Abstract
Objective
Folates exist as a fluctuating pool of polyglutamated metabolites that may serve as a clinical marker of MTX activity. This study evaluates circulating folate content and folate polyglutamate distribution in Juvenile Idiopathic Arthritis (JIA) patients and in a cell culture model based on MTX exposure and folate supply.
Methods
Blood, plasma and red blood cell (RBC) measurements of MTX and folates were obtained from previously published data sets and additional sample analysis for JIA patients receiving (n=98) and not receiving (n=78) MTX therapy. Erythroblastoid cells maintained in culture were exposed to MTX and grown under varying levels of folic acid supplementation. Samples were analyzed for cellular folate and MTX content.
Results
Circulating folate levels were lower in JIA patients receiving MTX, with reduced levels of blood, plasma and RBC 5-methyl-tetrahydrofolate (5mTHF) (p<0.0001). Average polyglutamate chain-length (Gluavg) of RBC 5mTHF was elevated in JIA patients receiving MTX (5.63±0.15 vs. 5.54±0.11, p<0.0001) and correlated with both RBC MTX accumulation (p=0.02) and reduced plasma 5mTHF levels (p=0.008). MTX exposure and folate deprivation in erythroblastoid cells resulted in a depletion of bioactive folate species that was associated with a shift to higher Gluavg values for several species, most notably tetrahydrofolate (THF) and 5,10-methylenetetrahydrofolate (CH2-THF). Increased Gluavg resulted from the depletion of short-chain and the accumulation of long-chain glutamate species.
Conclusion
Folate content and polyglutamate distribution are responsive markers of MTX activity and folate supply in vivo and in vitro, and may provide novel clinical markers of pharmacologic activity of MTX.
INTRODUCTION
Methotrexate (MTX) therapy continues to be a cornerstone in the treatment of Juvenile Idiopathic Arthritis (JIA). However, response to the drug remains variable and unpredictable with 30-40% of JIA patients failing to achieve an adequate therapeutic response (1, 2). In addition, MTX therapy is limited by a delayed onset of drug action, and potentially significant gastrointestinal, liver and hematologic toxicities (3, 4). Therefore, a need exists for the identification of clinical biomarkers to guide MTX therapy to maximize early therapeutic response while mitigating drug-associated toxicities.
The majority of efforts to identify clinical biomarkers for MTX therapy have focused on measures of drug disposition. Despite a high degree of inter-individual variability in plasma pharmacokinetics, plasma MTX has not been found to be consistently associated with drug response in inflammatory arthritis (5-7), and is of limited utility as a tool for therapeutic drug monitoring in these patients. The realization that MTX is metabolized intracellularly through addition of glutamate residues, similar to endogenous folates, forming pharmacologically active species has resulted in efforts to correlate clinical response with MTX polyglutamate concentrations in red blood cells (RBCs) (8). Although, associations of MTX polyglutamate levels with therapeutic response and toxicity have been observed, these finding haven't been consistently demonstrated (9-16), and the long accumulation half-life of MTX in RBCs may limit its early predictive capabilities (12, 17, 18). Recognizing the limitation of pharmacokinetic monitoring in MTX therapy, recent efforts have begun to evaluate the role of pharmacogenomic and pharmacodynamic markers in guiding therapy (14, 15, 19-21).
Although the pharmacologic basis of MTX activity in inflammatory arthritis remains incompletely understood, MTX exposure results in numerous measurable biochemical changes that may provide useful pharmacodynamic markers to guide drug therapy (22). In particular, MTX is a potent inhibitor of dihydrofolate reductase (DHFR) resulting in the depletion of biologically active reduced folate species and the corresponding accumulation of inactive oxidized folate species (23-25). Reduced serum and RBC folate levels have been consistently associated with MTX toxicity and more recently failure to respond to therapy in rheumatoid arthritis (19, 26, 27). However, these relationships haven't been examined in childhood arthritis and the clinical utility of these measurements is yet to be determined.
Animal studies have also shown that either dietary depletion of folates or exposure to MTX results not only in the depletion of reduced folates, but also a shift in the polyglutamate distribution profile of intracellular folates in favor of long-chain polyglutamate species (23, 28, 29). Although the mechanism is unclear, it has been hypothesized that folate depletion secondary to MTX exposure results in reduced turnover of residual folates with an increased residence time for the remaining folate species, resulting in increased glutamation by folylpolyglutamate synthase (FPGS) (23). These findings suggest that folate polyglutamate distribution may be a responsive pharmacodynamic marker of MTX activity. However, these findings haven't been replicated in human subjects.
In this study we hypothesize that circulating folate concentrations and polyglutamate distribution profiles represent pharmacodynamic markers of MTX activity and in the future may provide a means to guide drug therapy in the treatment of JIA. To evaluate this hypothesis this study analyzes samples from two cohorts of JIA patients and utilizes an erythroblastoid cell model to evaluate the relationship between MTX therapy and folate homeostasis.
PATIENTS AND METHODS
In vivo studies
Patient samples and data were acquired from two previous cross-sectional cohorts of JIA patients that have in part been reported previously (30, 31). Patient blood samples were separated into plasma and RBC fractions and analyzed for folate content and glutamate distribution. Only patients with a complete folate profile were included in this analysis. Studies were conducted under the approval of the Children's Mercy Kansas City Institutional Review Board.
