Abstract
Background
Caloric restriction (CR) increases maximal life span in short-lived organisms, and its effects are being explored in nonhuman primates. The objectives of this study were to determine the feasibility of prolonged CR in nonobese adults and to compare the effects of CR- and exercise-induced weight loss on body composition and abdominal adiposity.
Methods
A randomized, controlled trial was conducted with 48 healthy, nonobese women and men, aged 57 ± 1 (mean ± standard error [SE]) years, with body mass index 27.3 ± 0.3 kg/m2. Participants were randomly assigned to a 20% calorically-restricted diet (CR, n = 19), exercise designed to produce a similar energy deficit (EX, n = 19), or a healthy lifestyle control group (HL, n = 10) for 1 year. Assessments included weight, body composition by dual-energy x-ray absorptiometry, abdominal adipose tissue by magnetic resonance imaging, and energy intake by doubly labeled water.
Results
The average level of CR achieved by the CR group was 11.5 ± 2.1%, and the EX group completed 59 ± 6.7% of their prescribed exercise. Weight changes were greater (p ≤ .0005) in the CR (−8.0 ± 0.9 kg) and EX (−6.4 ± 0.9) groups as compared to the HL group (−1.3 ± 0.9 kg), corresponding to reductions of 10.7%, 8.4%, and 1.7% of baseline weights, respectively. Whole-body fat mass and visceral and subcutaneous abdominal adipose tissue decreased significantly (p < .005) and comparably in the CR and EX groups, but did not change in the HL group.
Conclusions
CR for 1 year was feasible, but the level of CR achieved was less than prescribed. CR and exercise were equally effective in reducing weight and adiposity.
Caloric restriction (CR) slows the aging process and increases maximal life span in various short-lived organisms (1), and has numerous physiologic benefits in nonhuman primates (2,3). However, little is known regarding the effects of long-term CR with good nutrition in nonobese humans. Preliminary information regarding physiologic adaptations to CR was obtained from eight adults living in Biosphere 2 (4), a self-contained ecologic mini-world in which an unplanned food shortage caused weight losses of 10%–25% by the end of 2 years. Cross-sectional data on the effects of longer-term CR were obtained from men and women who had been following a CR diet voluntarily for 6 years (5,6). These individuals had dramatically lower body mass index (BMI), body fat, blood pressure, total and LDL cholesterol, C-reactive protein, and carotid artery intima media thickness (5), as well as greater cardiac function (6), relative to healthy controls. Although these studies in humans were not prospective, they set the stage for controlled CR trials in humans. A set of feasibility studies for human CR, referred to as CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy), was completed recently. In this article we report on the feasibility and body composition effects of a 1-year, randomized, controlled trial of CR at the Washington University School of Medicine CALERIE site. We hypothesized that weight loss induced by exercise would result in proportionately greater fat loss than weight loss induced by CR.
Methods
Participants
Healthy, nonobese men and postmenopausal women, aged 50–60 years with a BMI between 23.5 and 29.9 kg/m2, were recruited from the St. Louis metropolitan area using electronic mail, direct mailings, television interviews, and newspaper and radio advertisements. Exercise training more than twice per week was exclusionary, as were smoking, recent weight loss, eating disorder symptoms, and medications that would affect the outcome measures. This study was approved by the Washington University School of Medicine Human Studies Committee and the General Clinical Research Center Scientific Advisory Committee. Written, informed consent was obtained from each participant.
Study Design
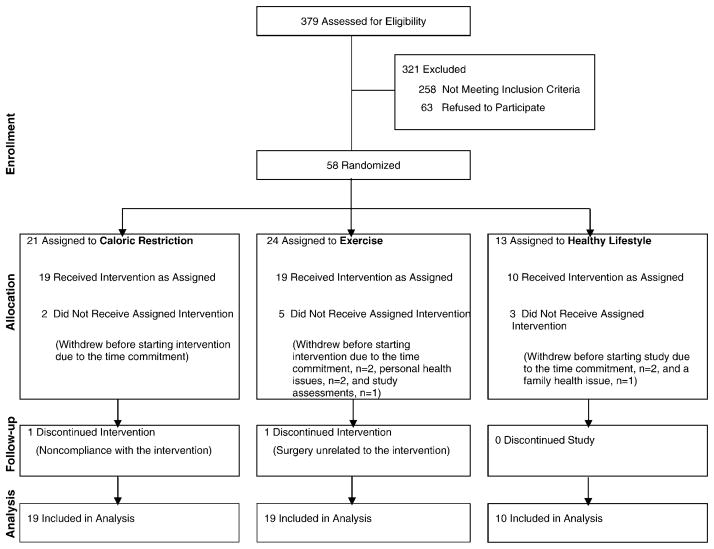
After a series of screening visits, participants were randomly assigned to the CR, exercise (EX), or healthy lifestyle (HL, control) group in a 2:2:1 sequence. Forty-eight adults (30 women, 18 men) began the intervention, and their data are included in these analyses (Figure 1).
