ABSTRACT
Purpose
To analyze changes in myopia, astigmatism, and anisometropia after laser treatment of retinopathy of prematurity (ROP), including aggressive posterior retinopathy of prematurity (APROP), in Mainland Chinese children.
Methods
This was a retrospective study of children who had laser treatment for threshold or type 1 prethreshold ROP between January 2004 and October 2012 and age-matched control subjects with spontaneously regressed type 2 prethreshold ROP. One hundred fifteen eyes of 60 patients were included as the laser-treated group, which were further subdivided into APROP and non-APROP groups. Thirty-seven eyes of 20 patients who were diagnosed during the same period were included as the control group. Between 12 and 36 months postnatal age (PA) (mean [±SD], 22.9 [±8.1] months), cycloplegic retinoscopy was performed to measure refractive outcomes. A general linear model was used to analyze refractive changes among different groups at each PA.
Results
After adjusting for PA and the correlation between right and left eyes, the magnitude and proportion of astigmatism (p = 0.04 and p = 0.004, respectively) and myopia (p < 0.0001 and p = 0.006, respectively) were greater in the laser-treated group than in the control group. The differences in myopia were even greater in children with APROP than those with non-APROP, whereas the differences in astigmatism were not. Eyes with APROP had higher prevalence of high myopia and spherical anisometropia than the control (p = 0.002 and p = 0.02, respectively) and the non-APROP groups (p < 0.0001 and p = 0.04, respectively).
Conclusions
Children with laser treatment for ROP, including APROP, tended to have higher myopia, astigmatism, and anisometropia, which may progress to amblyopia. These findings highlight the need for regular refractive screening after laser treatment of ROP.
Key Words: retinopathy of prematurity, aggressive posterior retinopathy of prematurity, laser treatment, myopia, astigmatism, anisometropia, amblyopia
Retinopathy of prematurity (ROP) is a proliferative vascular disorder of the retina that can lead to visual impairment or complete vision loss in premature infants.1 Retinal photocoagulation using a diode laser is an effective and safe treatment that could prevent most of these unfavorable outcomes of ROP.2,3 However, significant refractive errors are a concerning and frequently associated finding in ROP patients, with or without treatment.4–6 Sahni et al.6 reported that the mean spherical equivalent (SE) was lower in a group of 81 eyes at 36 months after laser treatment compared with that in 34 spontaneously regressing eyes (−2.40 diopters [D] vs. −0.22 D, respectively). Nevertheless, data regarding the refractive status of Chinese patients with ROP are limited. It has been reported that Chinese adults and children have a higher incidence of myopia and astigmatism than those in other regions.7–12 Therefore, the refractive error of Chinese patients with ROP is of interest.
Aggressive posterior retinopathy of prematurity (APROP) is an uncommon, rapidly progressing, and severe form of ROP that was first described in 2005.13 Several studies have reported that despite prompt and aggressive treatment, the progression of APROP to retinal detachment is common.14,15 However, few studies have examined the refractive status of eyes with APROP after successful treatment and anatomical normalization. Serious refractive error may lead to additional visual loss if it is not corrected in a timely manner,16 limiting the usefulness of previous efforts. Therefore, it is important to determine the refractive outcomes for this unique type of ROP.
This study is the first to assess refractive errors, including myopia, astigmatism, and anisometropia, after laser treatment of ROP in Mainland Chinese children. These results were compared with age-matched control subjects with spontaneously regressed type 2 prethreshold ROP. We also compared the refractive errors of children with APROP with those of the control group and with those of other laser-treated types of the ROP (non-APROP) group. The present research provides essential data on refractive errors of Mainland Chinese children with ROP, including those with APROP, and will help eye care professionals develop an optimal strategy to prevent visual loss in children with ROP.
METHODS
Ethics Statement
This study was approved by the review board and the ethics committee of the Eye and ENT Hospital of Fudan University. This study adhered to the tenets of the Declaration of Helsinki and was given exempt status by our institutional review board for the retrospective and anonymous design of this study.
Patients
According to the Early Treatment for Retinopathy of Prematurity (ETROP) multicenter trial, laser treatment should be considered in threshold or type 1 prethreshold ROP, including any stage of ROP in zone I with plus disease, stage 3 ROP in zone I without plus disease, and stage 2 or 3 ROP in zone II with plus disease.2,3 According to these criteria, we identified premature patients who underwent diode laser photocoagulation for threshold or type 1 prethreshold ROP at the Eye and ENT Hospital of Fudan University between January 2004 and October 2012; these patients were included as the laser-treated group. According to the definition of APROP as widespread retinovascular tortuosity and dilation without proportional lesions in the peripheral retina,13 the laser-treated group was further subdivided into APROP and non-APROP groups. The control group included patients diagnosed as having type 2 prethreshold ROP, which should be followed conservatively unless they progress to threshold or type 1 prethreshold ROP. In the ETROP trial,2,3 type 2 prethreshold ROP was defined as stage 1 or 2 ROP in zone I without plus disease and as stage 3 ROP in zone II without plus disease. According to these criteria, we identified patients who were diagnosed as having type 2 prethreshold ROP during the same period as that of the laser-treated group; these patients were included as the control group.
