Abstract
Background
Aldehyde dehydrogenase 2 (ALDH2), a critical enzyme for the detoxification of alcohol, is associated with many types of cancers. To verify the relationship of ALDH2 rs671 G>A polymorphism and esophageal cancer (EC), we performed a meta-analysis of a total of 31 published data including 8,510 patients and 16,197 controls.
Methods
The pooled odds ratio (OR) and the 95% confidence interval (CI) were calculated using a fixed or random-effects model. Heterogeneity (PH), publication bias, and sensitivity analysis were also determined.
Results
Although a protective effort was found in the rs671 homozygote comparison (AA/GG: OR=0.69; 95% CI=0.48–0.98), the heterozygote comparison was apparently associated with the risk of EC, particularly in the Chinese population (AG/GG: OR=1.39; 95% CI=1.03–1.87). Alcohol consumption remarkably increased this risk, especially in the AG genotype. Drinking men with the AG genotype appeared to show a higher risk (AG/GG: OR=4.39; 95% CI=1.24–6.55) than drinking women.
Conclusion
The present meta-analysis provided advanced information regarding the association of the ALDH2 A>G polymorphism and EC. Taken together, insights from this study suggested an enhanced effect on the development of EC through a genetic–environmental interaction.
Keywords: aldehyde dehydrogenase-2, single nucleotide polymorphism, SNP, esophageal cancer, EC, meta-analysis
Introduction
Esophageal cancer (EC) is a major type of cancer with high morbidity and mortality worldwide. In 2014, 18,170 new EC cases and 15,450 EC deaths were projected to occur in the United States.1 EC is a multistep, multifactorial disease that involves a complex interplay between genetic and environmental factors.2 However, the complex etiology of EC is not fully elucidated. Alcohol consumption has been considered as a group 1 risk factor for EC.3 Alcohol in the human body is oxidized to acetaldehyde which, in turn, is oxidized to harmless acetate by aldehyde dehydrogenases.4 In fact, ethanol and its metabolite acetaldehyde are vital human carcinogens.5 A speculation for the effect of alcohol on EC progression suggested that alcohol also enhanced tobacco carcinogens.6 Recently, cumulative evidence has shown that gene polymorphisms play important roles in the carcinogenic potential of acetaldehyde. Biomarkers for early diagnosis are important to decide therapeutic options, improve treatment efficiency, and predict prognosis.7
Aldehyde dehydrogenase 2 (ALDH2), located at chromosomes 12q24.2, is a major enzyme for acetaldehyde elimination, and its polymorphism determines blood acetaldehyde concentrations after alcohol consumption.8 Rs671 G>A (also named Glu487Lys, with the glutamate corresponding to *1 allele, and lysine corresponding to *2 allele) is a nonsynonymous single nucleotide polymorphism (SNP) located in the 12th exon of the ALDH2 gene, which is highly prevalent among the East Asian population.9 The SNP inactivates ALDH2, leading to a high level of acetaldehyde in the blood, which is considered to increase the susceptibility to carcinogenesis.10 Increasing evidence suggests that the rs671 polymorphism is correlated to many types of cancer, such as head and neck cancer,11 gastric cancer,12 colorectal cancer,13 and EC.14
To date, numerous studies have investigated the role of the rs671 G>A polymorphism in the etiology of EC. However, the results of these studies are controversial and inconclusive. In the present study, we conducted a comprehensive meta-analysis to clarify the effects of ALDH2 genotypes on the risk of EC. In consideration of the extensive role of the rs671 G>A polymorphism in EC through the influence of other factors, our study also included alcohol drinking, sex, region, and the source of controls.
Materials and methods
Identification and eligibility of relevant studies
PubMed, Embase, MEDLINE, and the Chinese Biomedical Database (CBM) were searched using several search terms: “ALDH2”; “aldehyde dehydrogenase-2”; “polymorphism”; and “esophagus”; “oesophagus”; and “carcinoma or cancer or neoplasm or tumour or tumor”. The literature search was limited to published English manuscripts and was updated until 2013. In the event that studies featured overlapping published data, we selected the most recent studies that included a large number of subjects. The studies selected for our meta-analysis must meet the following criteria: 1) evaluation of the ALDH2 polymorphism and EC risk; 2) the use of a case-control design; and 3) available genotype frequency.
This study was a meta-analysis; all the studies we explored provided ethics statements and a statement of informed consent.
Data extraction
Two investigators independently extracted the published data according to the following subjects: the original investigators; the year of publication; the genotyping method used; the numbers of genotyped cases and controls; the country of origin; ethnicity; value of the Hardy–Weinberg equilibrium (HWE); the source of the control groups (population-or hospital-based controls); and alcohol-drinking status. We reclassified alcohol-drinking status as never-drinkers = non-/rare/never-drinkers; exdrinkers = stop drinking alcohol more than 1 year; light drinkers =1–350 g/week of alcohol; and heavy drinkers ≥350 g/week of alcohol. Different country descents were categorized as the People’s Republic of China, Japan, Cape Town, and Thailand. For studies including subjects of different original groups, data were extracted separately for each country group.
Statistical analysis
The correlation between the ALDH2 rs671 polymorphism and the EC risk was assessed by the odds ratio (OR) and the 95% confidence interval (CI). Since the polymorphism is distributed randomly during gamete formation, according to the concept of Mendelian randomization, the pooled ORs were only obtained from the combination of individual studies by heterozygote comparison (GA/GG) or homozygote comparison (AA/GG). The significance of pooled ORs was determined using a Z-test. Both the Cochran’s Q statistic for testing heterogeneity (PH) and the I2 statistic for quantifying the proportion of the total variation due to heterogeneity were calculated to estimate the heterogeneity included in the selected published data.15,16 If the PH value of the Q test was <0.05, indicating a lack of heterogeneity across studies, the summary OR estimate of each study was calculated using the fixed-effects model (Mantel–Haenszel method)17 or the random-effects model (DerSimonian and Laird method).18 Stratified analysis was performed to evaluate other environmental factors, such as alcohol-drinking status (non-/rare/never-drinkers, exdrinkers, light drinkers, and heavy drinkers), country, sex, and the source of controls. Sensitivity analysis was completed to testify the stability of the results by deleting a single study in the meta-analysis each time to show the influence of the individual dataset to the pooled OR. Furthermore, funnel plots and Egger’s linear regression test were applied to assess the potential publication bias.19 All analyses were performed via Stata software (version 10.0; StataCorp LP, College Station, TX, USA) using two-sided P-values.
