Abstract
Background and Objectives:
To compare open versus totally intracorporeal robotic-assisted radical cystectomy, bilateral extended pelvic lymph node dissection, and Studer urinary diversion in bladder cancer patients.
Methods:
A retrospective comparison of open (n = 42) versus totally intracorporeal (n = 32) robotic-assisted radical cystectomy, bilateral extended pelvic lymph node dissection, and Studer urinary diversion was performed concerning patient demographic data, operative and postoperative parameters, pathologic parameters, complications, and functional outcomes.
Results:
Patient demographic data and the percentages of patients with pT2 disease or lower and pT3–pT4 disease were similar between groups (P > .05). Positive surgical margin rates were similar between the open (n = 1, 2.4%) and robotic (n = 2, 6.3%) groups (P > .05). Minor and major complication rates were similar between groups (P > .05). Mean estimated blood loss was significantly lower in the robotic group (412.5 ± 208.3 mL vs 1314.3 ± 987.1 mL, P < .001). Significantly higher percentages of patients were detected in the robotic group regarding bilateral neurovascular bundle–sparing surgery (93.7% vs 64.3%, P = .004) and bilateral extended pelvic lymph node dissection (100% vs 71.4%, P = .001). The mean lymph node yield was significantly higher in the robotic group (25.4 ± 9.7 vs 17.2 ± 13.5, P = .005). The number of postoperative readmissions for minor complications was significantly lower in the robotic group (0 vs 7, P = .017). Better trends were detected in the robotic group concerning daytime continence with no pad use (84.6% vs 75%, P > .05) and severe daytime incontinence (8.3% vs 16.6%, P > .05). No significant differences were detected regarding postoperative mean International Index of Erectile Function scores between groups (P > .05).
Conclusions:
Robotic surgery has the advantages of decreased blood loss, better preservation of neurovascular bundles, an increased lymph node yield, a decreased rate of hospital readmissions for minor complications, and a better trend for improved daytime continence when compared with the open approach.
Keywords: Robotic radical cystectomy, Open versus robotic, Intracorporeal, Studer pouch, Comparison
INTRODUCTION
Open radical cystectomy (RC) with urinary diversion is currently accepted as the gold-standard surgical approach in the management of muscle-invasive bladder cancer and for patients with high-grade, recurrent, noninvasive tumors.1
After the introduction of the da Vinci S 4-arm surgical robot (Intuitive Surgical, Sunnyvale, California), robot-assisted radical cystectomy (RARC) is more frequently being performed, although the number of centers performing intracorporeal Studer urinary diversion after RARC is very limited currently. We have recently reported our experience and technique related to neurovascular bundle (NVB)–sparing RARC and intracorporeal urinary diversion including 12 and 27 cases.2,3
In this article we retrospectively report comparative outcomes of open versus totally intracorporeal RARC, bilateral pelvic lymph node dissection (PLND), and Studer urinary diversion in bladder cancer patients.
MATERIALS AND METHODS
Between December 2009 and January 2013, we performed open RC, bilateral PLND, and Studer pouch urinary diversion in 42 patients (41 men and 1 woman) and RARC, bilateral extended PLND, and intracorporeal Studer pouch urinary diversion in 32 patients (29 men and 3 women) for invasive bladder cancer. Anterior pelvic exenteration was also performed in female patients. Overall, 5 different surgeons have performed both the open and robotic cases.
The da Vinci S 4-arm surgical robot was used for RARC procedures. Patients with a history of abdominopelvic radiotherapy and major abdominal surgery were excluded from undergoing the robotic approach. We have recently published our initial experience and surgical technique in detail regarding RARC and intracorporeal urinary diversion for invasive bladder cancer.2,3
The mean age was 61.4 ± 10 years (range, 41–80 years) in patients who underwent the open approach and 62.2 ± 10.6 years (range, 41–80 years) in those who underwent the robotic approach. Comparative patient demographic data of both groups are presented in Table 1. Complications that occurred during the perioperative (30-day) period and within 31 to 90 days of surgery were graded according to the modified Clavien system.4
Table 1.
Comparison of Demographic Data in Open Group Versus Robotic Group
| Open Group (n = 42) | Robotic Group (n = 32) | P Value | |
|---|---|---|---|
| Male/female | 41/1 | 29/3 | .310 |
| Age, mean ± SD (range), y | 61.4 ± 10 (41–80) | 62.2 ± 10.6 (41–80) | .742 |
| BMIa, mean ± SD (range), kg/m2 | 24.8 ± 2.1 (21–29) | 25.7 ± 3.3 (19–32) | .162 |
| ASAa score, n | |||
| I | 13 | 4 | .094 |
| II | 23 | 15 | .639 |
| III | 6 | 13 | .015 |
| IV | 0 | 0 | - |
| Preoperative IIEFa score, mean ± SD (range) | 31.6 ± 21.9 (5–67) | 32.8 ± 21.1 (5–62) | .866 |
| Previous abdominal surgery, n (%) | |||
| Laparoscopic | 1 (2.3) | 2 (6) | .575 |
| Openb | 9 (21.4) | 6 (21.2) | > .99 |
| Endourologic (TURPa) | 1 (2.3) | 2 (9) | .575 |
ASA = American Society of Anesthesiologists; BMI = body mass index; IIEF = International Index of Erectile Function; TURP = transurethral resection of prostate.
