Abstract
Caloric restriction (CR) results in fat loss; however, it may also result in loss of muscle and thereby reduce strength and aerobic capacity (V̇O2 max). These effects may not occur with exercise-induced weight loss (EX) because of the anabolic effects of exercise on heart and skeletal muscle. We tested the hypothesis that CR reduces muscle size and strength and V̇O2 max, whereas EX preserves or improves these parameters. Healthy 50- to 60-yr-old men and women (body mass index of 23.5–29.9 kg/m2) were studied before and after 12 mo of weight loss by CR (n = 18) or EX (n = 16). Lean mass was assessed by dual-energy X-ray absorptiometry, thigh muscle volume by MRI, isometric and isokinetic knee flexor strength by dynamometry, and treadmill V̇O2 max by indirect calorimetry. Both interventions caused significant decreases in body weight (CR: −10.7 ± 1.4%, EX: −9.5 ± 1.5%) and lean mass (CR: −3.5 ± 0.7%, EX: −2.2 ± 0.8%), with no significant differences between groups. Significant decreases in thigh muscle volume (−6.9 ± 0.8%) and composite knee flexion strength (−7.2 ± 3%) occurred in the CR group only. Absolute V̇O2 max decreased significantly in the CR group (−6.8 ± 2.3%), whereas the EX group had significant increases in both absolute (+15.5 ± 2.4%) and relative (+28.3 ± 3.0%) V̇O2 max. These data provide evidence that muscle mass and absolute physical work capacity decrease in response to 12 mo of CR but not in response to a similar weight loss induced by exercise. These findings suggest that, during EX, the body adapts to maintain or even enhance physical performance capacity.
Keywords: diet, training, energy deficit, cardiovascular, sarcopenia
WEIGHT LOSS IN OVERWEIGHT and obese individuals is beneficial with respect to reducing the risk of developing cardiovascular disease, diabetes, and some cancers. Although caloric restriction (CR) is an effective means for achieving weight loss, the negative energy balance induced by CR may result not only in reductions in fat mass but also in catabolism of skeletal muscle and myocardium, thus affecting the capacity to perform physical work. Indeed, previous studies have demonstrated that CR-induced weight loss is associated with decreases in muscle size (6, 7, 13, 14) and strength (8, 10). Furthermore, because fat-free mass is a strong determinant of aerobic exercise capacity (V̇O2 max) (5), CR may also decrease V̇O2 max.
An increase in exercise energy expenditure, without a compensatory increase in food intake, is also an effective means for achieving weight loss (12, 14, 15). Negative energy balance occurs in humans and animals during catastrophic events, such as escaping from environmental disasters (floods, forest fires, earthquakes), avoiding predators (for example, when an animal’s territory is invaded by another, more powerful species), or escaping from or participating in war. In this context, the ability to preserve or improve strength and V̇O2 max, despite a negative energy balance, might have been selected for during the course of evolution because it provided a survival advantage. Although the preservation of strength and V̇O2 max may not be important in modern society for the purposes of avoiding predators, for example, decrements in strength and V̇O2 max may be predisposing factors for physical frailty in late life (3, 4).
The purpose of the present study was to test the hypothesis that CR results in decreases in muscle size and strength and in V̇O2 max, whereas a similar energy deficit induced by increasing exercise energy expenditure without changing energy intake does not alter muscle size or strength and likely increases V̇O2 max. The data reported in this paper were obtained as part of a larger investigation of the feasibility of CR in healthy volunteers (CALERIE: Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) (12).
METHODS
Participants
Fifty- to sixty-year-old men and women who had body mass index values in the high-normal to overweight range (i.e., 23.5–29.9 kg/m2) were recruited from the St. Louis, Missouri, metropolitan area. Candidates for the study were excluded if they had 1) a history of diabetes or a fasting blood glucose value ≥126 mg/dl, 2) a history or clinical evidence of coronary artery disease, stroke, or lung disease, 3) a resting blood pressure (BP) ≥170 mmHg systolic or 100 mmHg diastolic, or 4) a recent history or evidence of malignancy. Furthermore, all candidates had to be nonsmokers and sedentary (defined as exercising <20 min per day twice per week during the 6 mo before baseline testing). Women had to be postmenopausal. All participants gave their informed, written consent to participate in the study, which was approved by the Human Studies Committee and the General Clinical Research Center Advisory Committee at Washington University School of Medicine.
The participants were randomly assigned, with stratification for sex, to the CR, EX, or a control group in a 2:2:1 ratio. Among the 19 subjects in the CR and EX groups who started the intervention, 1 from each group dropped out of the study and 18 in each group completed the study. For reasons that included technical problems during testing, participant refusal to perform tests, and failure to achieve maximal exercise during the treadmill exercise test, data from some subjects were not included in the present analyses. Furthermore, because of the reasons described above, complete outcome data were only available on 4 of the 10 participants who were randomized to the control group; therefore, the control group data were not included in the analyses for the present report. In total, data from 34 subjects (CR: n = 18; EX: n = 16) are included in the present report. However, sample sizes for some outcomes are smaller because of missing data; specific sample size information for each outcome is as indicated.