The MTX treatment cohort consisted of 98 subjects (66 females) with JIA on a stable dose and route of administration of MTX for at least 3 months (30). The non-MTX treated cohort consisted of 93 subjects with juvenile arthritis, 78 of which were specifically diagnosed with JIA (48 females) (31). Subjects with JIA were verified by the International League of Associations for Rheumatology Edmonton 2001 criteria (32). Subjects receiving MTX were younger (median [IQR] age of 116 [65, 160] vs 144 [96,184] months, p=0.01) and more likely to be receiving folate supplementation (47% vs. 24% of patients, p=0.003), as compared to the non-MTX treated group. Folate supplementation was defined as 1 mg/day dosing of folic acid in MTX treated patients and a daily multivitamin that contained folic acid at a recommended daily allowance (400mcg) in the non-MTX treated patients. No additional information regarding dietary folate intake was obtained. At the time of the study, disease activity was similar between groups with active arthritis in at least one joint in 64% of patients in the non-MTX group and 54% of the MTX group (p=0.2). There was no statistical difference in the median number of swollen joints, tender joints, erythrocyte sedimentation rate, use of NSAIDs, anti-TNF-α agents, or oral steroids between cohorts (Table 1).
Table 1.
Demographics of two cross-sectional JIA cohorts.
| MTX Cohort n=98 | non-MTX Cohort n=78 | P value | |
|---|---|---|---|
| Female | 66 (67%) | 48 (62%) | 0.4 |
| Age Median (IQR) months | 116 (65, 160) | 144 (96, 184) | 0.01 |
| Folate supplementation | 46 (47%) | 19 (24%) | 0.003 |
| Presence of active arthritis | 53 (54%) | 50 (64%) | 0.2 |
| No. swollen joints Median (IQR) | 0 (0,3) | 1 (0,2) | 0.3 |
| No. tender joints Median (IQR) | 0 (0,1) | 0 (0,1) | 0.2 |
| ESR Median (IQR) mm/hr | 11(8, 21) | 12.5 (8, 28) | 0.3 |
| Use of NSAIDs | 42 (43%) | 38 (49%) | 0.5 |
| Use of anti-TNF-α agents | 31 (32%) | 19 (24%) | 0.3 |
| Use of oral steroids | 7 (7%) | 6 (8%) | 1.0 |
| MTX dose Median (IQR) mg/kg | 0.5 (0.3, 0.7) | N/A | N/A |
| Duration of MTX Median (IQR) months | 17 (9, 46) | N/A | N/A |
In vitro studies
Because erythrocytes were the primary cellular compartment for in vivo analysis, the erythroblastoid K562 cell line (Coriell Cell Repository, GM05372) was chosen for these studies. K562 cells actively transport and metabolize MTX to its polyglutamated metabolites (33-35). Cells were maintained in RPMI-1640 medium (Life Technologies, 61870-127) supplemented with 10% fetal bovine serum (Atlanta Biologicals, S11150) in a 37°C and 5% CO2 controlled incubator at a density between 2×105 and 1×106 cells/mL. All experiments were conducted within 6-passages.
Cells were treated with MTX over 24-hours as previously published (35). Folic acid (Sigma, F7876) was added to folic acid-free RPMI-1640 (Life Technologies, 27016-021) to achieve concentrations between 0 and 20 μM, and supplemented with 10% fetal bovine serum. Cells were maintained in 2 μM folic acid-supplemented media prior to experimentation. On the day of experimentation, cells were re-suspended in media containing supplemental folic acid concentrations of 0, 0.02, 0.2, 2 (control) and 20 μM. After 24-hours, cells were evaluated to ensure no significant differences in viability by trypan blue staining and cell counting. Cell samples were obtained for each treatment condition for analysis.
Cells were washed thrice with 4°C D-PBS and stored at −80°C until analysis. Protein content was determined using the micro-BCA method (Thermo Scientific, 23235). Samples were re-suspended in extraction buffer consisting of 40% acetonitrile, 40% methanol and 20% 0.1M phosphate-buffered water at pH 7.4 containing 0.1% 2-mercaptoethanol and 1% sodium ascorbate. Samples were vortexed and centrifuged at 16,100xg for 3-minutes. Sample supernatant was analyzed for folate content and normalized to sample protein content or sample cell count.
Analytical Methodology
Whole blood, plasma and cellular concentrations of 5,10-methenyl-tetrahydrofolate (CH=THF), 5-methyl-tetrahydrofolate (5mTHF), and polyglutamate distribution profiles for 5mTHF were determined using previously established methods (36). Patient total folate was calculated as the sum of 5mTHF and CH=THF. In addition, intracellular concentrations of folic acid (FA), dihydrofolate (DHF), tetrahydrofolate (THF) and 5,10-methylene-tetrahydrofolate (CH2THF) were determined in K562 cells using the previously described method. Polyglutamate distribution profiles for these species were determined by extrapolation of the mass fragmentation pattern as previously described for 5mTHF (36). To minimize exposure of intracellular folate and MTX polyglutamates to extracellular conjugases, K562 cells were prepared in a manner similar to the published method for RBCs (36). Following exposure to MTX, K562 cells were separated from conjugase-containing media, washed and maintained at −80 °C. Samples were reconstituted under denaturing conditions prior to analysis. Monoglutamate forms were found to represent only a small fraction of the intracellular folate pool. Average polyglutamate chain length (Gluavg) was determined by multiplying each polyglutamate species by the number of attached glutamate residues and dividing their sum by total cellular folate. Cellular levels of MTX and MTX polyglutamates were determined in patient RBCs and K562 cells using a previously published procedure involving ion-pair UPLC/MS/MS (35, 37, 38).
Statistical analyses were conducted by Spearman's rank correlation analysis, simple linear regression (SLR) analysis, Wilcoxon's rank sum test, or Student's t test analysis, as appropriate using JMP 10 (SAS Institute Inc.).
RESULTS
MTX therapy and folates in JIA
Concentrations of 5mTHF and CH=THF were measured in blood, plasma and RBCs (Table 2), and 5mTHF represented median [IQR] values of 93 [90, 96], 93 [90, 96] and 98 [94, 99] % of the total folate pool, respectively. Total folate and 5mTHF concentrations were significantly lower in the MTX treated group. In contrast, concentrations of CH=THF were lower in blood and RBCs, but median plasma concentrations were observed to be 6.7-fold higher in the MTX treatment group. Although age and dietary folate supplementation differed between the two cohorts, neither variable was found to be independently associated with folate levels. Males tended to have increased RBC concentrations of total folate compared to females (927 [646,1293] vs. 790 [574, 1110] nmol/L, p=0.04).