Previous abdominal surgery included partial cystectomy (n = 1), abdominal surgery for peptic ulcer (n = 2), inguinal hernia repair (n = 3), laparotomy for ileus (n = 1), and appendectomy (n = 2) in the open group and included inguinal hernia repair (n = 1), splenectomy (n = 1), appendectomy (n = 3), and cholecystectomy (n = 1) in the robotic group.
Overall, 42 patients who underwent open RC, bilateral PLND, and Studer pouch reconstruction for bladder cancer at our institution were included for retrospective comparison with the robotic approach. The group undergoing open RC, bilateral PLND, and Studer pouch reconstruction and the group undergoing RARC, bilateral extended PLND, and intracorporeal Studer pouch reconstruction for bladder cancer were compared in terms of patient demographic data, operative and postoperative parameters, pathologic parameters, and complications. For the comparison of functional outcomes (urinary continence and erectile function), patients who had completed 9 months of postoperative follow-up were selected and compared.
Postoperative Functional Evaluation
Daytime incontinence and nighttime incontinence were evaluated and are presented at latest follow-up for each patient. Daytime urinary incontinence was measured as none (0–1 security pad per day), mild (1–2 pads per day), moderate (3 pads per day), or severe (>3 pads per day) as described by Lantz et al.5 Nighttime urinary incontinence was measured as good (dry with no protection), fair (dry with 1 awakening), or poor (wet, leakage, and incontinence during sleep) as described by Kulkarni et al.6
Erectile function was assessed using the International Index of Erectile Function (IIEF) scores described by Rosen et al7 as follows: severe dysfunction, IIEF score <7; moderate dysfunction, IIEF score of 7 to 12; mild to moderate dysfunction, IIEF score of 13 to 18; mild dysfunction, IIEF score of 19 to 24; or no dysfunction, IIEF score >24. All patients with preoperative erectile function (IIEF score >7) were instructed to use oral phosphodiesterase type 5 inhibitors after removal of the urethral catheter.
Statistical Analysis
Statistical analysis was performed with the SPSS program (version 15.0; IBM, Armonk, New York). The Student t test was used for parametric comparisons of data. The Fisher exact test and χ2 test were used to compare categorical data between the groups.
RESULTS
Outcomes were evaluated and are presented retrospectively. Among the patients who underwent RARC, the mean operative time was 9.77 ± 1.27 hours (range, 7.05–12.45 hours) and mean estimated blood loss was 415 ± 227.5 mL (range, 100–1200 mL). Overall, 29 patients (58%) had organ-confined disease and 21 patients (42%) had local extravesical disease. The mean lymph node (LN) yield was 22.09 ± 10.6. Positive soft-tissue margins were detected in 3 patients (6%). Operative, postoperative, and pathologic parameters are shown in Tables 2 and 3. Minor complications (grades 1 and 2) were detected in 38 patients and 13 patients during the 0- to 30-day (perioperative) period and 31- to 90-day period, respectively. Major complications (grades 3–5) were detected in 15 patients and 8 patients during the 0- to 30-day (perioperative) period and 31- to 90-day period, respectively. Complications according to the modified Clavien system are presented in Table 4.
Table 2.
Comparison of Operative and Postoperative Parameters in Open Group Versus Robotic Group
| Open Group (n = 42) | Robotic Group (n = 32) | P Value | |
|---|---|---|---|
| Bilateral NVBa sparing, n (%) | 27 (64.3) | 30 (93.7) | .004 |
| Unilateral (left) NVB sparing, n (%) | 0 | 1 (3) | .432 |
| Non—NVB sparing, n (%) | 15 (35.7) | 1 (3) | .001 |
| Bilateral extended lymph node dissection, n (%) | 30 (71.4) | 32 (100) | .001 |
| Bilateral standard lymph node dissection, n (%) | 12 (28.6) | 0 | .001 |
| Operative time, median ± SD (range), h | 9.20 ± 1.86 (4.00–12.00) | 9.76 ± 1.29 (7.05–12.45) | .154 |
| Anomalies detected during surgery, n (%) | |||
| Ureteral duplication | 0 | 2 (6.2) | .184 |
| APAa detected and preserved | 0 | 2 (6.2) | .184 |
| Estimated blood loss, mean ± SD (range), mL | 1314.3 ± 987.1 (200–4500) | 412.5 ± 208.3 (100–800) | < .001 |
| Time to intake of liquid diet, mean ± SD (range), d | 4.10 ± 1.53 (1–9) | 3.69 ± 1.65 (1–9) | .276 |
| Time to resumption of regular diet, mean ± SD (range), d | 7.07 ± 2.06 (3–14) | 6.78 ± 2.25 (3–13) | .567 |
| Time to ambulation, mean ± SD (range), d | 1.79 ± 0.6 (1–3) | 2.13 ± 0.9 (1–5) | .064 |
| Lodge drain removal time, mean ± SD (range), d | 11 ± 5.02 (8–35) | 10.1 ± 4.60 (5–30) | .444 |
| Length of hospital stay, mean ± SD (range), d | 18.8 ± 10.6 (9–56) | 17.4 ± 9.8 (8–62) | .548 |
APA = accessory pudendal artery; NVB = neurovascular bundle.
Table 3.