Figure 1.
Consort diagram depicting the flow of participants through the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE) trial at Washington University School of Medicine.
CR Intervention
The objective of the CR intervention was to decrease daily energy intake by 16% for the initial 3 months of the intervention and by 20% for the remaining 9 months. The CR prescriptions were calculated from baseline energy intake as determined by the doubly labeled water method over a 4-week period. Each participant received a prescription of total calories to consume daily (and number of calories to remove relative to their baseline intake), but the macronutrient composition was flexible to accommodate individual preferences. Meals were provided from the General Clinical Research Center metabolic kitchen for 5 consecutive days at week 4 (16% CR) and after the 3-month time point (20% CR) to educate participants on the appropriate serving sizes. Participants attended individual and/or group meetings led by a registered dietitian and behavioral psychologist weekly for the first 6 months of the intervention, and less frequently throughout the last 6 months. The curriculum addressed using food scales, reading nutrition labels, modifying recipes and meals to contain fewer calories, incorporating meal replacements, and using various behavior modification strategies from the LEARN manual (7). CR participants were instructed to set weekly behavioral goals and to attend weekly weigh-in sessions throughout the intervention, and were encouraged to use food diaries or the BalanceLog program (HealtheTech, Inc., Golden, CO) on Palm handhelds (Palm, Inc., Sunnyvale, CA) as a means of daily monitoring to enhance adherence. A multivitamin with mineral supplement was given to participants in all groups.
EX Intervention
The goal of the exercise intervention was to induce an energy deficit comparable to the CR intervention by increasing daily energy expenditure through exercise without changing caloric intake. To enable adaptation to exercise, the EX prescription began at ~16% and then increased to 20%. Exercise physiologists and trainers worked with EX participants individually to establish and monitor their exercise routines, which could be performed in our exercise facility (containing treadmills, a track, cycle ergometers, rowing ergometers, elliptical machines, and stairclimbers), a health club, participants’ homes, or outdoors. All EX participants were instructed to attend weekly weigh-in sessions throughout the intervention. Because this was not a training study, exercise intensity was not prescribed, and the number of weekly exercise sessions was individualized.
Healthy Lifestyle
The HL group received general information about a healthy diet and was offered free yoga classes, but did not receive a diet or exercise prescription, and had minimal contact with our research team. This group served as a control.
Anthropometry and Body Composition
Body weight was measured in duplicate after an overnight fast, with the participant wearing a hospital gown. Baseline body weight was calculated as the mean of five weekly weights measured during the 4-week baseline period. Weights at months 1, 3, 6, 9, and 12 represent the mean of three weekly weights obtained at the beginning, middle, and end of each 2-week assessment period. Height was measured to the nearest 0.1 cm. BMI was calculated as weight/height2 (kg/m2). Whole-body fat mass (FM), fat-free mass (FFM), and %FM were assessed by dual-energy x-ray absorptiometry (DXA) (software version 11.2, Delphi W; Hologic Corporation, Waltham, MA); reported values represent the mean of 2–3 DXA scans at baseline and 2 DXA scans at the follow-up time points.
Abdominal visceral and subcutaneous adipose tissue volumes were quantified using proton magnetic resonance imaging (MRI) at baseline and after 1 year. Ten serial 10 mm axial images were acquired using a 1.5-T superconducting magnet (Siemens, Iselin, NJ), beginning at L1 (identified by the origin of the psoas muscle) and moving downward. Baseline and 1-year images were batch-analyzed using Hippo software (8). Slice-level fat volumes were summed for total volumes of visceral and subcutaneous adipose tissue.
Total Energy Expenditure
Total energy expenditure (TEE) was measured during 2-week periods using the doubly labeled water (DLW) method (9). Two baseline urine samples were collected, after which an oral dose of DLW was administered (0.20 g of H218O, 0.12 g of 2H2O per kg of total body water). Postdose urine samples were collected at 4.5 hours, 6 hours, 7 days (two samples), and 14 days (two samples), and were analyzed in duplicate for 18O and 2H abundance by isotope ratio mass spectrometry in the Mass Spectrometry Laboratory at Pennington Biomedical Research Center (10).