Table 2.
Circulating folate concentrations in JIA patients receiving or not receiving MTX therapy. Statistical comparisons were made by Wilcoxon's rank sum analysis.
| (-) MTX (Median [IQR]) |
(+) MTX (Median [IQR]) |
P-value |
||
|---|---|---|---|---|
| Total Folate (nmol/L) | Blood | 450 [300, 534] | 297 [218, 371] | < 0.0001 |
| Plasma | 43 [27, 63] | 29 [23, 41] | < 0.0001 | |
| RBC | 1161 [707, 1367] | 728 [539, 926] | < 0.0001 | |
| CH=THF (nmol/L) | Blood | 24 [15, 37] | 21 [11, 33] | 0.09 |
| Plasma | 0.3 [0.3, 0.4] | 2.0 [0.6, 2.5] | < 0.0001 | |
| RBC | 67 [36, 102] | 51 [27, 84] | 0.03 | |
| 5mTHF (nmol/L) | Blood | 404 [274, 495] | 265 [187, 336] | < 0.0001 |
| Plasma | 43 [26, 63] | 27 [21, 40] | < 0.0001 | |
| RBC | 1033 [646, 1252] | 643 [454, 829] | < 0.0001 |
When the groups were stratified by MTX use, differences were observed in the effect of folate supplementation upon folate concentrations. In the non-MTX treated group, subjects receiving folate supplementation through a daily multivitamin had higher total folate concentrations in their blood (505 [443, 542] vs 434 [279, 530] nmol/L, p=0.03), plasma (51 [42, 75] vs 37 [26, 50] nmol/L, p=0.003) and RBCs (1239 [1133, 1387] vs 1101 [662, 1331] nmol/L, p=0.07), but this increase in folate concentration was not observed in the MTX treatment group, despite the use of much higher doses of folic acid (1 mg/day).
Using RBC MTX disposition data previously published for the MTX treatment cohort (20), increased accumulation of MTX in RBCs was associated with reductions in circulating folate levels (Table 3). Increased MTX (MTXGlutotal) concentrations in RBCs was associated with reduced total folate and 5mTHF in blood, as well as plasma concentrations of total folate, 5mTHF and CH=THF. MTX subspecies analysis revealed that accumulation of short-chain MTX polyglutamates (MTXGlu1-2) correlated with a reduction in total folate and 5mTHF concentrations in all compartments; while accumulation of long-chain MTX polyglutamates (MTXGlu3-5) correlated with reduced plasma concentrations of total folate, 5mTHF and CH=THF. Additionally, increased MTX dose was associated with reduced 5mTHF concentrations in blood (r2=0.12, p=0.0004), plasma (r2=0.05, p=0.03) and RBCs (r2=0.07, p=0.007).
Table 3.
Correlation of RBC MTX with circulating folate concentrations in JIA patients.
| MTX Disposition in RBCs |
||||
|---|---|---|---|---|
| MTXGlUtotal (nmol/L) |
MTXGlu1-2 (nmol/L) |
MTXGlu3-5 (nmol/L) |
||
| Total Folate (nmol/L) | Blood | −0.23* | −0.33** | −0.18 |
| Plasma | −0.36*** | −0.33** | −0.33** | |
| RBC | −0.19 | −0.29** | −0.15 | |
| CH=THF (nmol/L) | Blood | +0.08 | −0.08 | +0.13 |
| Plasma | −0.22* | −0.01 | −0.22* | |
| RBC | +0.09 | −0.08 | +0.13 | |
| 5mTHF (nmol/L) | Blood | −0.24* | −0.32*** | −0.20 |
| Plasma | −0.34*** | −0.31** | −0.31** | |
| RBC | −0.20 | −0.29** | −0.17 | |
Values represent Spearman's rank correlation coefficients
p<0.05
p<0.01
p<0.001.
MTX therapy and folate polyglutamates in JIA
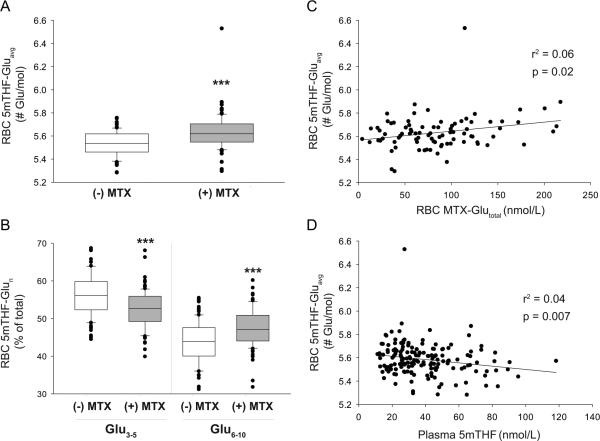
Folate polyglutamate levels in RBCs were determined for the most abundant species, 5mTHF. Polyglutamate distribution of 5mTHF was expressed as the average number of glutamate residues per molecule (Gluavg). RBC 5mTHF-Gluavg was found to be increased in JIA patients receiving MTX therapy (Figure 1A). This shift in 5mTHF polyglutamate distribution is represented by a decrease in the percent composition of the short-chain species (5mTHF-Glu3-5) and an increase in long-chain species (5mTHFGlu6-10) (Figure 1B).
Figure 1.