Except for patients with unfavorable structural outcomes, further treatments, or incomplete data, all other patients were enrolled in this study. As described in previous studies, unfavorable structural outcomes included posterior retinal detachment, retinal fold involving the macula, retrolental tissue, or serious complications of laser treatment, including glaucoma, cataract, hyphema, and macular burns.17–19 Further treatment included cryotherapy, scleral buckling, vitrectomy, and intravitreal anti–vascular endothelial growth factor (VEGF) therapy. Incomplete data means no appropriate refractive outcomes were acquired.
Appropriate patients of every group were chosen according to their medical history and RetCam II (Clarity Medical System, Pleasanton, CA) photographs taken during fundus examination in the initial and each follow-up visit. Two experienced ophthalmologists (XH and H-DS) classified each patient after reviewing their medical history and RetCam II photographs.
Laser Photocoagulation
The laser treatment was performed by the same qualified ophthalmologist (XH) within 24 hours of the diagnosis of threshold or type 1 prethreshold ROP. The avascular retina, usually from the ridge to the ora serrata, was treated using a Nd:YAG 532-nm ophthalmic laser (Alcon, Fort Worth, TX) at 100 to 300 mW with a half-spot interval between each laser spot (Fig. 1). By the end of the session, the laser burns expanded to a near-confluent pattern. By 2 weeks after treatment, the scars had usually expanded to confluence (Fig. 2). Supplementary treatment was applied to the skipped areas if plus disease or fibrovascular proliferation persisted for 2 weeks after the primary treatment. Although the same therapeutic regimen was used in both APROP and non-APROP treatment, more laser spots were delivered in eyes with APROP that is characterized by posterior locations.
FIGURE 1.
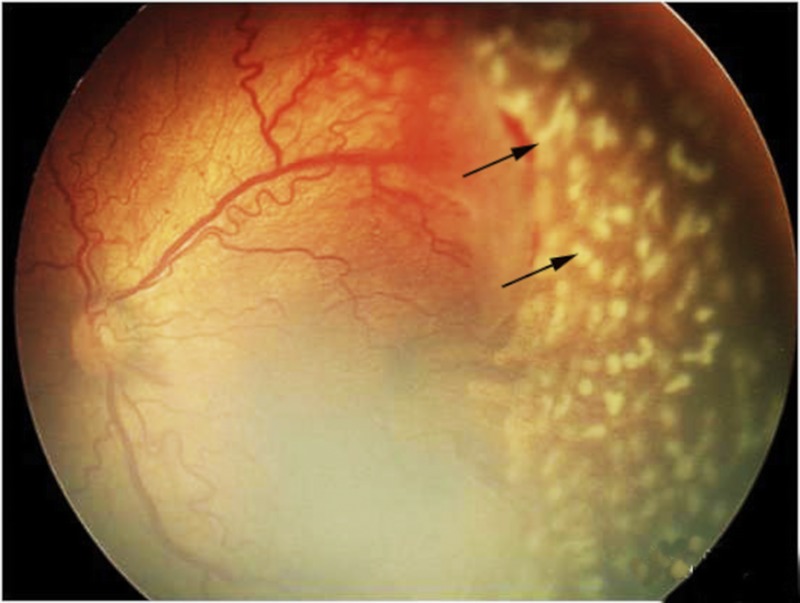
Fundus photograph to illustrate laser spots (arrows) during the treatment of laser photocoagulation.
FIGURE 2.
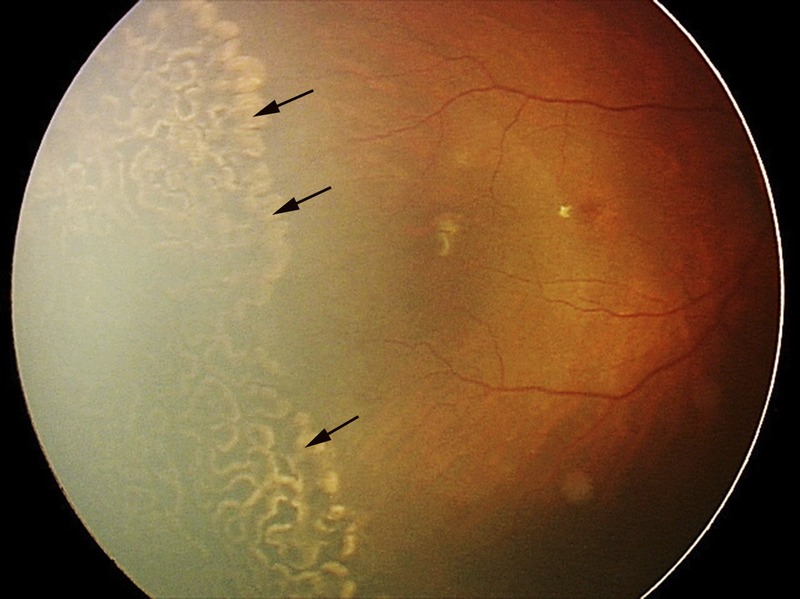
Fundus photograph to illustrate laser scars (arrows) after the treatment of laser photocoagulation.
