Abstract
Argon cluster sputtering of an organic multilayer reference material consisting of two organic components, 4,4′-bis[N-(1-naphthyl-1-)-N-phenyl- amino]-biphenyl (NPB) and aluminium tris-(8-hydroxyquinolate) (Alq3), materials commonly used in organic light-emitting diodes industry, was carried out using time-of-flight SIMS in dual beam mode. The sample used in this study consists of a ∽400-nm-thick NPB matrix with 3-nm marker layers of Alq3 at depth of ∽50, 100, 200 and 300 nm. Argon cluster sputtering provides a constant sputter yield throughout the depth profiles, and the sputter yield volumes and depth resolution are presented for Ar-cluster sizes of 630, 820, 1000, 1250 and 1660 atoms at a kinetic energy of 2.5 keV. The effect of cluster size in this material and over this range is shown to be negligible. © 2014 The Authors. Surface and Interface Analysis published by John Wiley & Sons Ltd.
Keywords: SIMS, organic depth profiling, argon cluster, ToF-SIMS, Ar-GCIB
Introduction
Depth profiling of organic materials in SIMS has always suffered from the fact that the intensity of a characteristic organic secondary ion is rapidly diminished because of damage accumulation from high sputtering doses. This could be circumvented to some extent by low energy Cs,1–3 O2,1–3 Xe3 or C604 sputtering. However, these methods could not be applied in general to a wide range of different organic materials. With the invention and recent commercial availability of argon gas cluster ion beams (GCIB) for SIMS, organic materials can now be depth-profiled while retaining the molecular information.5 This is of great interest for organic electronic industries dealing with organic multilayer systems such as organic light-emitting diodes (OLED). The National Physical Laboratory (NPL) is developing certified organic reference materials suitable for the calibration and testing of techniques and methods for quantitative surface chemical analysis. Besides the already well investigated OML reference material, consisting of a thick Irganox 1010 and thin Irganox 3114 marker layers, an additional organic reference material especially suitable for OLED industry has been developed because it consists of the OLED relevant compounds 4,4′-bis[N-(1-naphthyl-1-)-N-phenyl- amino]-biphenyl (NPB) and aluminium tris-(8-hydroxyquinolate) (Alq3) (structures; Fig.1a and b). The so-called ONA reference material has been produced at NPL by alternate vacuum evaporation of NPB and Alq3 in the same manner as the previously described Irganox-based OML reference material.6 It consists of a ∽400-nm-thick NPB matrix with 3-nm marker layers of Alq3 at depths of ∽50, 100, 200 and 300 nm on a flat silicon wafer. This is shown schematically in Fig.1c.
Figure 1.
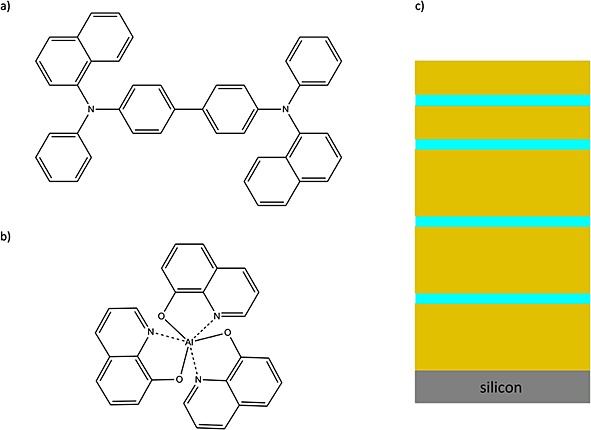
Molecular structure of the a) NPB in the matrix layers and b) Alq3 in the thin marker layer of the ONA reference material by NPL. c) Schematic of the ONA multilayer material.
In this paper, we present the first results on a systematic investigation of the ONA reference material by dual beam depth profiling. The effect of the Ar-cluster size on the sputter yield volume and the depth resolution is studied.
