are capable of forming hydrogen bonds like those present in 1,2-amino alcohols. It has been shown28 that sphingosine micelles are in fact aggregates of five-membered cyclic structures linked by intra and intermolecular hydrogen bonds. Hence, the presence of vicinal amino alcohol moieties in SPH and SPC molecules determines their ability to undergo radiation-induced destruction proceeding via the formation of nitrogen-centered radicals (2).
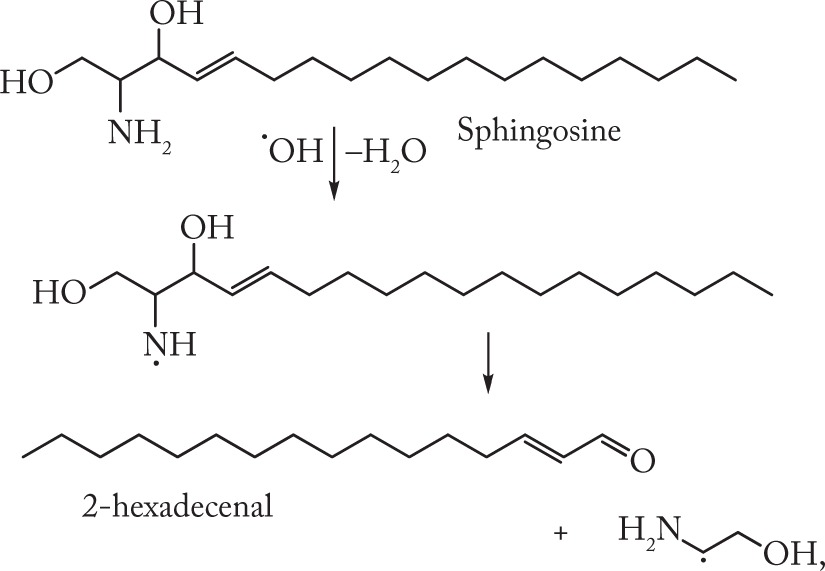 |
(2) |
In the nitrogen-centered radicals formed from SM, the acyl group, due to its electron withdrawing (−M) mesomeric effect, reduces electron density at the nitrogen atom, thereby preventing the C–C bond destruction, as evidenced by the absence of Hex among the products of radiation- and HOCl-induced transformations of SM.
The nitrogen-centered radicals can be obtained from SM and other N-acetylated species by means of photochemical removal of the acyl group. We have shown earlier11 that photolysis of aqueous dispersions of ceramide, SM, and galactocerebroside was accompanied by the formation of Hex, as demonstrated by the following reactions:
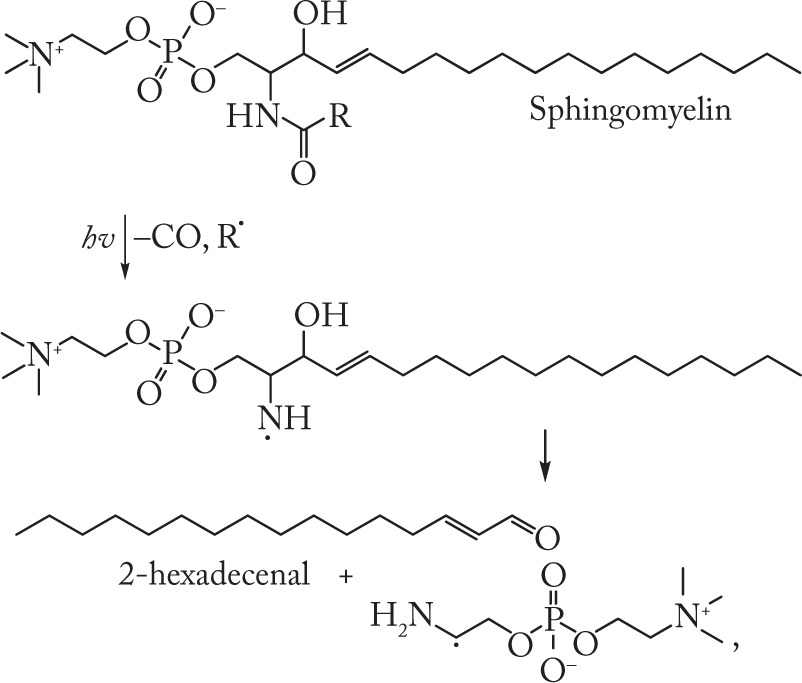 |
(3) |
The presence of Hex among the photolysis products formed from SM, ceramide and galactocerebroside may serve as confirmation of the ability of nitrogen-centered radicals to undergo fragmentation involving C–C bond cleavage.11
Another effective method for the generation of nitrogen-centered radicals from nitrogen-containing organic compounds is the reaction of the latter with HOCl29–31:
| (4) |
So, the formation of nitrogen-centered radicals from chloramines obtained by reaction of phosphatidyl ethanolamine with HOCl has been established using electron paramagnetic resonance (ESR).31
HOCl is characterized by powerful bactericidal and cytotoxic properties because it is a strong oxidizer and a source of RCS.23 The source of HOCl in a living organism is reaction (5), ie, two-electron oxidation of chloride ions by hydrogen peroxide, which is catalyzed by MPO.24,25
| (5) |
Our experiments with SPH, SPC, S1P, and SM treated with HOCl, either added as a reagent or produced by the MPO/H2O2/Cl− system, have shown that the starting lipids yield mono- and di-N-chlorinated derivatives (Fig. 2). In cases of SPH, SPC, and S1P, the chloramines formed are unstable and decompose homolytically to give aminyl radicals, which in turn undergo fragmentation to give Hex (Figs. 3 and 4).