Influence of MTX therapy and plasma folates on 5mTHF polyglutamate distribution in JIA patients. Comparison of (A) RBC 5mTHF-Gluavg and (B) glutamate distribution of RBC 5mTHF between JIA patients currently receiving or not receiving MTX therapy by Wilcoxon's rank sum analysis (***, p<0.001). Relationship of RBC 5mTHF-Gluavg with (C) RBC MTX-Glutotal and (D) plasma 5mTHF concentrations in JIA patients was evaluated by linear regression analysis.
RBC 5mTHF-Gluavg was found to positively correlate with RBC concentrations of MTX (Figure 1C). This relationship was further assessed by evaluating the relationship of specific MTX glutamate subspecies with 5mTHF-Gluavg. Increased 5mTHF-Gluavg was associated with increased RBC concentrations of MTXGlu2 (r2=0.06; p=0.02), MTXGlu3 (r2=0.09; p=0.005) and MTXGlu4 (r2=0.05; p=0.04). No associations were observed between RBC 5mTHF-Gluavg and dietary folate supplementation, age or gender. However, increased RBC 5mTHF-Gluavg was associated with reduced plasma concentrations of 5mTHF (Figure 1D).
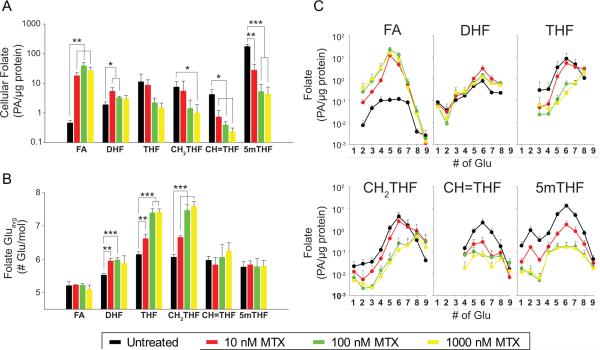
In vitro evaluation of MTX and folates
Intracellular folates were measured in K562 cells following exposure to MTX (Figure 2). MTX caused a concentration-dependent accumulation of oxidized folates, DHF and FA, and the depletion of reduced folates: THF, CH2THF, CH=THF and 5mTHF (Figure 2A). Using previously published measures of MTX disposition in these samples (35), cellular accumulation of MTX was associated with the depletion of CH2THF, CH=THF and 5mTHF, as well as the accumulation of FA and DHF (Supplemental Table 1). Changes in intracellular folate concentrations were consistently found to be most strongly associated with the accumulation MTXGlu1-2, rather than MTXGlu3-7.
Figure 2.
Effect of MTX on intracellular folate content and polyglutamate distribution in K562 cells. Intracellular folate (A) content (B) Gluavg and (C) glutamate distribution profiles were determined in K562 cells exposed to MTX at concentrations between 0 and 1000 nM for 24-hours. Data represents mean ± SD from three independent experimental evaluations (*, p<0.05; **, p<0.01; ***, p<0.001 by Student's t test).
MTX exposure resulted in a concentration-dependent shift in Gluavg for several of the measured folate species (Figure 2B). An increase in Gluavg was observed for DHF, THF, CH2THF and CH=THF; however, unlike the in vivo data, Gluavg for 5mTHF remained unchanged. The largest shifts in Gluavg were observed for THF and CH2THF. Folate polyglutamate distribution profiles were created by plotting the absolute abundance of each glutamate sub-species under each treatment condition (Figure 2C). Increased Gluavg for THF, CH2THF and CH=THF results from depletion of short-chain species, and accumulation of long-chain species. Shifts in Gluavg were found to positively correlate with the cellular accumulation of MTX (Supplemental Table 1), and were more strongly associated with the accumulation of MTXGlu1-2 than MTXGlu3-7.
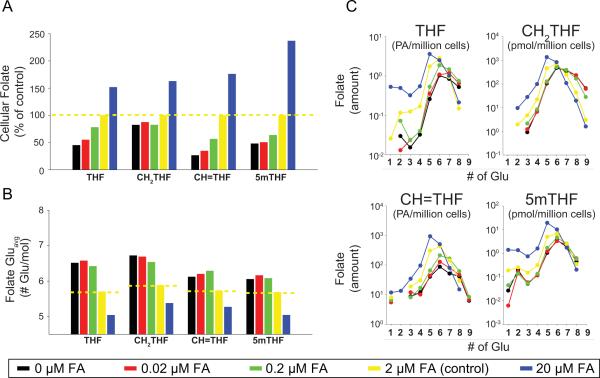
Folate depletion through reduced concentrations of supplemental folic acid resulted in a depletion of the reduced folate pool, represented by reductions in THF, CH2THF, CH=THF and 5mTHF (Figure 3). Reductions in cellular folate content were associated with increased Gluavg for the folate species (Figure 3B). Cellular folate concentration and Gluavg displayed a concentration-dependent relationship with folic acid supplementation, similar to that observed following MTX exposure. Similar to MTX, the largest shift in Gluavg was observed for THF and CH2THF. However, unlike MTX exposure, reduced folic acid supplementation also increased Gluavg for CH=THF and 5mTHF. Folate polyglutamate distribution profiles for reduced levels of folic acid supplementation are similar to those for MTX and reveal that increases in folate Gluavg occurs through the cellular depletion of short-chain glutamates as well as an increase in cellular concentrations of the long-chain species (Figure 3C).
Figure 3.
Effect of exogenous folic acid supplementation on intracellular folate content and polyglutamate distribution in K562 cells. Intracellular folate (A) content (B) Gluavg and (C) glutamate distribution profiles were determined in K562 cells grown for 24-hours with supplemental folic acid concentrations ranging from 0 to 20 μM. Data represent a single experimental evaluation.