Experimental
The ONA reference material was constructed using a similar architecture and method to the OML reference materials used in two VAMAS interlaboratory studies.5,6 Briefly, 10 × 10 mm silicon wafers with ∽20-nm-thick oxide were extensively cleaned using isopropanol and placed in an Edwards Auto306 vacuum coater (Crawley, Surrey, UK) facing two crucibles containing NPB (99% pure) and Alq3 (99.995% pure) powders, both purchased from Sigma-Aldrich and used without further purification other than the vacuum sublimation described here. The crucibles were heated and the deposition monitored by a quartz crystal microbalance (QCM), which had been calibrated using an M2000DI spectroscopic ellipsometer (Woollam, Nebraska).
All depth profiles were performed in dual beam mode on a TOF.SIMS 5 instrument (ION-TOF GmbH, Münster, Germany) of the reflectron-type, equipped with a 30 keV bismuth liquid metal ion gun (LMIG) as primary ion source, a 20 keV argon gas cluster ion source both mounted at 45° with respect to the sample surface and an electron flood gun. The LMIG was operated at 0.5 μA emission current in the so-called high current bunched mode (high mass resolution and low lateral resolution). Bi3+ was selected as primary ion by appropriate mass filter settings. Primary and sputter ion currents were directly determined at 200 µs cycle time (i.e. a repetition rate of 5.0 kHz) using a Faraday cup located on a grounded sample holder. To decrease the target current of the LMIG, the pulse width of the Bi3+ ion pulse was reduced. Operation conditions with these settings comprised a target current of 0.13 pA for the selected primary ion. Scanning area for analysis was 80 × 80 µm2 with 64 × 64 pixels. The sputter area for each measurement was adjusted for each Ar-cluster size (630, 820, 1000, 1250 and 1660) to compensate for different sputter currents resulting in a constant areic dose rate of 9 × 1015 ions/(m2s). The areic dose rate is calculated from the quotient of the sputtering beam current and the sputtered area.
Surface charging was compensated by flooding with low energy electrons. The vacuum in the analysis chamber was in the range of 10−9 mbar during all measurements. ToF-SIMS depth profiles were acquired in positive ion mode. The mass scale was internally calibrated using a number of well defined and easily assignable secondary ions (C2H5+, C3H7+and C4H9+) keeping the error in calibration for all spectra below 10 ppm.
Results and discussion
Several investigations of organic layers and multilayer have already been carried out with Ar GCIBs, and the best results concerning depth resolution were achieved at an argon beam energy of 2.5 keV.5,7 Therefore, our systematic study of the ONA multilayer reference material provided by NPL was started at this energy. The first depth profiles were carried out at standard operation conditions using Ar1000 clusters for sputtering and 30 keV Bi3+ primary ions at full ion fluence in interlaced mode. Using these conditions, however, an additional crater development at the impact site of the primary ions was observed. This resulted in a broadening of characteristic secondary ions of the marker layer Alq3 in the depth profile. To approach efficient operating conditions, the subsequent experiments were carried out at reduced primary ion fluence, i.e. the target current of the primary ions, and hence, the areic dose of the measuring area was reduced. The GCIB sputter conditions were also changed to noninterlaced mode using 2 s sputter time per cycle. Further, different Ar-cluster sizes were included in the study to account for different energy per atom in the cluster (i.e. E/n = 4.0, 3.0, 2.5, 2.0 and 1.5 eV). These changes in sputter and analysis condition significantly improved the depth profile as no additional crater development by the LMIG was observed. Additionally, the depth resolution of all four marker layers was quite consistent, with a slight decrease for the last marker layer, conforming to the observations for the OML reference material used in a recent VAMAS study.5
To further enhance the depth resolution, 30 keV Bi5+ primary ions were used (note that because the target current for Bi5+ primary ions is much lower, two scans, i.e. two shots per pixel, were used per cycle to keep the areic dose rate comparable with Bi3+), but no improvement could be observed (Table1). Therefore, Bi3+ primary ions for analysis were maintained while decreasing the LMIG energy to 15 keV (keeping the areic dose rate at the same value as for the previous experiments), because there is evidence of the influence of the primary ion energy on the information depth and hence the depth resolution.5,8 The sputter time of the Ar-GCIB was successively reduced from 2 to 1 s and finally changing to interlaced mode. The result of these experimental set-ups is compiled in Table1. A detailed compilation of the experimental set-up and current results can be found in the supporting information. Supporting information is given on the SIA website.