Results
Published data selection
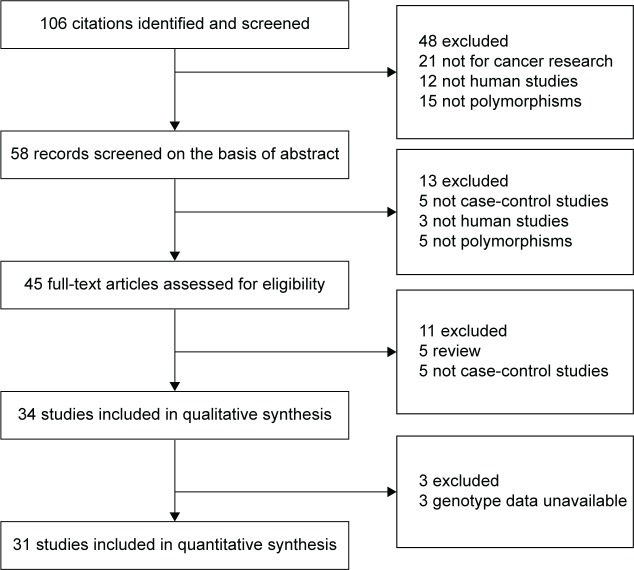
Over 106 published literatures relevant to the search terms were obtained. After selection by title screening, clinical data quality check, and abstract review, a total of 31 eligible studies were selected (Figure 1), which contained 8,510 cases and 16,197 controls in the pooled analyses.14,20–49 The characteristics of selected studies with a case-control design were summarized in Table 1 – 15 from the People’s Republic of China, 14 from Japan, one from Thailand, and one from Cape Town. All of the EC cases were histologically and/or pathologically confirmed by licensed physiologists. The polymerase chain reaction–restriction fragment length polymorphism assay was performed in 16 of the 31 studies. In addition, controls were mainly matched based on sex and age, of which eleven studies were population-based and 20 studies were hospital-based. The distributions of genotypes in the controls of most studies were consistent with HWE, with the exception of seven studies23–29 that were tested by the sensitivity analyses.
Figure 1.
Diagram of the relevant literature search and selection procedure.
Table 1.
Characteristics of the literature included in the meta-analysis
| Author | Year | Country | Ethnicity | Controls | Genotyping method | Cases
|
Controls
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | GG | AG | AA | Total | GG | AG | AA | HWE | ||||||
| Yokoyama et al30 | 2006 | People’s Republic of China | Asian | HB | PCR-RFLP | 52 | 26 | 22 | 4 | 421 | 223 | 167 | 22 | 0.19 |
| Boonyaphi et al23 | 2002 | Thailand | Asian | HB | APLP | 202 | 161 | 40 | 1 | 261 | 215 | 40 | 6 | 0.02 |
| Ding et al31 | 2009 | People’s Republic of China | Asian | PB | PCR | 221 | 90 | 89 | 42 | 191 | 114 | 66 | 11 | 0.70 |
| Chao et al21 | 2000 | People’s Republic of China | Asian | HB | PCR | 88 | 40 | 46 | 2 | 434 | 294 | 126 | 14 | 0.91 |
| Guo et al32 | 2008 | People’s Republic of China | Asian | HB | PCR-RFLP | 80 | 37 | 43 | 0 | 480 | 252 | 195 | 33 | 0.57 |
| Li et al24 | 2008 | Cape Town | African | PB | PCR | 237 | 212 | 18 | 7 | 268 | 248 | 17 | 3 | 0.00019 |
| Yang et al33 | 2005 | Japan | Asian | HB | PCR-CTPP | 165 | 38 | 126 | 1 | 494 | 254 | 195 | 45 | 0.39 |
| Yang et al34 | 2007 | People’s Republic of China | Asian | PB | PCR-CTPP | 191 | 90 | 98 | 3 | 198 | 108 | 76 | 14 | 0.89 |
| Yokoyama et al25 | 2001 | Japan | Asian | PB | PCR-RFLP | 90 | 0 | 40 | 50 | 577 | 0 | 520 | 57 | 2.09E-37 |
| Yokoyama et al22 | 2002 | Japan | Asian | HB | PCR | 234 | 63 | 169 | 2 | 634 | 341 | 250 | 43 | 0.76 |
| Lee et al35 | 2008 | People’s Republic of China | Asian | HB | PCR-RFLP | 406 | 111 | 281 | 14 | 656 | 325 | 286 | 45 | 0.09 |
| Cai et al36 | 2006 | People’s Republic of China | Asian | PB | PCR-RFLP | 205 | 119 | 61 | 25 | 394 | 214 | 160 | 20 | 0.31 |
| Ding et al26 | 2002 | People’s Republic of China | Asian | PB | PCR | 208 | 120 | 64 | 24 | 207 | 133 | 59 | 15 | 0.03 |
| Itoga et al27 | 2004 | Japan | Asian | PB | PCR-RFLP | 74 | 0 | 51 | 23 | 241 | 0 | 188 | 53 | 3.18E-39 |
| Matsuo et al37 | 2001 | Japan | Asian | HB | PCR-CTPP | 102 | 35 | 66 | 1 | 241 | 126 | 96 | 19 | 0.91 |
| Yokoyama et al20 | 1996 | Japan | Asian | HB | PCR | 69 | 27 | 42 | 0 | 83 | 71 | 12 | 0 | 0.48 |
| Yokoyama et al38 | 2006 | Japan | Asian | PB | PCR-RFLP | 42 | 24 | 18 | 0 | 273 | 248 | 25 | 0 | 0.43 |
| Hori et al49 | 1997 | Japan | Asian | HB | PCR | 91 | 20 | 68 | 3 | 71 | 30 | 36 | 5 | 0.18 |
| Wu et al39 | 2004 | People’s Republic of China | Asian | HB | PCR-RFLP | 134 | 32 | 99 | 3 | 237 | 120 | 105 | 12 | 0.07 |
| Gu et al40 | 2012 | People’s Republic of China | Asian | HB | MALDI | 380 | 225 | 144 | 11 | 378 | 219 | 137 | 22 | 0.93 |
| Wu et al41 | 2012 | People’s Republic of China | Asian | PB | Taqman | 799 | 523 | 243 | 33 | 1,027 | 645 | 337 | 45 | 0.91 |
| Li et al28 | 2011 | People’s Republic of China | Asian | PB | PCR-RFLP | 226 | 76 | 129 | 21 | 246 | 41 | 164 | 41 | 1.70E-10 |