CR intervention
The objective of the CR intervention was to decrease energy intake by 16% during the first 3 mo and by 20% during the remaining 9 mo. Study dietitians met with participants on a weekly basis to weigh them and teach them strategies for reducing portion sizes, replacing high-energy-dense foods with food of lower energy density, and daily self-monitoring of dietary intake with the use of either 7-day food diaries or personal digital assistant diet-tracking software. The CR group participants attended ~85% of the weekly meetings with the dietitians (12). Subjects with little or no weight loss were asked to frequently maintain food diaries, which the dietitians used to develop personalized dietary recommendations. Additional details about the CR intervention, including data to reflect adherence, have been reported previously (12, 20). However, in brief, average energy intake during the CR intervention was ~12% lower than baseline as determined by doubly labeled water, and physical activity levels remained unchanged according to physical activity recall questionnaires (12).
Exercise intervention
The goal of the EX intervention was to induce the same energy deficit as was induced by the CR intervention by increasing exercise energy expenditure by 16% of baseline total daily energy expenditure for the first 3 mo and by 20% for the subsequent 9 mo without changing energy intake. On a weekly basis, the participants met with exercise trainers for body weight measurements and to receive updated gross exercise energy expenditure goals. The participants attended ~85% of the weekly meetings with the trainers (12). The energy expenditure goals were based on 16 or 20% of total daily energy expenditure plus an estimate of how much energy would have been expended in the absence of exercise; further details have been described previously (20). The participants exercised either in our facility or on their own while wearing heart rate monitors (S610, Polar Electro, Kempele, Finland). These monitors gave the subjects feedback regarding their daily and weekly gross exercise energy expenditure. Although the participants were advised that frequent vigorous endurance exercise would be the most time-efficient way to meet their exercise energy expenditure goals, specific information about exercise frequency, duration, and intensity were not prescribed. For participants who showed little or no weight loss during the intervention, the heart rate monitor data were first examined to assess compliance with the exercise recommendations, and individualized exercise plans were then developed as needed to improve compliance. If exercise compliance was acceptable, individuals with little or no weight loss were asked to record food diaries to determine whether energy intake had increased; as necessary, individualized diet plans were provided to encourage the participants to maintain energy intake at baseline levels. As reported previously (12, 20), the participants exercised ~6 days/wk for ~60 min per session at ~70% of maximal heart rate (HRmax), and this resulted in ~320 kcal/day gross exercise energy expenditure (or ~230 kcal/day net exercise energy expenditure). Walking, elliptical machine exercise, cycling, and running were the most commonly used exercise modes, and none of the subjects used strength training as a means for achieving their exercise energy expenditure goals. According to doubly labeled water-based estimates, average energy intake during the EX intervention did not change from baseline (12, 20).
Protein intake
Protein intake was assessed by use of 7-day food diaries and computerized nutrient analysis (Nutrition Data System for Research, versions 4.05, 4.06, and 5.0; Nutrition Coordination Center, University of Minnesota, Minneapolis, MN) as described previously (12, 20). Dietary data were collected before and during the intervention (i.e., at baseline and at 1, 3, 6, 9, and 12 mo), and data collected during the intervention were averaged for each individual to reflect year-long average protein intake.
Body weight and lean body mass
Body weight was measured in duplicate in the morning, after an overnight fast, while the participant was wearing only underwear and a hospital gown. Baseline and final body weights were calculated as the mean of multiple weekly weights (up to 5 at baseline and up to 3 at 12 mo), except when used for the calculation of relative V̇O2 max (i.e., ml·kg−1·min−1), for which body weight was measured immediately before the exercise test. Lean body mass (i.e., whole body mass minus fat mass and bone mineral mass) was measured by dual-energy X-ray absorptiometry (Delphi W, Hologic, Waltham, MA; software version 11.2). Lean mass for each subject was calculated as the mean of up to three assessments at baseline and one or two assessments at 12 mo.
Thigh muscle volume and average cross-sectional area
MRIs of both thighs were obtained at baseline and at the end of the intervention. Ten transverse images, 10 mm thick, were acquired just superior to the patella with no intersection gap between slices with a 1.5-T superconducting MRI instrument (Siemens, Iselin, NJ) and a T1-weighted pulse sequence. Muscle volume and cross-sectional area (CSA) were quantified in each slice by Analyze Direct software (version 5.0; Mayo Clinic, Rochester, MN), which distinguishes muscle from other tissues based on pixel brightness. Volume data for the 10 slices were summed to represent muscle volume in the 10-cm longitudinal region of interest. Average CSA was based on the CSA values from all slices in the region of interest. Muscle size data are reported as the sum of values from the right and left thighs. When muscle volume data were used to normalize strength data (as described below), only data from the right thigh were used.
Muscle strength
Tests for concentric isokinetic and isometric knee flexor and knee extensor strength were performed at baseline and at the end of the intervention with a Cybex dynamometer (Ronkonkoma, NY). Many of the subjects were limited by patellar dysfunction during the knee extension test. Therefore, only knee flexion data are reported. The subjects were seated with the back supported and hips flexed at 120° and were secured to the seat of the dynamometer with thigh and pelvis straps. All tests were performed on the right leg. The isokinetic tests were performed at an angular velocity of 60°/s. The isometric tests were performed with the knee flexed at 45°. The best of three maximal voluntary efforts for each test component (i.e., isokinetic knee flexion and isometric knee flexion) was used as the measure of absolute strength. Strength data were also reported relative to body weight and relative to right thigh muscle volume as a measure of muscle-specific force. A composite strength score was calculated as the sum of the isometric and isokinetic knee flexion tests.
