Abstract
Background
Antioxidant donor pretreatment is one of the pharmacologic strategy proposed to prevent renal ischemia-reperfusion injuries and delayed graft function (DGF). The aim of the study was to investigate whether a donor pretreatment with N-acetylcysteine (NAC) reduces the incidence of DGF in adult human kidney transplant recipients.
Methods
In this randomized, open-label, monocenter trial, 160 deceased heart-beating donors were allowed to perform 236 renal transplantations from September 2005 to December 2010. Donors were randomized to receive, in a single-blind controlled fashion, 600 mg of intravenous NAC 1 hr before and 2 hr after cerebral angiography performed to confirm brain death. Primary endpoint was DGF defined by the need for at least one dialysis session within the first week or a serum creatinine level greater than 200 μmol/L at day 7 after kidney transplantation.
Results
The incidence of DGF was similar between donors pretreated with or without NAC (39/118; 33% vs. 30/118; 25.4%; P = 0.19). Requirement for at least one dialysis session was not different between the NAC and No NAC groups (17/118; 14.4% vs. 14/118; 11.8%, P = 0.56). The two groups had comparable serum creatinine levels, estimated glomerular filtration rates, and daily urine output at days 1, 7, 15, and 30 after kidney transplantation as well as at hospital discharge. No difference in recipient mortality nor in 1-year kidney graft survival was observed.
Conclusion
Donor pretreatment with NAC does not improve delayed graft function after kidney transplantation.
A prospective, randomized, single-blind study of donor treatment with N-acetylcysteine prior to procurement shows that antioxidant treatment does not influence early renal allograft function for organs from brain-dead donors. Alternative approaches will be necessary to prevent delayed graft function.
Delayed graft function (DGF) remains a complication of kidney transplantation that occurs in 20 to 35% of recipients.1-5 The mechanism of early renal transplant dysfunction remains incompletely understood. Nevertheless, ischemia-reperfusion plays a crucial role, leading to cell deaths, inflammatory response, immunologic activation, tissue fibrosis and impaired renal microcirculation.3-5 Reperfusion is characterized by an exacerbated oxidative stress due to a higher generation of oxygen free radicals.3,6-10 Administration of contrast-medium is practiced in some countries to confirm the diagnosis of brain death before organ procurement. Several factors contribute to consider deceased donors at higher risk for developing contrast-induced acute kidney injury (CI-AKI).11-13 The pathophysiology of this complication has many similarities with the DGF’s one.12-14
Delayed graft function is associated with an increased risk for acute graft rejection, chronic allograft failure and impaired long-term renal function after kidney transplantation.4,15-17 Consequently, many pharmacologic strategies, especially antioxidant molecules, were proposed to limit ischemia-reperfusion renal injuries without real clinical positive effect.3,18-22 Scarce clinical trials have evaluated the impact of various drugs in preventing DGF with conflicting results.23-27 A donor pretreatment with low-dose dopamine reduced the need for dialysis within the first 7 days after kidney transplantation,23 whereas DGF was not reduced with high repeated doses of epoetin25 nor with a unique dose of steroids.24
Thanks to its thiol-group, N-acetylcysteine (NAC) is able to regenerate glutathione stores and scavenges oxygen-free radicals.9,28-31 In addition to its antioxidant properties, NAC reduces ischemia-reperfusion damages by improving renal perfusion and by decreasing cell apoptosis.8,32 Controversy persists whether NAC affects creatinine levels because of modifications in muscular creatinine production.33,34 Thus, the real “protective” effect of NAC on renal function remains questioned. Several recent meta-analysis reported a protective effect of NAC for preventing the development of CI-AKI.35-37 In a randomized controlled trial, Koc et al.38 found that a prophylactic high dose of NAC reduced the occurrence of CI-AKI after coronary procedure. The KDIGO group suggests to administer NAC with an intravenous isotonic crystalloid in patients at risk to prevent CI-AKI.39 The impact of a pretreatment with NAC on kidney graft function has been evaluated in two animal models of kidney transplantation.22,40 N-acetylcysteine did not improve serum creatinine (SCr) levels nor histologic damages 24 hr after transplantation as compared with normal saline infusion.40 Lin et al.22 have shown that SCr and blood urea nitrogen (BUN) were lower at day 3 after renal transplantation. None of these studies assessed the incidence of DGF.
Considering its experimental antioxidant properties and vasodilatory effects, NAC might reduce ischemia-reperfusion injuries of kidney grafts. Our goal was therefore to investigate the effectiveness of a donor pretreatment with NAC at reducing the occurrence of DGF after kidney transplantation.
RESULTS
Baseline Characteristics
Of 251 patients evaluated for eligibility, 217 underwent randomization. Twenty-four of the 106 donors assigned to NAC treatment and 33 of the 111 donors assigned to No NAC treatment were excluded for various reasons (see Figure 1). Thus, a total of 82 and 78 donors in the NAC and No NAC groups, respectively, underwent kidney harvesting. One kidney per donor was transplanted in the study center with a frequency of 56% in the NAC group and 48.7% in the No NAC group (P = 0.350). Finally, 118 recipients received a kidney graft in each group, completed the follow-up study period and were analyzed (Figure 1).