Comparison of Pathologic Parameters in Open Group Versus Robotic Group
| Open Group (n = 42) | Robotic Group (n = 32) | P Value | |
|---|---|---|---|
| Pathologic stage (pT) | |||
| Organ-confined disease (≤pT2), n (%) | 26 (62) | 19 (59.3) | > .99 |
| pT0 | 9 | 6 | |
| pTa | 1 | 0 | |
| Primary CISa | 1 | 1 | |
| pT1b | 4 | 2 | |
| pT2a | 3 | 6 | |
| pT2b | 8 | 4 | |
| Local extravesical disease (pT3–4), n (%) | 16 (38) | 13 (40.6) | > .99 |
| pT3a | 10 | 6 | |
| pT3b | 0 | 2 | |
| pT4a | 5 | 5 | |
| pT4b | 1 | 0 | |
| Occurrence of inadvertent bladder entry, n (%) | 0 | 0 | |
| Staging, n (%) | |||
| pN0 | 27 (64.2) | 23 (71.8) | .618 |
| pN+ | 15 (35.7) | 9 (28.1) | |
| LNa yield, mean ± SD (range) | 17.2 ± 13.5 | 25.4 ± 9.7 | .005 |
| LN yield after exclusion,c mean ± SD (range) | 20.4 ± 14.6 | 25.4 ± 9.7 | .118 |
| LN yield range | 4–81 | 8–46 | |
| LN involvement stratified by pT stage, n (%) | 15 (35.7) | 9 (28.1) | |
| pT1 or less | 1 (2.4) | 1 (3.1) | .913 |
| pT2 | 3 (7.1) | 2 (6.3) | |
| pT3–4 | 11 (26.2) | 6 (18.8) | |
| Positive soft-tissue surgical margins, n (%) | 1 (2.4) | 2 (6.3) | .575 |
| Incidental prostate adenocarcinoma, n (%) | 18 (42.9) | 7 (21.9) | .083 |
CIS = carcinoma in situ; LN = lymph node.
Concomitant carcinoma in situ was present in 2 patients.
Comparison of LN yields between open and robotic groups when patients with standard LN dissection were excluded in the open radical cystectomy group.
Table 4.
Comparison of 0- to 30-Day and 31- to 90-Day Complications According to Modified Clavien Classification in Open Group Versus Robotic Group
| Open Group (n = 42) |
Robotic Group (n = 32) |
P Value |
||||
|---|---|---|---|---|---|---|
| 0–30 d (Perioperative) | 31–90 d | 0–30 d (Perioperative) | 31–90 d | 0–30 d | 31–90 d | |
| Grade of complication according to modified Clavien system | ||||||
| Minor complication (grade 1 and 2), n | 27 | 6 | 20 | 5 | > .99 | > .99 |
| Major complication (grade 3–5), n | 13 | 5 | 6 | 2 | .422 | .212 |
| Readmission rate for minor complications, n | 4 | 7 | 2 | 0 | .693 | .017 |
| Readmission rate for major complications, n | 2 | 3 | 1 | 1 | > .99 | .629 |
Adjuvant chemotherapy was offered to patients who had pT3b–pT4 and/or LN metastasis.
Comparisons of patient demographic data, operative and postoperative parameters, pathologic parameters, complications according to the modified Clavien classification, urinary continence, and erectile function between the open and robotic groups are presented in Tables 1–6. The groups were similar in terms of patient demographic data (Table 1). The number of patients who underwent bilateral NVB-sparing surgery was significantly higher in the robotic group (27 [64.3%] vs 30 [93.7%], P = .004) (Table 2). The number of patients who underwent bilateral extended PLND was significantly higher in the robotic group (30 [71.4%] vs 32 [100%], P = .004) (Table 2). Mean estimated blood loss was significantly lower in the robotic group (412.5 ± 208.3 mL vs 1314.3 ± 987.1 mL, P < .001) (Table 2). Transfusion was performed in 18 patients (56%) in the robotic groups versus 40 patients (95%) in the open group (P < .001). The mean LN yield was significantly higher in the robotic group (25.4 ± 9.7 vs 17.2 ± 13.5, P = .005) (Table 3). Although minor and major complications were similar between the groups, the robotic group had a significantly decreased readmission rate for minor complications during 31 to 90 days postoperatively (Table 4).
Table 5.
Comparison of Postoperative Urinary Continence Outcomes of Patients Who Have Completed Postoperative 9-Month Follow-Up of Neurovascular Bundle–Sparing in Open Group Versus Robotic Group
| M/Fa | FUa, mo | NVBa Sparing | Postoperative Daytime Incontinence |
Postoperative Nighttime Incontinence |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | Good | Fair | Poor | ||||
| Open group (n = 12) | 11/1 | 41.8 ± 14 (15–64) | Bilateral: 15 (100%) Unilateral: 0 (0%) NNSa: 0 (0%) |
9 (75%) | 1 (8.3%) | 0 (0%) | 2 (16.6%) | 7 (58.3%) | 5 (41.6%) | 0 (0%) |
| Robotic group (n = 13) | 11/2 | 26.1 ± 8.3 (9–39) | Bilateral: 12 (92.3%) Unilateral: 1 (8.7%) NNS: 0 (0%) |
11 (84.6%) | 1 (8.3%) | 0 (0%) | 1 (8.3%) | 6 (46.1%) | 4 (30.7%) | 3 (23%) |
| P Value | .645 | > .99 | < .001 | .593 | .695 | .688 | .220 | |||
There were no statistically significant differences detected between the groups (open and robotic) regarding all parameters related to daytime and nighttime urinary incontinence. However, there was a trend toward improved daytime continence with no pad use and decreased rates of severe daytime incontinence.
FU, follow-up; M/F = male/female; NNS = non–nerve sparing; NVB = neurovascular bundle.
Table 6.