Fundus Examination
Before the examination, the infants’ pupils were dilated to a minimum of 7 mm with three to six instillations of eye drops containing 0.5% cyclopentolate hydrochloride and 0.5% phenylephrine hydrochloride every 10 minutes. Fundus images were acquired at the initial and each follow-up visit. Examinations were routinely performed at 3 to 4 weeks of postnatal age (PA) or at a postmenstrual age of 31 weeks, and every week or fortnight thereafter until the disease regressed. For laser-treated infants, follow-up examinations were performed biweekly to assess the anatomical outcomes for 3 to 4 months after treatment and then every 3 months for 12 months, and every 6 months thereafter. All photographs were reviewed to detect acute-phase ROP and adverse structural outcomes by two experienced ophthalmologists (XH and H-DS), which were the key elements to determine grouping.
Refractive Examination
Rapid biological changes in optical elements, including axial length, corneal curvature, and lens power, normally occur during the first year of life. Hence, in this retrospective study, refractive outcomes were measured between the PA of 12 and 36 months. To reduce the difference of PA, the data recorded at a PA closest to 24 months were used for data analysis and the mean (±SD) PA was 22.9 (±8.1) months (median, 22 months; range, 12 to 36 months). Cycloplegic retinoscopy was performed to measure refractive outcomes by a masked examiner (X-ZL) using a streak retinoscope20,21 (66 Vision Technology, Suzhou, Jiangsu, China) after cyclopleging the patient with the drop regimen described above. To allow us to compare our results with those of the ETROP trial, we used the same definitions of refractive errors. Myopia and high myopia were defined as SE less than or equal to −0.25 D and SE less than or equal to −5.00 D, respectively. Astigmatism and high astigmatism were defined as plus cylindrical degree (CD) greater than or equal to +1.00 D and greater than or equal to +2.00 D, respectively.22–26 Because the risk of anisometropic amblyopia is reportedly increased in eyes with spherical anisometropia greater than or equal to 2.00 D and cylindrical anisometropia greater than or equal to 1.50 D, a spherical difference between two eyes of greater than or equal to 2.00 D was defined as spherical anisometropia.27 Similarly, a cylindrical difference between two eyes of greater than or equal to 1.50 D was defined as cylindrical anisometropia.27
Data Collecting and Recording
The examiner who viewed fundus photographs was masked to refractive error determination; the examiner who performed retinoscopy was masked to treatment group. The detection of ROP and retinoscopy were performed independently by different persons as described above. In the end, data of every participant were collected and recorded using a codename. Data included acute-phase ROP, treatment, sex, gestational age (GA), birth weight (BW), PA, and refractive outcomes.
Statistical Analysis
In this study, data from both eyes of each subject were used for refractive comparisons and the statistical analysis accounted for the correlation between eyes (except for measures of anisometropia). The unit of analysis was the patient for anisometropia, sex (sex ratio [SR]), BW, and GA. Cylindrical degree, SE, BW, GA, and PA are presented as the mean ± SD. Categorical variables are presented as the number and percentage. The distributions of BW and GA were compared between two groups using the Student t test. The differences in refractive outcomes, including CD, SE, and the prevalence of astigmatism, high astigmatism, myopia, and high myopia, were compared at each PA point among each group using a general linear model (a general linear model is appropriate for normal or non–normally distributed data). For the comparing of prevalence of anisometropia and SR, the χ2 test was performed. Statistical significance was accepted at p values of less than 0.05. SAS software version 9.2 (SAS Institute Inc, Cary, NC) was used for all statistical analyses.
RESULTS
Patient Characteristics
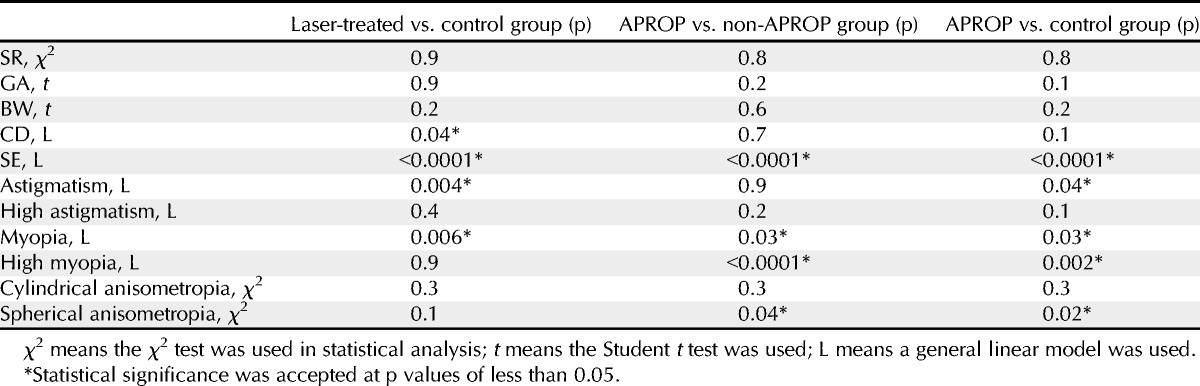
The laser-treated group (115 eyes of 60 children) was further subdivided into APROP (13 eyes of 7 children) and non-APROP groups (102 eyes of 53 children). The control group included 37 eyes of 20 children. The characteristics of each group are outlined in Table 1. Sex ratio, GA, and BW were not significantly different between the control and the laser-treated groups (p = 0.9, p = 0.9, and p = 0.2, respectively) or between the APROP and non-APROP groups (p = 0.8, p = 0.2, and p = 0.6, respectively). Moreover, SR, GA, and BW were not significantly different between the control and APROP groups (p = 0.8, p = 0.1, and p = 0.2, respectively) or between the control and non-APROP groups (p = 0.9, p = 0.8, and p = 0.3, respectively) (Table 3).