Physiological responses to maximal treadmill exercise
We previously reported V̇O2 max data as an index of adherence to the EX intervention (12, 20). For the present study, however, because V̇O2 max is reported as a physiological outcome, only data from subjects whose baseline and final tests met the criteria for “true” V̇O2 max, as described below, were included. Metabolic responses to treadmill exercise were measured by indirect calorimetry (True Max 2400, ParvoMedics, Salt Lake City, UT) during a graded treadmill exercise test. The incremental test started at a speed determined, during a warm-up period, to elicit ~70% of age-predicted HRmax and remained constant throughout the test, and grade was increased by 1–2% every 1–2 min. The test continued until the subject could no longer exercise because of exhaustion or until other conditions, such as ECG changes or development of symptoms, made it unsafe to continue. Metabolic data were generated every 15 s, and the highest average oxygen consumption (V̇O2) value from two consecutive 15-s data points was designated peak V̇O2. Peak V̇O2 was considered to be “true” V̇O2 max if two of the following three conditions were met: 1) peak respiratory exchange ratio (RER) was ≥1.10, 2) measured HRmax was ≥ predicted HRmax minus 10 [where predicted HRmax = 208 – 0.7 × age (19)], and 3) V̇O2 increase was ≤150 ml/min between the last two stages of the test. BP, measured by auscultation, and 12-lead ECG were monitored throughout the test. HRmax was determined from the maximal exercise ECG and was based on ≥5 R-R normal sinus cardiac cycles. Maximal exercise rate pressure product (RPP) was calculated as the product of HRmax and maximal exercise systolic BP. Maximal exercise oxygen pulse was calculated as V̇O2 max divided by HRmax.
Statistical analyses
Between-group comparisons of the changes in the outcome variables were performed by analyses of covariance with the baseline to 12-mo change as the dependent variable (absolute change and percent change from baseline), treatment group as the independent variable, and baseline value as a covariate. Furthermore, percent change in body weight was included as a covariate in analyses of all outcome data (except body weight) so the results can be evaluated as if the weight changes in the two groups were exactly equal. Age and sex were not included as covariates in these models because the sex distribution was not different between the groups and because small variations in age, within the relatively narrow 50- to 60-yr age range, would not be expected to affect the physiological responses to CR or EX. Significance of the within-group changes were based on the least squares means from the analyses of covariance. Associations between changes in selected variables were assessed by Pearson correlations. Analyses were performed with SAS software, version 9.1.3 of the SAS System for Linux (SAS Institute, Cary, NC). All statistical tests were two-tailed, and significance was accepted at P ≤ 0.05. Data are presented as least squares means ± SE, unless noted otherwise.
RESULTS
Participants
The male and female distribution was similar in the CR (7 men, 11 women) and EX (6 men, 10 women) groups. Mean age (±SD) for both groups was in the middle to upper end of the targeted age range for the study (CR: 55.2 ± 3.4 yr; EX: 59.4 ± 1.0 yr). Baseline body mass index (mean ± SD) was 27.1 ± 2.5 kg/m2 in the CR group and 27.0 ± 1.8 kg/m2 in the EX group, reflecting the fact that most of the participants were overweight.
Protein intake
Absolute protein consumption (g/day) decreased significantly in the CR group but not in the EX group (Table 1). When expressed relative to body weight, protein consumption did not change in the CR group but increased significantly in the EX group (Table 1). The between-group comparisons of changes in absolute and relative protein consumption were both marginally significant.
Table 1.
Protein consumption, lean mass, and thigh muscle size
| CR (n = 18) | EX (n = 16) | Between Group P Value | |
|---|---|---|---|
| Protein consumption | |||
| Baseline, g/day | 88±6 | 83±5 | |
| Average during intervention, g/day | 80±4 | 87±6 | |
| Change, g/day | −7.3±3* | 3.1±3 | 0.03 |
| Change, % | −5.9±4 | 4.7±4 | 0.06 |
| Protein consumption | |||
| Baseline, g·kg−1·day−1 | 1.11±0.05 | 1.09±0.04 | |
| Average during intervention, g·kg−1·day−1 | 1.11±0.06 | 1.23±0.07 | |
| Change, g·kg−1·day−1 | 0.003±0.04 | 0.14±0.05* | 0.05 |
| Change, % | 1.7±4 | 13.1±4* | 0.06 |
| Lean mass | |||
| Baseline, kg | 49.1±2.4 | 47.9±2.8 | |
| Final, kg | 47.4±2.4 | 46.8±2.6 | |
| Change, kg | −1.6±0.3* | −1.2±0.3* | 0.41 |
| Change, % | −3.4±0.7* | −2.3±0.7* | 0.31 |
| Thigh muscle volume | |||
| Baseline, cm3 | 1529±80 | 1516±81 | |
| Final, cm3 | 1413±67 | 1532±86 | |
| Change, cm3 | −110±15* | 7.95±17 | <0.0001 |
| Change, % | −6.9±0.8* | 0.49±1.0 | <0.0001 |
| Thigh muscle CSA | |||
| Baseline, cm2 | 191±10 | 190±10 | |
| Final, cm2 | 177±8.4 | 192±11 | |
| Change, cm2 | −13.8±1.8* | 0.99±2.2 | <0.0001 |
| Change, % | −6.9±0.8* | 0.49±1.0 | <0.0001 |
Values are means ± SE except for change data, which are least squares means ± SE from the analysis of covariance (ANCOVA) with baseline value and percent change in weight as covariates. For protein consumption data, “average during intervention” reflects the mean of data collected at 1, 3, 6, 9, and 12 mo during the intervention and sample sizes in the exercise-induced weight loss (EX) group are 1 less than listed in table header. Thigh muscle size data represent the sum of the right and left thighs, and sample sizes for the caloric restriction (CR) and EX groups are 1 and 4 less than listed in table header, respectively. CSA, cross-sectional area.