Energy Intake
Energy intake was assessed by DLW and food diaries. Using DLW, energy intake at baseline was assumed to equal TEE, because participants were weight stable. For each 3-month interval, energy intake was calculated as the average TEE (e.g., average of 3- and 6-month TEE for the 3–6 month interval) with adjustment for changes in FM and FFM during that interval. The energy values of FM and FFM were assumed to be 9.3 and 1.1 kcal/g, respectively. The %CR was calculated as the percent reduction in energy intake from baseline.
Seven-day food diaries were used to estimate self-reported intake. Participants received detailed instructions on how to weigh, measure, and record all food and beverages consumed. Research dietitians reviewed the diaries with participants and then analyzed them using Nutrition Data System for Research (NDS-R) (software versions 4.05, 4.06, and 5.0; Nutrition Coordinating Center, University of Minnesota, Minneapolis).
Exercise Adherence
Adherence to the EX intervention was estimated using heart rate (HR) monitors (S610; Polar Electro Oy, Kempele, Finland), which EX participants were instructed to wear during each exercise session and bring in for downloading during weekly meetings with the exercise trainers. The HR monitors store minute-by-minute HR values and exercise duration, and calculate energy expenditure for each session using participant-specific values for maximal oxygen uptake (VO2max), maximum HR (HRmax), weight, height, age, and sex. Participants also were asked to report any exercise sessions that were not recorded on their HR monitors.
Physical Activity
A modified version of the Stanford Seven-Day Physical Activity Recall Questionnaire (PAR) (11,12) was used among all participants to quantify the amount of time spent sleeping and engaged in light, moderate, hard, and very hard physical activities, which corresponded to increasing metabolic equivalents (METs). Physical activity data are presented as MET-h/d above rest, where rest is assumed to equal 24 MET-h/d.
VO2max
VO2max was determined by indirect calorimetry during an incremental exercise test to exhaustion (13). Participants walked on a level treadmill at a pace that elicited 60%–70% of age-predicted maximal heart rate for a 5-minute warm-up. The speed was then set at the fastest comfortable pace, and the grade was increased 1%–2% every 1–2 minutes until volitional exhaustion, electrocardiographic changes, or other abnormalities that rendered it unsafe to continue.
Statistical Analyses
Intention-to-treat analyses were performed using SAS software, version 9.1.3 of the SAS System for Linux (SAS Institute Inc., Cary, NC), with inclusion of all participants who provided follow-up data at any time point. Longitudinal changes between and within groups were tested with mixed model repeated-measures analyses of variance; when interactions between group and time point were significant, contrasts assessing the equality of changes from baseline to 6 months and baseline to 1 year were examined. For outcomes assessed at two time points only (MRI and VO2), analysis of covariance and paired t tests were used. All body composition outcomes were adjusted for gender and age. Statistical tests were two-tailed, with significance accepted at p ≤ .05. Data are presented as the least square mean (for repeated measures) or mean (for measures collected at a single time point) ± standard error.
Results
Demographic characteristics are shown in Table 1. Forty-six participants (96%) completed the study; one woman dropped out at 6 months due to inability to adhere to the CR prescription, and one man dropped out of the EX group at 9 months for medical reasons unrelated to the intervention (Figure 1). Ten eligible individuals withdrew before completing baseline testing (n = 7 for study issues; n = 3 for personal issues). Attendance at the weekly weigh-in sessions with the dietitians (CR group) and exercise trainers (EX group) was comparable, averaging 84.6 ± 2.8% of the total intervention weeks in the CR group and 85.0 ± 3.2% in the EX group. Adverse events among CR participants included hunger and constipation, whereas EX participants experienced muscle soreness and joint pain. One serious adverse event occurred in CR and one in EX; both were deemed unrelated to the interventions.
Table 1.
Baseline Characteristics and Intervention Prescriptions
| Variable | CR (N = 19) | EX (N = 19) | HL (N = 10) |
|---|---|---|---|
| Age, y* | 55.6 ± 0.8 | 58.8 ± 0.6 | 56.0 ± 0.9 |
| Gender | |||
| Female | 12 (63%) | 12 (63%) | 6 (60%) |
| Male | 7 (37%) | 7 (37%) | 4 (40%) |
| Race | |||
| African American/Black | 0 | 1 (5%) | 2 (20%) |
| White | 17 (89%) | 17 (90%) | 7 (70%) |
| Other | 2 (11%) | 1 (5%) | 1 (10%) |
| Education | |||
| < College degree | 4 (21%) | 6 (32%) | 6 (60%) |
| College degree | 4 (21%) | 4 (21%) | 2 (20%) |
| Graduate school | 11 (58%) | 9 (47%) | 2 (20%) |
| Body mass index, kg/m2 | 27.2 ± 0.6 | 27.2 ± 0.4 | 27.9 ± 0.4 |
| Total daily energy expenditure, kcal/d | 2493 ± 114 | 2508 ± 94 | 2550 ± 138 |
| Prescribed reduction in energy intake, kcal/d | 524 ± 33 | 0 | 0 |
| Prescribed increase in energy expenditure, kcal/d | 0 | 563 ± 18 | 0 |
Notes: Values represent mean ± standard error or N (% of participants).
p = .007 for difference between groups.
