Summary
Arginine methylation is an important post-translational protein modification that modulates protein function for a wide range of biological processes. PIWI proteins, a subclade of the Argonaute family proteins, contain evolutionarily conserved symmetrical dimethylarginines (sDMAs). It has become increasingly apparent that the sDMAs of PIWI proteins serve as binding elements for TUDOR-domain containing proteins and that sDMA-dependent protein interactions play crucial roles in the biogenesis and function of PIWI-interacting RNAs (piRNAs). We describe a method for detecting PIWI sDMAs and purifying PIWI/piRNA complexes using anti-sDMA antibodies.
Keywords: PIWI, piRNA, Arginine methylation, Symmetrical dimethylarginine (sDMA), Y12, SYM10, SYM11
1. Introduction
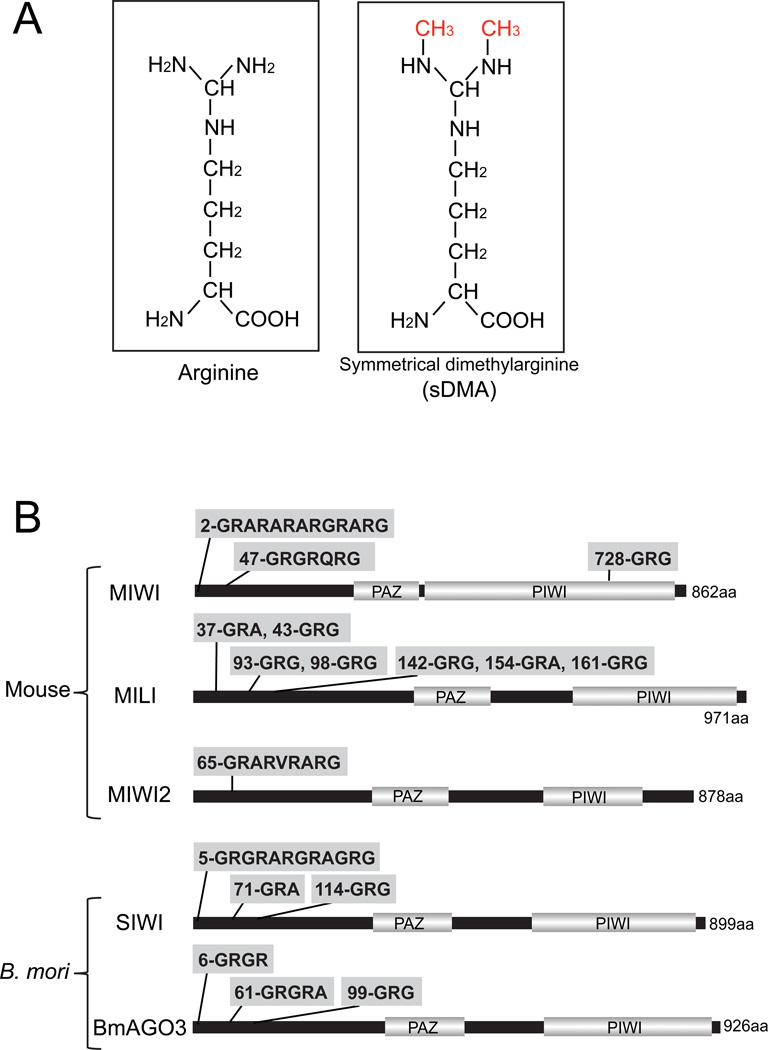
Arginine methylation is an important post-translational protein modification that plays crucial roles in numerous biological processes, such as structural remodeling of chromatin, signal transduction, mRNA splicing and DNA repair [1–5]. Arginine methylation is mediated by two types of protein methyltransferases (PRMTs): type I enzymes (e.g., PRMT1) catalyze asymmetrical dimethylarginine (aDMA) in which two methyl groups are placed on one of the terminal nitrogen atoms of the guanidino group, whereas type II enzymes (e.g., PRMT5) catalyze symmetrical dimethylarginine (sDMA, Fig 1A) in which one methyl group is placed on each of the terminal nitrogens [1–5]. sDMA modifications occur in “sDMA motifs” comprising arginines flanked by glycines (GRG) or alanines (GRA or ARG), which are often found as repeats. sDMAs are known to specifically bind to TUDOR domains of proteins and regulate protein–protein interactions [1–5]. For example, in mammals, sDMAs of Sm proteins, components of small nuclear ribonucleoproteins (snRNPs), promote their binding to the TUDOR domain of survival of motor neuron (SMN) protein, which facilitates snRNP assembly [6,7].