TABLE 1.
Characteristics of patients in the control and the laser-treated groups
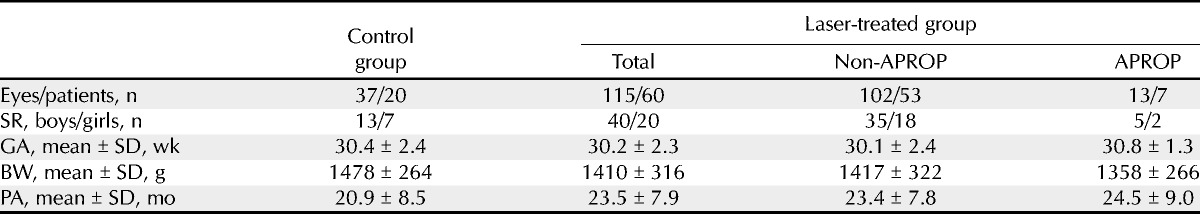
TABLE 3.
p Values in univariate analysis between the laser-treated and control groups, between the APROP and non-APROP groups, and between the APROP and control groups
The mean (±SD) PA of the laser-treated group was 23.5 (±7.9) months (median, 23 months; range, 12 to 36 months) and that of the control group was 20.9 (±8.5) months (median, 20 months; range, 12 to 36 months). Within the laser-treated group, the APROP group presented a mean (±SD) PA of 24.5 (±9.0) months (median, 23 months; range, 12 to 36 months) and the non-APROP group presented a mean (±SD) PA of 23.4 (±7.8) (median, 23 months; range, 12 to 36 months).
CD and SE
The mean CD and SE of each of the groups are presented in Table 2. After adjusting for PA and the correlation between right and left eyes, the CD was significantly greater in the laser-treated group than in the control group (p = 0.04). However, the CD was not significantly different between the APROP and non-APROP groups (p = 0.7) or between the APROP and control groups (p = 0.1). As for SE, it was significantly lower in the laser-treated group than in the control group (p < 0.0001). The SE of the APROP group was significantly lower than that of the non-APROP group (p < 0.0001). The SE of the APROP (p < 0.0001) and non-APROP (p < 0.0001) groups was also significantly lower than that of the control group (Table 3).
TABLE 2.
Refractive error of patients in the control and the laser-treated groups
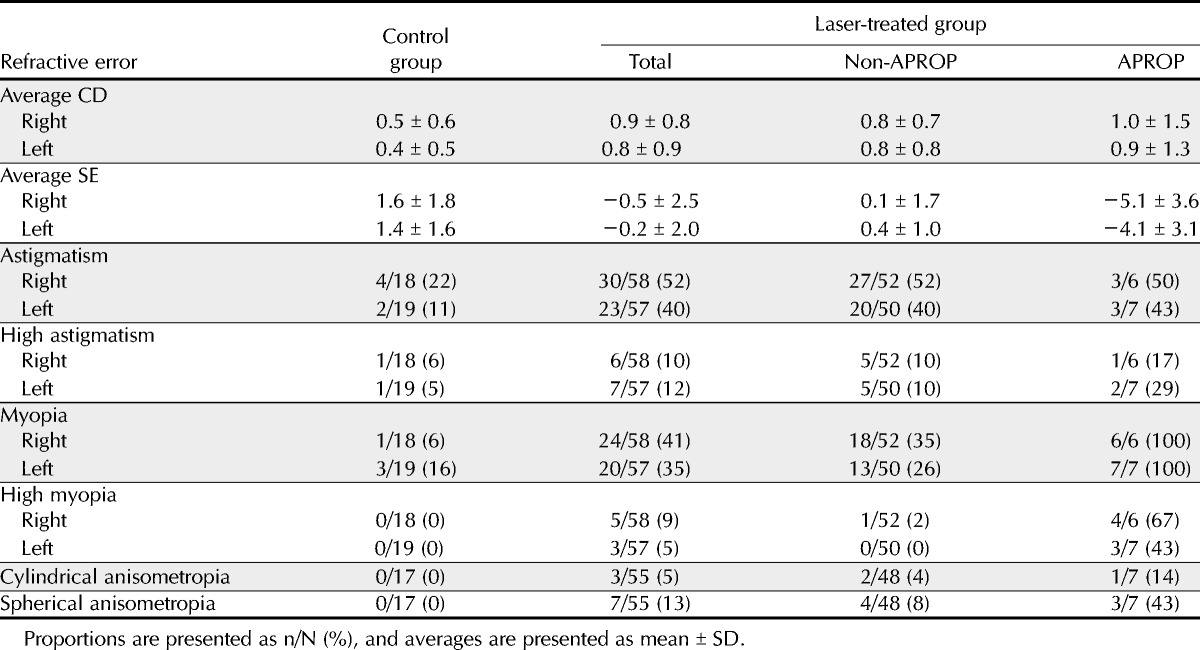
Prevalence of Astigmatism and High Astigmatism
Table 2 shows the prevalence of astigmatism and high astigmatism in each of the groups. After adjusting for PA and the correlation between right and left eyes, the prevalence of astigmatism was significantly greater in the laser-treated group than in the control group (p = 0.004; odds ratio [OR], 2.0; 95% confidence interval [CI], 1.6 to 10.9). However, the prevalence of astigmatism was similar between the APROP and non-APROP groups (p = 0.9). Both the APROP (p = 0.04; OR, 2.1; 95% CI, 1.1 to 19.1) and non-APROP groups (p = 0.003; OR, 2.1; 95% CI, 1.6 to 11.2) presented a significantly higher prevalence of astigmatism than the control group (Table 3).