A study by Ford et al32 indicated that 2-hexadecenal quickly accumulated in high-density lipoproteins (HDL-associated SPC and S1P) treated with an MPO/RCS generating system. The authors proposed the heterolytic mechanism of 2-hexadecenal formation.
Based on our results and on the data obtained,29–31 we propose the following scheme (6) for the HOCl-induced formation of Hex from SPH, SPC, and S1P:
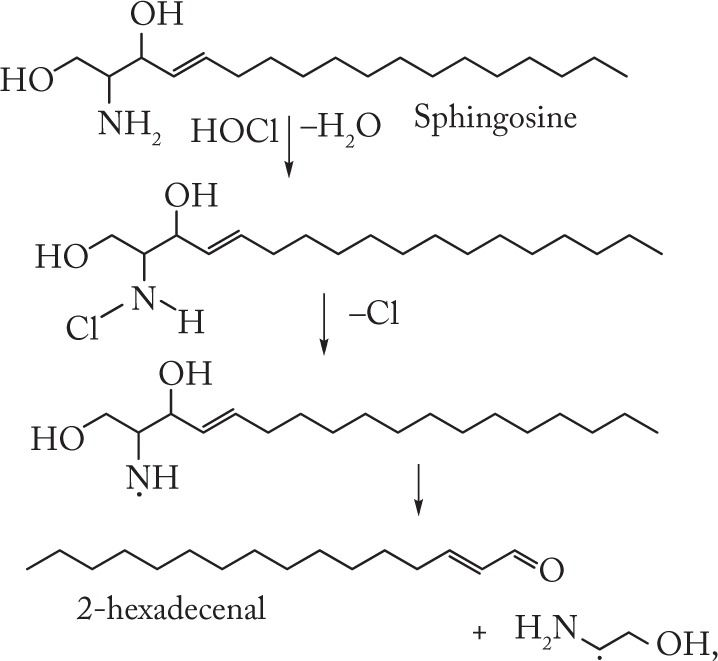 |
(6) |
In recent years, the biological role of MPO-mediated reactions that causes damage to biomolecules has been extensively studied.24,25,33,34 On one hand, the hypochlorous acid that is generated in MPO-dependent-reactions produces a nonspecific bactericidal action in many inflammatory diseases;24,25 on the other, however, increased production of HOCl on activation of the MPO-mediated halogenating cycle in a living organism causes damage to the tissues and leads to the development of a number of cardiovascular diseases, in particular atherosclerosis.34 These adverse effects are due to the accumulation of toxic products of HOCl-induced oxidation and chlorination reactions taking place in the course of various pathophysiological processes. It is noteworthy that Hex promotes modifications of the cell cytoskeleton and takes part in the induction of apoptosis.15,16 Taking these facts into account, one may expect the existence of a relationship between HOCl-induced formation of Hex and the development of a number of pathological events.
In order to study whether these reactions also occur in biosystems, we explored the effects of RCS in cultured cells. Recently, Lüth et al developed a new method for the quantification of the (2E)-hexadecenal in different cell lines and human plasma.22 We used this modified method (HPLC/fluorescence detector) for analysis of (2E)-hexadecenal in cultured cells after HOCl treatment. Our findings demonstrate that the treatment of C6 glioma and HEK 293 cells with a NaOCl solution leads to Hex formation in an amount ~3 nmol per 106 cells (Fig. 5). During the experiment on cell cultures, high concentrations of oxidant were used to identify the degradation products by using available methods. HOCl generated in vivo will target primarily thioethers as well as sulfhydryl and amino groups. However, during the functioning of MPO by the halogenating cycle near biomembranes, HOCl may also interact with various lipids, including sphingolipids.