CR = caloric restriction; EX = exercise; HL = healthy lifestyle.
Body Weight and Composition
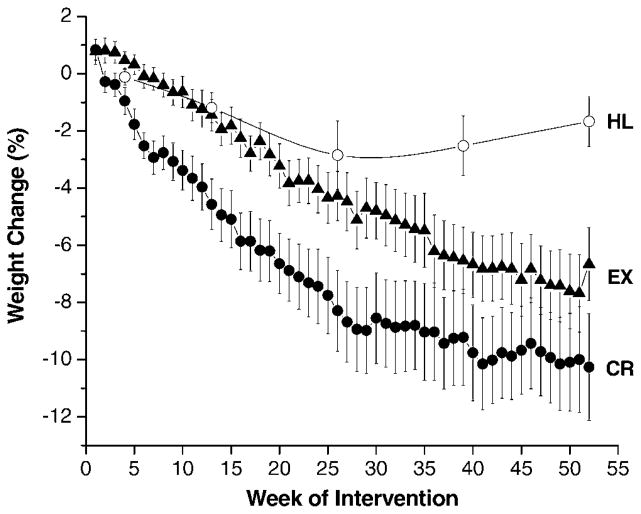
At baseline, most participants were overweight, with a mean BMI of 27.3 ± 0.3 kg/m2, and body fat of 39 ± 1% in women and 25 ± 1% in men. After 1 year, weight losses were 8.0 ± 0.9 kg in the CR group, 6.4 ± 0.9 kg in the EX group, and 1.3 ± 0.9 kg in the HL group (Table 2), which corresponded to reductions of 10.7%, 8.4%, and 1.7% of baseline body weights, respectively. The time course of weight loss is depicted in Figure 2. There was no correlation between the amount of weight lost and the number of weekly weigh-in sessions attended. BMI values after 1 year were 24.4 ± 0.6 kg/m2, 25.0 ± 0.5 kg/m2, and 27.4 ± 0.5 kg/m2 in the CR, EX, and HL groups, respectively (p < .0001 CR vs HL; p=.0005 EX vs HL). As with the decreases in weight and BMI, reductions in whole-body FM were significantly greater in the CR and EX groups than in the HL group, with no significant difference observed between the CR and EX interventions (Table 2). FM comprised 77% of total weight loss in CR and 87% in EX (median values, p = .31 between groups by Wilcoxon’s test).
Table 2.
Body Weight and Body Composition
| Variable | Time Point | CR | EX | HL | p Between Groups After 1 Year |
|---|---|---|---|---|---|
| Weight, kg | Baseline | 78.5 ± 2.3 | 77.5 ± 2.4 | 81.9 ± 3.7 | |
| 3 mo | 73.8 ± 2.3 | 75.4 ± 2.4 | 80.9 ± 3.7 | ||
| 6 mo | 71.7 ± 2.3* | 73.3 ± 2.4* | 79.8 ± 3.7† | ||
| 9 mo | 70.9 ± 2.3 | 71.9 ± 2.4 | 80.0 ± 3.7 | <.0001 CR vs HL | |
| 1 y | 70.5 ± 2.3* | 71.0 ± 2.4* | 80.7 ± 3.7 | .0005 EX vs HL | |
| % Fat mass | Baseline | 33.1 ± 1.1 | 31.7 ± 1.0 | 32.4 ± 1.0 | |
| 3 mo | 30.5 ± 1.2 | 29.6 ± 1.0 | 31.6 ± 1.0 | ||
| 6 mo | 28.7 ± 1.2* | 27.9 ± 1.0* | 31.4 ± 1.1 | ||
| 9 mo | 27.8 ± 1.2 | 27.1 ± 1.0 | 31.7 ± 1.0 | <.0001 CR vs HL | |
| 1 y | 27.7 ± 1.2* | 26.7 ± 1.0* | 32.4 ± 1.0 | <.0001 EX vs HL | |
| Fat mass, kg | Baseline | 25.9 ± 1.2 | 24.4 ± 1.3 | 26.3 ± 1.0 | |
| 3 mo | 22.4 ± 1.2 | 22.0 ± 1.3 | 25.2 ± 1.0 | ||
| 6 mo | 20.6 ± 1.2* | 20.3 ± 1.3* | 24.9 ± 1.0† | ||
| 9 mo | 19.8 ± 1.2 | 19.3 ± 1.3 | 25.0 ± 1.0 | <.0001 CR vs HL | |
| 1 y | 19.7 ± 1.2* | 18.9 ± 1.3* | 25.9 ± 1.0 | <.0001 EX vs HL | |
| Fat-free mass, kg | Baseline | 53.6 ± 1.1 | 53.9 ± 1.0 | 56.9 ± 1.1 | |
| 3 mo | 52.2 ± 1.1 | 54.0 ± 1.0 | 56.9 ± 1.1 | ||
| 6 mo | 51.9 ± 1.1† | 53.9 ± 1.0 | 56.4 ± 1.1 | ||
| 9 mo | 52.0 ± 1.1 | 53.2 ± 1.0 | 56.1 ± 1.1 | ||
| 1 y | 51.9 ± 1.1† | 53.0 ± 1.0 | 56.2 ± 1.1 |
Notes: Values represent least square means ± standard error; body composition measures were adjusted for gender and age. p between groups after 1 year reflects the equality of changes from baseline to 1 year by statistical contrasts, only when overall p ≤ .05.