Comparison of Postoperative Erectile Function Outcomes of Patients With Mild or No Preoperative Erectile Dysfunction Who Have Completed Postoperative 9-Month Follow-Up of Neurovascular Bundle–Sparing in Open Group Versus Robotic Group
| Preopa Erectile Function Status According to IIEFa Score | Open Group |
Robotic Group |
P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Preop IIEF Score | NVBa Sparing | Postopa PDE5-Ia Use | Mean IIEF Score at Latest FUa | Mean Preop IIEF Score | NVB Sparing | Postop PDE5-I Use | Mean IIEF Score at Latest FU | ||
| Mild dysfunction (IIEF score of 19–24) (n = 2) | 19 | Bilateral: 1 (100%) Unilateral: 0 (0%) NNS: 0 (0%) |
0 (0%) | 19 | 20 | Bilateral: 1 (100%) Unilateral: 0 NNS: 0 |
0 (0%) | 5 | > .05b |
| No dysfunction (IIEF score >24) (n = 13) | 46.5 ± 11.3 (33–63) | Bilateral: 6 (100%) Unilateral: 0 (0%) NNS: 0 (0%) |
3 (50%) | 22 ± 18.7 (5–42) | 53.4 ± 7.4 (42–62) | Bilateral: 6 (85.7%) Unilateral: 1 (14.3%) NNS: 0 |
2 (28.5%) | 13.6 ± 13.6 (5–42) | > .05b |
FU = follow-up; IIEFS = International Index of Erectile Function Score; NNS = non–nerve sparing; NVB = neurovascular bundle; PDE5-I = phosphodiesterase type 5 inhibitor; Postop = postoperative; Preop = preoperative.
P value for comparison of IIEF scores.
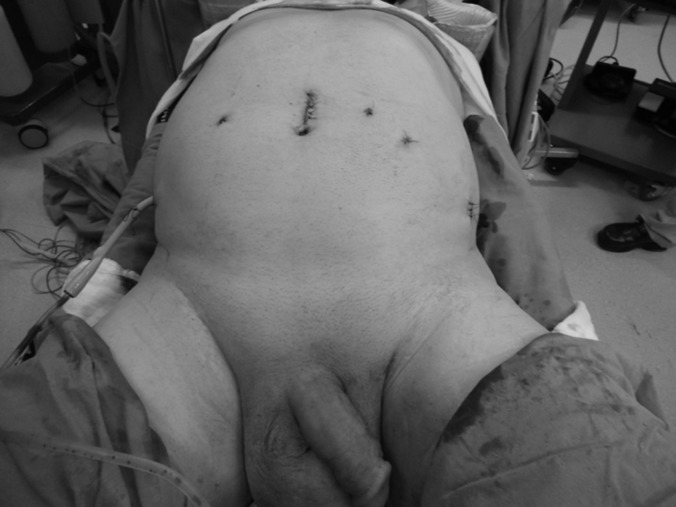
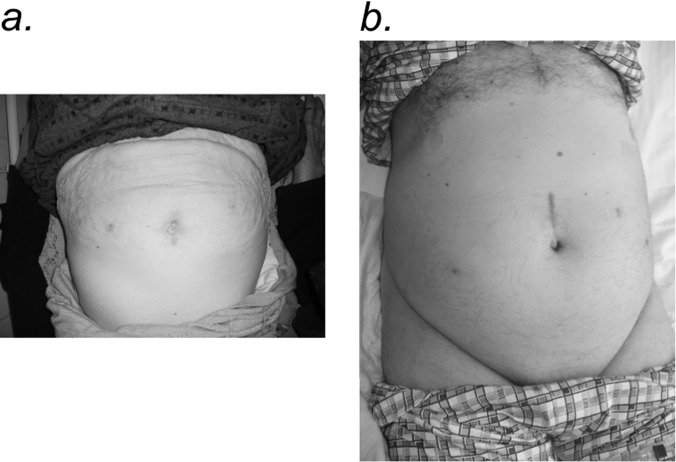
Figures 1–5 show trocar placement sites and the appearance of the bilaterally preserved NVBs in the pelvis after RARC, bilateral extended PLND, and completed robotic intracorporeal Studer pouch reconstruction. Figure 5 shows the immediate postoperative abdominal appearance of a patient who underwent RARC, bilateral extended PLND, and intracorporeal Studer pouch urinary reconstruction. Figures 6a and 6b show healed surgical wounds after RARC and intracorporeal Studer pouch urinary reconstruction in a 72-year-old female patient and a 65-year-old male patient, respectively.
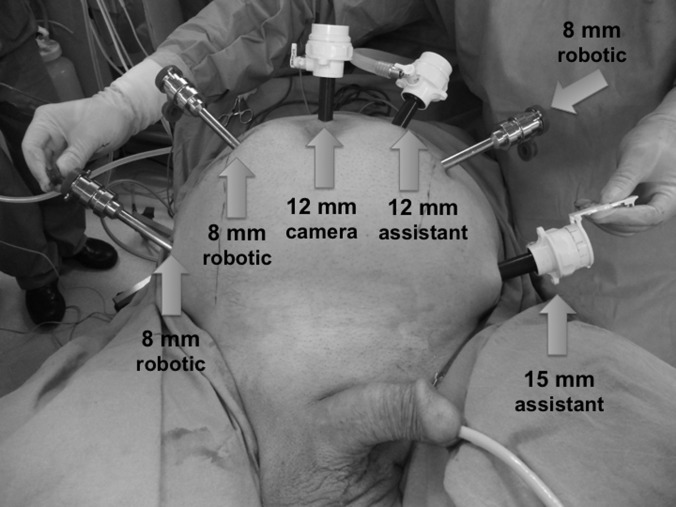
Figure 1.
