Metronidazole vaginal gel 1.3% once daily for 1, 3, or 5 days provides similar efficacy, safety, and tolerability as metronidazole vaginal gel 0.75% once daily for 5 days.
Key Words: bacterial vaginosis, metronidazole gel
Abstract
Objective
Metronidazole vaginal gel (MVG) 0.75% is a US Food and Drug Administration–approved, 5-day treatment for bacterial vaginosis (BV). This study tested the hypothesis that a shorter treatment course at a higher dose (MVG 1.3%) would yield similar efficacy to 5 days of MVG 0.75%.
Materials and Methods
This phase 2, multicenter, randomized, controlled, investigator-blinded, dose-ranging study enrolled women with a clinical diagnosis of BV. Patients were assigned to MVG 1.3% once daily for 1, 3, or 5 days or MVG 0.75% once daily for 5 days. The therapeutic cure rate, requiring clinical and bacteriological cure, at the end-of-study visit was determined for the per-protocol population. A Kaplan-Meier analysis was used to estimate median time-to-symptom resolution.
Results
In total, 255 women (mean age = 35 y) were enrolled. The per-protocol population included 189 patients. The therapeutic cure rate was higher in the 1-day (13/43, 30.2%), 3-day (12/48, 25.0%), and 5-day (16/49, 32.7%) MVG 1.3% groups versus the MVG 0.75% group (10/49, 20.4%). Median time-to-resolution of fishy odor was shorter in the 3 MVG 1.3% groups versus the MVG 0.75% group. The 5-day MVG 1.3% group had the lowest rate of symptom return. No clinically important differences were observed in adverse events across treatment groups; most events were mild or moderate in intensity and considered unrelated to treatment. Similar results were found in the modified intent-to-treat population.
Conclusions
Metronidazole vaginal gel 1.3% applied once daily for 1, 3, or 5 days showed similar efficacy, safety, and tolerability as MVG 0.75% once daily for 5 days.
Bacterial vaginosis (BV), the most common cause of abnormal vaginal discharge, is characterized by a reduction in lactobacilli and an increase in other gram-negative and gram-positive anaerobes.1,2 In the 2001–2004 US National Health and Nutrition Examination Survey studies, an estimated 29% of patients aged 14 to 49 years and more than 50% of African American women were positive for BV based on the Nugent criteria.3 In addition, women reporting more frequent sexual activity and/or new sexual partners have shown an increased incidence of BV.2,4–6 Symptoms of BV include vaginal discharge and a fishy odor; however, many women with BV are asymptomatic.7
Metronidazole is equally effective administered intravaginally or orally, although intravaginal administration is associated with significantly fewer adverse events (AEs).8 In clinical studies, metronidazole vaginal gel (MVG) 0.75% dosed once daily for 5 days was as effective as MVG 0.75% dosed twice daily for 5 days and had a similar safety profile.9,10 Metronidazole vaginal gel 0.75%, applied once or twice daily for 5 days, is approved by the US Food and Drug Administration for the treatment of patients with BV. A new formulation of MVG offers a higher concentration of metronidazole (1.3%) with a shorter treatment course and similar efficacy as MVG 0.75%. This study evaluated the efficacy and safety of MVG 1.3% once daily for 1, 3, or 5 days versus MVG 0.75% once daily for 5 days in the treatment of patients with BV.
MATERIALS AND METHODS
Study Design
This phase 2, multicenter, randomized, controlled, investigator-blinded, dose-ranging study was conducted at 20 investigational centers in the United States. Bacterial vaginosis was confirmed at baseline during pelvic examination using the following criteria: (1) off-white (milky or gray), thin, homogeneous discharge; (2) clue cells representing 20% or greater of the total epithelial cells on microscopic examination of saline wet mount at 100× magnification; (3) vaginal pH of 4.7 or greater; and (4) positive 10% KOH whiff test. Evaluations occurred at baseline, during 1 posttreatment telephone call between days 8 and 10 and at the end-of-study/test-of-cure (EOS/TOC) visit between days 21 and 30.