p ≤ .0001,
p ≤ .05 for change within group by contrasts for baseline to 6 mo and baseline to 1 y.
CR = caloric restriction; EX = exercise; HL = healthy lifestyle.
Figure 2.
Body weight changes throughout the 1-year interventions. Symbols represent mean values (black circles, caloric restriction [CR]; black triangles, exercise [EX]; open circles, healthy lifestyle [HL]) ± standard error.
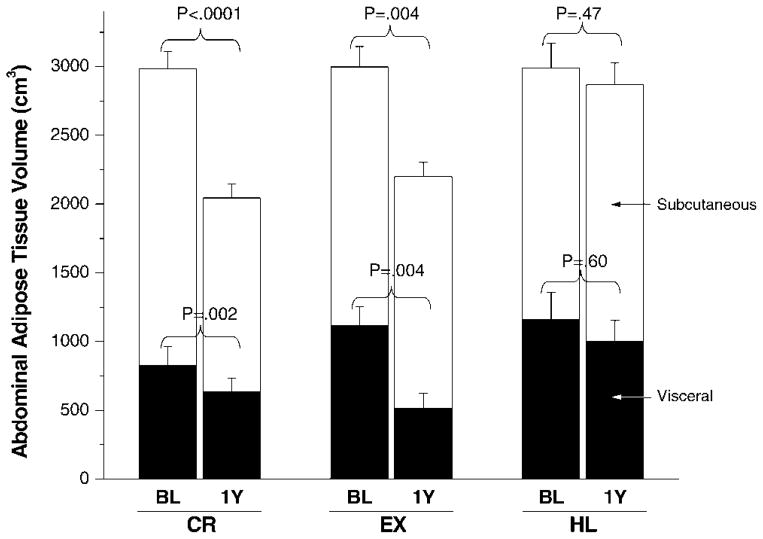
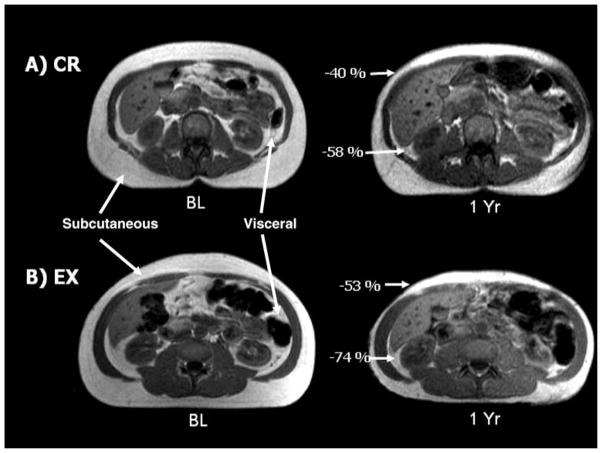
Based on MRI analyses, visceral and subcutaneous abdominal adipose tissue decreased significantly in the CR and EX groups, but not in the HL group (Figure 3). Although it appears that exercise caused a greater reduction in visceral fat than did CR, the relative changes were comparable (−39% in EX, −37% in CR), and the absolute changes were not statistically different between CR and EX (p =.46) when the baseline differences were accounted for. MRI images from two adherent participants (1 CR, 1 EX) are shown in Figure 4 to highlight the dramatic losses of abdominal adipose tissue in response to the interventions.
Figure 3.