P ≤ 0.05 vs. zero by ANCOVA with Tukey’s adjustment for multiple comparisons.
Body weight and lean body mass
As reported previously by our group for a slightly larger sample (12, 20), body weight decreased significantly in both the CR (−8.1 ± 1.1 kg, −10.7 ± 1.4%) and EX (−7.7 ± 1.2 kg, −9.5 ± 1.5%) groups, and these changes were not different between groups whether expressed in absolute terms (P = 0.80) or as a percentage (P = 0.58). Likewise, lean body mass decreased significantly in both groups, and these changes were not different between groups (Table 1).
Thigh muscle volume
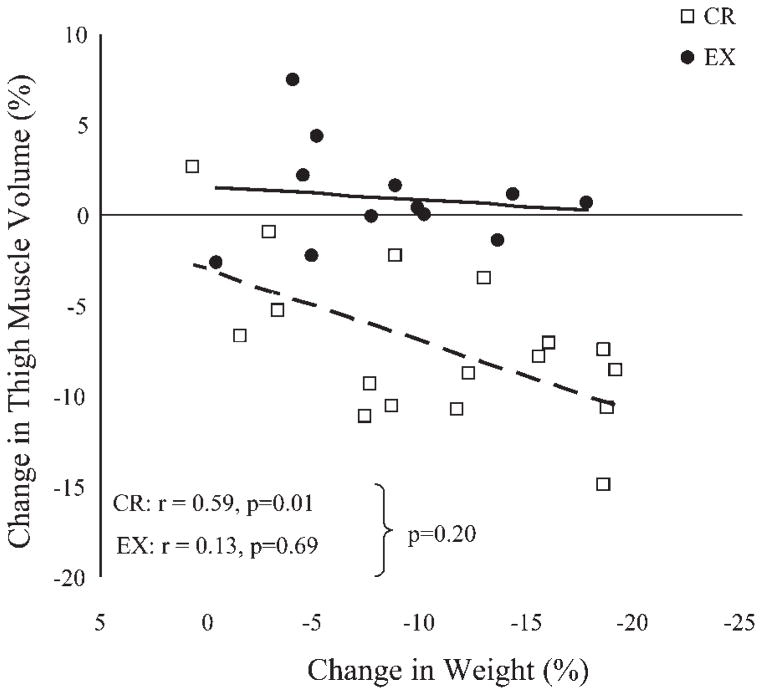
Thigh muscle volume and average thigh muscle CSA decreased significantly in the CR group but not in the EX group, and these changes were significantly different between groups (Table 1). In the CR group, the decrease in thigh muscle volume correlated with the magnitude of change in body weight, whereas in the EX group there was no evidence for such a relationship (Fig. 1). The comparison of correlation coefficients between groups did not achieve statistical significance.
Fig. 1.
Relationship (Pearson correlations) between the magnitude of weight loss and the magnitude of change in thigh muscle volume (sum of right and left thighs) in the caloric restriction group (CR) and the exercise group (EX).
Muscle strength
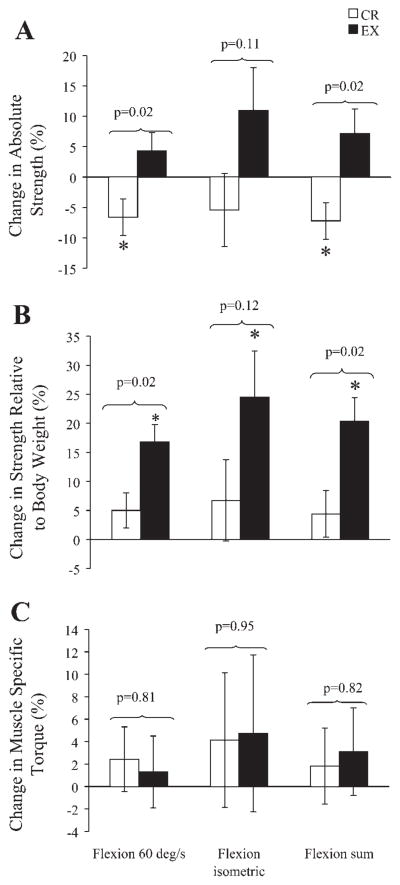
Isokinetic knee flexion torque decreased significantly in the CR group and remained unchanged in the EX group (Table 2 and Fig. 2). The between-group comparison of these changes was significant when expressed as a percentage. Isometric knee flexion did not change significantly in either the CR or EX groups (Table 2 and Fig. 2). Composite knee flexion strength decreased significantly in the CR group and remained unchanged in the EX group; these changes were statistically different between groups whether expressed in absolute terms or as a percentage (Table 2 and Fig. 2).