DISCUSSION
Consistent with studies of MTX therapy in rheumatoid arthritis, we found that JIA patients on MTX therapy have a pronounced reduction in circulating folate concentrations (26, 27, 39-43). Reductions in folates occurred despite supplementation with folic acid in 47% of patients receiving MTX. This finding is in contrast to studies that have found folic acid supplementation to prevent reductions in RBC and plasma folates that occur secondary to MTX therapy (19, 42). Although the reason for this apparent discrepancy is unclear, it is important to point out that this study differed in a number of ways from the previous studies, including: its cross-sectional design, patient population (i.e. pediatric), UPLC/MS/MS based method for folate analysis, and variability in folic acid supplementation. There may have been underreporting of folic acid adherence, an increase in folate needs and utilization in a developing and growing child compared to adults, or most likely differences in the ability to quantify folate species that may contribute to these differences. Evaluations are currently underway to validate these findings in an independent prospective cohort.
Observed differences in circulating folate levels were primarily driven by differences in the major circulating folate species, 5mTHF. A similar magnitude reduction in median 5mTHF concentrations were observed in both the plasma and RBC compartments (>30%), suggesting the pools were at equilibrium at the time of sampling; a less likely scenario is that the folate pools are exchangeable despite RBC folates consisting mostly of poorly exchangeable polyglutamates. In contrast to other studies, we were able to detect the minor folate species, CH=THF, and despite a 24% reduction in RBC levels in the MTX treatment group, plasma levels were markedly elevated compared to the control group. Animal and cell-based studies have found that concentrations of formylated THF, which is represented by CH=THF, are often preserved in relationship to other folate species and may represent direct inhibition of aminoimidazole carboxamide ribonucleotide transformylase (AICART) for which the formylated species is a co-factor (23, 25, 44, 45). Although these findings may support this hypothesis, it must be noted that AICART is an intracellular enzyme and one would expect to see these effects on intracellular folates, but not necessarily on plasma folates as observed here.
Based on animal studies, MTX exposure causes a shift in cellular folate polyglutamate distribution in favor of higher-order polyglutamates (23). The current findings represent the first to demonstrate these shifts occur in patients treated with MTX. Specifically, a significant increase in Gluavg for 5mTHF was observed in RBCs from JIA patients treated with MTX. However, it must be noted that this shift represents approximately one-tenth of a glutamate residue on average between the two groups, which may limit its clinical significance and utility as a biomarker, however, taken as an average, this still reveals a notable shift in polyglutamate distribution. Similar to findings in the animal models, patients receiving MTX have an increased fractional composition of long-chain glutamates and a decreased fractional composition of the short-chain species. Furthermore, animal studies have demonstrated that cellular folate polyglutamate distribution is sensitive to dietary folate supply, with extreme dietary folate depletion resulting in distribution profiles akin to those observed following exposure to MTX (28, 29, 46). Folate supplementation was not independently found to be associated with 5mTHF-Gluavg in RBCs. However, plasma 5mTHF concentrations, which may be a more appropriate measure of bioavailable folate supply, were found to be inversely associated with 5mTHF-Gluavg. Therefore, the polygutamate distribution of RBC 5mTHF in JIA patients appears to be related to both MTX exposure and folate supply.
By concurrently measuring circulating folate levels, folate polyglutamate distribution and MTX disposition we were able to evaluate the potential for pharmacokinetic-pharmacodynamic relationships. We found that both circulating plasma folate concentrations and RBC 5mTHF-Gluavg are significantly associated with the disposition of MTX in RBCs. For circulating plasma folate concentrations, the strongest correlation was observed with short-chain MTX polyglutamates (MTXGlu1-2), rather than the long-chain species (MTXGlu3-5) and may be consistent with recent studies that have observed a relationship between RBC MTXGlu2 and clinical response (11). However, for 5mTHF-Gluavg the strongest association was observed for the most abundant intracellular species, MTXGlu3. Regardless, these pharmacodynamic effects are most closely associated with the RBC accumulation of short to medium chain MTX species, in contrast to a recent study in JIA patients that has found clinical response to be most closely associated with RBC concentrations of long-chain MTX polyglutamates (10).
To further establish the observed relationship between MTX exposure and changes in folate homeostasis in vivo, cell-based studies were undertaken. In line with previous studies and our in vivo findings, MTX exposure resulted in a concentration-dependent re-distribution of intracellular folates with the depletion of reduced folate species and the concomitant accumulation of oxidized folate species (25, 45). Accompanying changes in cellular folates was a concentration-dependent shift in Gluavg of some, but not all, of the measured folate species. In particular, DHF, THF and CH2THF experienced a significant increase in Gluavg while CH=THF and 5mTHF were not significantly impacted. These findings are consistent with an increase in FPGS activity targeting its primary substrate, THF (47, 48). The corresponding, and similar, increase in Gluavg for CH2THF suggests these two reduced folate pools may be exchangeable under these conditions. Meanwhile, the lack of a significant increase in Gluavg for CH=THF and 5mTHF suggests that these pools are poorly exchangeable with THF. Increased Gluavg for DHF may represent the oxidation of long-chain reduced folates and inhibition of DHFR. In addition, DHF has been shown to be a good substrate for FPGS and may represent increased FPGS activity (47, 48). The lack of change in Gluavg for 5mTHF and FA is reflected by the maintenance of their polyglutamate distribution profiles despite dramatic decreases in the cellular 5mTHF pool and accumulation of FA. Since FA is known to be a poor substrate for FPGS, it is likely that accumulating FA polyglutamates represent the oxidation of folate polyglutamates, rather than glutamation of exogenously supplied folic acid (49).