Eligible patients were randomly assigned 1:1:1:1 to receive MVG 1.3% once daily for 1 day, MVG 1.3% once daily for 3 consecutive days, MVG 1.3% once daily for 5 consecutive days, or MVG 0.75% once daily for 5 consecutive days. Patients were instructed on the proper technique for applying MVG 1.3% or 0.75% at bedtime.
Randomization was achieved using a computer-generated block-randomization schedule, which permitted balanced distribution of patients to 1 of 4 treatment groups, stratified by investigational site. Drug supply kits were packaged and numbered in sequential order according to the randomization schedule and distributed across study sites. Treatment assignments were concealed from investigators and study coordinators, and an independent unblinded drug-dispensing coordinator was available at each site and was responsible for assigning and dispensing supply kits to patients in sequential order according to their enrollment date.
The study was conducted in accordance with the Declaration of Helsinki and was consistent with the ethical principles of the Good Clinical Practice guidelines of the International Conference on Harmonisation and applicable regulatory requirements, including approval by appropriate institutional review boards. Written informed consent was obtained from all patients.
Patients
Women (≥18 y) generally in good health and with a clinical diagnosis of BV were eligible to participate in the study. A negative urine pregnancy test was required for all women of childbearing potential before initiation of study treatment. All participants agreed to abstain from sexual intercourse for the first 7 days of the study and to use study-provided, nonlubricated condoms when engaging in sexual intercourse after day 7. Patients who were pregnant, lactating, menstruating, or planning to become pregnant during the study were excluded, as were those who experienced a clinically important medical event (e.g., stroke, myocardial infarction) within 90 days of baseline. Also excluded were women who had other known or suspected infectious causes of vulvovaginitis, such as candidiasis, trichomoniasis infection with Chlamydia trachomatis, or Neisseria gonorrhoeae, or any other vulvovaginal condition that could confound interpretation of results.
Intravaginal products, including douches, feminine deodorant sprays, spermicides, lubricated condoms, tampons, and diaphragms, as well as alcohol were not allowed during the study. Use of disulfiram, systemic corticosteroids, and antifungal or antimicrobial (systemic or intravaginal) treatment within 14 days of randomization was also not permitted. Patients using anticoagulation therapy with warfarin or those treated or planning to be treated for cervical intraepithelial neoplasia or cervical carcinoma were ineligible to participate, as were those with a primary or secondary immunodeficiency or a previous hypersensitivity reaction to oral or topical metronidazole or any form of parabens. Patients who had participated in a clinical study or taken an experimental medication or device within 30 days also were excluded.
Assessments
The primary efficacy measure was the proportion of patients with therapeutic cure, defined as a clinical and a bacteriological cure, at the EOS/TOC visit. Clinical cure was defined as: (1) absence of an off-white (milky or gray), thin, homogenous discharge; (2) absence of clue cells in the saline wet mount; (3) vaginal pH less than 4.7; and (4) a negative 10% KOH whiff test. Bacteriological cure was defined as a Nugent score of less than 4. The Nugent scoring system uses microscopy to assess the relative quantity of bacteria on a Gram-stained vaginal smear, for which scores of 7 to 10 are consistent with BV (4 to 6 indicate intermediate flora and 0 to 3 normal flora). Therapeutic cure required confirmation from the investigator that the patient no longer required treatment for BV.
Secondary efficacy measures included the proportion of patients with clinical cure at the EOS/TOC visit, proportion of patients with bacteriological cure at the EOS/TOC visit, time to resolution of symptoms (i.e., abnormal discharge and odor), percentage of patients with return of symptoms, pelvic examination results at the EOS/TOC visit, leakage of MVG, and patient EOS questionnaire on treatment satisfaction.
Safety assessments were conducted at baseline, during the post-treatment telephone call, and at the EOS/TOC visit. The frequencies of AEs, serious AEs, treatment-emergent AEs (TEAEs), and AEs leading to study discontinuation were recorded.
Data Analysis
Sample size estimation was based on providing a precise estimate for therapeutic cure rates. Assuming a cure rate of 51.6% in each group, a sample size of 45 patients was considered sufficient to provide an estimate with a 95% confidence interval of 15%. To allow for a 25% ineligibility assessment or dropout rate, a sample size of 60 per group was planned for enrollment and randomization.