The prevalence of high astigmatism was not significantly different between the control and the laser-treated groups (p = 0.4) or between the APROP and non-APROP groups (p = 0.2). Moreover, the prevalence of high astigmatism was also similar between the control and APROP groups (p = 0.1) and between the control and non-APROP groups (p = 0.5) (Table 3).
Prevalence of Myopia and High Myopia
The prevalence of myopia and high myopia in each group is presented in Table 2. After adjusting for PA and the correlation between right and left eyes, the prevalence of myopia was significantly greater in the laser-treated group than in the control group (p = 0.006; OR, 2.2; 95% CI, 1.5 to 14.5). Moreover, the APROP group presented a higher prevalence of myopia than the control (p = 0.03; OR, 1.6; 95% CI, 1.1 to 19.0) and the non-APROP groups (p = 0.03; OR, 2.0; 95% CI, 1.3 to 15.1) (Table 3).
The prevalence of high myopia did not reach statistical significance between the control and the laser-treated groups (p = 0.9). However, the prevalence of high myopia was significantly greater in the APROP group than in the control group (p = 0.002; OR, 1.9; 95% CI, 1.1 to 17.8) and in the non-APROP group (p < 0.0001; OR, 12.8; 95% CI, 3.8 to 43.9) (Table 3).
Prevalence of Cylindrical and Spherical Anisometropia
There were 17 infants with binocular spontaneous regression in the control group. Of 55 infants who underwent binocular laser treatment, 48 infants were classified as non-APROP and 7 infants were classified as APROP. Table 2 demonstrates the prevalence of anisometropia in each of these groups. In comparison to the prevalence of cylindrical anisometropia, there was no significant difference between the control and the laser-treated groups (p = 0.3) or between the APROP and the non-APROP groups (p = 0.3). Moreover, the prevalence of cylindrical anisometropia was similar between the APROP and the control groups (p = 0.3) and between the non-APROP and the control groups (p = 0.5) (Table 3).
As shown in Table 2, the prevalence of spherical anisometropia was significantly greater in the APROP group than in the control group (p = 0.02) and in the non-APROP group (p = 0.04). However, the prevalence of spherical anisometropia was similar between the control and the laser-treated groups (p = 0.1) or between the control and the non-APROP groups (p = 0.3) (Table 3).
DISCUSSION
We have studied refractive outcomes of Mainland Chinese children with ROP, including those with APROP. After adjusting for PA and the correlation between right and left eyes, eyes with regressed ROP after laser treatment presented greater astigmatism and myopia than eyes with spontaneously regressed ROP. Moreover, eyes with APROP had highest risk of high myopia and spherical anisometropia, which may progress to amblyopia.