Fig 1. sDMA motifs in PIWI proteins.
(A) Chemical structure of arginine and sDMA.
(B) The structures of PIWI proteins (MIWI, MILI and MIWI2 for mouse; SIWI and BmAGO3 for Bombyx mori) are shown with sDMA motifs comprising GRG or GRA/ARG sequences. PAZ and PIWI are the two major protein motifs that exist in the Argonaute family proteins.
Arginine methylation and its mediated protein-protein interactions have attracted increased attention as key molecular factors in small regulatory RNA pathway in the germline. PIWI proteins, a subclade of the Argonaute family proteins, are predominantly expressed in the germline and bind to 25–31 nucleotide (nt) PIWI-interacting RNAs (piRNAs) to form PIWI-ribonucleoproteins (piRNPs) [8–10]. Mice express three PIWI proteins, MIWI, MILI and MIWI2 [11–13], and Bombyx mori (silkworm) expresses two PIWI proteins, SIWI and BmAGO3 [14]. piRNPs play critical roles in germline development by regulating transposons and other targets to maintain genome integrity. PIWI proteins contain evolutionarily conserved sDMAs that are synthesized by PRMT5 [15]. Putative sDMA motifs are typically clustered at the N-terminus of PIWI proteins (Fig 1B); in mouse and Drosophila, sDMA positions were further determined by mass spectrometry [16–19]. Several members of the TUDOR domain-containing protein family, such as Spindle-E, Tudor, Krimper, and Tejas in Drosophila [19–23] and TDRD1-9 in mice [16–18,24–26], have recently been studied for their PIWI interactions and functional involvements in piRNA biogenesis and function [27,28].
Here, we describe a method for detecting sDMA modifications of PIWI proteins. There are three highly-specific anti-sDMA antibodies: Y12, SYM10 and SYM11. Y12 is a monoclonal antibody that was generated from a hybridoma of lupus erythmatosus-like syndrome mice developing autoantibodies against Sm proteins [29,30]. The epitope that Y12 recognizes on Sm proteins comprises sDMAs of the proteins [31]. SYM10 and SYM11 are polyclonal antibodies derived from rabbit serum immunized with peptides containing sDMAs, K(sDMA)G(sDMA)G(sDMA)G(sDMA)G and KAAILKAQVAA(sDMA)G(sDMA)G(sDMA)GMG(sDMA)G, respectively [32,33]. We describe an utilization of these antibodies to detect sDMAs of MIWI and MILI, which were purified from mouse testicles, and SIWI and BmAGO3, which were transiently expressed and purified from BmN4, a Bombyx-mori ovary derived cultured cell line [34].
In addition, we describe a method to purify piRNP using immunoprecipitation with the Y12 antibody. For piRNA purification and identification, piRNPs are typically purified by anti-PIWI immunoprecipitation. However, we previously reported successful piRNP purification using Y12 immunoprecipitation for mouse testicles and Xenopus oocytes [15]; we here demonstrate that it can also be used for BmN4 cells. Our results suggest that the Y12 antibody can be widely used to purify piRNPs for identifying piRNA sequences in various organisms for which antibodies against PIWI proteins have not yet been generated.
2. Materials
Recombinant protein G agarose beads (Invitrogen)
Anti-Flag® M2-agarose from mouse (Sigma)
Y12 antibody (mouse monoclonal, a gift from G. Dreyfuss, University of Pennsylvania; Note 1)
SYM10 antibody (rabbit polyclonal, Millipore)
SYM11 antibody (rabbit polyclonal, Millipore)
Anti-MILI antibody (mouse monoclonal clone 17.8 [15]; Note 1)
Non-immune mouse and rabbit serum
Mouse testicle (Pel-Freez Biochemicals)
Lysis buffer: 20 mM Tris-HCl (pH 7.4); 200 mM NaCl; 2.5 mM MgCl2; 0.5% NP-40; 0.1% Triton X-100; one tablet of Complete protease inhibitor EDTA-free (Roche) per 50 mL of lysis buffer.
7 mL Dounce tissue grinder (Wheaton)
Bioruptor sonication system (Diagenode)
BmN4 cell line (a gift from S. Katsuma, University of Tokyo)
Expression plasmids for Flag-SIWI and Flag-BmAGO3: The N-terminal Flag/His-tagged SIWI or BmAGO3 were cloned into a pIZ/V5-His vector (a gift from S. Katsuma, University of Tokyo) [34].