| Cui et al42 | 2009 | Japan | Asian | HB | Illumina | 1,066 | 314 | 735 | 17 | 2,762 | 1,545 | 1,038 | 179 | 0.79 |
| Gao et al14 | 2013 | People’s Republic of China | Asian | PB | Taqman | 2,104 | 119 | 785 | 125 | 2,265 | 1,155 | 903 | 207 | 0.11 |
| Wang et al43 | 2011 | People’s Republic of China | Asian | HB | PCR-CTPP | 81 | 36 | 42 | 3 | 163 | 85 | 60 | 18 | 0.15 |
| Chen et al44 | 2006 | People’s Republic of China | Asian | HB | PCR-RFLP | 330 | 92 | 228 | 10 | 592 | 294 | 257 | 41 | 0.13 |
| Yokoyama et al45 | 1998 | Japan | Asian | HB | PCR-RFLP | 87 | 41 | 46 | 0 | 487 | 443 | 44 | 0 | 0.29 |
| Oze et al46 | 2010 | Japan | Asian | HB | TaqMan | 265 | 67 | 197 | 1 | 530 | 277 | 210 | 43 | 0.72 |
| Yokoyama et al47 | 2006 | Japan | Asian | HB | PCR-RFLP | 33 | 14 | 19 | 0 | 556 | 484 | 72 | 0 | 0.11 |
| Yokoyama et al48 | 2003 | Japan | Asian | HB | PCR-RFLP | 65 | 28 | 37 | 0 | 206 | 174 | 32 | 0 | 0.23 |
| Yokoyama et al29 | 2007 | Japan | Asian | HB | PCR-RFLP | 40 | 10 | 30 | 0 | 55 | 32 | 23 | 0 | 0.05 |
Abbreviations: HWE, Hardy–Weinberg equilibrium; HB, hospital base; PCR-RFLP, polymerase chain reaction–restriction fragment length polymorphism; APLP, amplified product length polymorphism; PB, population base; PCR, polymerase chain reaction; PCR-CTPP, polymerase chain reaction with the confronting two-pair primers; MALDI, matrix-assisted laser desorption/ionization; Illumina, Illumina™ Golden Gate bead-based genotyping platform.
Sensitivity analysis and publication bias
In order to reflect the influence of the individual dataset to the pooled ORs, we deleted a single study involved in the meta-analysis each time, but the corresponding pooled ORs were not altered significantly (data not shown). Although the genotype distributions in seven studies did not follow HWE, the corresponding pooled ORs were not materially altered by the included studies, suggesting that the results obtained from the meta-analysis were stable. Begg’s funnel plot and Egger’s test were performed to assess the publication bias of the literature. As shown in Figure 2, the shape of the funnel plots seemed asymmetrical in the heterozygote comparison, but not in the homozygote comparison, suggesting the presence of publication bias. Then, the Egger’s test was adopted to provide statistical evidence of funnel plot asymmetry. As expected, the results showed obvious evidence of publication bias for AG/GG (t=2.08; P=0.047), but not for AA/GG (t=0.42; P=0.682). To adjust for this bias, a trim-and-fill method developed by Duval and Tweedie50 was implemented. Meta-analysis with or without the trim-and-fill method did not draw different conclusions, indicating that our results were statistically robust.
Figure 2.
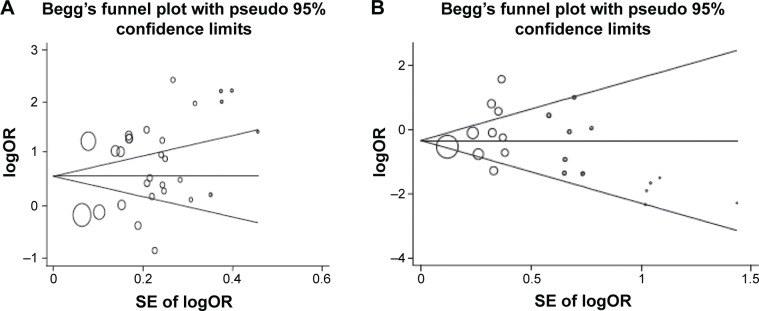
Begg’s funnel plot of the publication bias test.
Notes: (A) GA/GG comparison; (B) GA/GG comparison. Each point represents a separate study for the indicated association. Horizontal line, effect size.
Abbreviations: logOR, natural logarithm of the odds ratio; SE, standard error.
Significances in heterogeneity
Although a number of studies focused on determination of the ALDH2 rs671 polymorphism contributing to EC, the published data still remained unclear. By evaluating the correlation between the ALDH2 rs671 polymorphism and the susceptibility to EC, the present study identified a high relationship in heterozygote comparison (AG/GG: PH<0.001; I2=95.1%), as compared to the homozygote comparison (AA/GG: PH<0.001; I2=74.8%). After being stratified by drinking status, the significant study heterogeneity in the AG genotype persisted among both heavy drinkers (PH<0.001; I2=87.4%) and light drinkers (PH<0.001; I2=83.0%). In the AA genotype, it was kept among the never-/rare drinkers (PH=0.024; I2=54.7%). The overall test of heterogeneity from the given pooled stratified data verified a remarkable significant effect of heterogeneity on EC risk (AG/GG: PH<0.001, I2=87.4%; and AA/GG: PH=0.043, I2=42.3%).