As shown in Table 3, the magnitude and prevalence of astigmatism were similar in the APROP and non-APROP groups, which suggest that the severity of ROP is not associated with astigmatism. The significant differences observed between the control and the laser-treated groups suggest that laser treatment is associated with astigmatism. However, because the laser-treated group also had more severe ROP than the control group, it is possible that the difference in the severity of ROP is larger between these groups than between the APROP and non-APROP groups. Accordingly, we could only conclude that patients with severe ROP who undergo laser treatment tend to have more severe astigmatism. The study also revealed that there was no significant difference in the prevalence of high astigmatism between each group, which suggests that neither ROP nor laser treatment is associated with high astigmatism. These results show some differences to the ETROP trial, which analyzed the prevalence of astigmatism at 6 and 9 months and at 2 and 3 years between the early treated and the conventionally managed groups and found no significant difference between the two groups at every age. The ETROP trial also made conclusions that the prevalence of astigmatism was unrelated to severity of acute-phase ROP, as indicated by zone of disease and presence versus absence of plus disease.23,24
Our research showed that the SE was lowest and the prevalence of myopia was highest in the APROP group, followed by the non-APROP group and the control group (Table 3). These results suggest that infants who underwent laser treatment, especially those with APROP, tended to have higher myopia. However, it is difficult to infer whether it was the severity of the disease or treatment status that affected myopia in our patients. In terms of high myopia, only the APROP group presented higher prevalence than the control and the non-APROP groups, which suggests that APROP disease is related to high myopia. Many previous reports have shown that the prevalence of myopia is related to the severity of acute-phase and cicatricial-phase ROP.22,25,26,28 Quinn et al.28 reported that the prevalence of myopia was greater in eyes with moderate or severe acute-phase ROP compared with eyes with mild ROP in six age groups between 3 months and 5.5 years old. The results of the ETROP trial confirmed that conventionally managed eyes, in which ROP progresses to require ablative treatment, are more likely to develop myopia and high myopia than conventionally managed eyes in which ROP regressed, in seven age groups between 6 months and 6 years old. The ETROP trial also demonstrated that the prevalence rates of myopia and high myopia are greater in eyes with retinal residua than in eyes without.22,25,26
Comparing our results with those of other studies performed outside of China, we discovered that the laser-treated and control eyes in our study presented with lower myopia than age-matched eyes in other studies, although the severity and prevalence of astigmatism were similar.6,24,25,29 These findings are particularly interesting because prior reports suggested that the prevalence rates of myopia and astigmatism were greater in Chinese adults and children than those of other countries.7–12 Comparisons between our study and other studies, especially the ETROP trial, revealed several differences. Children in our study, like those reported in Indian populations,30 presented with a preponderance of male subjects, a heavier BW, a greater GA, and different ethnic composition compared with those reported by the ETROP group.31 It has been reported that infants with ROP in developing countries tend to be heavier and more mature.14 These differences might contribute to the discrepancies in refractive outcomes. However, without conducting further studies, we cannot make a final conclusion.
Aggressive posterior retinopathy of prematurity is characterized by its posterior location, prominence of plus disease, and the ill-defined nature of the retinopathy.13 Despite extensive and appropriate laser treatment, the disease often progresses to retinal detachment.14,32–34 In our study, APROP eyes with successful laser treatment and favorable structural outcomes tended to have serious refractive errors. As shown in Table 3, the prevalence of high myopia and spherical anisometropia was greater in the APROP group than in the control and non-APROP groups. Refractive errors, such as hyperopia, myopia, astigmatism, and anisometropia, are related to amblyopia, which is a modifiable source of vision loss.27,35–37 After all possible surgical and other therapy has been completed, continued attention to refractive correction and possible amblyopia treatment is needed to maximize the visual outcome of children born early, especially those with ROP including APROP. A search of PubMed yielded few reports about refractive errors in this population, indicating that refractive outcomes need more attention from researchers and clinicians alike. Recently, several groups reported positive effects of first-line anti-VEGF agent for APROP.38–42 In a 1-year follow-up study, a single intravitreal injection of bevacizumab was associated with less myopia and less astigmatism as compared with conventional retinal laser coagulation.3 Accordingly, anti-VEGF therapy might be an attractive option for infants with APROP.43 Large-scale clinical trials are necessary to evaluate safety and long-term efficacy of intravitreal injection of bevacizumab before widespread use is advised.44
Our study has several limitations that must be mentioned. First, it was a retrospective study without a randomized control group. The laser-treated and control groups were not matched for severity for ethical reasons. However, the study was rationally designed by excluding eyes with unfavorable structural outcomes and those requiring further treatment and limiting control subjects to type 2 prethreshold ROP. Second, the numbers of infants are relatively small, especially in the APROP group, which may reduce the strength of some statements. There were two reasons for the small sample. It was uncommon for infants with APROP to be successfully treated with laser photocoagulation alone. Considering the report of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity trial,42 we started to perform intravitreal injection of bevacizumab for infants with APROP recently. Therefore, it is difficult to enlarge members of the APROP group with laser treatment alone.
In conclusion, infants who underwent laser treatment for threshold or type 1 ROP tended to have higher myopia and astigmatism than control patients with type 2 ROP that did not require treatment. Children with laser treatment for APROP tended to have serious refractive errors, including high myopia and spherical anisometropia. Because refractive errors, especially serious ones, often progress to amblyopia, regular screening of refractive outcomes is important for the early detection and proper treatment of refractive errors.
Xin Huang
Department of Ophthalmology
Eye and ENT Hospital of Fudan University
No. 83 Fenyang Rd
Shanghai 200031
China
e-mail: xinhuang66@gmail.com
ACKNOWLEDGMENTS
The research was supported by the Shanghai Natural Science Foundation Project (13ZR1405900). The founding organization had no role in the design or conduct of this research. The authors would like to express their very great appreciation to Dr. Jia Yu and Kang Xue for expert technical assistance of fundus examinations; they also appreciate statistician She-Chang Li for his help with the statistical analysis.