The safety population included all randomized patients who applied any amount of MVG; these data were used for all safety analyses. Data from the intent-to-treat (ITT) population, which included all randomized patients, was used to summarize patient disposition, demographic and baseline characteristics, medical history, and prior and concomitant medications. The modified ITT (mITT) population included all randomized patients who received study medication, returned for at least 1 postbaseline assessment, had a negative test result for C. trachomatis and N. gonorrhoeae, and had a Gram stain slide Nugent score of 4 or higher at baseline.
The per-protocol population included patients from the mITT population who satisfied all inclusion criteria, started MVG on the day of randomization or within 2 days of randomization, were compliant with study medication, and had the full TOC evaluation between days 20 and 31. Data from this population were used for the primary analyses. Patients whose EOS visit occurred before day 21 were included in the per-protocol population if data indicated that the patient was a clinical failure for BV without another specified cause (i.e., C. trachomatis, N. gonorrhoeae). Patients who had missing primary efficacy data were counted as treatment failures for the primary efficacy assessment and included in the mITT population, which was used for supportive efficacy analyses.
For proportion variables, data were summarized by treatment group with 95% confidence intervals. Kaplan-Meier analyses were used to estimate the median time to resolution of symptoms with 95% confidence intervals according to the Greenwood formula. Kaplan-Meier survival curves were plotted for the time to resolution of symptoms for all treatment groups. This dose-ranging study was not powered to detect differences between treatment groups; therefore, no formal statistical testing was performed to compare treatments.
RESULTS
Patients
A total of 255 patients were randomly assigned to treatment and included in the ITT population from 2 February to 26 April 2010. Overall, 234 patients (92%) completed the study and 21 (8%) discontinued early (see Figure 1). Baseline demographics and patient characteristics were similar across treatment groups (see Table 1).
FIGURE 1.
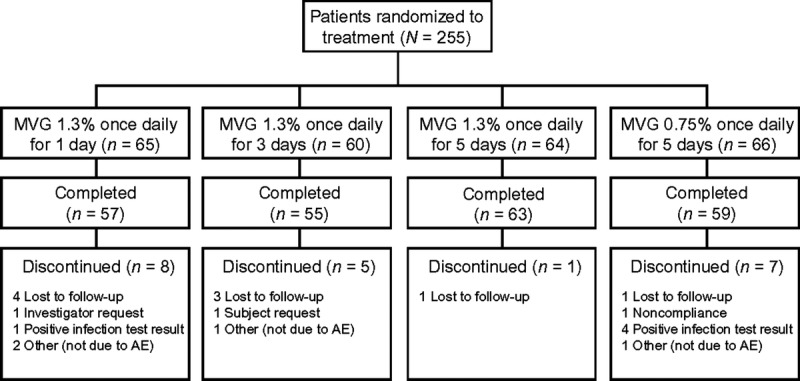
Disposition of patients. AE, adverse event; MVG, metronidazole vaginal gel.
TABLE 1.
Baseline Demographics and Patient Characteristics: Intent-to-Treat Population
With the exception of 1 patient, all patients (n = 254) were included in the safety population. The mITT population included 228 patients; 23 (85%) of the 27 patients excluded from this population had baseline Nugent scores that were not consistent with a diagnosis of BV. The per-protocol population included 189 patients; 30 (45%) of the 66 patients excluded from this population did not have Nugent scores for days 20 and 31.