Enhancement of the effect of alcohol consumption on EC risk
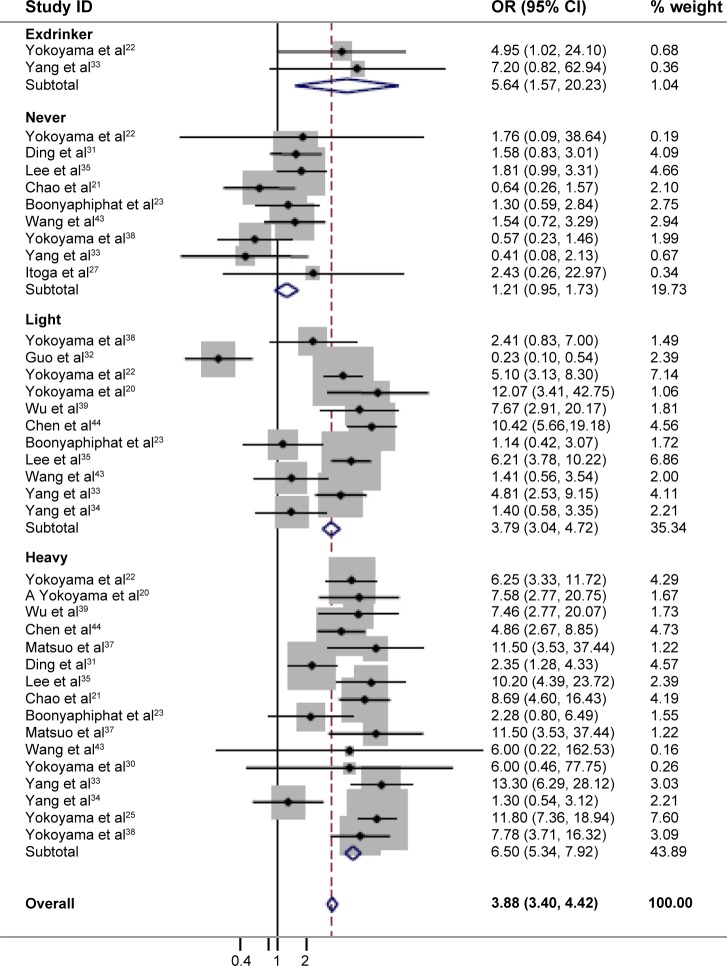
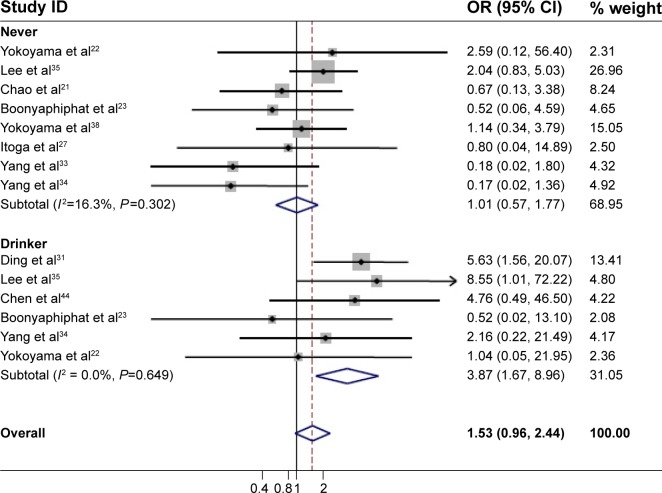
Since alcohol consumption appeared to be an important factor for the development of EC, we were particularly interested in the detection of a combined effect of the genetic factor and alcohol factor. As shown in Table 2, a significant protective effect was found in the homozygote comparison (AA/GG: OR=0.69, 95% CI=0.48–0.98, PH<0.001, I2=74.8%). In contrast, the heterozygote comparison demonstrated a remarkably increased risk (AG/GG: OR=2.34, 95% CI=1.75–3.13, PH<0.001, I2=95.1%). In particular, further stratifying the analyses yielded an increased EC risk in AG/GG with alcohol consumption (Table 3) (non-/rare drinkers: OR=1.21, 95% CI=0.95–1.73, PH=0.330, I2=12.8%; light drinkers: OR=3.79, 95% CI=3.04–4.72, PH<0.001, I2=87.4%; and heavy drinkers: OR=6.50; 95% CI=5.34–7.92, PH<0.001, I2=64.4%, respectively). The meta-regression analysis showed that the OR values for the AG genotype significantly went up by increasing the amount of alcohol consumed (P<0.001; Figure 3). In contrast, although a significant protective effect was found in the homozygote comparison in total, a remarkably increased risk was found in alcohol consumption subgroups (OR=3.87, 95% CI=1.67–8.96, PH=0.649, I2=0.00%; Figure 4). These results suggested that the interaction between genotype and alcohol consumption led to a synergic effect on EC risk.
Table 2.
Stratified analyses of the ALDH2 rs671 polymorphism on esophageal cancer
| Variables | Na | Cases/controls | AA versus GG
|
GA versus GG
|
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Pb | I2 (%) | OR (95% CI) | Pb | I2 (%) | |||
| Total | 31 | 8,510/16,197 | 0.69 (0.48–0.98)c | <0.001 | 74.8 | 2.34 (1.75–3.13)c | <0.001 | 95.1 |
| Countries | ||||||||
| People’s Republic of China | 15 | 5,505/7,880 | 0.86 (0.57–1.30)c | <0.001 | 78.9 | 1.39 (1.03–1.87)c | <0.001 | 92.2 |
| Japan | 14 | 2,566/7,773 | 0.32 (0.21–0.49) | 0.485 | 0 | 4.79 (3.80–6.05)c | <0.001 | 71.2 |
| Source of control | ||||||||
| HB | 20 | 7,074/10,419 | 0.48 (0.37–0.62) | 0.143 | 28.5 | 3.14 (2.46–4.00)c | <0.001 | 87 |
| PB | 11 | 1,436/5,782 | 1.08 (0.58–2.04)c | <0.001 | 88.9 | 1.14 (0.83–1.55)c | <0.001 | 87.5 |
| Sex | ||||||||
| Female | 4 | 842/2,853 | 0.76 (0.33–1.76) | 0.111 | 60.6 | 1.39 (1.08–3.08) | 0.362 | 0 |
| Male | 7 | 1,376/4,732 | 1.36 (1.15–1.61)c | <0.001 | 80.7 | 4.39 (1.24–6.55)c | <0.001 | 97.5 |
Notes:
N of comparisons.
P-value for the Q test for heterogeneity.
The random-effects model was used when the P-value for the heterogeneity test was <0.05; otherwise, the fixed-effects model was used.
Abbreviations: ALDH2, aldehyde dehydrogenase 2; N, number; OR, odds ratio; CI, confidence interval; PB, population base; HB, hospital base.
Table 3.
Risk of EC associated with ALDH2 AG versus GG according to country and sex, stratified by alcohol-drinking status
| Variables | Non-/rare drinkers
|
Light drinkers
|
Heavy drinkers
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | ||||||
| Total | 1.21 (0.95–1.73) | 0.330 | 12.8 | 3.79 (3.05–4.72)b | <0.001 | 87.4 | 6.50 (5.34–7.92)b | <0.001 | 64.4 | |||||
| Countries | ||||||||||||||
| People’s Republic of China | 1.62 (1.20–2.18) | 0.123 | 42.4 | 3.24 (2.46–4.26)b | 0 | 93.7 | 5.71 (3.39–9.60)b | 0.004 | 58.3 | |||||
| Japan | 0.65 (0.23–1.54) | 0.562 | 0 | 5.06 (3.35–7.65) | 0.159 | 45.6 | 7.81 (5.03–12.13) | 0.853 | 0 | |||||
| Sex | ||||||||||||||
| Female | 1.03 (0.57–1.84) | 0.109 | 61.1 | 1.75 (1.87–3.53) | 0.456 | 0 | 3.02 (1.79–32.41) | 0.673 | 0 | |||||
| Male | 2.08 (1.35–3.26) | 0.461 | 0 | 4.58 (2.71–5.67)b | 0 | 93.3 | 8.64 (3.39–13.45) | 0.114 | 46.3 | |||||
Notes: P-value of the Q test for heterogeneity.