Exogenous supply of folic acid was also found to be directly and concentration-dependently related to intracellular reduced folate concentrations and folate Gluavg. These similarities are believed to represent the pharmacologically-induced folate-depleted state caused by MTX through the inhibition of DHFR. However, unlike MTX, folate depletion doesn't appear to selectively increase Gluavg for THF and CH2THF, but also has a marked effect on CH=THF-Gluavg and 5mTHF-Gluavg. This finding would support inhibition of folate interconversion in the selective effects of MTX on THF-Gluavg and CH2THF-Gluavg (50). Examination of the glutamate distribution profiles shows that redistribution under both conditions of MTX exposure and folate depletion occurs from concurrent depletion of short-chain species and accumulation of long-chain species. These findings support the hypothesis that increased folate glutamate chain-length occurs as a result of the folate depletion that accompanies exposure to MTX, despite differences in the affected folate isoforms. Whether this increase in chain length represents increased FPGS catalytic activity, or an increased residence time of the folate species cannot be determined by this data. However, the observation that MTX can act as an inhibitor of FPGS may support the latter (49).
We conclude that MTX therapy in the treatment of JIA is associated with the depletion of circulating folates and a corresponding shift in the glutamate distribution profile of RBC 5mTHF. Based on in vitro studies, we further conclude that intracellular folate levels and glutamate distribution profiles display a concentration-dependent sensitivity to both MTX exposure and folate supply. However, due to the cross-sectional design of this study and lack of clinical response data we were unable to evaluate the clinical utility of these measurements, or how they compare to other biomarkers, such as RBC MTX polyglutamates. Regardless, as we strive to fully comprehend the mechanisms of action of one of the most commonly used disease modifying agents in adults and children; we must begin by characterizing the effects of MTX upon its known endogenous folate pathway target. Future prospective clinical studies will aim to evaluate the relationship of these pharmacodynamic markers of MTX activity with therapeutic and toxic response in JIA, and can then lead the way for future studies to evaluate the utility of folate supplementation upon MTX efficacy and toxicity, and the identification of improved cellular biomarkers of drug response.
ACKNOWLEDGEMENTS
The authors would like to thank Drs. Mark Hoeltzel and Andrew Lasky, and Mrs. Chelsey Smith for their assistance in patient recruitment and sample collection for this study.
This work was supported through grants from the American College of Rheumatology Research Foundation, Children's Mercy Kansas City, and the National Institute of Child Health and Human Disease [T32-HD069038].
Footnotes
The authors report no financial conflicts of interest in regards to the work presented in this manuscript.
REFERENCES
- 1.Lambert CM, Sandhu S, Lochhead A, Hurst NP, McRorie E, Dhillon V. Dose escalation of parenteral methotrexate in active rheumatoid arthritis that has been unresponsive to conventional doses of methotrexate: a randomized, controlled trial. Arthritis Rheum. 2004;50:364–71. doi: 10.1002/art.20167. [DOI] [PubMed] [Google Scholar]
- 2.Ruperto N, Murray KJ, Gerloni V, Wulffraat N, de Oliveira SK, Falcini F, et al. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum. 2004;50:2191–201. doi: 10.1002/art.20288. [DOI] [PubMed] [Google Scholar]
- 3.Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, et al. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children's Study Group. N Engl J Med. 1992;326:1043–9. doi: 10.1056/NEJM199204163261602. [DOI] [PubMed] [Google Scholar]
- 4.Becker ML, Rose CD, Cron RQ, Sherry DD, Bilker WB, Lautenbach E. Effectiveness and toxicity of methotrexate in juvenile idiopathic arthritis: comparison of 2 initial dosing regimens. J Rheumatol. 2010;37:870–5. doi: 10.3899/jrheum.090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafforgue P, Monjanel-Mouterde S, Durand A, Catalin J, Acquaviva PC. Lack of correlation between pharmacokinetics and efficacy of low dose methotrexate in patients with rheumatoid arthritis. J Rheumatol. 1995;22:844–9. [PubMed] [Google Scholar]
- 6.Ravelli A, Di Fuccia G, Molinaro M, Ramenghi B, Zonta L, Regazzi MB, et al. Plasma levels after oral methotrexate in children with juvenile rheumatoid arthritis. J Rheumatol. 1993;20:1573–7. [PubMed] [Google Scholar]
- 7.Hiraga Y, Yuhki Y, Itoh K, Tadano K, Takahashi Y, Mukai M. Pharmacokinetics and efficacy of low-dose methotrexate in patients with rheumatoid arthritis. Mod Rheumatol. 2004;14:135–42. doi: 10.1007/s10165-004-0280-y. [DOI] [PubMed] [Google Scholar]
- 8.Baugh CM, Krumdieck CL, Nair MG. Polygammaglutamyl metabolites of methotrexate. Biochem Biophys Res Commun. 1973;52:27–34. doi: 10.1016/0006-291x(73)90949-2. [DOI] [PubMed] [Google Scholar]
- 9.de Rotte MC, den Boer E, de Jong PH, Pluijm SM, Bulatovic Calasan M, Weel AE, et al. Methotrexate polyglutamates in erythrocytes are associated with lower disease activity in patients with rheumatoid arthritis. Ann Rheum Dis Published Online First. 2013 Decemeber; doi: 10.1136/annrheumdis-2013-203725. doi: 10.1136/ard.2013.203725. [DOI] [PubMed] [Google Scholar]