Primary Efficacy Outcome
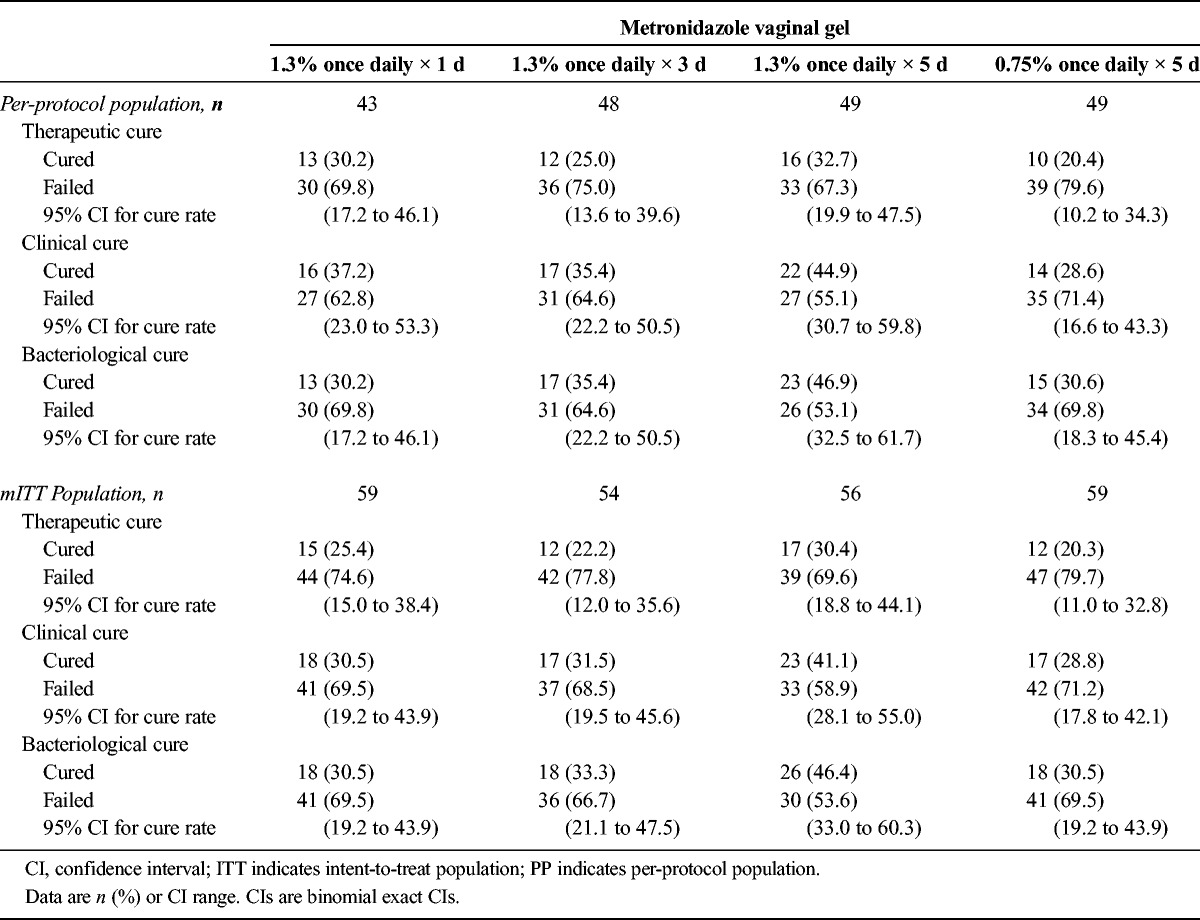
In the mITT and per-protocol populations, the therapeutic cure rate was numerically higher in all 3 MVG 1.3% groups compared with the MVG 0.75% group (see Table 2). Similarly, clinical cure rates were numerically higher in the MVG 1.3% groups compared with the MVG 0.75% group. Bacteriological cure rates were numerically higher only in the 3- and 5-day MVG 1.3% groups compared with the 1-day MVG 1.3% and MVG 0.75% groups. For all the efficacy analyses, the differences were not statistically significant.
TABLE 2.
Summary of Cure Rates at Test of Cure/End of Study: Per-Protocol and Intent-to-Treat Populations
Secondary Efficacy Outcomes
In the per-protocol population, the median time to resolution of symptoms (i.e., abnormal discharge and fishy odor) was 5 days in the MVG 1.3% groups and 6 days in the MVG 0.75% group (see Figure 2). The median time to resolution of abnormal discharge was 3 days in all groups. The median time to resolution of fishy odor was 2 days in the MVG 1.3% groups and 3 days in the MVG 0.75% group. None of these observed differences were statistically significant. No difference was observed in the median time to resolution of symptoms between the per-protocol and mITT populations.