The random-effects model was used when the P-value for the heterogeneity test was <0.05; otherwise, the fixed-effects model was used. We reclassified alcohol-drinking status according to data from the different studies by approximately measuring alcohol intake levels as never-drinkers = no, never, and rare; light drinkers =1–350 g/week of alcohol; and heavy drinkers ≥350 g/week alcohol.
Abbreviations: EC, esophageal cancer; ALDH2, aldehyde dehydrogenase 2; OR, odds ratio; CI, confidence interval.
Figure 3.
Association of the ALDH2 AG genotype and the risk of EC.
Notes: We reclassified alcohol-drinking status according to data from the different studies by approximately measuring alcohol intake levels as never-drinkers = no, never, and rare; exdrinkers = stopped drinking alcohol for more than 1 year; light drinkers =1–350 g/week of alcohol; heavy drinkers ≥350 g/week of alcohol.
Abbreviations: ID, identifier; OR, odds ratio; CI, confidence interval; ALDH2, aldehyde dehydrogenase 2; EC, esophageal cancer.
Figure 4.
Association of the ALDH2 AA genotype with the risk of EC.
Notes: Never-drinkers: no, never, and rare. Drinkers: all those who drink alcohol.
Abbreviations: ID, identifier; OR, odds ratio; CI, confidence interval; ALDH2, aldehyde dehydrogenase 2; EC, esophageal cancer.
The effects of other factors on EC risk
In addition, the analysis stratified by country showed that a decreased risk in the AA genotype was observed in the Japanese population (Table 2) (OR=0.32, 95% CI=0.21–0.49, PH=0.485, I2=0.00%), but not in the Chinese population (OR=0.86, 95% CI=0.57–1.30, PH<0.001, I2=78.9%). Interestingly, in the AG genotype, a high risk of EC was observed among light and heavy drinkers in both countries (Table 3) (light drinkers: OR=3.24, 95% CI=2.46–4.26, PH=0, I2=93.7%; heavy drinkers: OR=5.71, 95% CI=3.39–9.60, PH=0.035, I2=58.3% in the People’s Republic of China; and light drinkers: OR=5.06, 95% CI=3.35–7.65, PH=0.159, I2=45.6%; heavy drinkers: OR=7.81, 95% CI=5.03–12.13, PH=0.853, I2=0.00% in Japan, respectively). However, an increased risk was also found in Chinese non-/rare drinkers (OR=1.62, 95% CI=1.20–2.18, PH=0.123, I2=42.4%), but not in Japanese non-/rare drinkers (OR=0.65, 95% CI=0.23–1.54, PH=0.562, I2=0.00%). The results indicated that the interaction of genotype and alcohol drinking is modulated by the regional factor.
Furthermore, the subgroup analysis by sex showed that significant increased risks were found in the two genetic types among males (Table 2) (AA/GG: OR=1.36, 95% CI=1.15–1.61, PH<0.001, I2=80.7%; and AG/GG: OR=4.39, 95% CI=1.24–6.55, PH<0.001, I2=97.5%). In particular, men with the AG genotype were highly susceptible to EC dependent on alcohol-drinking status than were those with the GG genotype (non-/rare drinkers: OR=2.08, 95% CI=1.35–3.26, PH=0.46, I2=0.00%; light drinkers: OR=4.58, 95% CI=2.71–5.67, PH=0, I2=93.3%; and heavy drinkers: OR=8.64, 95% CI=3.39–13.45, PH=0.114, I2=46.3%, respectively). However, the increased risk was only found in AG females (OR=1.39, 95% CI=1.08–3.08, PH=0.362, I2=0.00%), and the risk was further increased by alcohol-drinking status (non-/rare drinkers: OR=1.03, 95% CI=0.57–1.84, PH=0.109, I2=61.1%; light drinkers: OR=1.75, 95% CI=1.87–3.53, PH=0.456, I2=0.00%; and heavy drinkers: OR=3.02, 95% CI=1.79–32.41, PH=0.673, I2=0.00%, respectively), although the risk was lower than in males (Table 3). Overall, sex also affected the interaction of genotype and alcohol drinking.
Moreover, in consideration of the control source, investigations with hospital-based controls showed an elevated risk in the heterozygote comparison (AG/GG: OR=3.14, 95% CI=2.46–4.00, PH<0.001, I2=87.0%) (Table 2). However, a protective effort was found in the homozygote comparison (AA/GG: OR=0.48, 95% CI=0.37–0.62, PH=0.143, I2=28.5%). No significant associations were found in the studies with population-based controls in both genetic comparisons (AG/GG: OR=1.14, 95% CI=0.83–1.55, PH<0.001, I2=87.5%; and AA/GG: OR=1.08, 95% CI=0.58–2.04, PH<0.001, I2=88.9%).
Discussion
Acetaldehyde, an active intermediate in ethanol metabolism, is able to adduct DNA or protein, and ALDH2 is thought to play a key role in clearing acetaldehyde generated from alcohol consumption.51,52 It has been reported that the mutant ALDH2A allele encodes a catalytically inactive subunit and causes a high blood level of acetaldehyde, which may contribute to susceptibility to carcinogenesis.53 In particular, ALDH2 AG and AA SNPs led to increases in blood acetaldehyde concentrations that were six- and 19-fold higher, respectively, than in the GG wild type after drinking the same amount of alcohol.54 The ALDH2 mutation is also associated with increased exposure of the upper digestive tract mucosa to salivary acetaldehyde from drinking alcohol.55 After a moderate dose of oral alcohol intake, the salivary acetaldehyde levels in Asians with the inactive ALDH2 AG genotype was two to three times higher than in those with the active ALDH2 GG genotype, which resulted in higher levels of acetaldehyde-related DNA adducts in their lymphocytes.56
Although numerous epidemiological studies demonstrated the effects of the ALDH2 rs671 polymorphism on the risk of EC, the results were conflicting and inconclusive based on different combinative sets of genetic and environmental factors. Li et al and Ding et al reported that the rs671 AA genotype is associated with an increased risk of EC.28,31 Additionally, several studies showed that rs671 AG heterogeneity contributes to the increased risk of EC.34,39 However, Gao et al and Chen et al predicted that the rs671 polymorphism is not associated with EC risk, and it may even reduce the risk.32,44 Furthermore, Yokoyama et al and Wang et al indicated that the rs671 polymorphism significantly increases the risk of EC in Chinese females, but not in Japanese females.30,43 To clarify relationship of the rs671 G>A polymorphism and the development of EC, the present study organized the published data from 31 case-control studies with 8,510 cases and 16,197 controls and reanalyzed the data to ascertain the correlation between the polymorphism and the increased EC risk via a meta-analysis. This comprehensive study investigated whether the polymorphism plays a causal role in the development of EC by combining many other environmental contributive factors, such as drinking habits, sex, countries, and the source of controls.