- 10.Bulatovic Calasan M, den Boer E, de Rotte MC, Vastert SJ, Kamphuis S, de Jonge R, et al. Methotrexate polyglutamates in erythrocytes are associated with lower disease activity in juvenile idiopathic arthritis patients. Ann Rheum Dis Published Online First. 2013 Nov 28; doi: 10.1136/annrheumdis-2013-203723. doi: 10.1136/ard.2013.203723. [DOI] [PubMed] [Google Scholar]
- 11.Hobl EL, Jilma B, Erlacher L, Duhm B, Mustak M, Broll H, et al. A short-chain methotrexate polyglutamate as outcome parameter in rheumatoid arthritis patients receiving methotrexate. Clin Exp Rheumatol. 2012;30:156–63. [PubMed] [Google Scholar]
- 12.Stamp LK, Barclay ML, O'Donnell JL, Zhang M, Drake J, Frampton C, et al. Effects of changing from oral to subcutaneous methotrexate on red blood cell methotrexate polyglutamate concentrations and disease activity in patients with rheumatoid arthritis. J Rheumatol. 2011;38:2540–7. doi: 10.3899/jrheum.110481. [DOI] [PubMed] [Google Scholar]
- 13.Stamp LK, O'Donnell JL, Chapman PT, Zhang M, James J, Frampton C, et al. Methotrexate polyglutamate concentrations are not associated with disease control in rheumatoid arthritis patients receiving long-term methotrexate therapy. Arthritis Rheum. 2010;62:359–68. doi: 10.1002/art.27201. [DOI] [PubMed] [Google Scholar]
- 14.Dervieux T, Greenstein N, Kremer J. Pharmacogenomic and metabolic biomarkers in the folate pathway and their association with methotrexate effects during dosage escalation in rheumatoid arthritis. Arthritis Rheum. 2006;54:3095–103. doi: 10.1002/art.22129. [DOI] [PubMed] [Google Scholar]
- 15.Dervieux T, Furst D, Lein DO, Capps R, Smith K, Caldwell J, et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann Rheum Dis. 2005;64:1180–5. doi: 10.1136/ard.2004.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelis-Stoforidis P, Vajda FJ, Christophidis N. Methotrexate polyglutamate levels in circulating erythrocytes and polymorphs correlate with clinical efficacy in rheumatoid arthritis. Clin Exp Rheumatol. 1999;17:313–20. [PubMed] [Google Scholar]
- 17.Schroder H. In vivo methotrexate kinetics and metabolism in human hematopoietic cells. Clinical significance of methotrexate concentrations in erythrocytes. Dan Med Bull. 1990;37:22–40. [PubMed] [Google Scholar]
- 18.Hendel J, Nyfors A. Pharmacokinetics of methotrexate in erythrocytes in psoriasis. Eur J Clin Pharmacol. 1984;27:607–10. doi: 10.1007/BF00556900. [DOI] [PubMed] [Google Scholar]
- 19.de Rotte MC, de Jong PH, Pluijm SM, Calasan MB, Barendregt PJ, van Zeben D, et al. Association of low baseline levels of erythrocyte folate with treatment nonresponse at three months in rheumatoid arthritis patients receiving methotrexate. Arthritis Rheum. 2013;65:2803–13. doi: 10.1002/art.38113. [DOI] [PubMed] [Google Scholar]
- 20.Becker ML, Gaedigk R, van Haandel L, Thomas B, Lasky A, Hoeltzel M, et al. The effect of genotype on methotrexate polyglutamate variability in juvenile idiopathic arthritis and association with drug response. Arthritis Rheum. 2011;63:276–85. doi: 10.1002/art.30080. [DOI] [PubMed] [Google Scholar]
- 21.Becker ML, Leeder JS. Developmental pharmacogenetics in pediatric rheumatology: utilizing a new paradigm to effectively treat patients with juvenile idiopathic arthritis with methotrexate. Hum Genom Proteomics Published Online. 2010 Jun 22; doi: 10.4061/2010/257120. doi: 10.4061.2010.257120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan ES, Cronstein BN. Mechanisms of action of methotrexate. Bull Hosp Joint Dis. 2013;71(Suppl 1):S5–8. [PubMed] [Google Scholar]
- 23.Selhub J, Seyoum E, Pomfret EA, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991;51:16–21. [PubMed] [Google Scholar]
- 24.Kremer JM, Galivan J, Streckfuss A, Kamen B. Methotrexate metabolism analysis in blood and liver of rheumatoid arthritis patients. Association with hepatic folate deficiency and formation of polyglutamates. Arthritis Rheum. 1986;29:832–5. doi: 10.1002/art.1780290703. [DOI] [PubMed] [Google Scholar]
- 25.Allegra CJ, Fine RL, Drake JC, Chabner BA. The effect of methotrexate on intracellular folate pools in human MCF-7 breast cancer cells. Evidence for direct inhibition of purine synthesis. J Biol Chem. 1986;261:6478–85. [PubMed] [Google Scholar]
- 26.Andersen LS, Hansen EL, Knudsen JB, Wester JU, Hansen GV, Hansen TM. Prospectively measured red cell folate levels in methotrexate treated patients with rheumatoid arthritis: relation to withdrawal and side effects. J Rheumatol. 1997;24:830–7. [PubMed] [Google Scholar]
- 27.Morgan SL, Baggott JE, Vaughn WH, Young PK, Austin JV, Krumdieck CL, et al. The effect of folic acid supplementation on the toxicity of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 1990;33:9–18. doi: 10.1002/art.1780330102. [DOI] [PubMed] [Google Scholar]
- 28.Varela-Moreiras G, Selhub J. Long-term folate deficiency alters folate content and distribution differentially in rat tissues. J Nutr. 1992;122:986–91. doi: 10.1093/jn/122.4.986. [DOI] [PubMed] [Google Scholar]
- 29.Cassady IA, Budge MM, Healy MJ, Nixon PF. An inverse relationship of rat liver folate polyglutamate chain length to nutritional folate sufficiency. Biochim Biophys Acta. 1980;633:258–68. doi: 10.1016/0304-4165(80)90411-0. [DOI] [PubMed] [Google Scholar]