Insect-Xpress medium (LONZA)
Sf-900™ III SFM (1×), liquid (Invitrogen)
ESCORT transfection reagent (Sigma)
NuPAGE LDS sample buffer (Invitrogen)
β-Mercaptoethanol
NuPAGE 4%–12% Bis-Tris gel (Invitrogen)
NuPAGE MOPS SDS running buffer (Invitrogen)
SilverQuest staining kit (Invitrogen)
Nitrocellulose/filter paper; 0.45 µm pore size (Invitrogen)
Transfer buffer: 62.5 mM Tris; 18 mM Glycine; 20% Methanol
TE70 ECL semi-dry transfer unit (GE Healthcare)
PBS (TEKNOVA)
PBST: PBS containing 0.1% Tween 20
Blocking solution: 5% non-fat dry milk in PBST
ECL anti-rabbit IgG, horseradish peroxidase linked F(ab’) 2 fragment from donkey (GE Healthcare)
ECL anti-mouse IgG, horseradish peroxidase linked F(ab’) 2 fragment from sheep (GE Healthcare)
ECL plus western blotting detection system (GE Healthcare)
ChemiDoc™ XRS+ system (Bio-Rad)
Trizol (Invitrogen)
Glycogen (Ambion)
3 M NaOAc, pH5.5 (Ambion)
Isopropanol (Sigma)
Centrifugal evaporator (myVac)
Alkaline phosphatase, calf intestinal; CIP (NEB)
T4 Polynucleotide Kinase; T4 PNK (NEB)
ATP [γ-32P] (American Radiolabeled Chemicals)
15% PAGE solution with 7 M Urea (1L): 420.42 g Urea (Sigma); 376 mL 40% acrylamide and bis-acrylamide solution (19:1, Bio-Rad); 100 mL Ultrapure™ 10×TBE buffer (Invitrogen) and MilliQ water to prepare 1L. After filtration, store at 4°C (protect from light).
Ammonium persulfate (Sigma)
Ultrapure™ TEMED (Invitrogen)
SE-400 electrophoresis system (Hoefer)
2×Loading buffer for Urea PAGE: 5.4 g Urea (Sigma); 6 mg Bromophenol blue (Sigma); 6 mg Xylene cyanol (Sigma) and MilliQ water for 10 mL.
Phosphor autoradiography plate (Kodak)
Molecular Imager PharosFX System (Bio-Rad)
3. Methods
3-1 Purification of MIWI and MILI from mouse testicles by immunoprecipitation
3-1-1 Preparation of antibody-bound agarose beads
Wash protein G agarose beads (10 µL bed volume) three times with 1 mL of lysis buffer.
Add either anti-MIWI (10 µL), anti-MILI (2.5 µL), non-immune mouse serum (NMS, negative control) or non-immune rabbit serum (NRS, negative control) to the beads in 700 µL of lysis buffer.
Rotate for 1 h at room temperature (RT).
Discard the buffer containing antibody and wash five times with 1 mL of lysis buffer.
3-1-2 Preparation of mouse testicle lysate
Use one mouse testicle per immunoprecipitation (500 µL of lysis buffer). Homogenize testicles in lysis buffer with a Dounce tissue grinder in a cold room (4°C).
Sonicate the homogenate using the Biorupter sonication system according to manufacturer’s instructions (on: 5 sec, off: 7 sec, cycles: 14, strength level: medium).
Centrifuge the lysate at 20,000 g for 10 min at 4°C. Collect the supernatant.
3-1-3 Immunoprecipitation
Add the testicle lysate to the prepared beads with antibodies and rotate for 1 h at 4°C.
Wash the beads five times with 1 mL of lysis buffer.
3-1-4 Visualization of purified proteins on SDS-PAGE by silver-staining
Add 30 µL of NuPAGE LDS sample buffer containing 10% β-Mercaptoethanol to the beads and incubate at 70°C for 15 min.
Run 10 µL of immunoprecipitate samples on NuPAGE 4%–12% Bis-Tris gel.