FIGURE 1.
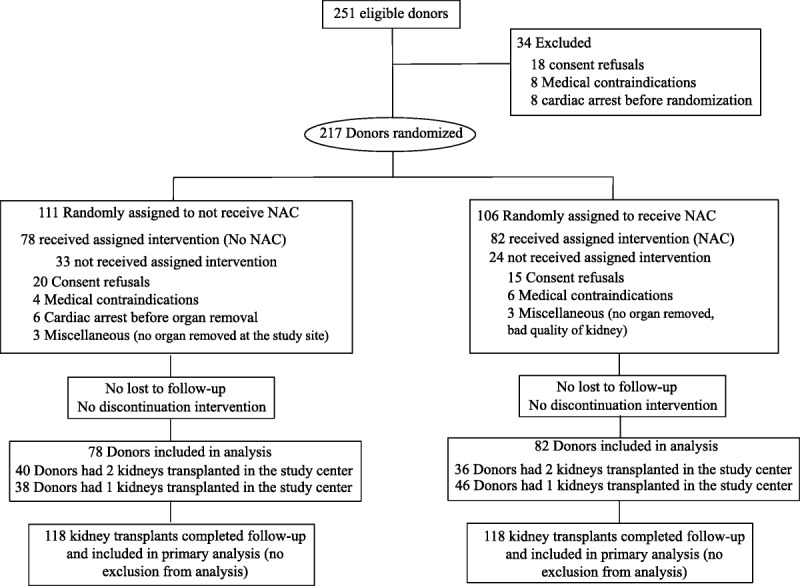
Flow diagram for study enrollment and randomization of donors and recipients.
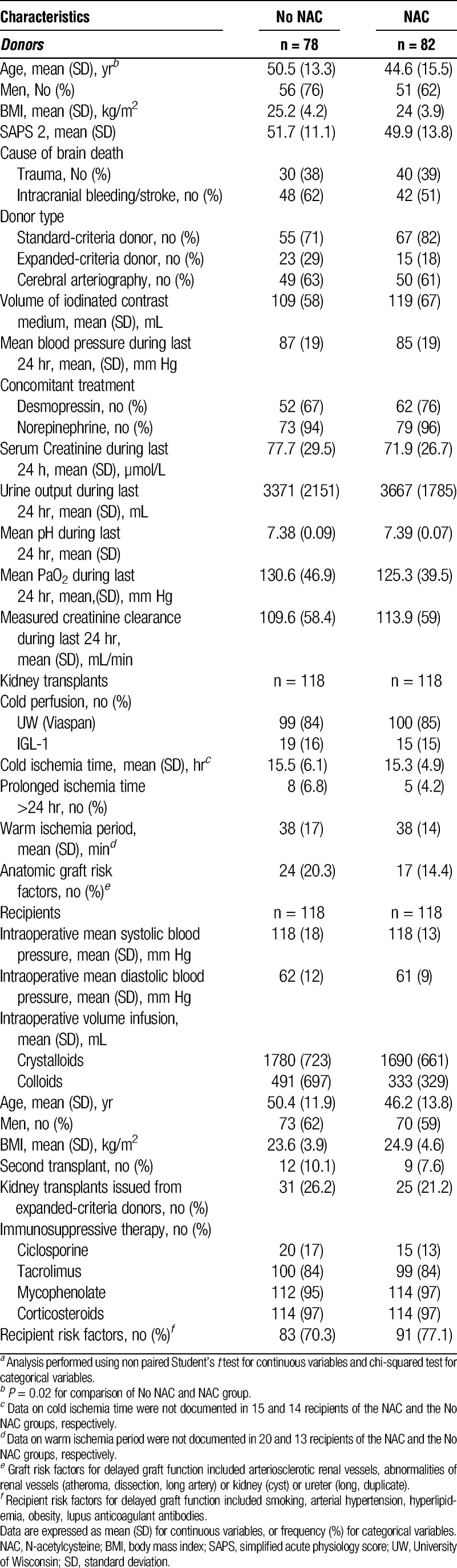
Baseline and demographic characteristics of donors were similar in the two groups, except for age. Patients were significantly younger in the NAC group (P = 0.02). The number of donors who met the current United Network for Organ Sharing definition for expanded-criteria donor (ECD) kidney was comparable in the NAC and No NAC groups (15/82 [18.3%] vs. 23/78 [29.4%]; P = 0.105). Both groups were comparable with respect to hemodynamic and oxygenation conditions and renal function (Table 1). Factors conditioning kidney function and preservation before transplantation (flushing perfusion, ischemia time) were comparable between the two groups. Demographic, clinical recipients’ characteristics, and immunosuppressive therapy were similar in both groups. Major risk factors of DGF related to the donor (number of ECD), to kidney transplants conditions (prolonged ischemia time >24 hr), and to recipients (number of second transplant) were comparable in both groups (Table 1).
TABLE 1.