FIGURE 2.
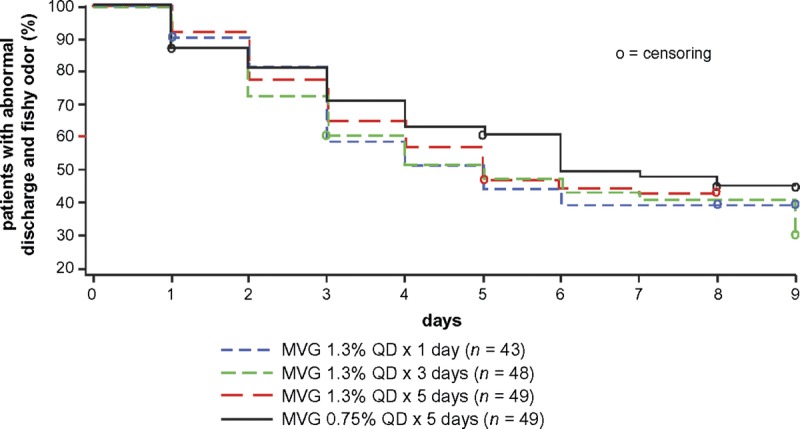
Kaplan-Meier analysis of the time to resolution of symptoms (abnormal discharge and fishy odor): per-protocol population.
In the per-protocol population, the proportion of patients whose symptoms initially resolved and subsequently returned was lowest in the 5-day MVG 1.3% group. Abnormal discharge and fishy odor returned in fewer patients in the 5-day MVG 1.3% group compared with the other groups (see Table 3). Similarly, abnormal discharge alone and fish odor alone returned in fewer patients in the 5-day MVG 1.3% group compared with the other groups.
TABLE 3.
Percentage of Patients Whose Symptoms Returned: Per-Protocol Population

Most patients (181/189, 96%) reported absent or mild vulvovaginal itching, irritation, and inflammation at baseline, with no important changes observed at the EOS/TOC visit. The majority of patients in the per-protocol (119/189, 63%) and mITT (138/228; 61%) populations experienced minimal to no leakage of MVG; however, a greater proportion of patients in the MVG 0.75% group reported minimal to no leakage compared with the MVG 1.3% groups.
On the EOS patient questionnaire, most patients in all treatment groups reported no return of BV symptoms (57%–76%). Regardless of treatment group, most patients indicated that MVG was “very easy to apply (138/189, 73.0%),” they were “very satisfied” with the treatment (102/189, 54.0%), and they preferred MVG for future symptoms of BV (114/189, 60.3%). More than half of the patients in the MVG 1.3% groups were “very satisfied” with the treatment compared with 44.9% (22/49 patients) in the MVG 0.75% group. The 1-day MVG 1.3% group had the highest ratings for “very easy to apply” (38 patients [88.4%]), “very convenient” (length of treatment; 35 patients [81.4%]), and “very satisfied” (27 patients [62.8%]). The 3-day MVG 1.3%, 5-day MVG 1.3%, and MVG 0.75% groups rated treatment as “convenient” (17 [35.4%], 22 [44.9%], and 22 [44.9%] patients, respectively) and “very convenient” (22 [45.8%], 16 [32.7%], and 17 [34.7%] patients, respectively).