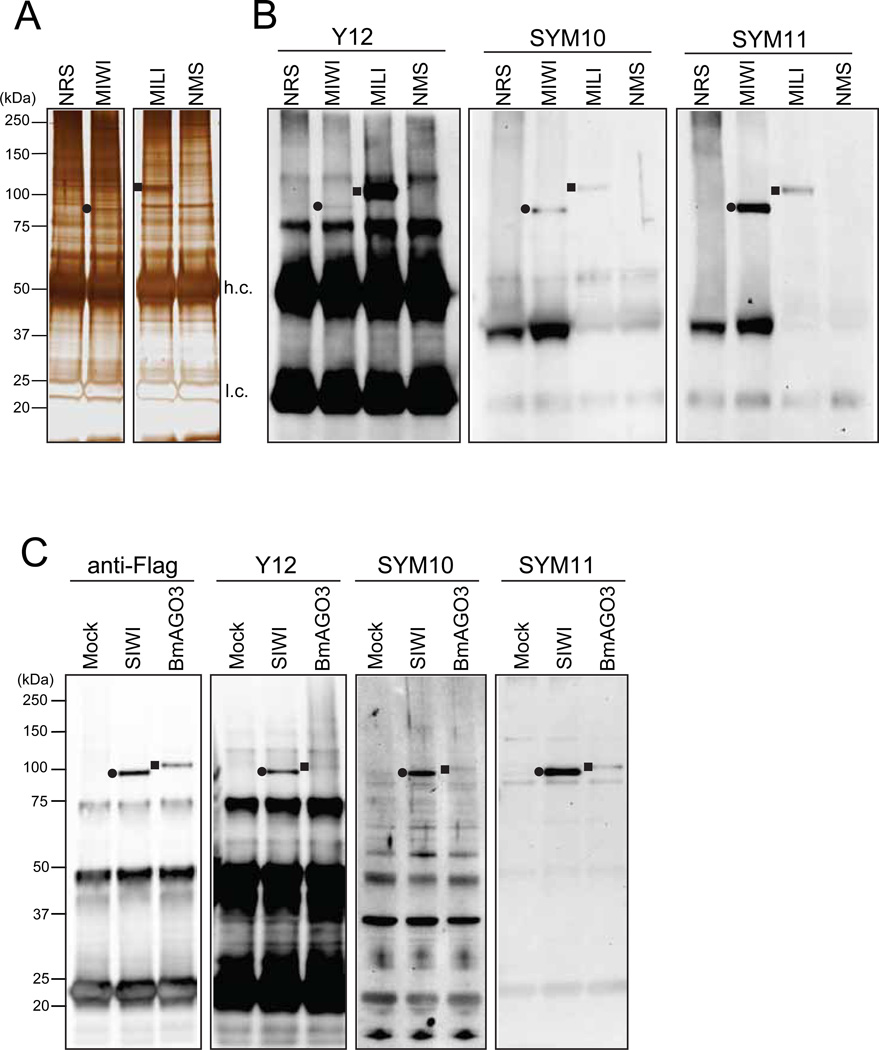
Stain with SilverQuest staining kit according to the manufacturer’s instructions (Fig 2A).
Fig 2. Detection of PIWI sDMAs by western blot with anti-sDMA antibodies.
(A) MIWI and MILI immunoprecipitates from mouse testicles were visualized by silver staining (NMS: non-immune mouse serum; NRS: non-immune rabbit serum). The filled circle and square indicate the bands for purified MIWI and MILI, respectively. h.c. and l.c. indicate antibody heavy and light chains, respectively.
(B) Immunoprecipitates from mouse testicles were probed on western blots with the indicated antibodies. The Y12, SYM10 and SYM11 antibodies recognized both MIWI (filled circle) and MILI (filled square), which indicated that MIWI and MILI contain sDMAs.
(C) Flag-SIWI or Flag-BmAGO3 expressed in BmN4 cells were immunopurified with an anti-Flag antibody (Mock: the sample from BmN4 cells with no transient protein expression) and probed on western blots with the indicated antibodies. All these antibodies recognized SIWI (filled circle) and BmAGO3 (filled square), indicating the presence of sDMAs in SIWI and BmAGO3.
3-2 Purification of SIWI and BmAGO3 from BmN4 cells by immunoprecipitation
3-2-1 Transient expression of Flag-SIWI and Flag-BmAGO3 in BmN4 cells
Spread and culture 5×106 BmN4 cells on a 10 cm dish in Insect X-press medium at 27°C for 24 h, then change the medium with 5 mL of Sf-900 medium.
Gently mix 13 µg of expression plasmid with 40 µL of ESCORT reagent and 650 µL of Sf-900 medium by pipetting, and then incubate at RT for 15 min.
Add this mixture to cells, incubate at 27°C for 6 h, and add 5 mL of Sf-900 medium.
After three days, collect cells with a scraper and wash the cells twice with 1 mL of PBS.
3-2-2 Preparation of BmN4 cell lysate
Resuspend cells in lysis buffer (5×106 cells in 200 µL of lysis buffer per immunoprecipitation).
Sonicate the suspension and collect the supernatant as described in 3-1-2.
3-2-3 Anti-Flag Immunoprecipitation
Wash anti-Flag agarose beads (10 µL bed volume) three times with 1 mL of lysis buffer.