Demographic and baseline characteristics of donors and recipentsa
Endpoints
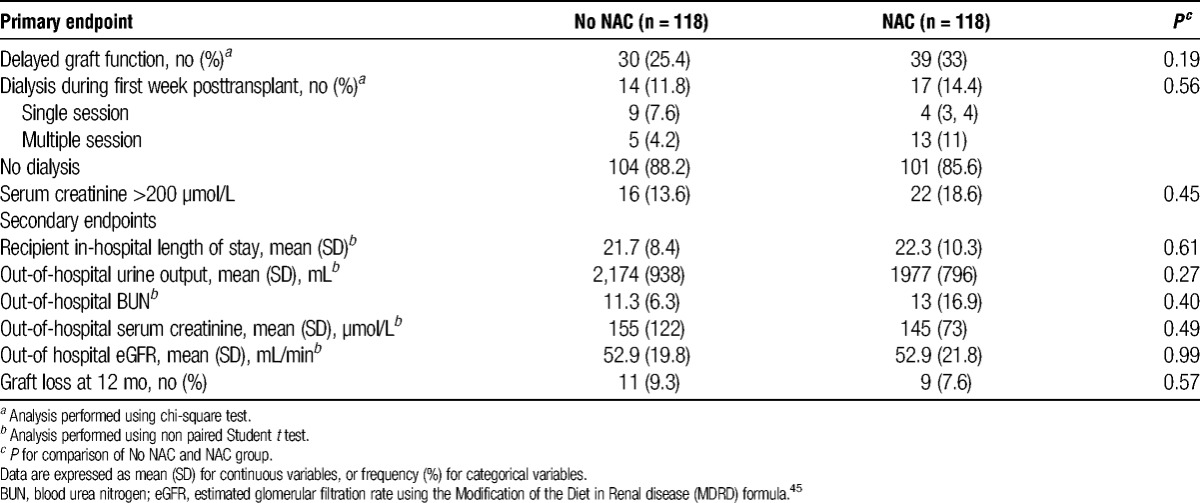
The incidence of DGF was comparable between the NAC and No NAC groups (39/118 [33%, 95% confidence interval [95% CI] 24%–42%] vs. 30/118 [25.4%, 95% CI 17%–34%]; P = 0.19) (Table 2). Dialysis during the first 7 days after transplantation was required in the same proportion of recipients of the two groups (17/118 [14.4%, 95% CI 8%–21%] vs. 14/118 [11.8%, 95% CI 6%–18%]; P = 0.56, respectively, in the NAC and No NAC groups) (Table 2). We did not observe any adverse effect related to NAC administration.
TABLE 2.
Results for primary and secondary endpoints
At 1-day posttransplantation, SCr level, estimated glomerular filtration rate (eGFR), and daily urine production did not differ between the two groups. Serum creatinine level decreased comparably in both groups (P = 0.65) and within the first week after transplantation (P < 0.0001). The post hoc analysis showed that these variables did not change from day 7 to day 30 in both groups (Figure 2A). Both groups showed a comparable increased eGFR (P = 0.82) from day 1 to day 7 (P = 0.0001) and remained stable within the first month posttransplantation (Figure 2B). Daily urine production was unchanged at days 1, 7, 14 and 30 after renal transplantation (P = 0.17) and similar in the two groups (P = 0.82) (Figure 3).
FIGURE 2.
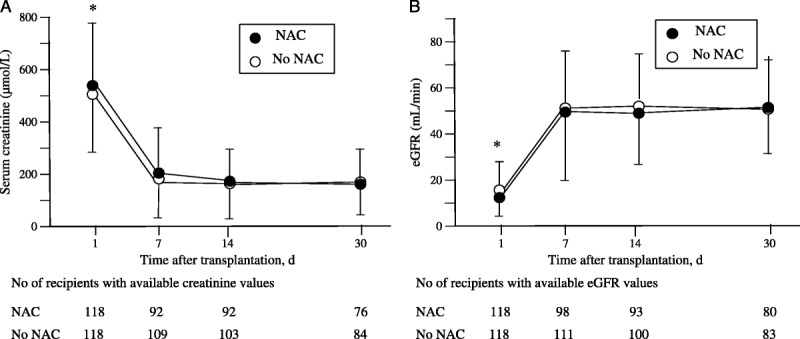
Time course of serum creatinine (A), eGFR (B) in the first month after renal transplantation assessed at days 1, 7, 14, and 30. Data markers represent the mean and the error bars, SD. Numbers of values at days 7, 14, and 30 were smaller than the number of recipients because of missing data and of patients discharged from hospital. A, The two-way analysis for repeated measurements showed that the factor time was significant (P < 0.0001), whereas both the factor group and the interaction between group and time were not significant (respectively, P = 0.653 and P = 0.472). *P < .0001 day 1 vs. days 7, 14, and 30 in both groups (post hoc analysis F Scheffe test). B, eGFR was calculated using the MDRD formula.39 The two-way analysis for repeated measurements showed that the factor time was significant (P < 0.0001), whereas both the factor group and the interaction between group and time were not significant (respectively P = 0.826 and P = 0.531). MDRD, Modification of the Diet in Renal Disease; eGRF, estimated glomerular filtration rate; SD, standard deviation.