Table 2.
Isokinetic and isometric knee flexor strength
| CR (n = 15) | EX (n = 12) | Between Group P Value | |
|---|---|---|---|
| Torque (raw) | |||
| Peak torque at 60°/s | |||
| Baseline, N·m | 98±6 | 80±8 | |
| Final, N·m | 91±6 | 83±7 | |
| Change, N·m | −6.1±3* | 1.2±3 | 0.11 |
| Change, % | −6.6±3* | 4.3±3 | 0.02 |
| Peak torque at 0°/s | |||
| Baseline, N·m | 95±8 | 73±7 | |
| Final, N·m | 88±7 | 79±7 | |
| Change, N·m | −5.9±4 | 4.7±5 | 0.16 |
| Change, % | −5.4±6 | 11.0±7 | 0.11 |
| Sum of 0 and 60°/s peak torques | |||
| Baseline, N·m | 193±14 | 152±14 | |
| Final, N·m | 178±13 | 162±14 | |
| Change, N·m | −13.3±6* | 7.5±7 | 0.04 |
| Change, % | −7.2±3* | 7.2±4 | 0.02 |
| Torque relative to body weight | |||
| Peak torque at 60°/s | |||
| Baseline, N·m−1·kg −1 | 1.24±0.06 | 1.06±0.08 | |
| Final, N·m−1·kg−1 | 1.29±0.06 | 1.23±0.08 | |
| Change, N·m−1·kg−1 | 0.05±0.04 | 0.16±0.04* | 0.06 |
| Change, % | 5.0±3 | 16.8±3* | 0.02 |
| Peak torque at 0°/s | |||
| Baseline, N·m−1·kg −1 | 1.19±0.08 | 0.97±0.07 | |
| Final, N·m−1·kg−1 | 1.24±0.08 | 1.17±0.09 | |
| Change, N·m−1·kg−1 | 0.05±0.06 | 0.20±0.07* | 0.16 |
| Change, % | 6.7±7 | 24.5±8* | 0.12 |
| Sum of 0 and 60°/s peak torques | |||
| Baseline, N·m−1·kg −1 | 2.43±0.13 | 2.03±0.14 | |
| Final, N·m−1·kg−1 | 2.53±0.13 | 2.40±0.17 | |
| Change, N·m−1·kg−1 | 0.08±0.08 | 0.40±0.09* | 0.02 |
| Change, % | 4.4±4 | 20.4±4* | 0.02 |
Values are are means ± SE except for change data, which are least squares means ± SE from the ANCOVA with baseline value and percent change in weight as covariates.
P ≤ 0.05 within group by ANCOVA with Tukey’s adjustment for multiple comparisons.
Fig. 2.
Changes in absolute muscle strength (A), strength relative to body weight (B), and muscle-specific torque (C) for the knee flexor muscles. Data are least-squares means ± SE after adjustment for baseline values and percent change in body weight. Muscle-specific torque was calculated as absolute torque divided by muscle volume and was based on strength and muscle volume data from the right leg only. *P ≤ 0.05 within group by ANCOVA with Tukey’s adjustment for multiple comparisons.
All indexes of knee flexor strength relative to body weight remained unchanged from baseline in the CR group and increased significantly in the EX group (Table 2 and Fig. 2). The changes in isokinetic flexion strength and composite knee flexion strength were significantly different between groups. The changes in isometric flexion torque were not significantly different between groups; however, it is noteworthy that the mean values for these changes were similar to those seen for isokinetic knee flexion. When strength data were reported relative to muscle volume, no changes from baseline were evident in either study group, and none of the between group differences was significant (Table 3 and Fig. 2).
Table 3.
Isokinetic and isometric muscle specific torque (torque ÷ thigh muscle volume)
| CR (n = 14) | EX (n = 11) | Between Group P Value | |
|---|---|---|---|
| Peak torque at 60°/s | |||
| Baseline, N·m−1·cm−3 | 0.128±0.007 | 0.106±0.008 | |
| Final, N·m−1·cm−3 | 0.127±0.006 | 0.109±0.007 | |
| Change, N·m−1·cm−3 | 0.002±0.003 | −0.001±0.004 | 0.60 |
| Change, % | 2.43±2.8 | 1.30±3.2 | 0.81 |
| Peak torque at 0°/s | |||
| Baseline, N·m−1·cm−3 | 0.121±0.008 | 0.098±0.007 | |
| Final, N·m−1·cm−3 | 0.122±0.008 | 0.103±0.008 | |
| Change, N·m−1·cm−3 | 0.003±0.006 | 0.002±0.007 | 0.92 |
| Change, % | 4.13±6.0 | 4.74±7.0 | 0.95 |
| Sum of 0 and 60°/s peak torques | |||
| Baseline, N·m−1·cm−3 | 0.249±0.014 | 0.204±0.014 | |
| Final, N·m−1·cm−3 | 0.248±0.013 | 0.212±0.014 | |
| Change, N·m−1·cm−3 | 0.003±0.008 | 0.004±0.009 | 0.96 |
| Change, % | 1.82±3.4 | 3.11±3.9 | 0.82 |
Values are means ± SE except for change data, which are least squares means ± SE from the ANCOVA with baseline value and percent change in weight as covariates. Data were calculated using right leg data for both strength and muscle volume.
