Abstract
Tan Yue, Wen Xiaosa, Qi Ruirui, Shi Wencai, Xin Hailiang, and Li Min. The effects of Portulaca oleracea on hypoxia-induced pulmonary edema in mice. High Alt Med Biol 16:43–51, 2015—Portulaca oleracea L. (PO) is known as “a vegetable for long life” due to its antioxidant, anti-inflammatory, and other pharmacological activities. However, the protective activity of the ethanol extract of PO (EEPO) against hypoxia-induced pulmonary edema has not been fully investigated. In this study, we exposed mice to a simulated altitude of 7000 meters for 0, 3, 6, 9, and 12 h to observe changes in the water content and transvascular leakage of the mouse lung. It was found that transvascular leakage increased to the maximum in the mouse lung after 6 h exposure to hypobaric hypoxia. Prophylactic administration of EEPO before hypoxic exposure markedly reduced the transvascular leakage and oxidative stress, and inhibited the upregulation of NF-kB in the mouse lung, as compared with the control group. In addition, EEPO significantly reduced the levels of proinflammatory cytokines and cell adhesion molecules in the lungs of mice, as compared with the hypoxia group. Our results show that EEPO can reduce initial transvascular leakage and pulmonary edema under hypobaric hypoxia conditions.
Key Words: : hypoxia, nuclear factor kappa B, oxidative stress, Portulaca oleracea, pulmonary edema
Introduction
Repeated immune or non-immune insults to the lung may increase pulmonary vascular permeability, and induce pulmonary edema. Increased pulmonary vascular permeability may arouse the inflammatory process, leading to pulmonary edema and worsening hypoxia in acute respiratory distress syndrome (ARDS) as a vicious cycle. Under hypoxic conditions, imbalance of forces that drive and remove water into and out of airspace has been recognized as two fundamental mechanisms of pulmonary edema (Staub, 1974). When the alveolar epithelium is exposed to a high altitude environment, damaged vascular endothelial cells reduce the bioavailability of endogenous vasodilator molecules (Duplain et al., 1999; Ingram et al., 2010) and increase the expression of cytokines and adhesion molecules. In addition, reactive oxygen species (ROS) formed in the mitochondria contributes to pulmonary injury (Guzy et al., 2006; Waypa et al., 2008). Despite these findings, inflammation does not seem to initiate the events leading to human high-altitude pulmonary edema (HAPE) (Swenson et al., 2002). During hypoxia exposure, oxidative stress is characterized by increased production of oxidants and/or decreased concentrations of antioxidants and antioxidant enzymes. Oxidants may damage tissues either by direct oxidation of key biological molecules (i.e., lipid peroxidation, DNA damage) or by alteration of transcription factors, such as nuclear factor-kB (Toledano et al., 1991). The normal cell gene expression profile is altered by activating transcriptional factors (Kunsch et al., 1999), resulting in severe pulmonary vascular permeability. It would be useful to find substances that could reduce pulmonary edema and oxidative stress seen in mice under hypoxic conditions.
Recent pharmacological studies (Chandel et al., 2000) have shown that Portulaca oleracea L. (PO) has analgesic, anti-inflammatory, and antioxidant activities. Previous studies showed that flavonoids, coumarins, monoterpene glycoside, and alkaloids were the major bioactive components in PO (Awad, 1994; Sakai et al., 1996). About 10% of ethanol extract of Portulaca oleracea (EEPO) possessed analgesic and anti-inflammatory activities as compared with synthetic drugs. Some other studies also reported that PO could be used to reduce the incidence of cardiovascular disease (Liu et al., 2000) and the expression of TNF-α in vascular endothelial cells (Lee et al., 2012). Our previous studies confirmed that the ethanol extract of EEPO could prolong the survival and reduce oxidative stress-induced injuries in mice (Wang et al., 2007; Chen et al., 2009; Wanyin et al., 2012). However, little is known about the protective effect of PO against pulmonary edema. The aim of this study was to determine whether EEPO could attenuate hypoxia-induced lung injury and explore the action mechanism of EEPO underlying this effect, if any.
Materials and Methods
Experimental animals and treatment
The experiment was carried out with male Bal b/c mice weighing 19–24 g (Super-B&K Laboratory Animal Corp., Shanghai, China). All animal procedures were done strictly in accordance with international ethical guidelines and the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, and were approved by the Experimental Animal Administration Committee of the Second Military Medical University (Shanghai, China). The animals were maintained in the animal house of the institute at 24°±1°C with a 12–12 h light dark cycle with unrestricted access to food and water.