Table 1.
Compilation of the results for the ONA reference material for different liquid metal ion gun and 2.5 keV Ar gas cluster ion beams measuring conditions
| LMIG | Ar-GCIB | Sputter yield volume | Depth resolution | ||
|---|---|---|---|---|---|
| Cluster size | Sputter time (s) | Averageb | SD | ||
| (s) | (nm3) | (nm) | (nm) | ||
| Bi3+ 30 keV | 620 | 2 | 21.96 | 9.93 | 0.41 |
| 830 | 2 | 21.15 | 10.28 | 1.27 | |
| 1000 | 2 | 20.61 | 10.70 | 1.06 | |
| 1250 | 2 | 19.62 | 10.88 | 1.15 | |
| 1660 | 2 | 17.07 | 10.50 | 1.62 | |
| Bi5+ 30 keV |
620 | 2 | 20.78 | 10.60 | 0.48 |
| 830 | 2 | 21.63 | 10.33 | 0.30 | |
| 1000 | 2 | 20.88 | 10.33 | 0.48 | |
| 1250 | 2 | 20.87 | 10.78 | 0.29 | |
| 1660 | 2 | 20.07 | 9.95 | 0.37 | |
| Bi3+ 15 keV | 620 | 2 | 21.77 | 9.93 | 0.54 |
| 830 | 2 | 20.86 | 10.08 | 0.61 | |
| 1000 | 2 | 20.10 | 10.08 | 0.51 | |
| 1250 | 2 | 20.05 | 9.98 | 0.89 | |
| 1660 | 2 | 18.39 | 9.93 | 0.39 | |
| Bi3+ 15 keV | 620 | 1 | n.a.c | n.a.c | n.a.c |
| 830 | 1 | 21.99 | 9.75 | 0.75 | |
| 1000 | 1 | 20.48 | 10.08 | 0.91 | |
| 1250 | 1 | 20.33 | 10.03 | 1.02 | |
| 1660 | 1 | 18.35 | 9.83 | 0.96 | |
| Bi3+ 15 keV | 620 | int.a | 19.84 | 10.33 | 1.00 |
| 830 | int.a | 19.38 | 9.85 | 0.84 | |
| 1000 | int.a | 20.29 | 10.45 | 0.70 | |
| 1250 | int.a | 20.00 | 10.10 | 0.61 | |
| 1660 | int.a | 19.13 | 10.10 | 1.48 | |
LMIG, liquid metal ion gun; GCIB, gas cluster ion beams.
Interlaced mode.
Average of the four marker layer.
Omitted due to unstable operating conditions.
Throughout the OLED like ONA reference material, very intense and stable molecular, quasi-molecular and characteristic fragment ions for both substances could be detected. The interfaces between the organic and metal-organic layers are described by a fast rise of Alq3 specific signals and a slow drop of NPB-specific signals. The depth profiles (Fig.2a) were fitted with the Dowsett response function.9 The sputter yield volume YV for cluster sizes from 630 to 1660 atoms at a kinetic energy of 2.5 keV was determined (while keeping the aeric doses of the sputter and analysis beam constant) by plotting the known central depth versus the sputter dose (Fig.2b) required to obtain the relevant maximum in the [Alq3 + H]+ (C27H19N3O3Al, m/z = 460.1242) quasi-molecular secondary ion intensity.5 Such plots yield a steady increasing straight line with excellent correlation coefficients (R > 0.99) after linear fitting confirming constant sputter yield volumes. The depth resolution for each layer is expressed as the full width at half maximum and calculated from the Dowsett response function fitted to the data. The achieved average depth resolution and standard deviations are summarised in Table1. Taking the uncertainty of measurement expressed as standard deviation into account, it can be seen that the depth resolution seems to be independent on the Ar-cluster sizes used so far at a kinetic energy of 2.5 keV. No significant change in depth resolution was observed. Also, the sputter yield volume is reasonably constant at 20.07 ± 1.06 nm3 using the values for the 15 keV Bi3+ primary ions.