Add the 200 µL of lysate to the beads and adjust total volume to 500 µL with lysis buffer so that the beads are not stacked within the tube during rotation.
Rotate for 1 h at 4°C, and then prepare samples for SDS-PAGE as described in 3-1-4.
3-3 Detection of PIWI sDMAs by western blots using anti-sDMA antibodies
3-3-1 SDS-PAGE and Transfer
Run 4 µL of immunoprecipitate samples on NuPAGE 4%–12% Bis-Tris gel.
Immerse the gel, nitrocellulose membrane and filter paper in transfer buffer and perform transfer procedure with TE70 ECL semi-dry transfer unit according to the manufacturer’s instructions (run at 90 mA for 45 min).
3-3-2 Immunoblotting and detection
Rinse the membrane with MilliQ water, and then incubate the membrane with blocking buffer at 70 rpm for 1 h at RT.
Discard the blocking buffer and rinse the membrane twice with PBST.
Incubate the membrane with a primary antibody solution (anti-Flag 1:1000; Y12 1:500; SYM10 1:1000; and SYM11 1:1000; each diluted in blocking buffer) at 4°C overnight.
Rinse the membrane briefly and wash three times with PBST for 10 min.
Incubate the membrane with a secondary antibody solution (1:5000; diluted in PBST) at RT for 1 h.
Rinse the membrane with PBST, wash three times with PBST for 10 min, and immerse in PBS.
Detect bands using ECL-Plus detection solution and ChemiDoc according to the manufacturer’s instructions (see Fig 2B, 2C and Note 2).
3-4 Isolation of piRNPs from BmN4 cells by immunoprecipitation with Y12 antibody
3-4-1 Immunoprecipitation with Y12 antibody
Prepare protein G agarose beads (10 µL bed volume) bound to Y12 (5 µL) as described in 3-1-1.
Prepare BmN4 cell lysate (1×107 cells per immunoprecipitation) as described in 3-2-2.
Add the lysate to the beads and rotate at 4°C for 2 h.
Wash the beads five times with 1 mL of lysis buffer.
3-4-2 Isolation of piRNAs
Add 500 µL of Trizol reagent to the immunoprecipitate beads and vortex for 30 sec.
Add 100 µL of chloroform, vortex for 15 sec, and let stand at RT for 2 min.
Centrifuge at 20,000 g for 30 min at 4°C. Collect the upper aqueous phase (carefully exclude the interphase).
Add 2 µL of glycogen (5 mg/mL), vortex briefly, add 350 µL of isopropanol, and vortex again. Cool the tube at −20°C for 20 min.
Centrifuge at 20,000 g for 30 min at 4°C. Carefully remove the supernatant and dry the pellet for 1 min with a centrifugal evaporator.
Dissolve the pellet in 14 µL of MilliQ water with thorough pipetting.
Store the piRNA solution at −80°C.
3-4-3 5’-end radiolabeling of isolated piRNAs
To remove the 5’-end phosphate of piRNAs, incubate 7 µL of the isolated piRNA solution with 0.5 µL of CIP, 2 µL of 10×buffer and 10.5 µL MilliQ water (total 20 µL) at 37°C for 30 min.
Adjust the total volume to 100 µL with MilliQ water, add 100 µL of phenol and thoroughly vortex.
Centrifuge at 20,000 g for 5 min at RT and then carefully collect the upper phase.
Add 2 µL of glycogen (5 mg/mL), 10 µL of 3M NaOAc and briefly vortex. Then, add 275 µL of chilled 100% ethanol, vortex well and cool the tube at −80°C for 30 min.
Centrifuge at 20,000 g for 30 min at 4°C. Carefully remove the supernatant and dry the pellet for 1 min with a centrifugal evaporator.
Dissolve the pellet with 7.5 µL of MilliQ water with thorough pipetting.
For 5’-end labeling of piRNAs, incubate 7.5 µL of dephosphorylated piRNA solution with 1 µL of [γ-32P]ATP, 0.5 µL of T4 PNK and 1 µL of 10×buffer (total 10 µL) for 1 h at 37°C.
3-4-4 Separation and detection of labeled piRNAs
Set up the gel apparatus (SE-400 system) using 18×24 cm glass plate and 0.75 mm thick combs.
Add 150 µL of 10% ammonium persulfate and 10 µL of TEMED to 30 mL of 15% 7M Urea PAGE solution. Mix gently and immediately pour into the apparatus.
Run the gel at 300 V for 30 min and wash the wells with a syringe to remove accumulated urea in the wells.