Trocar placement sites for robot-assisted radical cystectomy, bilateral extended pelvic lymph node dissection, and intracorporeal Studer pouch urinary reconstruction.
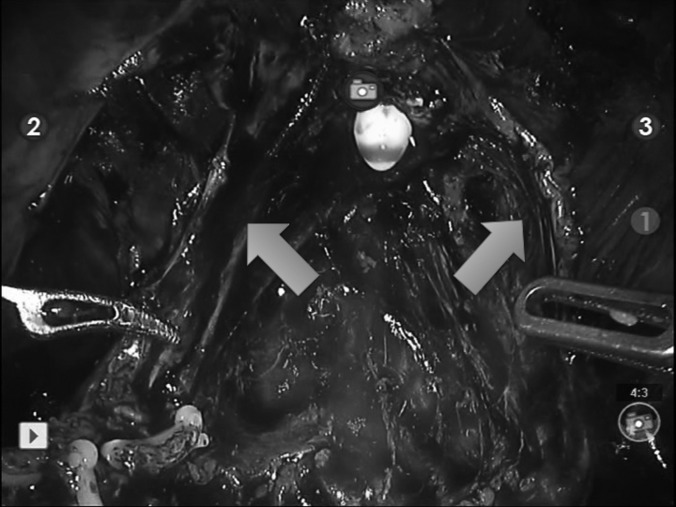
Figure 2.
Bilaterally preserved neurovascular bundles in pelvis (arrows) after robot-assisted radical cystectomy.
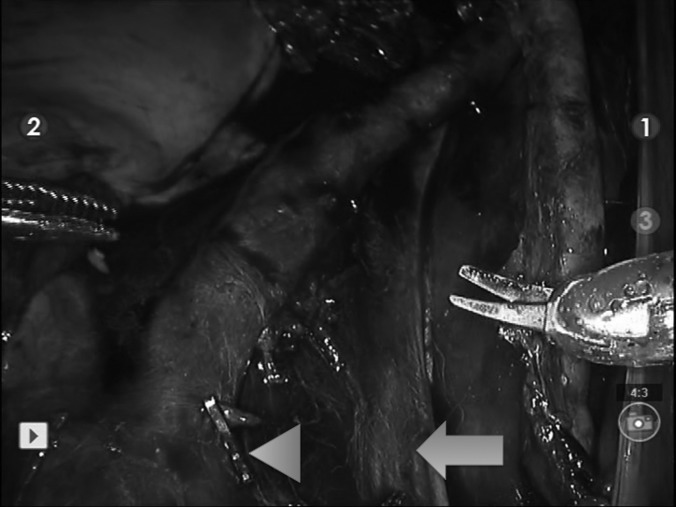
Figure 3.
The abdominal aorta and common iliac arteries are seen with the right external and internal branches and accompanying major venous vessels after completion of robotic bilateral extended lymph node dissection. The arrowhead indicates the abdominal aorta with an endoclip (Ethicon Endo-Surgery, Inc., Cincinnati, Ohio). The arrow indicates the inferior vena cava.
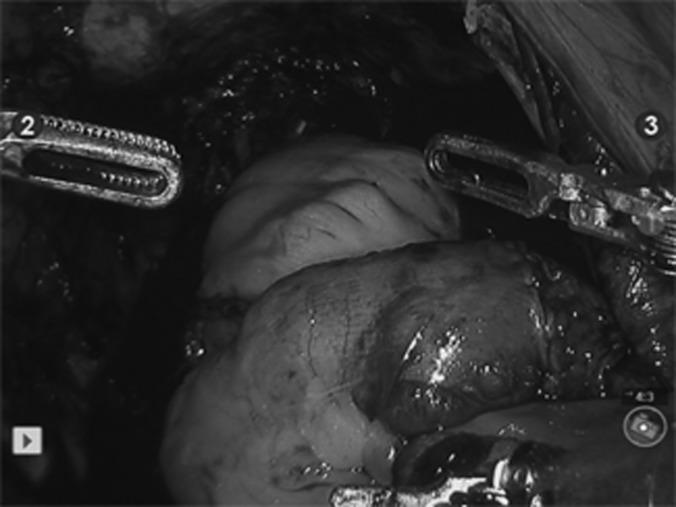
Figure 4.
Completed robotic intracorporeal Studer pouch.
Figure 5.
Immediate postoperative abdominal appearance of a patient who underwent robot-assisted radical cystectomy, bilateral extended pelvic lymph node dissection, and intracorporeal Studer pouch urinary reconstruction. The lodge drain inserted through the 8-mm-sized robotic port site in the right side of the abdomen should be noted.
Figure 6.
a, Healed surgical wounds 4 months postoperatively in a 72-year-old female patient who underwent robot-assisted radical cystectomy (RARC), anterior pelvic exenteration, bilateral extended pelvic lymph node dissection, and intracorporeal Studer pouch urinary reconstruction. b, Healed surgical wounds 6 months postoperatively in a 65-year-old male patient who underwent RARC, bilateral extended pelvic lymph node dissection, and intracorporeal Studer pouch urinary reconstruction.
DISCUSSION
Although open RC with urinary diversion is the gold-standard surgical treatment in the management of muscle-invasive bladder cancer and for patients with high-grade, recurrent, noninvasive tumors,1 RARC is increasingly being performed with mostly extracorporeal or less frequently intracorporeal urinary diversion.