FIGURE 3.
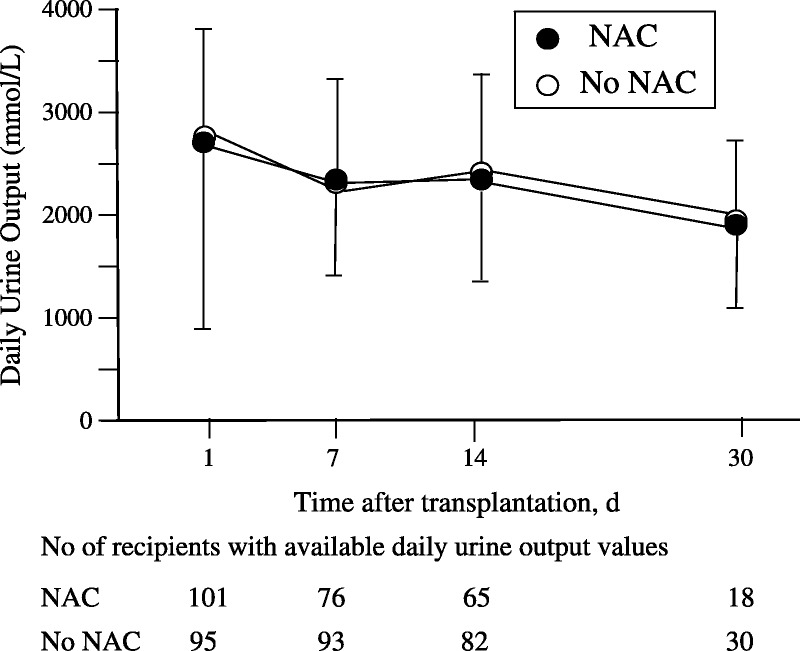
Time course of daily urine production in the first month after renal transplantation assessed at days 1, 7, 14, and 30. * P < 0.0001 day 1 vs. days 7, 14, and 30 in both groups (post hoc analysis F Scheffe test). C, The two-way analysis for repeated measurements showed that the factor time and the factor group were not significant (respectively P = 0.820 and P = 0.172).
There was no significant between-group difference in out-hospital daily urine production, BUN, SCr level, and eGFR (Table 2). The proportion of kidney graft losses at 1 year after transplantation was comparable in the NAC and No NAC groups (9 [7.6%] vs. 11 [9.3%]; P = 0.57]. The in-hospital length of stay of recipients was also comparable in the two groups. All recipients, but one, were surviving 1 year after transplantation. Kidney graft nephrectomy was performed within the first year in seven (5.9%) and eight (6.8%) patients, respectively, in the No NAC group and the NAC group.
DISCUSSION
The current study shows that a donor pretreatment with NAC failed to reduce the incidence and duration of DGF as compared with a control group. This result was associated with the absence of improvement in short-term renal graft function assessed by SCr, eGFR, and daily urine output within the first month after transplantation, and in 1 year recipients and graft function survival.
Early renal graft dysfunction has several deleterious consequences. It has been demonstrated to be an independent risk factor for short-term acute transplant rejection and long-term chronic allograft dysfunction.4,15,16 Delayed graft function is also associated with an increased morbimortality in recipients. Most of those risk factors are related to donors’ and recipients’ conditions and management, and to organs’ preservation conditions.10 Consequently, major therapeutic goals aim to optimize tissue organ quality by maintaining renal oxygenation and perfusion, by shortening the ischemia period, by improving the quality of preservation solutions, and by preventing transplant immune rejection.