The results obtained from the present meta-analysis verified that the ALDH2 AG genotype increased the risk of EC by approximately 35% compared to the GG wild-type, indicating that the high level of acetaldehyde may be a contributor for the development of EC. The EC risk in the AG genotype was significantly increased by alcohol consumption (Figure 2), but there was no increase in the EC risk without alcohol drinking, suggesting a combinative effect from the interaction of genetic and environmental factors. The AG genotype did not increase the EC risk unless alcohol was consumed, which was consistent with previous results from the study of AG genotype on the risks of head and neck cancer by Boccia et al.11 Interestingly, the meta-analysis indicated that the ALDH2 AA genotype highly reduced the risk of EC by approximately 30% compared to the GG wild-type. Individuals bearing the ALDH2 AA genotype appeared to develop a severe reaction after the intake of small amounts of alcohol; therefore, this genotype protected against EC by leading to the avoidance of alcohol consumption. Although the proportion of drinkers in the AA individuals was very small, the AA genotype still highly increased the EC risk by alcohol consumption (OR=3.87, 95% CI=1.67–8.96), as compared to the GG genotype.
The subgroup analysis in regard to country demonstrated that the ALDH2 AG genotype was highly correlated to the risk of EC in Chinese and Japanese individuals with light and heavy drinking; however, the increased EC risk was only found in Chinese non-/rare drinkers, but not in Japanese individuals from the same group. The potential reason for the regional difference may be due to the amount of alcohol drinking. Over the past 40 years, there has been a nine-fold increase in the per capita alcohol consumption in the People’s Republic of China, but only a two-fold increase in Japan. Other factors, such as selection bias, different matching criteria, differences in the environmental and lifestyle context (including dietary habits), and tobacco smoking, have also had insufficient statistical power to generate slight differences in the development of EC.
With respect to sex, our results indicated that the drinking women with the ALDH2 AG genotype shared a relatively lower risk for the development of EC than did the drinking men. The AG genotype is associated with the development of EC in non-/rare drinking males, but not in females. Two case-control studies that focused on the cancer risk in women suggested that the risk of EC is significantly related to the interaction of genotype and alcohol-drinking status, especially in heavy female drinkers with the ALDH2 AG genotype.30,43 The difference in the risk of developing EC between men and women is considered to be due to better health-related life habits in women than in men – for instance, a higher intake of fruits and vegetables and a lower frequency of smoking.43 In addition, hormonal factors may also play an important role in cancer susceptibility. Recent studies from England and Switzerland demonstrated that hormone replacement therapy and long-term breastfeeding reduces the risk of EC.57,58
We verified the correlation of the ALDH2 rs671 polymorphism and the risk of EC using both hospital-based controls and population-based controls because some biases may exist in hospital-based studies. For example, the hospital-based controls may represent samples of an ill-defined reference population instead of the general population, particularly when the genotypes investigated were associated with the disease under hospital-based controls. Thus, representative cancer-free control subjects are important to reduce biases in such genotype-associated studies.
Some limitations of the meta-analysis may have affected the objectivity of the study and should be considered when interpreting the results. Firstly, we reclassified alcohol-drinking status according to data from the different studies by approximately measuring alcohol intake levels as non-/rare drinking, light drinking, and heavy drinking. Due to this, we did not have access to individual-level data; this classification may cause heterogeneity in our meta-analysis. Secondly, although smoking is a critical factor for the risk of developing EC, and given that drinking likely enhances the development of EC risk caused by smoking, the influence of smoking has been limited because related information was missing in most of the studies. Thirdly, the overall outcomes were based on unadjusted estimates; thus, a more precise evaluation should be conducted if more detailed individual data are available. In spite of certain limitations, the present meta-analysis provided significant advantages. First, substantial numbers of cases and controls were pooled from different studies, which significantly increased the statistical power of the analysis and prompted our meta-analysis to become more comprehensive and persuasive. Second, the quality of the case-control studies included in current meta-analysis was satisfactory and met our inclusion criteria. Third, we conclusively estimated the association between the ALDH2 rs671 G>A polymorphism and EC risk.
Conclusion
In conclusion, our meta-analysis suggested that the ALDH2 AA genotype reduced EC risk, whereas ALDH2 AG increased the risk of EC, and the effects of the ALDH2 AG genotype were associated with the level of alcohol consumption. The insight from this study predicts ALDH2 AG as a potential genetic marker in the etiology of EC. We suggest that more clinical studies including larger samples stratified by a genetic–environmental interaction need to be performed to fully clarify the roles of the ALDH2 polymorphisms in the etiology of EC.