Visceral (black bars) and subcutaneous (white bars) abdominal adipose tissue by magnetic resonance imaging (MRI) at baseline and after 1 year of intervention. CR, caloric restriction; EX, exercise; HL, healthy lifestyle. Final values were adjusted for baseline differences between groups. Values of p represent the change within group from baseline (BL) to 1 year. For comparison between groups, the following contrasts were significant: CR versus HL for visceral adipose tissue (p = .05), CR versus HL for subcutaneous adipose tissue (p = .02), and EX versus HL for visceral adipose tissue (p = .01).
Figure 4.
Magnetic resonance images of the abdomen at baseline (BL) and after the 1 year of caloric restriction (CR) (A) and exercise (EX) (B) interventions. Single-slice images are shown, although analyses were based on multislice volume estimates. The CR participant is a 54-year-old woman whose body weight decreased 12 kg (19%); the EX participant is a 60-year-old man whose weight decreased 16 kg (14%).
Adherence to CR
As shown in Table 3, energy intake decreased significantly (p < .0001) in the CR group during the first 6 months, as assessed by both DLW and food diaries, with adherence lower during the second half of the intervention. Averaged over the entire year, the CR group achieved 11.5 ± 2.1% CR, based on DLW. As instructed, the EX group did not restrict their energy intake, whereas the HL group had an average 2.5 ± 2.1% CR. Self-reported energy intake estimated from the food diaries was consistently lower than DLW-determined intake for all groups.
Table 3.
Energy Intake Estimated From DLW-Derived Energy Expenditure With DXA-Derived Changes in Body Energy Stores or 7-Day Food Diaries
| Energy Intake | Time Point or Interval | CR | EX | HL | Overall p Between Groups |
|---|---|---|---|---|---|
| Energy Intake by DLW/DXA, kcal/d | Baseline | 2541 ± 122 | 2471 ± 100 | 2550 ± 146 | |
| Baseline–3 mo | 2195 ± 123 | 2443 ± 100 | 2468 ± 147 | ||
| 3 mo–6 mo | 2156 ± 123* | 2435 ± 101 | 2450 ± 156 | ||
| 6 mo–9 mo | 2259 ± 123 | 2501 ± 102 | 2512 ± 154 | ||
| 9 mo–1 y | 2375 ± 124 | 2532 ± 103 | 2501 ± 151 | .01 | |
| Energy Intake by Food Diaries, kcal/d | Baseline | 2081 ± 105 | 2052 ± 103 | 2201 ± 151 | |
| 3 mo | 1739 ± 106 | 2100 ± 103 | 2075 ± 153 | ||
| 6 mo | 1766 ± 105* | 2104 ± 104 | 2149 ± 159 | ||
| 9 mo | 1812 ± 105 | 2132 ± 105 | 2139 ± 157 | ||
| 1 y | 1737 ± 106† | 2062 ± 107 | 2265 ± 156 | .01 |
Notes: Values represent least square means ± standard error from the mixed model. Overall p between groups reflects the equality of changes from baseline to 1 year (however, statistical contrasts between CR and EX or CR and HL did not reach significance).
p ≤ .0001,
p ≤ .005 for change within group by contrasts for baseline to 6 mo and baseline to 1 y.
DLW = doubly labeled water; DXA = dual energy x-ray absorptiometry; CR = caloric restriction; EX = exercise; HL = healthy lifestyle.
Adherence to EX
Based on heart rate monitor data, EX participants exercised 5.8 ± 0.6 sessions/wk, 62.5 ± 4.3 min/session, at an intensity of 72 ± 0% of HRmax, and expended 317 ± 39 kcal/d throughout the intervention, which corresponded to 58.7 ± 6.7% of the exercise prescription. This latter value is likely to be an underestimate, however, because HR data were not recorded for 12.8 ± 4.0% of the exercise sessions due to equipment failure or participants forgetting to use their monitors. The most frequently used modes of exercise were walking and/or jogging, using elliptical machines, and using cycle ergometers.
As shown in Table 4, results from the PAR questionnaire indicate that physical activity increased only in the EX group. Likewise, absolute VO2max increased only in the EX group, which contrasts the reduction observed in the CR group, providing additional evidence that weight loss was achieved by CR in the CR participants and by exercise in the EX participants.
Table 4.