P ≤ 0.05 within group by ANCOVA with Tukey’s adjustment for multiple comparisons.
Physiological responses to maximal treadmill exercise
CR resulted in a decrease in absolute V̇O2 max; however, when expressed per unit body weight or lean mass, no change in V̇O2 max was evident (Table 4). V̇O2 max increased in the EX group regardless of whether it was expressed in absolute terms or relative to body weight or lean mass. All of the changes in V̇O2 max, regardless of how it was expressed, were significantly different between the CR and EX groups.
Table 4.
Physiological responses to maximal treadmill exercise: V̇O2max RER, VE/VO2, and heart rate
| CR (n = 15) | EX (n = 14) | Between Group P Value | |
|---|---|---|---|
| Absolute V̇O2max | |||
| Baseline, ml/min | 2075±139 | 1965±158 | |
| Final, ml/min | 1949±148 | 2250±175 | |
| Change, ml/min | −133±46* | 293±47* | <0.0001 |
| Change, % | −6.8±2.3* | 15.5±2.4* | <0.0001 |
| V̇O2max relative to body weight | |||
| Baseline, ml·kg−1·min−1 | 26.3±1.2 | 25.5±1.4 | |
| Final, ml·kg−1·min−1 | 27.4±1.3 | 32.3±1.9 | |
| Change, ml·kg−1·min−1 | 0.9±0.7 | 7.0±0.7* | <0.0001 |
| Change, % | 3.9±2.9 | 28.3±3.0* | <0.0001 |
| V̇O2max relative to lean mass | |||
| Baseline, ml·kg lean−1·min−1 | 43.5±1.2 | 40.5±1.2 | |
| Final, ml·kg lean−1·min−1 | 42.0±1.3 | 47.7±1.5 | |
| Change, ml·kg lean−1·min−1 | −1.5±0.9 | 7.2±0.9* | <0.0001 |
| Change, % | −3.3±2.1 | 17.8±2.2* | <0.0001 |
| RER at maximal exercise | |||
| Baseline | 1.19±0.01 | 1.16±0.02 | |
| Final | 1.32±0.03 | 1.19±0.02 | |
| Change | 0.14±0.02* | 0.03±0.02 | 0.001 |
| Change, % | 11.7±1.8* | 2.49±1.8 | 0.002 |
| VE/V̇O2 at maximal exercise | |||
| Baseline | 30.1±1.1 | 29.9±1.1 | |
| Final | 33.0±1.5 | 30.0±0.9 | |
| Change | 2.9±0.95* | 0.17±0.99 | 0.06 |
| Change, % | 9.37±3.3* | 2.09±3.4 | 0.13 |
| HRmax | |||
| Baseline, beats/min | 170±2 | 173±3 | |
| Final, beats/min | 170±2 | 173±2 | |
| Change, beats/min | 0.34±1.5 | 0.92±1.5 | 0.79 |
| Change, % | 0.24±0.9 | 0.64±0.9 | 0.76 |
Values are means ± SE except for change data, which are least squares means ± SE from the ANCOVA with baseline value and percent change in weight as covariates. V̇O2max, maximal oxygen uptake; HRmax, maximal heart rate; RER, respiratory exchange ratio; VE/V̇O2, ventilatory equivalent for oxygen uptake.
P ≤ 0.05 within group by ANCOVA with Tukey’s adjustment for multiple comparisons.
The high values for maximal exercise RER and minute ventilation (V̇E)/V̇O2 observed during the treadmill tests (Table 4) indicate that the subjects achieved V̇O2 max. Both RER and V̇E/V̇O2 during maximal exercise increased in response to the CR intervention, and the increase in maximal exercise RER was significantly different from that in the EX group. Although there was a tendency for the change in maximal exercise V̇E/V̇O2 in the CR group to be different from that in the EX group, this comparison did not achieve significance. HRmax did not change in either group, and there were no between-group differences (Table 4).
Oxygen pulse decreased significantly in the CR group and increased significantly in the EX group, and these changes were significantly different between groups (Table 5). Systolic BP during maximal exercise was significantly lower after the 1-yr intervention in the EX group only, whereas diastolic BP during maximal exercise was significantly lower during the final test in the CR group only (Table 5). The changes in maximal exercise systolic and diastolic BP were not different between groups. RPP during maximal exercise did not change in either group, and there were no differences between groups (Table 5).
Table 5.