Recently, new pharmacologic strategies were proposed to prevent renal ischemia-reperfusion injuries and DGF.4,41 Subsequently, vasodilating agents, anti-inflammatory drugs, antioxidant agents, and modified small interfering RNA have been reported to improve organ dysfunction after ischemia-reperfusion in various experimental models. However, scarce clinical data remain conflicting.21,23-25 High doses of erythropoietin did not reduce the incidence of DGF in high-risk patients.25,26 Steroids pretreatment of organ donors was not able to reduce the occurrence of DGF despite a significant suppression of inflammation and immune response in kidney grafts.24 Low-dopamine deceased heart-beating donor pretreatment was found to decrease the need for dialysis after kidney transplantation in a randomized controlled study.23 The administration of low-dose dopamine in living donors was also reported to improve the early renal graft function assessed by urine output during the first hours after transplantation, while Scr and BUN did not differ.27
Among antioxidant drugs, NAC is safe and inexpensive. Moreover, it is commonly administered as a mucolytic agent and to treat acetaminophen poisoning. Experimental studies have shown that NAC improves ischemia-reperfusion injuries in various organs, such as the liver42 and the bowels.43 The impact of NAC has been widely evaluated during various renal ischemia-reperfusion conditions including CI-AKI and renal transplantation.7,22,28,29,36 The improvement of kidney injuries seems to result from several mechanisms associating decreased oxidative stress, nitric oxide-inhibiting effect leading to vasodilation, and preventive decrease in cytokine production and in apoptotic cell death.8,22,30,32,36,44 To our knowledge, only two animal studies evaluated the impact of a pretreatment with NAC on renal graft function and reported conflicting results.22,40 Fuller et al40 have found that histologic damages and Scr 24 hr after transplantation were comparable in rats pretreated with NAC as compared with those pretreated with mannitol or normal saline. However, NAC pretreated rats showed a better renal metabolism. Lin et al22 have shown that pretreated dogs with two antioxidant molecules improved urine output and peak SCr posttransplantation. These effects were associated with a lower tumor necrosis factor-α, higher inducible nitric oxide synthase concentrations, and a decreased apoptosis. To date, our clinical study is the first to test whether a NAC donor’s pretreatment was able to improve renal dysfunction after transplantation. Our data failed to confirm this hypothesis, showing that this strategy did not reduce the incidence of DGF in our population of donors and recipients. Our results cannot be related to differences in major risk factors for DGF between groups which are completely comparable (same number of ECD, second kidney transplantation, and prolonged cold ischemia time). These conflicting results could be explained by methodological heterogeneities including large variability in doses, timing and route for NAC administration, cold ischemia period, and parameters for kidney graft function assessment. As reported previously, an artefactual effect of NAC on SCr level could have interfered with our results.33 However, such a confounding effect seems unlikely because we used a single small dose of NAC.34 Moreover, NAC administration to the donor cannot affect muscular creatinine production in recipients.
There are several limitations with this study. First, this is a single-blinded randomized monocenter trial. However, because of blinding of intensivists, surgeons, and nephrologists in charge of the donors and the recipients, blinding of data management and analysis, it seems unlikely that the study design should induce any bias. Moreover, we choose to evaluate DGF as a primary endpoint because of its most consensual definition and assessment. Consequently, the requirement for dialysis within 7 days after transplantation would be unlikely to be influenced by any pretreatment ever known. Finally, we did not find any differences between groups in other biologic parameters of kidney graft function (SCr, daily urine production, and eGFR) at days 1, 7, 15, and 30 after transplantation and in 1-year kidney graft survival. Second, we did not analyze systematically graft biopsies that would allow us to identify acute transplant rejection and histologic injuries. Also, we did not measure accurately the renal blood flow nor biologic surrogate markers of oxidative stress to detect any hemodynamic or antioxidant effect of NAC. Thus, we cannot exclude any infraclinical vasodilating or antioxidant effect of NAC in our population. Third, we did not focus on recipients receiving graft from expanded-criteria donors associated with prolonged ischemia time.4,45 Consequently, we cannot extrapolate our data for this subgroup of high-risk kidney grafts. Fourth, we choose to administer the most usual and empirically suggested dose that is based on the preventive action of NAC for CI-AKI.11 However, the optimal dose of NAC to prevent kidney transplant dysfunction is still unknown.46 The putative benefit of a higher dose or a longer period of NAC administration remains to be evaluated.
In conclusion, this study shows that NAC pretreatment of deceased heart-beating organ donors neither reduce the incidence of renal DGF nor improve daily urine production, SCr, and eGFR within the first month after transplantation.
MATERIALS AND METHODS
Study Design and Patients
This prospective, randomized, controlled open-label, monocenter study was conducted between September 2005 and December 2010 at the university hospital of Nice (with a final follow-up on December 2011). The investigators, the allograft recipients, and the physicians, who provided cares and renal function assessment, were blinded regarding donor’s pretreatment. The protocol was approved by the local institutional ethics committee (05-053 CPP Sud Méditerrannée V), and a signed informed consent was obtained from the relatives of the donor and from the recipient.
Patients older than 18 years with all clinical signs of brain death were considered as eligible heart-beating donors. Patients presenting a preexisting chronic renal insufficiency (defined by a 24-hr measured creatinine clearance ≤ 30 mL/min) were not eligible. The diagnosis of brain death was legally declared after cerebral arteriography or computed tomography angiography. Exclusion criteria were consent refusals by the next-of-kin, medical contraindications for organ procurement, a presumed refusal organ donation, kidney graft stemming from another center, and medical complications before or during procurement leading to stop the procedure. A signed information was obtained from the donor’s relatives during the interview aiming to ask the presumed donor’s consent. Both the intensivist in charge of the patient and the local transplant coordinator were involved in this conversation which could be held before or after angiography. Thus, donors were excluded before or after randomization (i.e., angiography), in case of the absence of organ removal related to consent refusal, medical contraindications, or cardiac arrest.
Treatment and Randomization
One hour before the angiography diagnosis, brain-dead donors were randomly assigned in a 1:1 ratio using a computerized random-number generator list to receive or not receive NAC. The randomization code was not revealed to investigators, physicians who cared for renal transplantation and graft function. N-acetylcysteine (Fluimucil; Zambon France, France) was administered as an intravenous bolus of 600 mg 1 hr before and 2 hr after cerebral or computed tomography scan angiography.