Safety Outcomes
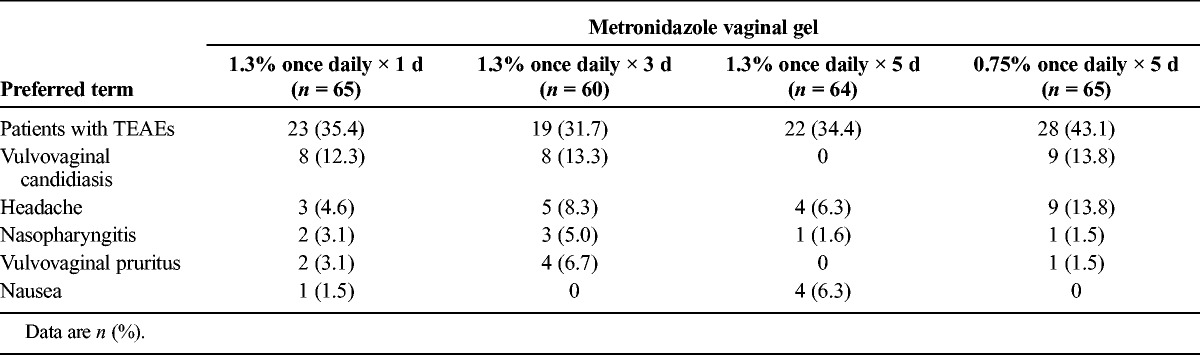
Overall, 92 patients (36.2%) were found to have AEs and TEAEs. No clinically important differences were observed in the incidence of AEs across treatment groups. The incidence of TEAEs was generally similar across treatment groups, with the exception of vulvovaginal candidiasis (see Table 4). Most AEs were mild or moderate in intensity and were assessed as unrelated to MVG. One patient in the 1-day MVG 1.3% group reported 2 severe AEs, vaginal burning and vulval edema beginning on day 3, which were determined to be likely related to treatment. In addition, another patient in the 1-day MVG 1.3% group reported a serious AE, hypoglycemia, which was determined to be unrelated to treatment. No deaths occurred during the study, and no discontinuations were related to TEAEs.
TABLE 4.
Most Frequent Treatment-Emergent Adverse Events Occurring in At Least 5% of Patients: Safety Population
DISCUSSION
The aim of this phase 2 study was to determine whether the efficacy, safety, and patient acceptability of MVG 1.3% applied over 1, 3, and 5 days were comparable to that of MVG 0.75% applied over 5 days. Overall, the therapeutic and clinical cure rates reported in the MVG 1.3% groups were numerically higher than those in the MVG 0.75% group, but the sample size in the trial was insufficient to demonstrate statistical superiority for any of the regimens. Bacteriological cure rates in the 1-day MVG 1.3% group were comparable to those in the MVG 0.75% group and numerically higher in the 3- and 5-day MVG 1.3% groups. Rates of resolution of symptoms were similar in all 4 treatment groups. Time to resolution of fishy odor was shorter in the MVG 1.3% groups than in the MVG 0.75% group. The 5-day MVG 1.3% group had the lowest rate of symptom return, while the 1-day MVG 1.3%, 3-day MVG 1.3%, and MVG 0.75% groups reported similar rates of symptom return. All 4 treatments had favorable tolerability and a low incidence of AEs, with no clinically important differences observed across treatment groups.
Although the cure rates were lower than what was estimated in the power analysis and those found in other studies of metronidazole gel,9 these results were not unexpected because more strict definitions for categories for cure were used. The definitions of cure and results from this study were similar to those found with oral tinidazole before its approval for BV in the United States.11 It should be emphasized, however, that differences in cure rates are difficult to compare across studies because the patient populations differ.
This study had a high rate of completion and relatively modest decrease in evaluable populations from enrollment to EOS, which were lower compared with historical studies. Because this initial dose-ranging study was powered to provide an estimate for the therapeutic cure rate but not to detect differences between treatment groups, no formal statistical testing was performed to compare treatments. Although the observed differences in cure rates were not statistically significant, the data suggest a dose-response relationship, with a trend toward higher cure rates with MVG 1.3% for 5 days. In addition, cure rates with MVG 1.3% for 1 day and for 3 days were comparable or better than MVG 0.75% for 5 days.
Achieving good patient compliance with multiday regimens can be challenging. In this study, patients reported that they were “very satisfied” with the 1-day application of MVG 1.3% and that this regimen was very convenient and very easy to apply. Overall, MVG 1.3% applied once daily for 1 day had the highest patient acceptability based on ease of application, convenience (length of treatment), and satisfaction with treatment. The 5-day MVG 1.3% regimen was rated highest for treatment preference of future BV symptoms. On the basis of the findings of this study, application of the MVG 1.3% dose for treatment of BV will be assessed in a phase 3 clinical study.