Physiological responses to maximal treadmill exercise: oxygen pulse, blood pressure, and rate pressure product
| CR (n = 15) | EX (n = 14) | Between Group P Value | |
|---|---|---|---|
| Oxygen pulse at maximal exercise | |||
| Baseline, ml/beat | 12.2±0.8 | 11.3±0.9 | |
| Final, ml/beat | 11.5±0.9 | 13.0±1.0 | |
| Change, ml/beat | −0.82±0.3* | 1.7±0.3* | <0.0001 |
| Change, % | −7.2±2.5* | 15.3±2.6* | <0.0001 |
| Systolic BP at maximal exercise† | |||
| Baseline, mmHg | 192±8.1 | 204±6.9 | |
| Final, mmHg | 186±5.7 | 188±6.4 | |
| Change, mmHg | −10.6±5.4 | −12.2±5.4* | 0.84 |
| Change, % | −4.44±2.4 | −4.97±2.4* | 0.88 |
| Diastolic BP at maximal exercise† | |||
| Baseline, mmHg | 92±2.6 | 93±2.9 | |
| Final, mmHg | 85±3.6 | 89±2.0 | |
| Change, mmHg | −7.04±2.9* | −3.74±2.9 | 0.44 |
| Change, % | −6.46±3.4 | −3.43±3.4 | 0.53 |
| RPP at maximal exercise† | |||
| Baseline, beats·min−1·mmHg | 32740±1497 | 35243±1104 | |
| Final, beats·min−1·mmHg | 31692±1063 | 32620±1187 | |
| Change, beats·min−1·mmHg | −1749±974 | −1922±974 | 0.90 |
| Change, % | −4.10±2.6 | −4.65±2.6 | 0.88 |
Values are means ± SE except for change data, which are least squares means ± SE from the ANCOVA with baseline value and percent change in weight as covariates.
Because of missing maximal exercise blood pressure (BP) data, CR group samples sizes for maximal systolic BP, diastolic BP, and RPP are 1 less than that listed in table header. RPP, rate pressure product.
P ≤ 0.05 within group by ANCOVA with Tukey;s adjustment for multiple comparisons.
Seven subjects (1 CR, 6 EX) were taking BP medications for hypertension during the study. Although the medications and dosages for these subjects did not change during the study, it is possible that the inclusion of these subjects might have affected the results of the hemodynamic outcomes. Therefore, we performed several subanalyses after excluding these subjects. Exclusion of the one subject who was taking a beta-blocker (EX group) did not affect the HRmax or oxygen pulse results. Exclusion of all seven subjects who were taking any BP medication did not alter the results for maximal exercise RPP or maximal exercise diastolic BP; however, the significant decrease in maximal exercise systolic BP for the EX group became nonsignificant (P = 0.26).
DISCUSSION
Results from the present study indicate that 12 mo of CR results in significant reductions in absolute thigh muscle mass, knee flexor strength, and V̇O2 max, whereas a similar 1-yr energy deficit induced by exercise completely preserves thigh muscle mass and strength and improves V̇O2 max. When strength and V̇O2 max are expressed relative to body weight, there are no decreases in response to CR. However, in response to EX, there were increases in body weight-related strength (17–24%) and V̇O2 max (28%), despite the fact that the exercise regimens were designed primarily for expending energy and not necessarily for aerobic training or strengthening per se.
Previous studies have demonstrated that a negative energy balance induced by CR results not only in loss of fat but also in loss of muscle (6, 7, 13, 14). Although we did not measure total muscle mass in the present study, it is likely that both CR and EX resulted in net loss of skeletal muscle because lean mass measured by dual-energy X-ray absorptiometry (i.e., total body mass minus fat and bone mass) decreased significantly in both groups. We measured thigh muscle size as a representative region of muscle that is involved in most modes of endurance exercise, including those that were commonly used in the present study. Thigh muscle size decreased in the CR group and was completely preserved in the EX group. It is likely that the changes in the CR group were due to loss of contractile protein, as opposed to noncontractile components of muscle such as lipid and connective tissue, because the decreases in size were paralleled by decreases in strength. As a result, there were no changes in muscle-specific torque (i.e., torque per unit muscle volume). Together, these findings suggest that endurance exercise protects against the energy deficit-induced loss of skeletal muscle size and strength, although it is likely that this is only true for muscles that are active during exercise.
Dietary protein consumption may affect the amount of lean or muscle mass that is lost during CR-induced weight loss (2, 9). As a consequence of the reduced food consumption in the CR group, there was a marginally significant decrease in absolute daily protein consumption. In contrast, absolute daily protein consumption in the EX group remained unchanged. However, as a result of the decreases in body weight in both groups, protein consumption relative to body weight was unchanged in the CR group and increased in the EX group. It is possible that the differences in protein consumption between the CR and EX groups might have been partly responsible for the differential effects of the weight loss interventions on muscle size and strength.
Absolute V̇O2 max decreased in response to CR in the present study. Because V̇O2 max is the product of HRmax, maximal exercise stroke volume (SVmax), and maximal exercise arterio-venous oxygen content difference [(a-v)O2max] and because HRmax did not change during the intervention, the decrease in V̇O2 max was due to decreases in SVmax and/or (a-v)O2max. In contrast to what was observed in the CR group, the energy deficit induced by exercise resulted in increases in V̇O2 max and oxygen pulse. Previous studies have shown that V̇O2 max increases in response to exercise training through increases in SVmax and (a-v)O2max (17, 18). To a large extent, the increases in SVmax are because of physiological hypertrophy of the myocardium (11, 16) and the increases in (a-v)O2max are because of increases in the capillary density and mitochondrial content in skeletal muscle (1). Therefore, despite the presence of a whole body catabolic state (i.e., weight loss), exercise may have had adaptive effects on skeletal muscle mitochondria and the myocardium. However, we do not have direct evidence to support this notion.