Add an equal volume of 2×loading buffer to the labeled piRNA solution.
Run 10 µL of the samples at 700 V until the dye (BPB) front reaches the bottom of the gel.
Disassemble glass plates and peel out one side of glass plate with a spatula. Cover the gel with a wrap and expose the gel to a phosphor autoradiography plate in a cassette for 1 h–overnight at −80°C.
Scan the plate using a PharosFX phosphor imager (see Fig 3, Note 2 and Note 3).
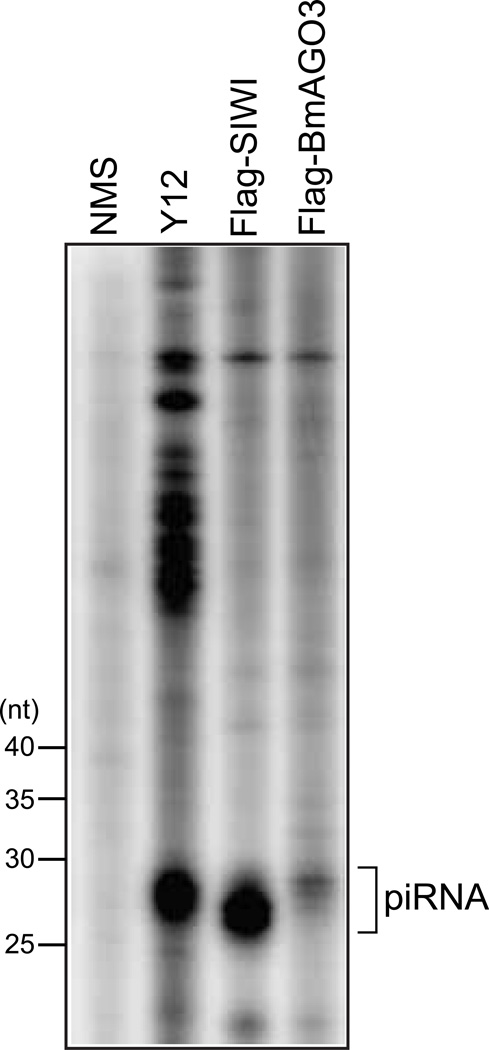
Fig 3. piRNP purification by immunoprecipitation with the Y12 antibody.
Y12 immunoprecipitate from BmN4 cells, and anti-Flag immunoprecipitates from BmN4 cells expressing Flag-SIWI or Flag-BmAGO3 were subjected to RNA extraction, 5’-end labeling of the extracted RNA, and denaturing PAGE. piRNAs were clearly observed in Y12 immunoprecipitate as well as PIWI’s, which indicated the successful purification of piRNPs with the Y12 antibody.
Acknowledgments
We are grateful to G. Dreyfuss for the Y12 antibody, to S. Katsuma for BmN4 and PIWI expression constructs, and to Z. Mourelatos for support and discussion. This work was supported by the Cedars-Sinai Medical Center Research Fund, Martz Translational Breast Cancer Research Fund, and a Grant for Basic Science Research Project from The Sumitomo Foundation (YK).
Footnotes
The Y12, anti-MIWI, and anti-MILI antibodies are commercially available from various distributors.
The affinities of the respective anti-sDMAs for PIWI proteins have certain differences. SYM10 and SYM11 recognize MIWI and SIWI well. This is because both of these PIWI proteins contain a long GRG/ARG/GRA repeat (Fig 1B) that is similar to the sequences of the peptides used for producing SYM10 and SYM11 [32,33]. In contrast, the Y12 antibody attaches to MILI much more strongly than to MIWI on western blot (Fig 2B) and in immunoprecipitation [15].
It has been reported that immunoprecipitation with SYM10 or SYM11 could be used to pull down various sDMA-containing proteins, including Sm proteins [32,33,35]. Based on these reports, we attempted to perform SYM10 and SYM11 immunoprecipitation using lysates from mouse testicles or Drosophila ovaries to purify piRNPs. However, despite using different lysis buffers containing different salt concentrations (100–200 mM), we were unable to detect PIWI proteins and piRNAs in these immunoprecipitates. It was observed that, among the anti-sDMA antibodies, the Y12 antibody was particularly useful for purifying piRNPs.