Figure 2.
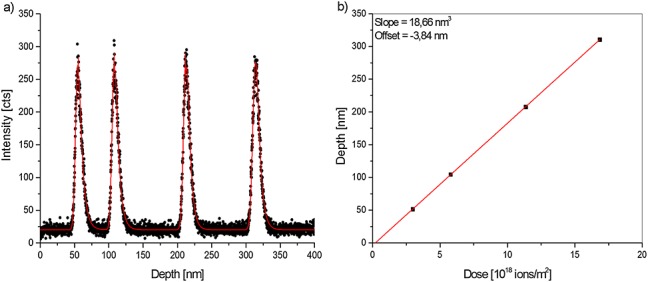
Typical depth profile of the ONA reference material showing the [Alq3 + H]+ secondary ion intensity (black dots) and a fit with four summed Dowsett response functions (red line); b) corresponding plot of the known central depth of each Alq3 layer against the sputter dose required to reach the maximum [Alq3 + H]+ secondary ion intensity (black squares) and the linear fit to the data (red line).
The sputtering yield data for the noninterlaced conditions is variable, which might be due to a higher uncertainty in the areic dose in these experiments. The procedure was refined for the final set of data using the interlaced mode. This does not invalidate the depth resolution results. In Fig.3, the sputter yield volume induced by the 2.5 keV Ar-cluster bombardment is presented using the data from the interlaced sputter mode of Table1. These results show, for the range of conditions investigated here (Fig.3a), that the yield is nearly independent on the cluster size used. However, at the moment, too few data are available to make this a general conclusion. In fact, it has been observed in a detailed study of several different organic materials that the sputter yield volume (YV) is decreasing with increasing Ar-cluster size (n) at a given kinetic energy (E).10 It is therefore expected to find this evident at an Ar-cluster size of n = 5000. Our latest (unpublished) results show a decrease of Yv around an Ar-cluster size of 1250. This is in principal agreement with the published data, which also show an independence of Yv at Ar-cluster sizes below 2000 (cf. results for HTM-1 in reference 10). The same publication also presents a universal equation for Ar gas cluster sputtering yields. The solid line in the graph of Fig.3b is the fit of the data using this general equation:
Figure 3.
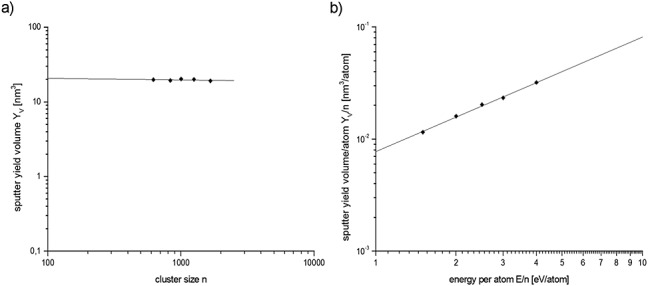
Dependence of a) the sputter yield volume on the cluster size for 2.5 keV clusters and b) the sputter yield volume per atom in the cluster on the kinetic energy per atom in the cluster. The solid line is a fit of the data to the universal equation for argon gas cluster sputter yields introduced by Seah.10
The data available so far yield after fitting B = 0.0120 nm3, A = 0.780 eV and q = 1.044. The presented data exhibit the very low standard deviation of 2.2% about this fit. These results therefore show excellent internal consistency of the data. The fitting parameters here are not significant and will change when data are available for lower E/n values when A and q may be properly defined. For most organic materials, the plot of the type shown in Fig.3b exhibits a downward trend to the slope q, but this may only occur below E/n = 1.10 Here, the lowest E/n value is 1.5 so the fitted values of A, B and q here have correlated uncertainties. The quality of the fit, however, does show the excellent quality of both the materials and procedures. The absolute values of 20 nm3 per ion at 2.5 keV is very similar to the absolute values for Irganox 1010 and the OLED material HTM-1.10 Because the erosion efficiency is a function of cluster size and kinetic energy, it is more suitable to express this relationship in a plot of sputter yield volume per atom in the cluster (YV/n) against the energy per atom (E/n) of the sputter projectile.11 The data from different sputter kinetic energies then fall on a single curve as exemplified in Fig.3b. A molecular dynamics simulation for solid benzene show that the sputter yield volume shows a linear dependence above a threshold of E/n = 1 eV with a strong nonlinear dependence below this value.11 Our data are so far in no contradiction to this conclusion.