REFERENCES
- 1. Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, Tung B. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics 2005; 116: 15– 23. [DOI] [PubMed] [Google Scholar]
- 2. Phelps DL. The Early Treatment for Retinopathy of Prematurity study: better outcomes, changing strategy. Pediatrics 2004; 114: 490– 1. [DOI] [PubMed] [Google Scholar]
- 3.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003; 121: 1684– 94. [DOI] [PubMed] [Google Scholar]
- 4. Yang CS, Wang AG, Sung CS, Hsu WM, Lee FL, Lee SM. Long-term visual outcomes of laser-treated threshold retinopathy of prematurity: a study of refractive status at 7 years. Eye (Lond) 2010; 24: 14– 20. [DOI] [PubMed] [Google Scholar]
- 5. Axer-Siegel R, Maharshak I, Snir M, Friling R, Ehrlich R, Sherf I, Shalev B, Sirota L, Weinberger D. Diode laser treatment of retinopathy of prematurity: anatomical and refractive outcomes. Retina 2008; 28: 839– 46. [DOI] [PubMed] [Google Scholar]
- 6. Sahni J, Subhedar NV, Clark D. Treated threshold stage 3 versus spontaneously regressed subthreshold stage 3 retinopathy of prematurity: a study of motility, refractive, and anatomical outcomes at 6 months and 36 months. Br J Ophthalmol 2005; 89: 154– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan CW, Zheng YF, Anuar AR, Chew M, Gazzard G, Aung T, Cheng CY, Wong TY, Saw SM. Prevalence of refractive errors in a multiethnic Asian population: the Singapore epidemiology of eye disease study. Invest Ophthalmol Vis Sci 2013; 54: 2590– 8. [DOI] [PubMed] [Google Scholar]
- 8. Sun J, Zhou J, Zhao P, Lian J, Zhu H, Zhou Y, Sun Y, Wang Y, Zhao L, Wei Y, Wang L, Cun B, Ge S, Fan X. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Invest Ophthalmol Vis Sci 2012; 53: 7504– 9. [DOI] [PubMed] [Google Scholar]
- 9. Lam CS, Lam CH, Cheng SC, Chan LY. Prevalence of myopia among Hong Kong Chinese schoolchildren: changes over two decades. Ophthalmic Physiol Opt 2012; 32: 17– 24. [DOI] [PubMed] [Google Scholar]
- 10. Dirani M, Chan YH, Gazzard G, Hornbeak DM, Leo SW, Selvaraj P, Zhou B, Young TL, Mitchell P, Varma R, Wong TY, Saw SM. Prevalence of refractive error in Singaporean Chinese children: the strabismus, amblyopia, and refractive error in young Singaporean Children (STARS) study. Invest Ophthalmol Vis Sci 2010; 51: 1348– 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saw SM, Goh PP, Cheng A, Shankar A, Tan DT, Ellwein LB. Ethnicity-specific prevalences of refractive errors vary in Asian children in neighbouring Malaysia and Singapore. Br J Ophthalmol 2006; 90: 1230– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan DS, Rao SK, Cheung EY, Islam M, Chew S, Lam DS. Astigmatism in Chinese preschool children: prevalence, change, and effect on refractive development. Br J Ophthalmol 2004; 88: 938– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005; 123: 991– 9. [DOI] [PubMed] [Google Scholar]
- 14. Sanghi G, Dogra MR, Das P, Vinekar A, Gupta A, Dutta S. Aggressive posterior retinopathy of prematurity in Asian Indian babies: spectrum of disease and outcome after laser treatment. Retina 2009; 29: 1335– 9. [DOI] [PubMed] [Google Scholar]
- 15. Vinekar A, Trese MT, Capone A., Jr Evolution of retinal detachment in posterior retinopathy of prematurity: impact on treatment approach. Am J Ophthalmol 2008; 145: 548– 55. [DOI] [PubMed] [Google Scholar]
- 16. Bakar N, Chen A, Noor A, Goh P. Comparison of refractive error and visual impairment between Native Iban and Malay in a formal government school vision loss prevention programme. Malays J Med Sci 2012; 19: 48– 55. [PMC free article] [PubMed] [Google Scholar]
- 17. Simons BD, Wilson MC, Hertle RW, Schaefer DB. Bilateral hyphemas and cataracts after diode laser retinal photoablation for retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 1998; 35: 185– 7. [DOI] [PubMed] [Google Scholar]
- 18. Noonan CP, Clark DI. Acute serous detachment with argon laser photocoagulation in retinopathy of prematurity. J AAPOS 1997; 1: 183– 4. [DOI] [PubMed] [Google Scholar]
- 19.Cryotherapy for Retinopathy of Prematurity Cooperative Group. The natural ocular outcome of premature birth and retinopathy. Status at 1 year. Arch Ophthalmol 1994; 112: 903– 12. [DOI] [PubMed] [Google Scholar]
- 20. Smith G, Haymes S. The streak retinoscopy pupil reflex in the presence of astigmatism. Ophthalmic Physiol Opt 2003; 23: 295– 305. [DOI] [PubMed] [Google Scholar]
- 21. Boeder P, Kolder HE. Neutralization at infinity in streak retinoscopy. Arch Ophthalmol 1984; 102: 1396– 9. [DOI] [PubMed] [Google Scholar]