It should be noted that the cellular levels of SPH and other lysosphingolipids are low. However, low level of Hex formation does not preclude the importance of this agent, since low basal cellular levels are found for a number of second messengers, including diacylglycerol and inositol trisphosphate.35
Thus, this study has resulted in the discovery of a new pathway that leads to the formation of bioactive Hex from sphingolipids during γ- and HOCl-induced destruction of the latter. The mechanism of this transformation includes generation of nitrogen-centered radicals from sphingolipids followed by fragmentation of these radicals through simultaneous rupture of C–C and O–H bonds. For the realization of such a process, the presence of a free amino group in the sphingolipid molecule is necessary to ensure the formation of nitrogen-centered radicals from the substrate on its interaction with ROS and RCS. The presence of an acyl group in the structure of SM prevents this molecule from γ- and HOCl-induced destruction to form Hex.
As mentioned above, Hex possesses a wide spectrum of biological activity.15,16 Therefore, any change in the sphingolipid/Hex ratio in a cell should play an important role in the signaling way switch-over mechanisms and, as a consequence, in the functioning of the cell. Hence, nonenzymatic reactions that lead to the formation of Hex in living organisms under conditions of halogenating stress may influence biological functions. The fact that S1P lyase is used as a therapeutic target while developing medicinal drugs for treatment of numerous pathologic conditions associated with S1P13,14 allows the assumption to be made that inhibitors of nonenzymatic pathways of Hex formation may also manifest pharmacological activity. Determining the full physiological significance of sphingolipid breakdown products promises to be an exciting area of investigation.
The results discussed above should be taken into account while conducting studies aimed at the development of novel medications intended for the prevention and treatment of diseases associated with the activation of free-radical reactions in living organisms.
Conclusion
The action of γ-radiation, HOCl, and MPO/H2O2/Cl− system on aqueous sphingolipid dispersions was found to produce Hex, which has a wide spectrum of biological activity. The obtained data allowed us to propose a free-radical mechanism for this process, in which the key stage is the fragmentation of nitrogen-centered radicals formed from the starting sphingolipids. Our studies have confirmed that such reactions of sphingolipid destruction can occur in living cells upon exposure to reactive chlorine species.
Footnotes
ACADEMIC EDITOR: Tim Levine, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: OS, AL, GS. Analyzed the data: AL, IE, NA. Wrote the first draft of the manuscript: OS, AL. Contributed to the writing of the manuscript: OS, AL, GS, IE, NA. Agree with manuscript results and conclusions: OS, AL, GS, IE, NA. Jointly developed the structure and arguments for the paper: OS, AL, GS. Made critical revisions and approved final version: OS, AL, GS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Brown RE. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J Cell Sci. 1998;111:1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50:S323–S328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50:S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS Lett. 2010;584:1887–1894. doi: 10.1016/j.febslet.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shadyro OI, Yurkova IL, Kisel MA. Radiation-induced peroxidation and fragmentation of lipids in a model membrane. Int J Radiat Biol. 2002;78:211–217. doi: 10.1080/09553000110104065. [DOI] [PubMed] [Google Scholar]
- 7.Shadyro O, Yurkova I, Kisel M, Brede O, Arnhold J. Formation of phosphatidic acid, ceramide and diglyceride on radiolysis of lipids: identification by MALDI-TOF mass spectrometry. Free Radic Biol Med. 2004;36:1612–1624. doi: 10.1016/j.freeradbiomed.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Shadyro OI, Yurkova IL, Kisel MA, Arnhold J. Free-radical fragmentation of galactocerebrosides: a MALDI-TOF mass spectrometry study. Chem Phys Lipids. 2005;134:41–49. doi: 10.1016/j.chemphyslip.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Yurkova IL, Stuckert F, Kisel MA, Shadyro OI, Arnhold J, Huster D. Formation of phosphatidic acid in stressed mitochondria. Arch Biochem Biophys. 2008;480:17–26. doi: 10.1016/j.abb.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Lisovskaya AG, Shadyro OI, Edimecheva IP. A new mechanism for photo- and radiation-induced decomposition of sphingolipids. Lipids. 2011;46:271–276. doi: 10.1007/s11745-010-3506-0. [DOI] [PubMed] [Google Scholar]