CONCLUSIONS
In this study, MVG 1.3% applied once daily for 1, 3, or 5 days showed similar efficacy, safety, and tolerability as MVG 0.75% applied once daily for 5 days. These findings support the development of MVG 1.3% as a safe and efficacious alternative to MVG 0.75% in patients with BV.
Footnotes
Writing and editorial assistance were provided by Peloton Advantage, LLC, and Scientific Connexions, funded by Medicis Pharmaceutical Corporation, a division of Valeant Pharmaceuticals.
Steven E. Chavoustie has received honoraria/speaking fees from Medicis Global Services Corporation. Arthur S. Waldbaum has received consulting fees from Medicis as payment for being an investigator in the study. Sharon F. Levy was employed by Graceway Pharma, original study sponsor, during the time of the study. After study completion, Dr. Levy has been employed by Prosoft Clinical, which has consulted on other projects with Valeant. Sharon L. Hillier has received no financial remuneration in the area of vaginitis; the University of Pittsburgh was compensated for the laboratory component of this study by Medicis and received NIH grant support. Dr. Hillier has served on advisory boards for Merck for antiretroviral drugs and has received from University of Toronto and State University of New York travel/accommodations/meeting expenses unrelated to the study in this article. Paul Nyirjesy has received consulting and research grants from Medicis Pharmaceutical Corporation. Mark Jacobs and Howard A. Reisman have no conflicts of interest to declare. This study was sponsored by Medicis Pharmaceutical Corporation, a division of Valeant Pharmaceuticals. Clinical Trial Registration: NCT01055106
REFERENCES
- 1. Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, et al. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG 2011; 118: 533– 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marrazzo JM. Interpreting the epidemiology and natural history of bacterial vaginosis: are we still confused? Anaerobe 2011; 17: 186– 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109: 114– 20. [DOI] [PubMed] [Google Scholar]
- 4. Fethers K, Marks C, Mindel A, Estcourt CS. Sexually transmitted infections and risk behaviours in women who have sex with women. Sex Transm Infect 2000; 76: 345– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fethers KA, Fairley CK, Morton A, Hocking JS, Hopkins C, Kennedy LJ, et al. Early sexual experiences and risk factors for bacterial vaginosis. J Infect Dis 2009; 200: 1662– 70. [DOI] [PubMed] [Google Scholar]
- 6. Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis 2008; 47: 1426– 35. [DOI] [PubMed] [Google Scholar]
- 7. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006; 55: 1– 94. [PubMed] [Google Scholar]
- 8. Brandt M, Abels C, May T, Lohmann K, Schmidts-Winkler I, Hoyme UB. Intravaginally applied metronidazole is as effective as orally applied in the treatment of bacterial vaginosis, but exhibits significantly less side effects. Eur J Obstet Gynecol Reprod Biol 2008; 141: 158– 62. [DOI] [PubMed] [Google Scholar]
- 9. Livengood CH, III, Soper DE, Sheehan KL, Fenner DE, Martens MG, Nelson AL, et al. Comparison of once-daily and twice-daily dosing of 0.75% metronidazole gel in the treatment of bacterial vaginosis. Sex Transm Dis 1999; 26: 137– 42. [DOI] [PubMed] [Google Scholar]
- 10. Soper DE, Livengood C, Sheehan KL, Fenner DE, Martens MG, Nelson AL, et al. Safety and efficacy comparison of two dosing regimens of MetroGel-Vaginal(R) in the treatment of bacterial vaginosis: a multicenter, randomized, single-blind, parallel comparison. Prim Care Update Ob Gyns 1998; 5: 150. [DOI] [PubMed] [Google Scholar]
- 11. Livengood CH, III, Ferris DG, Wiesenfeld HC, Hillier SL, Soper DE, Nyirjesy P, et al. Effectiveness of two tinidazole regimens in treatment of bacterial vaginosis: a randomised controlled trial. Obstet Gynecol 2007; 110 (2 Pt 1): 302– 9. [DOI] [PubMed] [Google Scholar]