The French Biomedicine Agency was responsible for the allocation of kidneys to recipients. Renal distribution was not changed, giving priority to national emergent indications of transplantation and considering waiting time, cold ischemia time, usual compatibility criteria (human leukocyte antigen mismatch). Before being nominated on the waiting list, the transplant candidates had to fulfil and accept the usual conditions and rules of transplantation that were necessary for a definitive validation by the Biomedicine Agency. Candidates had also to sign an informed consent which stipulates clearly that they cannot know the identity of the donor nor choose their organ. They were also informed of the possibility to receive a kidney procured by a donor who was possibly participating in a clinical trial. Therefore, all recipients older than 18 years and transplanted at the study site were eligible. On the day of kidney transplantation, they were specifically informed of this study and were excluded in case of written consent refusal, but they were not allowed to refuse renal transplantation.
Endpoints and Data Collection
The primary endpoint was the incidence of DGF defined by the need for at least one dialysis session within the first week after kidney transplantation and a SCr level greater than 200 μmol/L at day 7 after transplantation.
Secondary endpoints included the evolution of renal graft function within 30 days after transplantation assessed by SCr, daily urine output, and eGFR at days 1, 7, 14 and 30 after transplantation. Estimated GFR was calculated according to the Modified Diet in Renal Disease formula.47 Other secondary endpoints were the in-hospital length of stay and mortality of recipients, the 1-year renal graft survival, and incidence of detransplantation. Investigators collected the donors and recipients baseline demographic characteristics, kidney transplant characteristics, and follow-up data on the recipients. Most significant risk factors associated with DGF were collected: 1) the number of ECDs defined by the United Network for Organ Sharing criteria4,48,49; (2) the number of prolonged cold ischemia time defined by a delay >24 hr15; (3) and the number of recipients receiving a second renal transplantation.
Procedures
The study protocol did not modify the global procedures. Management of heart-beating donors was consistent with the French guidelines aiming to maintain an appropriate organ perfusion and oxygenation (mean arterial pressure ≥65 mm Hg, PaO2 ≥80 mm Hg, central venous pressure 8–14 cmH2O, hemoglobin >7 g/dL, hourly diuresis ≥0.5 mL/kg/min). Organs were perfused in a cold preservation solution until transplantation. Management of recipients was performed as usual by nephrologists. The protocol of immunosuppressive therapy was left to the discretion of the nephrologist and included an induction therapy with a biologic agent (interleukin-2-receptor antagonist or a lymphocyte-depleting agent for kidney transplant recipients at high immunologic risk). Tacrolimus (Prograf; Astellas Pharma, France), aiming a target blood concentration between 8 and 15 ng/mL, was the first-line calcineurin-inhibitor used. Tacrolimus or cyclosporine (Sandimmun; Novartis Pharma, France) (target blood concentration from 150 to 250 ng/mL) was started at the time of transplantation. The antiproliferative medication consisted in the administration of 2 g intravenous daily mycophenolate mofetil (Cellcept, Roche, France) or 1.44 g orally daily enteric-coated mycophenolate sodium (Myfortic, Novartis Pharma). High dose of methylprednisolone (Solumedrol, Pfizer, France) (10 mg) was administered before transplantation followed by decreasing doses in the perioperative and the early posttransplant periods.
Statistical Analysis
Based on the French Biomedicine Agency data, we assumed that approximately 30% of kidney grafts would experience a DGF.2 Therefore, the inclusion of enough donors for 118 kidney graft recipients was required in each group to detect a 50% reduction in the proportion of DGF with a statistical power of 80% and a two-sided significance level of 0.05. Because of the rules of renal distribution, one or two kidneys from one donor could be available for transplantation at the study site. Thus, we preassumed that donor inclusion would stop when each group of recipients reached 118. Because a mean of 50% kidney transplantations are realized in our study center, we calculated that time to complete our study will be 5 years.
Only organ donors who were successful in kidney transplantation in our study center were analyzed as well as all recipients receiving a kidney graft from donors of our study center. Analysis was conducted using a modified intention-to-treat way, with all the donors and recipients who met the inclusion criteria and completed the study. Descriptive statistics included frequencies (percentage and the 95% CI) for qualitative variables and mean (error standard) for continuous data. Comparison between groups was performed using the chi-square test and an unpaired Student t test when appropriate (StatView 5.0, SAS Institute, Cary, NC). A two-way analysis of variance for repeated measurements was used to evaluate the interaction between time and group, followed by Scheffe F tests (intragroup and intergroup comparison) as post hoc analysis. A P value less than 0.05 was considered statistically significant.
ACKNOWLEDGMENTS
The authors thank Jean Amiel, MD, Daniel Chevallier, MD, and Jacques Jourdan, MD, Urologic Unit, University Hospital of Nice, Nice, France, for their cooperation in optimizing kidney grafts during transplantation. The authors also thank Frédéric Demont, Audrey Leroy, and Anne Pierini, Coordinator nurses, Coordination of Procurement and Transplantation Unit, University Hospital of Nice, Nice, France, for their help in collecting all data. The authors are grateful to Aminata Diop (clinical research assistant) for collecting data and to all medical staff, staff nurses, (Intensive Care unit, rsity Hospital of Nice, Nice, France) who contributed to complete the study.