Physical Activity and Fitness at Baseline and After 1 Year of Intervention
| Variable | Time Point | CR | EX | HL | p Between Groups After 1 Year |
|---|---|---|---|---|---|
| Physical Activity from PAR: MET-h/d above rest | Baseline | 11.0 ± 0.6 | 10.4 ± 1.0 | 9.5 ± 1.4 | .002 CR vs EX |
| 1 y | 10.1 ± 0.5 | 14.3 ± 1.0† | 10.7 ± 1.3 | ||
| VO2max, L/min | Baseline | 2.11 ± 0.13 | 1.97 ± 0.15 | 2.18 ± 0.23 | <.0001 CR vs EX |
| 1 y | 1.95 ± 0.05* | 2.37 ± 0.05* | 1.95 ± 0.07 | <.0001 EX vs HL | |
| VO2max, ml · kg−1 · min−1 | Baseline | 26.5 ± 1.1 | 25.3 ± 1.4 | 26.0 ± 1.8 | .0003 CR vs EX |
| 1 y | 27.4 ± 1.0† | 32.8 ± 1.0* | 24.7 ± 1.3 | <.0001 EX vs HL |
Notes: Values represent least square means ± standard error. Rest was assumed to equal 24 MET-h/d; PAR data were analyzed using ranked values.
p ≤ .001,
p ≤ .05, for change within group from baseline to 1 y. p between groups after 1 year reflects the equality of changes from baseline to 1 y by statistical contrasts.
CR = caloric restriction; EX = exercise; HL = healthy lifestyle; PAR = Stanford Seven-Day Physical Activity Recall Questionnaire; MET = metabolic equivalent; VO2max = maximal oxygen uptake.
Discussion
CALERIE is the first clinical trial of CR in nonobese humans, and the present study is the first that directly compares the effects of a long-term, diet-induced energy deficit with a comparable exercise-induced energy deficit on whole-body and abdominal adiposity. Our results support the feasibility of long-term, modest CR, and demonstrate that 1 year of CR reduces whole-body adiposity and abdominal adipose tissue significantly and comparably to exercise. Novel features of this study, as compared to many previous studies of energy restriction, are the relatively long duration of the intervention and the focus on nonobese individuals.
Consistent with our observation that, compared to CR, exercise did not significantly enhance the mobilization of visceral adipose tissue stores, Ross and colleagues reported comparable reductions in visceral adipose tissue in response to 3 months of diet- or exercise-induced weight loss among men (14) and premenopausal women (15) with abdominal obesity. In contrast to our results, however, they found that exercisers lost more total FM than did dieters (14,15), and that female exercisers lost more abdominal subcutaneous adipose tissue than did female dieters (15). This discrepancy with our results may be explained by differences in study design, including the older age and lower BMI of our participants, or the longer duration of our intervention.
A potential adverse consequence of CR in the present and previous (14) studies is a reduction in FFM, which was accompanied in the present study by a small decrement in absolute VO2max (i.e., L/min). The clinical importance of these changes is unclear. As expected (16,17), exercise helped to preserve lean mass during weight loss and promoted increases in VO2max in the present study.
One of the most dramatic effects of life-prolonging CR in rodents is prevention of the large increase in body fat that normally occurs with advancing age in sedentary animals fed ad libitum. It has been hypothesized that maintenance of leanness is importantly involved in the mechanism by which CR slows aging (18). In support of this hypothesis, Blüher and colleagues (19) reported that adipocyte-specific insulin receptor knockout mice have lower FM and increased maximal life span despite normal food intake. As shown in the present study, exercise without CR can be as effective as CR alone in reducing body fat stores, and people who regularly engage in endurance exercise generally stay lean (20). However, although regular exercise helps protect against secondary aging (i.e., physiological declines and disease processes attributable to modifiable lifestyle factors), there is no evidence that exercise slows primary aging (i.e., physiological declines attributable to the aging process itself).
Research on rats, in which aging over the life span can be studied under controlled conditions, has shown clearly that maintenance of leanness by means of exercise does not slow primary aging (i.e., does not increase maximal life span). In these studies (21–23), male rats given access to running wheels were compared to sedentary animals in which food intake was restricted 30% to match their body weights to those of the runners. As in many previous studies, the CR rats had increases in average and maximal longevity. In contrast, the runners had a 10% increase in average longevity but no increase in maximal longevity (21–23), despite the observation that exercise prevents body fat accumulation and insulin resistance with advancing age more effectively than CR does (24,25). Therefore, although CR and exercise have many similar benefits, CR has a unique ability to slow primary aging in short-lived organisms. A comparison of long-term CR and exercise in humans may reveal the adaptive response unique to CR that could be involved in slowing primary aging.
A limitation of the present study is that adherence to the prescribed CR and EX regimens was less than 100%. It is difficult for adults in our society to follow a CR diet long-term, as evidenced by the high prevalence of overweight and obese individuals (26). Despite relatively high adherence to the CR diet for the first 6 months, participants in the present study were unable to maintain 20% CR during the final 6 months. Furthermore, the large volume of exercise needed to achieve a 20% increase in daily energy expenditure required approximately 90 minutes every day. Nevertheless, our results provide strong evidence that the CR group did follow a calorically-restricted diet throughout the intervention, and that the EX group lost weight through exercise and not by dieting. Importantly, our CR participants were responsible for purchasing and preparing their own meals, and we did not provide financial incentives for adherence or study completion. Another limitation is that a few HL participants lost a significant amount of weight by 6 months, which may be attributable to the fact that individuals who enrolled in this study were ready to make lifestyle changes, and were able to do so with minimal intervention from the research team. The low rate of study attrition (4%) is encouraging as we prepare to initiate phase 2 of the CALERIE trial.