References
- 1.Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005;18(3):263–272. doi: 10.1016/j.molcel.2005.04.003. doi:S1097-2765(05)01247-5 [pii] 10.1016/j.molcel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Krause CD, Yang ZH, Kim YS, Lee JH, Cook JR, Pestka S. Protein arginine methyltransferases: evolution and assessment of their pharmacological and therapeutic potential. Pharmacol Ther. 2007;113(1):50–87. doi: 10.1016/j.pharmthera.2006.06.007. doi:S0163-7258(06)00121-5 [pii] 10.1016/j.pharmthera.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Wolf SS. The protein arginine methyltransferase family: an update about function, new perspectives and the physiological role in humans. Cell Mol Life Sci. 2009;66(13):2109–2121. doi: 10.1007/s00018-009-0010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33(1):1–13. doi: 10.1016/j.molcel.2008.12.013. doi:S1097-2765(08)00856-3 [pii] 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackwell E, Ceman S. Arginine methylation of RNA-binding proteins regulates cell function and differentiation. Mol Reprod Dev. 2012;79(3):163–175. doi: 10.1002/mrd.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yong J, Wan L, Dreyfuss G. Why do cells need an assembly machine for RNA-protein complexes? Trends Cell Biol. 2004;14(5):226–232. doi: 10.1016/j.tcb.2004.03.010. S0962892404000844 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Meister G, Eggert C, Fischer U. SMN-mediated assembly of RNPs: a complex story. Trends in cell biology. 2002;12(10):472–478. doi: 10.1016/s0962-8924(02)02371-1. [DOI] [PubMed] [Google Scholar]
- 8.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94–108. doi: 10.1038/nrg2504. doi:nrg2504 [pii] 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10(2):126–139. doi: 10.1038/nrm2632. doi:nrm2632 [pii] 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 10.Farazi TA, Juranek SA, Tuschl T. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development. 2008;135(7):1201–1214. doi: 10.1242/dev.005629. doi:dev.005629 [pii] 10.1242/dev.005629. [DOI] [PubMed] [Google Scholar]
- 11.Kuramochi-Miyagawa S, Kimura T, Yomogida K, Kuroiwa A, Tadokoro Y, Fujita Y, Sato M, Matsuda Y, Nakano T. Two mouse piwi-related genes: miwi and mili. Mechanisms of development. 2001;108(1–2):121–133. doi: 10.1016/s0925-4773(01)00499-3. [DOI] [PubMed] [Google Scholar]
- 12.Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Developmental cell. 2002;2(6):819–830. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
- 13.Carmell MA, Girard A, van de Kant HJ, Bourc'his D, Bestor TH, de Rooij DG, Hannon GJ. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Developmental cell. 2007;12(4):503–514. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Kawaoka S, Minami K, Katsuma S, Mita K, Shimada T. Developmentally synchronized expression of two Bombyx mori Piwi subfamily genes, SIWI and BmAGO3 in germ-line cells. Biochem Biophys Res Commun. 2008;367(4):755–760. doi: 10.1016/j.bbrc.2008.01.013. doi:S0006-291X(08)00047-8 [pii] 10.1016/j.bbrc.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Kirino Y, Kim N, de Planell-Saguer M, Khandros E, Chiorean S, Klein PS, Rigoutsos I, Jongens TA, Mourelatos Z. Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nature cell biology. 2009;11(5):652–658. doi: 10.1038/ncb1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuter M, Chuma S, Tanaka T, Franz T, Stark A, Pillai RS. Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile. Nat Struct Mol Biol. 2009;16(6):639–646. doi: 10.1038/nsmb.1615. doi:nsmb.1615 [pii] 10.1038/nsmb.1615. [DOI] [PubMed] [Google Scholar]
- 17.Vagin VV, Wohlschlegel J, Qu J, Jonsson Z, Huang X, Chuma S, Girard A, Sachidanandam R, Hannon GJ, Aravin AA. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 2009;23(15):1749–1762. doi: 10.1101/gad.1814809. doi:gad.1814809 [pii] 10.1101/gad.1814809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Jin J, James DA, Adams-Cioaba MA, Park JG, Guo Y, Tenaglia E, Xu C, Gish G, Min J, Pawson T. Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc Natl Acad Sci U S A. 2009;106(48):20336–20341. doi: 10.1073/pnas.0911640106. doi:0911640106 [pii] 10.1073/pnas.0911640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishida KM, Okada TN, Kawamura T, Mituyama T, Kawamura Y, Inagaki S, Huang H, Chen D, Kodama T, Siomi H, Siomi MC. Functional involvement of Tudor and dPRMT5 in the piRNA processing pathway in Drosophila germlines. EMBO J. 2009;28(24):3820–3831. doi: 10.1038/emboj.2009.365. doi:emboj2009365 [pii] 10.1038/emboj.2009.