Footnotes
J.C.O. and C.I. contributed to the hypothesis forming, collected the data, performed statistical analysis, and prepared and reviewed the article. H.Q., P.J., and C.S.L. contributed to the data collection. E.C. contributed to the results interpretation and article writing.
The authors declare no funding and conflicts of interest.
clinicaltrials.gov.identifier NCT 00998972
REFERENCES
- 1.OPTN/SRTR 2011 Annual Data Report. Available at: http://srtr.transplant.hrsa.gov/annual_reports/2011/flash/01_kidney/index.html#/1/zoomed.
- 2.Agence de Biomédecine Activity Report 2012. Available at: http://www.agence-biomedecine.fr/annexes/bilan2012/donnees/organes/06-rein/synthese.htm (accessed January 30, 2012).
- 3. Perico N, Cattaneo D, Sayegh MH, et al. Delayed graft function in kidney transplantation. Lancet. 2004; 364: 1814. [DOI] [PubMed] [Google Scholar]
- 4. Peeters P, Vanholder R. Therapeutic interventions favorably influencing delayed and slow graft function in kidney transplantation: mission impossible? Transplantation. 2008; 86: S31. [DOI] [PubMed] [Google Scholar]
- 5. Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011; 11: 2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997; 63: 968. [DOI] [PubMed] [Google Scholar]
- 7. Tylicki L, Rutkowski B, Hörl WH. Antioxidants: a possible role in kidney protection. Kidney Blood Press Res. 2003; 26 (5-6): 303. [DOI] [PubMed] [Google Scholar]
- 8. Conesa EL, Valero F, Nadal JC, et al. N-acetyl-l-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001; 281: R730. [DOI] [PubMed] [Google Scholar]
- 9. DiMari J, Megyesi J, Udvarhelyi N, et al. N-acetylcysteine ameliorates ischemic renal failure. Am J Physiol. 1997; 272: F292. [DOI] [PubMed] [Google Scholar]
- 10. Koning OH, Ploeg RJ, van Bockel JH, et al. Risk factors for delayed graft function in cadaveric kidney transplantation: a prospective study of renal function and graft survival after preservation with University of Wisconsin solution in multi-organ donors. European Multicenter Study Group. Transplantation. 1997; 63: 1620. [DOI] [PubMed] [Google Scholar]
- 11.KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Contrast-induced AKI. Kidney Int. 2012; 2: 69. [Google Scholar]
- 12. McCullough PA. Acute kidney injury with iodinated contrast. Crit Care Med. 2008; 36: S204. [DOI] [PubMed] [Google Scholar]
- 13. McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008; 51: 1419. [DOI] [PubMed] [Google Scholar]
- 14. Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int. 2006; 100 (Suppl): S11. [DOI] [PubMed] [Google Scholar]
- 15. Tapiawala S, Tinckam K, Cardella C, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010; 21: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yarlagadda S, Coca S, Formica RJ, et al. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009; 24: 1039. [DOI] [PubMed] [Google Scholar]
- 17. Qureshi F, Rabb H, Kasike BL. Silent acute rejection during prolonged delayed graft function reduces kidney allograft survival. Transplantation. 2002; 74: 1400. [DOI] [PubMed] [Google Scholar]
- 18. Sterling KA, Tehrani T, Rudnick MR. Clinical significance and preventive strategies for contrast-induced nephropathy. Curr Opin Nephrol Hypertens. 2008; 17: 616. [DOI] [PubMed] [Google Scholar]
- 19. Kelly AM, Dwamena B, Cronin P, et al. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008; 148: 284. [DOI] [PubMed] [Google Scholar]
- 20. Harrison EM, Sharpe E, Bellamy CO, et al. Heat-shock protein 90-binding agents protect renal cells from oxidative stress and reduce kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2008; 295: 397. [DOI] [PubMed] [Google Scholar]
- 21. Requiao-Moura LR, Ferraz E, Matos AC, et al. Comparison of long-term effect of thymoglobulin treatment in patients with high risk of delayed graft function. Transplant Proc. 2012; 44: 2429. [DOI] [PubMed] [Google Scholar]
- 22. Lin A, Sekhon C, Sekhon B, et al. Attenuation of ischemia-reperfusion injury in a canine model of autologous renal transplantation. Transplantation. 2004; 78: 654. [DOI] [PubMed] [Google Scholar]
- 23. Schnuelle P, Gottmann U, Hoeger S, et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA. 2009; 302: 1067. [DOI] [PubMed] [Google Scholar]
- 24. Kainz A, Wilflingseder J, Mitterbauer C, et al. Steroid pretreatment of organ donors to prevent postischemic renal allograft failure. A randomized controlled trial. Ann Intern Med. 2010; 153: 222. [DOI] [PubMed] [Google Scholar]