Summary
Our results support the feasibility of long-term, albeit modest, CR, and provide evidence for beneficial changes in whole-body adiposity and abdominal visceral and subcutaneous adipose tissue that are comparable to exercise-induced alterations.
Acknowledgments
This research was supported by National Institutes of Health (NIH) Cooperative Agreement 5-U01-AG20487, General Clinical Research Center Grant RR00036, Diabetes Research and Training Center Grant DK20579, and Clinical Nutrition Research Unit Grant DK56341. E.P. Weiss was supported by Institutional National Research Service Award AG00078.
We are grateful to the study participants for their cooperation, and to the staff of the Applied Physiology Laboratory and nurses of the General Clinical Research Center at Washington University School of Medicine for their skilled assistance.
References
- 1.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 3.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol Biol Sci Med Sci. 2003;58A:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 4.Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol Biol Sci. 2002;57A:B211–B224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- 5.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 7.Brownell KD. The LEARN Program for Weight Management. Dallas, TX: American Health Publishing Company; 2000. [Google Scholar]
- 8.Lancaster JL, Ghiatas AA, Alyassin A, Kilcoyne RF, Bonora E, DeFronzo RA. Measurement of abdominal fat with T1-weighted MR images. J Magn Reson Imaging. 1991;1:363–369. doi: 10.1002/jmri.1880010315. [DOI] [PubMed] [Google Scholar]
- 9.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr. 1988;118:1278–1289. doi: 10.1093/jn/118.11.1278. [DOI] [PubMed] [Google Scholar]
- 10.DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA. Field use of D2 18O to measure energy expenditure of soldiers at different energy intakes. J Appl Physiol. 1989;67:1922–1929. doi: 10.1152/jappl.1989.67.5.1922. [DOI] [PubMed] [Google Scholar]
- 11.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 12.Bonnefoy M, Normand S, Pachiaudi C, Lacour JR, Laville M, Kostka T. Simultaneous validation of ten physical activity questionnaires in older men: a doubly labeled water study. J Am Geriatr Soc. 2001;49:28–35. doi: 10.1046/j.1532-5415.2001.49006.x. [DOI] [PubMed] [Google Scholar]
- 13.Kohrt WM, Malley MT, Coggan AR, et al. Effects of gender, age, and fitness level on the response of VO2max to training in 60- to 71-year-olds. J Appl Physiol. 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- 14.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 16.Mayo MJ, Grantham JR, Balasekaran G. Exercise-induced weight loss preferentially reduces abdominal fat. Med Sci Sports Exerc. 2003;35:207–213. doi: 10.1249/01.MSS.0000048636.46744.01. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163:1343–1350. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 18.Barzilai N, Gupta G. Revisiting the role of fat mass in the life extension induced by caloric restriction. J Gerontol Biol Sci. 1999;54A:B89–B96. doi: 10.1093/gerona/54.3.b89. [DOI] [PubMed] [Google Scholar]
- 19.Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 20.Ryan AS, Nicklas BJ, Elahi D. A cross-sectional study on body composition and energy expenditure in women athletes during aging. Am J Physiol. 1996;271(5 pt 1):E916–E921. doi: 10.1152/ajpendo.1996.271.5.E916. [DOI] [PubMed] [Google Scholar]
- 21.Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- 22.Holloszy JO, Schechtman KB. Interaction between exercise and food restriction: effects on longevity of male rats. J Appl Physiol. 1991;70:1529–1535. doi: 10.1152/jappl.1991.70.4.1529. [DOI] [PubMed] [Google Scholar]
- 23.Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59:826–831. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- 24.Craig BW, Garthwaite SM, Holloszy JO. Adipocyte insulin resistance: effects of aging, obesity, exercise and food restriction. J Appl Physiol. 1987;62:95–100. doi: 10.1152/jappl.1987.62.1.95. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence JC, Colvin J, Cartee GD, Holloszy JO. Effects of aging and exercise on insulin action in rat adipocytes are correlated with changes in fat cell volume. J Gerontol. 1989;44:B88–B92. doi: 10.1093/geronj/44.4.b88. [DOI] [PubMed] [Google Scholar]
- 26.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]