- 30.Becker ML, van Haandel L, Gaedigk R, Lasky A, Hoeltzel M, Stobaugh J, et al. Analysis of intracellular methotrexate polyglutamates in patients with juvenile idiopathic arthritis: effect of route of administration on variability in intracellular methotrexate polyglutamate concentrations. Arthritis Rheum. 2010;62:1803–12. doi: 10.1002/art.27434. [DOI] [PubMed] [Google Scholar]
- 31.Becker ML, van Haandel L, Gaedigk R, Thomas B, Hoeltzel MF, Lasky A, et al. Red blood cell folate concentrations and polyglutamate distribution in juvenile arthritis: predictors of folate variability. Pharmacogenet Genomics. 2012;22:236–46. doi: 10.1097/FPC.0b013e3283500202. [DOI] [PubMed] [Google Scholar]
- 32.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 33.Koizumi S. Impairment of methotrexate (MTX)-polyglutamate formation of MTX-resistant K562 cell lines. Jpn J Cancer Res. 1988;79:1230–7. doi: 10.1111/j.1349-7006.1988.tb01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matherly LH, Czajkowski CA, Angeles SM. Identification of a highly glycosylated methotrexate membrane carrier in K562 human erythroleukemia cells up-regulated for tetrahydrofolate cofactor and methotrexate transport. Cancer Res. 1991;51:3420–6. [PubMed] [Google Scholar]
- 35.Funk RS, van Haandel L, Becker ML, Leeder JS. Low-dose methotrexate results in the selective accumulation of aminoimidazole carboxamide ribotide in an erythroblastoid cell line. J Pharmacol Exp Ther. 2013;347:154–63. doi: 10.1124/jpet.113.206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Haandel L, Becker ML, Williams TD, Stobaugh JF, Leeder JS. Comprehensive quantitative measurement of folate polyglutamates in human erythrocytes by ion pairing ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2012;26:1617–30. doi: 10.1002/rcm.6268. [DOI] [PubMed] [Google Scholar]
- 37.van Haandel L, Becker ML, Leeder JS, Williams TD, Stobaugh JF. A novel high-performance liquid chromatography/mass spectrometry method for improved selective and sensitive measurement of methotrexate polyglutamation status in human red blood cells. Rapid Commun Mass Spectrom. 2009;23:3693–3702. doi: 10.1002/rcm.4300. [DOI] [PubMed] [Google Scholar]
- 38.van Haandel L, Becker ML, Williams TD, Leeder JS, Stobaugh JF. Measurement of methotrexate polyglutamates in human erythrocytes by ion-pair UPLC-MS/MS. Bioanalysis. 2011;3:2783–2796. doi: 10.4155/bio.11.288. [DOI] [PubMed] [Google Scholar]
- 39.Morgan SL, Oster RA, Lee JY, Alarcon GS, Baggott JE. The effect of folic acid and folinic acid supplements on purine metabolism in methotrexate-treated rheumatoid arthritis. Arthritis Rheum. 2004;50:3104–11. doi: 10.1002/art.20516. [DOI] [PubMed] [Google Scholar]
- 40.Hornung N, Ellingsen T, Stengaard-Pedersen K, Poulsen JH. Folate, homocysteine, and cobalamin status in patients with rheumatoid arthritis treated with methotrexate, and the effect of low dose folic acid supplement. J Rheumatol. 2004;31:2374–81. [PubMed] [Google Scholar]
- 41.Morgan SL, Baggott JE, Lee JY, Alarcon GS. Folic acid supplementation prevents deficient blood folate levels and hyperhomocysteinemia during longterm, low dose methotrexate therapy for rheumatoid arthritis: implications for cardiovascular disease prevention. J Rheumatol. 1998;25:441–6. [PubMed] [Google Scholar]
- 42.Morgan SL, Baggott JE, Vaughn WH, Austin JS, Veitch TA, Lee JY, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann Intern Med. 1994;121:833–41. doi: 10.7326/0003-4819-121-11-199412010-00002. [DOI] [PubMed] [Google Scholar]
- 43.Morgan SL, Baggott JE, Altz-Smith M. Folate status of rheumatoid arthritis patients receiving long-term, low-dose methotrexate therapy. Arthritis Rheum. 1987;30:1348–56. doi: 10.1002/art.1780301205. [DOI] [PubMed] [Google Scholar]
- 44.Allegra CJ, Hoang K, Yeh GC, Drake JC, Baram J. Evidence for direct inhibition of de novo purine synthesis in human MCF-7 breast cells as a principal mode of metabolic inhibition by methotrexate. J Biol Chem. 1987;262:13520–6. [PubMed] [Google Scholar]
- 45.Baram J, Allegra CJ, Fine RL, Chabner BA. Effect of methotrexate on intracellular folate pools in purified myeloid precursor cells from normal human bone marrow. J Clin Invest. 1987;79:692–7. doi: 10.1172/JCI112872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward GJ, Nixon PF. Modulation of pteroylpolyglutamate concentration and length in response to altered folate nutrition in a comprehensive range of rat tissues. J Nutr. 1990;120:476–84. doi: 10.1093/jn/120.5.476. [DOI] [PubMed] [Google Scholar]
- 47.Cichowicz DJ, Shane B. Mammalian folylpoly-gamma-glutamate synthetase. 2 Substrate specificity and kinetic properties. Biochemistry. 1987;26:513–21. doi: 10.1021/bi00376a025. [DOI] [PubMed] [Google Scholar]
- 48.Moran RG, Colman PD. Mammalian folyl polyglutamate synthetase: partial purification and properties of the mouse liver enzyme. Biochemistry. 1984;23:4580–9. doi: 10.1021/bi00315a011. [DOI] [PubMed] [Google Scholar]
- 49.Clarke L, Waxman DJ. Human liver folylpolyglutamate synthetase: biochemical characterization and interactions with folates and folate antagonists. Arch Biochem Biophys. 1987;256:585–96. doi: 10.1016/0003-9861(87)90616-3. [DOI] [PubMed] [Google Scholar]
- 50.Chabner BA, Allegra CJ, Curt GA, Clendeninn NJ, Baram J, Koizumi S, et al. Polyglutamation of methotrexate. Is methotrexate a prodrug? J Clin Invest. 1985;76:907–12. doi: 10.1172/JCI112088. [DOI] [PMC free article] [PubMed] [Google Scholar]