- 25. Martinez F, Kamar N, Pallet N, et al. High dose epoetin beta in the first weeks following renal transplantation and delayed graft function. Am J Transplant. 2010; 10: 1704. [DOI] [PubMed] [Google Scholar]
- 26. Sureshkumar KK, Hussain SM, To TY, et al. Effect of high dose erythropoietin on graft function after kidney transplantation: a randomized, double-blind clinical trial. Clin J Am Soc Nephrol. 2012; 7: 1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hosseinzadeh H, Golazri S, Abravesh M, et al. Effect of low dose dopamine on early graft function on living unrelated kidney donors. Urol J. 2012; 9: 389. [PubMed] [Google Scholar]
- 28. Sehirili AO, Sener G, Satiroglu H, et al. Protective effect of N-acetylcysteine on renal ischemia/reperfusion injury in the rat. J Nephrol. 2003; 16: 75. [PubMed] [Google Scholar]
- 29. Dobashi K, Singh I, Orak JK, et al. Combination therapy of N-acetylcysteine, sodium nitroprusside and phosphoramidon attenuates ischemia-reperfusion injury in rat kidneys. Mol Cell Biochem. 2002; 240: 2. [DOI] [PubMed] [Google Scholar]
- 30. Effrati S, Dishy V, Averbuck M, et al. The effect of N-acetylcysteine on renal function, nitric oxide, and oxidative stress after angiography. Kidney Int. 2003; 64; 2182. [DOI] [PubMed] [Google Scholar]
- 31. Nitescu N, Ricksten SE, Marcussen N, et al. N-acetylcysteine attenuates kidney injuries in rats subjected to renal ischaemia-reperfusion. Nephrol Dial Transplant. 2006; 21: 1240. [DOI] [PubMed] [Google Scholar]
- 32. Heyman SN, Goldfarb M, Shina A, et al. N-acetylcysteine ameliorates renal microcirculation: studies in rats. Kidney Int. 2003; 63: 634. [DOI] [PubMed] [Google Scholar]
- 33. Hoffmann U, Fischereder M, Kruger B, et al. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004; 15: 407. [DOI] [PubMed] [Google Scholar]
- 34. Moist L, Sontrop JM, Gallo K, et al. Effect of N-Acetylcysteine on serum creatinine and kidney function: results of a randomized controlled study. Am J Kidney Dis. 2010; 56: 643. [DOI] [PubMed] [Google Scholar]
- 35. Trivedi H, Daram S, Szabi A, et al. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am J Med 2009; 122: 874e9. [DOI] [PubMed] [Google Scholar]
- 36. Fishbane S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol. 2008; 3: 381. [DOI] [PubMed] [Google Scholar]
- 37. Brown JR, Block CA, Malenka DJ, et al. Sodium bicarbonate plus N-acetylcysteine prophylaxis: a meta-analysis. JACC Cardiovasc Interv. 2009; 2: 1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koc F, Ozdemir K, Kata MG, et al. N-acetylcysteine plus high-dose hydration versus high-dose hydration for the prevention of contrast-induced nephropathy: CASIS-A multicenter prospective controlled trial. Int J Cardiol. 2012; 155: 418. [DOI] [PubMed] [Google Scholar]
- 39.KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Summary of recommendation statements. Kidney Int. 2012; 2 (suppl): 8. [Google Scholar]
- 40. Fuller TF, Serkova N, Niemann CU, et al. Influence of donor pretreatment with N-acetylcysteine on ischemia-reperfusion injury in rat kidney grafts. J Urol. 2004; 171: 1296. [DOI] [PubMed] [Google Scholar]
- 41. Thompson JD, Kornbrust DJ, Foy JW, et al. Toxicological and phamacokinetic properties of chemically modified siRNAs targeting p53 following intravenous administration. Nucleic Acid Ther. 2012; 22: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sener O, Tosun O. Melatonin and NAC have beneficial effects during hepatic ischemia and reperfusion. Life Sci. 2003; 72: 2707. [DOI] [PubMed] [Google Scholar]
- 43. Montero EF, Akerahow MS. Intestinal ischemia and reperfusion injury in growing rats. Microsurgery. 2003; 23: 517. [DOI] [PubMed] [Google Scholar]
- 44. Thiele H, Hildebrand L, Schirdewahn C, et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC trial. J Am Coll Cardiol. 2010; 55: 2201. [DOI] [PubMed] [Google Scholar]
- 45. Johnston TD, Thacker LR, Jeon H, et al. Sensitivity of expanded-criteria donor kidneys to cold ischaemia time. Clin Transplant. 2004; 18 (suppl 12): 28. [DOI] [PubMed] [Google Scholar]
- 46. Van Praet JT, De Vriese AS. Prevention of contrast-induced nephropathy: a critical review. Curr Opin Nephrol Hypertens. 2007; 16: 336. [DOI] [PubMed] [Google Scholar]
- 47. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130: 461. [DOI] [PubMed] [Google Scholar]
- 48. Irish WD, Ilsley JN, Schnitzler MA, et al. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010; 10: 2279. [DOI] [PubMed] [Google Scholar]
- 49. Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003; 3 (suppl 4): 114. [DOI] [PubMed] [Google Scholar]