In our study 42 patients were included who underwent open RC, bilateral PLND, and Studer pouch reconstruction for bladder cancer for retrospective comparison with patients who underwent RARC, bilateral extended PLND, and intracorporeal Studer pouch urinary diversion (n = 32). No specific selection criteria were used in order to include open cases. Patient demographic data were similar in both groups (Table 1). The number of patients with an American Society of Anesthesiologists score of III was significantly higher in the robotic group (Table 1). Overall, 5 different surgeons have performed both the open and robotic cases, and the experience of each surgeon is differs. Therefore surgeon experience could certainly have an impact on the operative and postoperative parameters.
The number of studies comparing open versus robotic RC is very limited in the literature. Such studies, including our study, are mostly retrospective, with limited numbers of patients. Only 2 prospective randomized studies exist, with very limited numbers of patients, and in those series urinary diversion was performed at the discretion of the surgeon, mostly extracorporeally.8,9 Therefore, to our knowledge, our study is the first to perform a head-to-head comparison of these 2 particular groups retrospectively.
In our study the median operative time was similar in both groups (Table 2) (P > .05). Although some studies reported similar operative times between the open and robotic RC groups,9,10 others reported increased operative times in the robotic group.8,11–13 Obviously, factors such as surgeon experience, type of urinary diversion, and whether urinary diversion was performed by an extracorporeal or intracorporeal approach might have an impact on the operative time that needs to be taken into account in comparative studies. In our study the numbers of patients undergoing bilateral NVB-sparing surgery and bilateral extended PLND were significantly higher in the robotic group, and this might have increased the operative time in the robotic group. In addition, this might also suggest that the robotic approach enabled the console surgeon to perform NVB-sparing surgery rather than non–nerve-sparing surgery in a greater number of patients, which might have an impact particularly on the postoperative functional outcomes and, in turn, might be an advantage of robotic surgery.
In our study, mean estimated blood loss was significantly lower in the robotic group (412.5 ± 208.3 mL vs 1314.3 ± 987.1 mL, P < .001) (Table 2). Other authors have also reported decreased estimated blood loss8–13 and decreased transfusion rates with the robotic approach.9,11–13 In addition, in our series anatomic anomalies such as the presence of an accessory pudendal artery and ureteral duplication were detected only in the robotic group, with no such anatomic anomalies in the open group (Table 2); this finding might suggest that with magnified and 3-dimensional vision in robotic surgery, anatomic variations might be better detected and preserved. Other operative, postoperative, and pathologic parameters were similar in both groups (Tables 2 and 3). Therefore the robotic approach seems to carry the advantage of significantly decreased blood loss compared with open surgery.
The mean time to intake of a liquid diet, mean time to resumption of a regular diet, mean time to ambulation, mean lodge drain removal time, and mean length of hospital stay were similar in both groups in our series (Table 2). Some studies reported a quicker return of bowel function8,13 and decreased time to resumption of a regular diet in the robotic group.13,14 On the other hand, similar outcomes were reported by other studies between the groups in terms of time to resumption of a regular diet.9 Nix et al8 reported lower use of inpatient narcotics in their robotic group. A shorter hospital stay in patients undergoing the robotic approach was reported by some studies,9,13–15 whereas other studies reported similar durations.11,12 In our series, in patients undergoing the robotic approach, we were much more cautious regarding starting liquid and regular diets, and this might have prolonged these parameters, in addition to the mean drain removal time and length of hospital stay, because these patients were our initial totally intracorporeal Studer pouch reconstruction patients. However, our current policy is to have patients start liquid and regular diets as soon as possible after the totally intracorporeal robotic approach and to discharge the patients whenever possible. Therefore we expect that in our future experience, the totally intracorporeal approach might carry an advantage particularly related to these parameters compared with the open approach. Another speculation is that decreased fluid loss from the bowels in totally intracorporeal urinary diversion might have a positive impact on postoperative bowel function recovery. This might thus further decrease the time to intake of liquid and regular diets and length of hospital stay and warrants further research.
Surgical oncologic quality and efficacy include LN yield and surgical margins (SMs) in RC. A positive SM rate of <10%16,17 and an LN yield of >15 LNs18–20 are recommended for oncologic sufficiency in open RC. Other RARC publications reported positive SM rates between 0% and 8.9%21–24 and mean LN yields of 16 to 19.22,24,25 In our experience the mean LN yield in the open group and robotic group was 17.2 ± 13.5 and 25.4 ± 9.7, respectively, and was significantly higher in the robotic group (P < .05). However, all patients in the robotic group and most of the patients in the open group underwent bilateral extended LN dissection in our series. The extent of LN dissection was performed at the surgeon's discretion. When patients with standard LN dissection were excluded from the open series, the mean LN yield was similar between the groups, although there was an increased LN yield trend in the robotic group. On the other hand, no significant difference was detected in terms of positive SM rates between the 2 groups (P > .05) (Table 3). Other studies comparing open versus robotic approaches have reported similar positive SM rates8–10,12–15 and LN yields8–10,12–15 in their study groups. Sung et al11 detected a significantly increased mean LN yield in their robotic group.