Acknowledgments
The study was partly supported by grants from the National Natural Science Foundation of China (grant number: 81272469), the National 973 Basic Research Program of China (grant number: 2013CB911300), and the clinical special project for the Natural Science Foundation of Jiangsu Province (grant number: BL2012016). The grant comes from the Nanjing 12th Five-Year Key Scientific Project of Medicine to Dr Jinfei Chen, and the Natural Science Foundation of China (grant number: 81372199) to Dr Yong Xu.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004;4(11):850–860. doi: 10.1038/nrc1476. [DOI] [PubMed] [Google Scholar]
- 3.Baan R, Straif K, Grosse Y, et al. WHO International Agency for Research on Cancer Monograph Working Group Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8(4):292–293. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson CJ. The role of acetaldehyde in the actions of alcohol (update 2000) Alcohol Clin Exp Res. 2001;25(5 Suppl ISBRA):15S–32S. doi: 10.1097/00000374-200105051-00005. [DOI] [PubMed] [Google Scholar]
- 5.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. 2010;96:3–1383. [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan P, Boffetta P. Mechanistic considerations in the molecular epidemiology of head and neck cancer. IARC Sci Publ. 2004;(157):393–414. [PubMed] [Google Scholar]
- 7.Blot WJ. Alcohol and cancer. Cancer Res. 1992;52(7 Suppl):2119s–2123s. [PubMed] [Google Scholar]
- 8.Hsu LC, Bendel RE, Yoshida A. Direct detection of usual and atypical alleles on the human aldehyde dehydrogenase-2 (ALDH2) locus. Am J Hum Genet. 1987;41(6):996–1001. [PMC free article] [PubMed] [Google Scholar]
- 9.Luo HR, Israel Y, Tu GC, Eriksson CJ, Zhang YP. Genetic polymorphism of aldehyde dehydrogenase 2 (ALDH2) in a Chinese population: gender, age, culture, and genotypes of ALDH2. Biochem Genet. 2005;43(5–6):223–227. doi: 10.1007/s10528-005-5213-8. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Borinskaya S, Yoshimura K, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet. 2009;73(Pt 3):335–345. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boccia S, Hashibe M, Gallì P, et al. Aldehyde dehydrogenase 2 and head and neck cancer: a meta-analysis implementing a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev. 2009;18(1):248–254. doi: 10.1158/1055-9965.EPI-08-0462. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo K, Oze I, Hosono S, et al. The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of stomach cancer. Carcinogenesis. 2013;34(7):1510–1515. doi: 10.1093/carcin/bgt080. [DOI] [PubMed] [Google Scholar]
- 13.Landi S, Gemignani F, Moreno V, et al. Bellvitge Colorectal Cancer Study Group A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet Genomics. 2005;15(8):535–546. doi: 10.1097/01.fpc.0000165904.48994.3d. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, He Y, Xu J, et al. Genetic variants at 4q21, 4q23 and 12q24 are associated with esophageal squamous cell carcinoma risk in a Chinese population. Hum Genet. 2013;132(6):649–656. doi: 10.1007/s00439-013-1276-5. [DOI] [PubMed] [Google Scholar]
- 15.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37(3–4):256–266. [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoyama A, Muramatsu T, Ohmori T, Higuchi S, Hayashida M, Ishii H. Esophageal cancer and aldehyde dehydrogenase-2 genotypes in Japanese males. Cancer Epidemiol Biomarkers Prev. 1996;5(2):99–102. [PubMed] [Google Scholar]
- 21.Chao YC, Wang LS, Hsieh TY, Chu CW, Chang FY, Chu HC. Chinese alcoholic patients with esophageal cancer are genetically different from alcoholics with acute pancreatitis and liver cirrhosis. Am J Gastroenterol. 2000;95(10):2958–2964. doi: 10.1111/j.1572-0241.2000.02328.x. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama A, Kato H, Yokoyama T, et al. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and glutathione S-transferase M1 and drinking, smoking, and diet in Japanese men with esophageal squamous cell carcinoma. Carcinogenesis. 2002;23(11):1851–1859. doi: 10.1093/carcin/23.11.1851. [DOI] [PubMed] [Google Scholar]
- 23.Boonyaphiphat P, Thongsuksai P, Sriplung H, Puttawibul P. Lifestyle habits and genetic susceptibility and the risk of esophageal cancer in the Thai population. Cancer Lett. 2002;186(2):193–199. doi: 10.1016/s0304-3835(02)00354-3. [DOI] [PubMed] [Google Scholar]
- 24.Li DP, Dandara C, Walther G, Parker MI. Genetic polymorphisms of alcohol metabolising enzymes: their role in susceptibility to oesophageal cancer. Clin Chem Lab Med. 2008;46(3):323–328. doi: 10.1515/CCLM.2008.073. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama A, Muramatsu T, Omori T, et al. Alcohol and aldehyde dehydrogenase gene polymorphisms influence susceptibility to esophageal cancer in Japanese alcoholics. Alcohol Clin Exp Res. 1999;23(11):1705–1710. [PubMed] [Google Scholar]
- 26.Ding J, Li S, Wu J, et al. Alcohol dehydrogenase-2 and aldehyde dehydrogenase-2 genotypes, alcohol drinking and the risk of primary hepatocellular carcinoma in a Chinese population. Asian Pac J Cancer Prev. 2008;9(1):31–35. [PubMed] [Google Scholar]
- 27.Itoga S, Nanmoku T, Uchimoto T, et al. Comparative analyses of four different methods of genotyping ALDH2. Alcohol Clin Exp Res. 2004;28(8 Suppl Proceedings):117S–122S. doi: 10.1097/01.alc.0000133585.65144.61. [DOI] [PubMed] [Google Scholar]
- 28.Li QD, Li H, Wang MS, et al. Multi-susceptibility genes associated with the risk of the development stages of esophageal squamous cell cancer in Feicheng County. BMC Gastroenterol. 2011;11:74. doi: 10.1186/1471-230X-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokoyama A, Omori T, Tanaka Y, et al. p53 Protein accumulation, cancer multiplicity, and aldehyde dehydrogenase-2 genotype in Japanese alcoholic men with early esophageal squamous cell carcinoma. Cancer Lett. 2007;247(2):243–252. doi: 10.1016/j.canlet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama A, Kato H, Yokoyama T, et al. Esophageal squamous cell carcinoma and aldehyde dehydrogenase-2 genotypes in Japanese females. Alcohol Clin Exp Res. 2006;30(3):491–500. doi: 10.1111/j.1530-0277.2006.00053.x. [DOI] [PubMed] [Google Scholar]