Interpretation of the body weight-independent data from the present study would suggest that CR has adverse effects on strength and cardiovascular fitness and that this might decrease the capacity for physical performance. It is important to note, however, that because CR resulted in substantial weight loss, the absolute work requirement for many (but not all) common activities, such as climbing stairs, also decreased. Therefore, from this perspective, the decreases in strength and V̇O2 max are proportional to the reduction in body weight, and the capacity for weight-bearing exercise (i.e., walking, running, climbing) would not likely be impaired. In the same context, however, it should also be recognized that weight loss induced by exercise coincided with a preservation of absolute strength and an increase in absolute V̇O2 max and therefore likely increased the capacity for physical performance.
The present study has strengths and limitations. One of the strengths is our measurement methods; we used a strict treadmill protocol to assess “true” V̇O2 max, and we used volumetric MRI-based measurements for thigh muscle size. Another strength is that we did not exclude data from subjects who were noncompliant with the intervention protocols; the data, therefore, reflect the average responses of overweight, middle-aged men and women to these interventions, rather than the best-case scenario for fully compliant individuals. A weakness of our study is that our strength assessments were limited to the knee flexor muscles; therefore, we cannot conclude about the effects of these interventions on strength in other regions of the body. Furthermore, because the muscle-specific torque data were based on all muscles in the thigh, rather than the knee flexors alone, and because we did not consider the biomechanical aspects of knee flexor force transfer, our data are only an index of muscle-specific torque and not a direct measure of force generation per unit of muscle tissue. Another limitation is that this was a fairly intensive intervention that required the participants to visit our facility on a weekly basis for weight checks and consultation. Therefore, data from the present study cannot be used to deduce the benefits of less supervised CR and EX interventions. Finally, because we did not include a combined CR and EX intervention, which might be more practical to perform, we cannot determine whether the beneficial effects of exercise override the effects of CR on absolute V̇O2 max, muscle size, and muscle strength.
In summary, data from the present study provide evidence that CR results in reductions in muscle size and strength and in V̇O2 max, although these changes are proportional to the reduction in body weight. In contrast, similar weight loss induced by increasing exercise energy expenditure without changes in energy intake prevents the loss of muscle size and strength, at least in the exercised muscles, and increases V̇O2 max. Data from the EX group in the present study suggest that, in the presence of an overall negative energy balance, the body is capable of selectively preserving and/or synthesizing skeletal muscle and perhaps other tissues that are involved in oxygen uptake and thus maintaining the capacity for the performance of physical activity.
Acknowledgments
We are grateful to the study participants for cooperation and to the staff of the Applied Physiology Laboratory and the General Clinical Research Center at Washington University School of Medicine for skilled assistance.
GRANTS
This work was supported by National Institutes of Health Cooperative Agreement AG-20487, National Institutes of Health General Clinical Research Center Grant RR-00036, National Institute of Diabetes and Digestive and Kidney Diseases Research Training Center Grant DK-20579, and National Institute of Diabetes and Digestive and Kidney Diseases Clinical Nutrition Research Unit Grant DK-56341. E. P. Weiss was supported by National Institute on Aging Grant AG-00078.
References
- 1.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 2.Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr. 2003;78:31–39. doi: 10.1093/ajcn/78.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Hunt BE, Davy KP, Jones PP, Desouza CA, Van Pelt RE, Tanaka H, Seals DR. Role of central circulatory factors in the fat-free mass-maximal aerobic capacity relation across age. Am J Physiol Heart Circ Physiol. 1998;275:H1178–H1182. doi: 10.1152/ajpheart.1998.275.4.H1178. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–438. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Ross R. Effects of sex on the change in visceral, subcutaneous adipose tissue and skeletal muscle in response to weight loss. Int J Obes Relat Metab Disord. 1999;23:1035–1046. doi: 10.1038/sj.ijo.0801038. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MJ, Friedl KE, Frykman PN, Moore RJ. Loss of muscle mass is poorly reflected in grip strength performance in healthy young men. Med Sci Sports Exerc. 1994;26:235–240. doi: 10.1249/00005768-199402000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 10.Nindl BC, Friedl KE, Frykman PN, Marchitelli LJ, Shippee RL, Patton JF. Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int J Sports Med. 1997;18:317–324. doi: 10.1055/s-2007-972640. [DOI] [PubMed] [Google Scholar]
- 11.Pluim BM, Zwinderman AH, van der LA, van der Wall EE. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–344. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 12.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO The Washington University School of Medicine CALERIE Group. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–691. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 14.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 16.Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol. 2002;40:1856–1863. doi: 10.1016/s0735-1097(02)02478-6. [DOI] [PubMed] [Google Scholar]
- 17.Spina RJ, Ogawa T, Martin WH, III, Coggan AR, Holloszy JO, Ehsani AA. Exercise training prevents decline in stroke volume during exercise in young healthy subjects. J Appl Physiol. 1992;72:2458–2462. doi: 10.1152/jappl.1992.72.6.2458. [DOI] [PubMed] [Google Scholar]
- 18.Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation. 1994;89:1648–1655. doi: 10.1161/01.cir.89.4.1648. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 20.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]