- 11.Lisovskaya AG, Shadyro OI, Edimecheva IP. A novel pathway of photoinduced decomposition of sphingolipids. Photochem Photobiol. 2012;88:899–903. doi: 10.1111/j.1751-1097.2012.01148.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Veldhoven PP. Sphingosine-1-phosphate lyase. In: Merrill AH, Hannun YA, editors. Sphingolipid Metabolism and Cell Signaling. New York: Academic Press; 2000. pp. 244–254. [Google Scholar]
- 13.Kumar A, Saba J. Lyase to live by: sphingosine phosphate lyase as a therapeutic target. Expert Opin Ther Targets. 2009;13:1013–1025. doi: 10.1517/14728220903039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serra M, Saba J. Sphingosine-1-phosphate lyase, a key regulator of sphingosine-1-phosphate signaling and function. Adv Enzyme Reg. 2010;50:349–362. doi: 10.1016/j.advenzreg.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Byun H-S, Bittman R, Saba J. The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in JNK-dependent manner. Cell Signal. 2011;23:1144–1152. doi: 10.1016/j.cellsig.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upadhyaya P, Kumar A, Byun HS, Bittman R, Saba JD, Hecht SS. The sphingolipid degradation product trans-2-hexadecenal forms adducts with DNA. Biochem Biophys Res Commun. 2012;424:18–21. doi: 10.1016/j.bbrc.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Gong Y, Byun H-S, Bittman R. An improved two-step synthetic route to primary allylic alcohols from aldehydes. New J Chem. 2010;34:470–475. [Google Scholar]
- 18.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 19.Fricke H, Hart EJ. Chemical dosimetry. In: Attix TH, Roesch WC, editors. Radiation Dosimetry. New York: Academic Press; 1966. pp. 167–239. [Google Scholar]
- 20.Morris JC. The acid ionization constant of HOCl from 5 to 35°. J Phys Chem. 1966;70:3798–3805. [Google Scholar]
- 21.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 22.Lüth A, Neuber C, Kleuser B. Novel methods for the quantification of (2E)-hexadecenal by liquid chromatography with detection by either ESI QTOF tandem mass spectrometry or fluorescence measurement. Anal Chim Acta. 2012;722:70–79. doi: 10.1016/j.aca.2012.01.063. [DOI] [PubMed] [Google Scholar]
- 23.Yap YW, Whiteman M, Cheung NS. Chlorinative stress: an under appreciated mediator of neurodegeneration? Cell Signal. 2007;19:219–228. doi: 10.1016/j.cellsig.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 25.Davies MJ. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr. 2011;48:8–19. doi: 10.3164/jcbn.11-006FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 27.Sladkova AA, Lisovskaya AG, Sosnovskaya AA, Edimecheva IP, Shadyro OI. Destruction of amino alcohols and their derivatives on radiolysis and photolysis in aqueous solutions. Rad Phys Chem. 2014;96:229–237. [Google Scholar]
- 28.Sasaki H, Arai H, Cocco MJ, White SH. pH Dependence of sphingosine aggregation. Biophys J. 2009;96:2727–2733. doi: 10.1016/j.bpj.2008.12.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins CL, Davies MJ. Reaction of HOCl with amino acids and peptides: EPR evidence for rapid rearrangement and fragmentation reactions of nitrogen-centred radicals. J Chem Soc Perkin Trans. 1998;2:1937–1945. [Google Scholar]
- 30.Pattison DI, Hawkins CL, Davies MJ. Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: absolute rate constants, product analysis and computational modeling. Chem Res Toxicol. 2003;16:439–449. doi: 10.1021/tx025670s. [DOI] [PubMed] [Google Scholar]
- 31.Kawai Y, Kiyokawa H, Kimura Y. Hypochlorous acid-derived modification of phospholipids: characterization of aminophospholipids as regulatory molecules for lipid peroxidation. Biochemistry. 2006;45:14201–14211. doi: 10.1021/bi0610909. [DOI] [PubMed] [Google Scholar]
- 32.Brahmbhatt VV, Hsu FF, Kao JL, Frank EC, Ford DA. Novel carbonyl and nitrile products from reactive chlorinating species attack of lysosphingolipid. Chem Phys Lipids. 2007;145:72–84. doi: 10.1016/j.chemphyslip.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Malle E, Furtmuller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong ND, Gransar H, Narula J, et al. Myeloperoxidase, subclinical atherosclerosis, and cardiovascular disease events. JACC Cardiovasc Imaging. 2009;2:1093–1099. doi: 10.1016/j.jcmg.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Berridge MJ. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]