The robotic approach has also been compared with the open approach in terms of complications. Although the numbers of minor and major complications were greater in the open group than in the group undergoing the RARC approach in our series, no statistically significant differences were detected between the 2 groups (Table 4). In addition, the readmission rate for minor complications between 31 and 90 days postoperatively was significantly lower in the robotic group than in the open group in our series (Table 4). In a prospective study by Ng et al,10 RARC was found to be an independent predictor of fewer overall and major complications at 30 and 90 days postoperatively. The rate of major complications was significantly higher in the open RC group (n = 104) than in the RARC group (n = 83) (P = .03). Recently, Schumacher et al26 have reported on surgery-related complications of RARC with intracorporeal urinary diversion in a series of 45 patients according to the Clavien classification. Overall, fewer complications were observed between the groups over time, with a significant decrease in late versus early complications. In the prospective randomized clinical trial of Parekh et al,9 the complication rates of the groups undergoing the open approach (n = 20) and the robotic approach (n = 20) were not significantly different regarding Clavien grade 2 or greater complications. Other authors also did not find any significant differences between the open and robotic approaches in terms of complications.8,12,14,15,27 In the study of Sung et al,11 although the overall complication rates were similar between the open and robotic groups, the percentages of patients with grade 2 or greater complications and with multiple complications were significantly lower in the robotic group. They also reported a significantly decreased rate of wound problems in the robotic group. We think that better cosmetic results might be another advantage of the robotic approach, particularly in female patients (Figures 6a and 6b). Therefore, according to the published literature, the robotic approach does not seem to have an increased risk of complications compared with the open approach.
Using a nerve-sparing technique during open RC is suggested and is expected to preserve urinary continence and erectile function after surgery.28 Regarding the robotic approach, Menon et al29 stated that NVB-sparing RARC combines the oncologic principles of open surgery with the technical advantages of the surgical robot, which allows a precise, gentle, quick, and safe operation. In our series intra-fascial bilateral NVB-sparing RARC was performed in most cases.
Functional outcomes including postoperative continence and penile erection are not reported in detail after RARC in most of the articles in the literature. Among the available patients who have completed 9 months' follow-up, no significant differences were detected in terms of daytime and nighttime incontinence in those who underwent open or robotic intracorporeal Studer urinary diversion (Table 5). However, there was a trend toward improved daytime continence with no pad use and decreased rates of severe daytime incontinence that might be because of better preservation of the NVBs during the robotic approach compared with open surgery (Tables 5 and 6). With an increase in patient numbers in both groups and increase in follow-up, a statistically significant difference could have been expected.
Among the available patients who have completed 9 months' follow-up, no significant differences were detected in terms of erectile function in those with no erectile dysfunction or mild dysfunction preoperatively who underwent open or robotic intracorporeal Studer urinary diversion, although the robotic group had greater preservation of the NVBs (Table 6). However, the limited number of patients and short length of follow-up are the main limitations of our study that are expected to have an impact on the outcomes. Multicenter studies with increased numbers of patients and with longer follow-up periods would be needed to better investigate particularly the functional outcomes.
CONCLUSIONS
The evolving technique of NVB-sparing RARC with extended PLND and intracorporeal Studer pouch urinary reconstruction yielded excellent postoperative surgical and pathologic outcomes and satisfactory functional results despite being a complex surgical procedure. According to our experience, when compared with the open approach, robotic surgery has the advantages particularly of decreased blood loss, giving the console surgeon the chance to better preserve the NVBs; an increased LN yield; and decreased rates of hospital readmissions postoperatively. Though not statistically significant, there was a trend toward improved daytime continence with no pad use and decreased rates of severe daytime incontinence in the robotic group. A cosmetic advantage that includes only abdominal port-site incisions exists, particularly in female patients, compared with open surgery.
Contributor Information
Ali Fuat Atmaca, School of Medicine, Yildirim Beyazit University, Ankara, Turkey.; Department of Urology, Ankara Ataturk Training and Research Hospital, Ankara, Turkey.
Abdullah Erdem Canda, School of Medicine, Yildirim Beyazit University, Ankara, Turkey.; Department of Urology, Ankara Ataturk Training and Research Hospital, Ankara, Turkey.
Bahri Gok, Department of Urology, Ankara Ataturk Training and Research Hospital, Ankara, Turkey..
Ziya Akbulut, School of Medicine, Yildirim Beyazit University, Ankara, Turkey.; Department of Urology, Ankara Ataturk Training and Research Hospital, Ankara, Turkey.
Serkan Altinova, Department of Urology, Ankara Ataturk Training and Research Hospital, Ankara, Turkey..
Mevlana Derya Balbay, Department of Urology, Memorial Sisli Hospital, Istanbul, Turkey..