- 31.Ding JH, Li SP, Cao HX, et al. Polymorphisms of alcohol dehydrogenase-2 and aldehyde dehydrogenase-2 and esophageal cancer risk in Southeast Chinese males. World J Gastroenterol. 2009;15(19):2395–2400. doi: 10.3748/wjg.15.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo YM, Wang Q, Liu YZ, Chen HM, Qi Z, Guo QH. Genetic polymorphisms in cytochrome P4502E1, alcohol and aldehyde dehydrogenases and the risk of esophageal squamous cell carcinoma in Gansu Chinese males. World J Gastroenterol. 2008;14(9):1444–1449. doi: 10.3748/wjg.14.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CX, Matsuo K, Ito H, et al. Esophageal cancer risk by ALDH2 and ADH2 polymorphisms and alcohol consumption: exploration of gene-environment and gene-gene interactions. Asian Pac J Cancer Prev. 2005;6(3):256–262. [PubMed] [Google Scholar]
- 34.Yang SJ, Wang HY, Li XQ, et al. Genetic polymorphisms of ADH2 and ALDH2 association with esophageal cancer risk in southwest China. World J Gastroenterol. 2007;13(43):5760–5764. doi: 10.3748/wjg.v13.i43.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CH, Lee JM, Wu DC, et al. Carcinogenetic impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of the esophagus with regard to the consumption of alcohol, tobacco and betel quid. Int J Cancer. 2008;122(6):1347–1356. doi: 10.1002/ijc.23264. [DOI] [PubMed] [Google Scholar]
- 36.Cai L, You NC, Lu H, et al. Dietary selenium intake, aldehyde dehydrogenase-2 and X-ray repair cross-complementing 1 genetic polymorphisms, and the risk of esophageal squamous cell carcinoma. Cancer. 2006;106(11):2345–2354. doi: 10.1002/cncr.21881. [DOI] [PubMed] [Google Scholar]
- 37.Matsuo K, Hamajima N, Shinoda M, et al. Gene-environment interaction between an aldehyde dehydrogenase-2 (ALDH2) polymorphism and alcohol consumption for the risk of esophageal cancer. Carcinogenesis. 2001;22(6):913–916. doi: 10.1093/carcin/22.6.913. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama A, Mizukami T, Omori T, et al. Melanosis and squamous cell neoplasms of the upper aerodigestive tract in Japanese alcoholic men. Cancer Sci. 2006;97(9):905–911. doi: 10.1111/j.1349-7006.2006.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu CF, Wu DC, Hsu HK, et al. Relationship between genetic polymorphisms of alcohol and aldehyde dehydrogenases and esophageal squamous cell carcinoma risk in males. World J Gastroenterol. 2005;11(33):5103–5108. doi: 10.3748/wjg.v11.i33.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu H, Gong D, Ding G, et al. A variant allele of ADH1B and ALDH2, is associated with the risk of esophageal cancer. Exp Ther Med. 2012;4(1):135–140. doi: 10.3892/etm.2012.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M, Chang SC, Kampman E, et al. Single nucleotide polymorphisms of ADH1B, ADH1C and ALDH2 genes and esophageal cancer: a population-based case-control study in China. Int J Cancer. 2013;132(8):1868–1877. doi: 10.1002/ijc.27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui R, Kamatani Y, Takahashi A, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137(5):1768–1775. doi: 10.1053/j.gastro.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Ji R, Wei X, et al. Esophageal squamous cell carcinoma and ALDH2 and ADH1B polymorphisms in Chinese females. Asian Pac J Cancer Prev. 2011;12(8):2065–2068. [PubMed] [Google Scholar]
- 44.Chen YJ, Chen C, Wu DC, et al. Interactive effects of lifetime alcohol consumption and alcohol and aldehyde dehydrogenase polymorphisms on esophageal cancer risks. Int J Cancer. 2006;119(12):2827–2831. doi: 10.1002/ijc.22199. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama A, Ohmori T, Muramatsu T, et al. Short-term follow-up after endoscopic mucosectomy of early esophageal cancer and aldehyde dehydrogenase-2 genotype in Japanese alcoholics. Cancer Epidemiol Biomarkers Prev. 1998;7(6):473–476. [PubMed] [Google Scholar]
- 46.Oze I, Matsuo K, Hosono S, et al. Comparison between self-reported facial flushing after alcohol consumption and ALDH2 Glu504Lys polymorphism for risk of upper aerodigestive tract cancer in a Japanese population. Cancer Sci. 2010;101(8):1875–1880. doi: 10.1111/j.1349-7006.2010.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokoyama A, Omori T, Yokoyama T, et al. Risk of squamous cell carcinoma of the upper aerodigestive tract in cancer-free alcoholic Japanese men: an endoscopic follow-up study. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2209–2215. doi: 10.1158/1055-9965.EPI-06-0435. [DOI] [PubMed] [Google Scholar]
- 48.Yokoyama A, Yokoyama T, Muramatsu T, et al. Macrocytosis, a new predictor for esophageal squamous cell carcinoma in Japanese alcoholic men. Carcinogenesis. 2003;24(11):1773–1778. doi: 10.1093/carcin/bgg142. [DOI] [PubMed] [Google Scholar]
- 49.Hori H, Kawano T, Endo M, Yuasa Y. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and human esophageal squamous cell carcinoma susceptibility. J Clin Gastroenterol. 1997;25(4):568–575. doi: 10.1097/00004836-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 51.Harada S, Zhang S. New strategy for detection of ALDH2 mutant. Alcohol Alcohol Suppl. 1993;1A:11–13. doi: 10.1093/alcalc/28.supplement_1a.11. [DOI] [PubMed] [Google Scholar]
- 52.Fang JL, Vaca CE. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis. 1997;18(4):627–632. doi: 10.1093/carcin/18.4.627. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida A, Huang IY, Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc Natl Acad Sci U S A. 1984;81(1):258–261. doi: 10.1073/pnas.81.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizoi Y, Yamamoto K, Ueno Y, Fukunaga T, Harada S. Involvement of genetic polymorphism of alcohol and aldehyde dehydrogenases in individual variation of alcohol metabolism. Alcohol and Alcoholism. 1994;29(6):707–710. [PubMed] [Google Scholar]
- 55.Yokoyama A, Tsutsumi E, Imazeki H, et al. Salivary acetaldehyde concentration according to alcoholic beverage consumed and aldehyde dehydrogenase-2 genotype. Alcohol Clin Exp Res. 2008;32(9):1607–1614. doi: 10.1111/j.1530-0277.2008.00739.x. [DOI] [PubMed] [Google Scholar]
- 56.Väkeväinen S, Tillonen J, Agarwal DP, Srivastava N, Salaspuro M. High salivary acetaldehyde after a moderate dose of alcohol in ALDH2-deficient subjects: strong evidence for the local carcinogenic action of acetaldehyde. Alcohol Clin Exp Res. 2000;24(6):873–877. [PubMed] [Google Scholar]
- 57.Cheng KK, Sharp L, McKinney PA, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer. 2000;83(1):127–132. doi: 10.1054/bjoc.2000.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagergren J, Jansson C. Sex hormones and oesophageal adenocarcinoma: influence of childbearing? Br J Cancer. 2005;93(8):859–861. doi: 10.1038/sj.bjc.6602810. [DOI] [PMC free article] [PubMed] [Google Scholar]