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313(5785):320–324. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 21.Lim AK, Kai T. Unique germ-line organelle, nuage, functions to repress selfish genetic elements in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(16):6714–6719. doi: 10.1073/pnas.0701920104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil VS, Kai T. Repression of retroelements in Drosophila germline via piRNA pathway by the Tudor domain protein Tejas. Curr Biol. 2010;20(8):724–730. doi: 10.1016/j.cub.2010.02.046. doi:S0960-9822(10)00234-4 [pii] 10.1016/j.cub.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 23.Kirino Y, Vourekas A, Sayed N, de Lima Alves F, Thomson T, Lasko P, Rappsilber J, Jongens TA, Mourelatos Z. Arginine methylation of Aubergine mediates Tudor binding and germ plasm localization. RNA. 2010;16(1):70–78. doi: 10.1261/rna.1869710. doi:rna.1869710 [pii] 10.1261/rna.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Saxe JP, Tanaka T, Chuma S, Lin H. Mili interacts with tudor domain-containing protein 1 in regulating spermatogenesis. Curr Biol. 2009;19(8):640–644. doi: 10.1016/j.cub.2009.02.061. doi:S0960-9822(09)00877-X [pii] 10.1016/j.cub.2009.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima K, Kuramochi-Miyagawa S, Chuma S, Tanaka T, Nakatsuji N, Kimura T, Nakano T. Associations between PIWI proteins and TDRD1/MTR-1 are critical for integrated subcellular localization in murine male germ cells. Genes Cells. 2009;14(10):1155–1165. doi: 10.1111/j.1365-2443.2009.01342.x. doi:GTC1342 [pii] 10.1111/j.1365-2443.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 26.Shoji M, Tanaka T, Hosokawa M, Reuter M, Stark A, Kato Y, Kondoh G, Okawa K, Chujo T, Suzuki T, Hata K, Martin SL, Noce T, Kuramochi-Miyagawa S, Nakano T, Sasaki H, Pillai RS, Nakatsuji N, Chuma S. The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell. 2009;17(6):775–787. doi: 10.1016/j.devcel.2009.10.012. doi:S1534-5807(09)00434-1 [pii] 10.1016/j.devcel.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Siomi MC, Mannen T, Siomi H. How does the royal family of Tudor rule the PIWI-interacting RNA pathway? Genes Dev. 2010;24(7):636–646. doi: 10.1101/gad.1899210. doi:24/7/636 [pii] 10.1101/gad.1899210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Nott TJ, Jin J, Pawson T. Deciphering arginine methylation: Tudor tells the tale. Nat Rev Mol Cell Biol. 2011;12(10):629–642. doi: 10.1038/nrm3185. [DOI] [PubMed] [Google Scholar]
- 29.Lerner EA, Lerner MR, Janeway CA, Jr, Steitz JA. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisetsky DS, Lerner EA. Idiotypic analysis of a monoclonal anti-Sm antibody. Journal of immunology. 1982;129(4):1489–1492. [PubMed] [Google Scholar]
- 31.Brahms H, Raymackers J, Union A, de Keyser F, Meheus L, Luhrmann R. The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for anti-Sm autoantibodies. J Biol Chem. 2000;275(22):17122–17129. doi: 10.1074/jbc.M000300200. M000300200 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Boisvert FM, Cote J, Boulanger MC, Cleroux P, Bachand F, Autexier C, Richard S. Symmetrical dimethylarginine methylation is required for the localization of SMN in Cajal bodies and pre-mRNA splicing. The Journal of cell biology. 2002;159(6):957–969. doi: 10.1083/jcb.200207028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boisvert FM, Cote J, Boulanger MC, Richard S. A proteomic analysis of arginine-methylated protein complexes. Molecular & cellular proteomics : MCP. 2003;2(12):1319–1330. doi: 10.1074/mcp.M300088-MCP200. [DOI] [PubMed] [Google Scholar]
- 34.Kawaoka S, Hayashi N, Suzuki Y, Abe H, Sugano S, Tomari Y, Shimada T, Katsuma S. The Bombyx ovary-derived cell line endogenously expresses PIWI/PIWI-interacting RNA complexes. RNA. 2009;15(7):1258–1264. doi: 10.1261/rna.1452209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Zhang S, Zhang Y, Wang X, Li D, Li Q, Yue M, Zhang YE, Xu Y, Xue Y, Chong K, Bao S. Arabidopsis floral initiator SKB1 confers high salt tolerance by regulating transcription and pre-mRNA splicing through altering histone H4R3 and small nuclear ribonucleoprotein LSM4 methylation. The Plant cell. 2011;23(1):396–411. doi: 10.1105/tpc.110.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]