References:
- 1. Huang GJ, Stein JP. Open radical cystectomy with lymphadenectomy remains the treatment of choice for invasive bladder cancer. Curr Opin Urol. 2007;17:369–375. [DOI] [PubMed] [Google Scholar]
- 2. Canda AE, Atmaca AF, Altinova S, Akbulut Z, Balbay MD. Robot assisted laparoscopic nerve sparing radical cystectomy with bilateral extended lymph node dissection and intracorporeal urinary diversion for bladder cancer: initial experience in 27 cases. BJU Int. 2012;110(3):434–444. [DOI] [PubMed] [Google Scholar]
- 3. Akbulut Z, Canda AE, Ozcan MF, Atmaca AF, Ozdemir AT, Balbay MD. Robot assisted laparoscopic nerve sparing radical cystoprostatectomy with bilateral extended lymph node dissection and intracorporeal Studer pouch construction: outcomes of first 12 cases. J Endourol. 2011;25(9):1469–1479. [DOI] [PubMed] [Google Scholar]
- 4. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lantz AG, Saltel ME, Cagiannos I. Renal and functional outcomes following cystectomy and neobladder reconstruction. Can Urol Assoc J. 2010;4:328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kulkarni JN, Pramesh CS, Rathi S, Pantvaidya GH. Long-term results of orthotopic neobladder reconstruction after radical cystectomy. BJU Int. 2003;91(6):485–488. [DOI] [PubMed] [Google Scholar]
- 7. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830. [DOI] [PubMed] [Google Scholar]
- 8. Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010;57(2):196–201. [DOI] [PubMed] [Google Scholar]
- 9. Parekh DJ, Messer J, Fitzgerald J, Ercole B, Svatek R. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. J Urol. 2013;189(2):474–479. [DOI] [PubMed] [Google Scholar]
- 10. Ng CK, Kauffman EC, Lee MM, et al. A comparison of postoperative complications in open versus robotic cystectomy. Eur Urol. 2010;57(2):274–281. [DOI] [PubMed] [Google Scholar]
- 11. Sung HH, Ahn JS, Seo SI, et al. A comparison of early complications between open and robot-assisted radical cystectomy. J Endourol. 2012;26(6):670–675. [DOI] [PubMed] [Google Scholar]
- 12. Styn NR, Montgomery JS, Wood DP, et al. Matched comparison of robotic-assisted and open radical cystectomy. Urology. 2012;79(6):1303–1308. [DOI] [PubMed] [Google Scholar]
- 13. Knox ML, El-Galley R, Busby JE. Robotic versus open radical cystectomy: identification of patients who benefit from the robotic approach. J Endourol. 2013;27(1):40–44. [DOI] [PubMed] [Google Scholar]
- 14. Wang GJ, Barocas DA, Raman JD, Scherr DS. Robotic vs open radical cystectomy: prospective comparison of perioperative outcomes and pathological measures of early oncological efficacy. BJU Int. 2008;101:89–93. [DOI] [PubMed] [Google Scholar]
- 15. Kader AK, Richards KA, Krane LS, Pettus JA, Smith JJ, Hemal AK. Robot-assisted laparoscopic vs open radical cystectomy: comparison of complications and perioperative oncological outcomes in 200 patients. BJU Int. 2013;112(4):E290–E294. [DOI] [PubMed] [Google Scholar]
- 16. Herr H, Lee C, Chang S, Lerner S; Bladder Cancer Collaborative Group. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: a collaborative group report. J Urol. 2004;171(5):1823–1828. [DOI] [PubMed] [Google Scholar]
- 17. Skinner EC, Stein JP, Skinner DG. Surgical benchmarks for the treatment of invasive bladder cancer. Urol Oncol. 2007;25(1):66–71. [DOI] [PubMed] [Google Scholar]
- 18. Stein JP, Cai J, Groshen S, Skinner DG. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol. 2003;170(1):35–41. [DOI] [PubMed] [Google Scholar]
- 19. Leissner J, Hohenfellner R, Thüroff JW, Wolf HK. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int. 2000;85(7):817–823. [DOI] [PubMed] [Google Scholar]
- 20. Herr HW, Faulkner JR, Grossman HB, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004;15:22(14):2781–2789. [DOI] [PubMed] [Google Scholar]
- 21. Hellenthal NJ, Hussain A, Andrews PE, et al. Surgical margin status after robot assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. J Urol. 2010;184(1):87–91. [DOI] [PubMed] [Google Scholar]
- 22. Pruthi RS, Nielsen ME, Nix J, Smith A, Schultz H, Wallen EM. Robotic radical cystectomy for bladder cancer: surgical and pathological outcomes in 100 consecutive cases. J Urol. 2010;183(2):510–514. [DOI] [PubMed] [Google Scholar]
- 23. Khan MS, Elhage O, Challacombe B, Rimington P, Murphy D, Dasgupta P. Analysis of early complications of robotic-assisted radical cystectomy using a standardized reporting system. Urology. 2011;77(2):357–362. [DOI] [PubMed] [Google Scholar]
- 24. Guru KA, Sternberg K, Wilding GE, et al. The lymph node yield during robot-assisted radical cystectomy. BJU Int. 2008;102(2):231–234. [DOI] [PubMed] [Google Scholar]
- 25. Murphy DG, Challacombe BJ, Elhage O, et al. Robotic-assisted laparoscopic radical cystectomy with extracorporeal urinary diversion: initial experience. Eur Urol. 2008;54(3):570–580. [DOI] [PubMed] [Google Scholar]
- 26. Schumacher MC, Jonsson MN, Hosseini A, et al. Surgery-related complications of robot-assisted radical cystectomy with intracorporeal urinary diversion. Urology. 2011;77(4):871–876. [DOI] [PubMed] [Google Scholar]
- 27. Sterrett S, Mammen T, Nazemi T, et al. Major urological oncological surgeries can be performed using minimally invasive robotic or laparoscopic methods with similar early perioperative outcomes compared to conventional open methods. World J Urol. 2007;25(2):193–198. [DOI] [PubMed] [Google Scholar]
- 28. Schoenberg MP, Walsh PC, Breazeale DR, Marshall FF, Mostwin JL, Brendler CB. Local recurrence and survival following nerve sparing radical cystoprostatectomy for bladder cancer: 10-year followup. J Urol. 1996;155(2):490–494. [PubMed] [Google Scholar]
- 29. Menon M, Hemal AK, Tewari A, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92(3):232–236. [DOI] [PubMed] [Google Scholar]