- 22. Quinn GE, Dobson V, Davitt BV, Wallace DK, Hardy RJ, Tung B, Lai D, Good WV. Progression of myopia and high myopia in the Early Treatment for Retinopathy of Prematurity study: findings at 4 to 6 years of age. J AAPOS 2013; 17: 124– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davitt BV, Quinn GE, Wallace DK, Dobson V, Hardy RJ, Tung B, Lai D, Good WV. Astigmatism progression in the early treatment for retinopathy of prematurity study to 6 years of age. Ophthalmology 2011; 118: 2326– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davitt BV, Dobson V, Quinn GE, Hardy RJ, Tung B, Good WV. Astigmatism in the Early Treatment for Retinopathy of Prematurity Study: findings to 3 years of age. Ophthalmology 2009; 116: 332– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quinn GE, Dobson V, Davitt BV, Hardy RJ, Tung B, Pedroza C, Good WV. Progression of myopia and high myopia in the early treatment for retinopathy of prematurity study: findings to 3 years of age. Ophthalmology 2008; 115: 1058– 64.e1. [DOI] [PubMed] [Google Scholar]
- 26. Davitt BV, Dobson V, Good WV, Hardy RJ, Quinn GE, Siatkowski RM, Summers CG, Tung B. Prevalence of myopia at 9 months in infants with high-risk prethreshold retinopathy of prematurity. Early Treatment for Retinopathy of Prematurity Cooperative Group. Ophthalmology 2005; 112: 1564– 8. [DOI] [PubMed] [Google Scholar]
- 27. Afsari S, Rose KA, Gole GA, Philip K, Leone JF, French A, Mitchell P. Prevalence of anisometropia and its association with refractive error and amblyopia in preschool children. Br J Ophthalmol 2013; 97: 1095– 9. [DOI] [PubMed] [Google Scholar]
- 28. Quinn GE, Dobson V, Kivlin J, Kaufman LM, Repka MX, Reynolds JD, Gordon RA, Hardy RJ, Tung B, Stone RA. Prevalence of myopia between 3 months and 5 1/2 years in preterm infants with and without retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1998; 105: 1292– 300. [DOI] [PubMed] [Google Scholar]
- 29. Hsieh CJ, Liu JW, Huang JS, Lin KC. Refractive outcome of premature infants with or without retinopathy of prematurity at 2 years of age: a prospective controlled cohort study. Kaohsiung J Med Sci 2012; 28: 204– 11. [DOI] [PubMed] [Google Scholar]
- 30. Dhawan A, Dogra M, Vinekar A, Gupta A, Dutta S. Structural sequelae and refractive outcome after successful laser treatment for threshold retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 2008; 45: 356– 61. [DOI] [PubMed] [Google Scholar]
- 31. Good WV. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004; 102: 233– 48. [PMC free article] [PubMed] [Google Scholar]
- 32. Sanghi G, Dogra MR, Katoch D, Gupta A. Aggressive posterior retinopathy of prematurity: risk factors for retinal detachment despite confluent laser photocoagulation. Am J Ophthalmol 2013; 155: 159– 64.e2. [DOI] [PubMed] [Google Scholar]
- 33. Drenser KA, Trese MT, Capone A., Jr Aggressive posterior retinopathy of prematurity. Retina 2010; 30: S37– 40. [DOI] [PubMed] [Google Scholar]
- 34. Suk KK, Berrocal AM, Murray TG, Rich R, Major JC, Hess D, Johnson RA. Retinal detachment despite aggressive management of aggressive posterior retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 2010; 47 Online: e1– 4. [DOI] [PubMed] [Google Scholar]
- 35. Sapkota K, Pirouzian A, Matta NS. Prevalence of amblyopia and patterns of refractive error in the amblyopic children of a tertiary eye care center of Nepal. Nepal J Ophthalmol 2013; 5: 38– 44. [DOI] [PubMed] [Google Scholar]
- 36. Caca I, Cingu AK, Sahin A, Ari S, Dursun ME, Dag U, Balsak S, Alakus F, Yavuz A, Palanci Y. Amblyopia and refractive errors among school-aged children with low socioeconomic status in southeastern Turkey. J Pediatr Ophthalmol Strabismus 2013; 50: 37– 43. [DOI] [PubMed] [Google Scholar]
- 37. Polling JR, Loudon SE, Klaver CC. Prevalence of amblyopia and refractive errors in an unscreened population of children. Optom Vis Sci 2012; 89: e44– 9. [DOI] [PubMed] [Google Scholar]
- 38. Mintz-Hittner HA. Avastin as monotherapy for retinopathy of prematurity. J AAPOS 2010; 14: 2– 3. [DOI] [PubMed] [Google Scholar]
- 39. Hosseini H, Khalili MR, Nowroozizadeh S. Intravitreal injection of bevacizumab (Avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina 2009; 29: 562.author reply 562–4. [DOI] [PubMed] [Google Scholar]
- 40. Nowroozzadeh MH. Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina 2009; 29: 564– 5. [DOI] [PubMed] [Google Scholar]
- 41. Mintz-Hittner HA, Kuffel RR., Jr Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina 2008; 28: 831– 8. [DOI] [PubMed] [Google Scholar]
- 42. Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011; 364: 603– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harder BC, Schlichtenbrede FC, von Baltz S, Jendritza W, Jendritza B, Jonas JB. Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol 2013; 155: 1119– 24.e1. [DOI] [PubMed] [Google Scholar]
- 44. Wu WC, Kuo HK, Yeh PT, Yang CM, Lai CC, Chen SN. An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in Taiwan. Am J Ophthalmol 2013; 155: 150– 8. [DOI] [PubMed] [Google Scholar]