Conclusion
The preliminary results of our study show that the ONA reference material is suitable for the depth profiling with large argon clusters. Intense molecular, quasi-molecular and characteristic fragment secondary ions were detected for both substances and showed in the case of NPB a stable signal during the depth profiling of the thick matrix layer. The sputter yield volume was constant throughout all depth profiles shown by a straight line with excellent correlation coefficients for all experiments in a plot of the known central depths against the sputter dose to reach these depths. The achieved depth resolution so far, using the [Alq3 + H]+ secondary ion is in the range of approximately 10 nm. More data has to be acquired especially at larger Ar-cluster sizes and higher kinetic energies to establish the wider relationships.
Acknowledgments
The authors thank M. P. Seah from NPL for helpful discussions of the data and both Steve Spencer and Steve Smith from NPL for the manufacture of the samples. This work is partly funded by the European Union through the European Metrology Research Program (EMRP). The EMRP is jointly funded by the EMRP participating countries within EURAMET and the European Union. M. H. is grateful for financial support by BAM trough the Adolf-Martens fellowship program and by the Austrian Science Found (FWF) through the Erwin Schrödinger fellowship program (project number J 3471-N28).
Supporting Information
Supporting info item
References
- Cramer HG, Grehl T, Kollmer F, Moellers R, Niehuis E, Rading D. Appl. Surf. Sci. 2008;255:966. [Google Scholar]
- Mine N, Douhard B, Brison J, Houssiau L. Rapid Commun. Mass Spectrom. 2007;21:2680. doi: 10.1002/rcm.3135. [DOI] [PubMed] [Google Scholar]
- Wehbe N, Houssiau L. Anal. Chem. 2010;82:10052. doi: 10.1021/ac101696c. [DOI] [PubMed] [Google Scholar]
- Shard AG, Green FM, Brewer PJ, Seah MP, Gilmore IS. J. Phys. Chem. B. 2008;112:2596. doi: 10.1021/jp077325n. [DOI] [PubMed] [Google Scholar]
- Shard AG, Havelund R, Seah MP, Spencer SJ, Gilmore IS, Winograd N, Mao D, Miyayama T, Niehuis E, Rading D, Moellers R. Anal. Chem. (Washington, DC, U. S.) 2012;84:7865. doi: 10.1021/ac301567t. [DOI] [PubMed] [Google Scholar]
- Shard AG, Ray S, Seah MP, Yang L. Surf. Interface Anal. 2011;43:1240. [Google Scholar]
- Niehuis E, Möllers R, Rading D, Cramer HG, Kersting R. Surf. Interface Anal. 2013;45:158. [Google Scholar]
- Muramoto S, Brison J, Castner DG. Anal. Chem. 2011;84:365. doi: 10.1021/ac202713k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett MG, Rowlands G, Allen PN, Barlow RD. Surf. Interface Anal. 1994;21:310. [Google Scholar]
- Seah MP. J. Phys. Chem. C. 2013;117:12622. doi: 10.1021/jp408168z. [DOI] [PubMed] [Google Scholar]
- Postawa Z, Paruch R, Rzeznik L, Garrison BJ. Surf. Interface Anal. 2013;45:35. doi: 10.1002/sia.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