Preparation of EEPO
All the aerial parts of PO used in the experiment were collected from Henan Province, China, and identified by Professor Zheng Han-chen from the Second Military Medical University. The voucher specimens were preserved in the Department of Traditional Chinese Medicine of the university. The powdered aerial parts of PO were refluxed twice with 80% ethanol solution, each time for 1 h. The extract was concentrated under reduced pressure to 80 L and then centrifuged at 5000 rpm for 4 min. The precipitation part was used as test materials. The yield of dried EEPO was 1.5±0.03%.
Hypoxic exposure
The experiment was carried out in two steps.
Step One:
A total of 50 mice were raised in animal breeding rooms. After 5-day adaptation, the animals were randomized into five equal groups. Group 1 served as a control group; Groups 2, 3, 4, and 5 were exposed to hypoxia for 3, 6, 9, and 12 hours, respectively. The mice were exposed to a simulated altitude of 7000 m in a hypobaric chamber for different periods of time. The temperature of the hypobaric chamber was set at 24°±1°C and the humidity of the chamber was maintained at 60±2%. The animals were provided with adequate quantities of food and water during exposure to hypoxia. After hypoxic exposure, the animals were anesthetized and sacrificed. The lungs were then perfused with cold phosphate buffered saline (PBS) to determine pulmonary edema and vascular permeability.
Step Two:
An additional 150 mice were divided equally into five groups: the control group (Control), hypoxia group (Hypoxia), low-dose EEPO group (Hyp+L EEPO), medium-dose EEPO group (Hyp+M EEPO) and high-dose EEPO group (Hyp+H EEPO). All animals were weighed to modulate EEPO administration. The animals in the three EEPO groups were orally administered with 100, 200, and 400 mg/kg (body weight) EEPO in 0.5 mL 0.5% CMC-Na solution as described in our previous study (Chen et al., 2009). Animals in the control group were orally administered with 0.5 mL 0.5% CMC-Na solution. The administration was carried out once a day lasting 7 days. One hour after the last drug administration, the mice were exposed to a simulated altitude 7000 m for 6 h in a hypobaric chamber. Mice in the control group were not exposed to hypoxia. The hypobaric chamber's settings were set according to Step One.
Determination of pulmonary edema
Lung water content
After exposure to hypoxia, the animals were anesthetized and sacrificed, and lung tissues were excised en bloc after perfusion with physiological saline, and weighed for wet weight immediately. The samples were dried in a hot air oven at 80°C for 72 h. Later, the dried tissue was weighed. The wet-to-dry ratio was calculated with the following equation: ratio=wet weight/dry weight (Yoshinari, 2001).
Determination of vascular permeability
The vascular permeability assays were performed as previously described (Kolosova et al., 2008). Evans Blue dye at a dose of 20 mg/kg (body weight) was injected into the mice via the caudal vein 30 min before tissue collection. The mouse was then removed from the hypoxia chamber, anesthetized, and perfused with PBS. The lungs were removed and kept in an oven at 80°C for 72 h. The lungs were homogenized in 1 mL PBS and incubated with 2 volumes of formamide at 60°C for 24 h. The homogenate was then centrifuged at 5000 rpm for 30 min. The optical density (OD) of the supernatant was determined at 620 nm. The results are presented as OD per gram (OD/g) dry weight.
Concentration of total protein in bronchoalveolar lavage fluid
Bronchoalveolar lavage fluid (BALF) was collected as described by Kuo-Hsun Chiu et al (Chiu et al., 2010) with minor modifications. Briefly, after hypoxia exposure, mice were anesthetized with sodium pentobarbital intraperitoneally (0.01 mg/g body weight). 0.05 mL/g body weight normal saline (NS) was gently instilled into the lungs through the tracheal cannula. The supernatant was frozen at −80°C for subsequent assays. The concentration of total protein in the lavage fluid was measured (μg/μL) in a commercially available kit (Beyotime, China) according to the manufacturer's instructions.
Histopathology
After hypoxic exposure, the lungs were inflated with NS, and perfused with 4% paraformaldehyde. The excised lungs were fixed in 4% paraformaldehyde for 24 h at 4°C and the lung tissues were implanted with paraffin. Tissue damage was determined by staining the tissue sections with hematoxylin and eosin (HE). The lung damage was detected by lung injury scoring method (Su et al., 2003). The degree of microscopic injury was scored based on the following variables: alveolar and interstitial edema, neutrophil infiltration, and hemorrhage. The severity of injury was graded for each variable: no injury=0; injury to 25% of the field=1; injury to 50% of the field=2; injury to 75% of the field=3; and diffuse injury=4. All samples were analyzed by a pathologist blinded to the experimental protocol and the region of sampling. The three slides from each lung sample were randomly screened, and the mean was taken as the representative value of the sample.
Determination of oxidative stress
After hypoxic exposure, the animals were anesthetized and sacrificed and washed with PBS to collect the lung tissue and prepare the homogenate for determining biochemical parameters in different levels of EEPO and the control groups. The production of ROS in the lung homogenate was determined by DCFHDA (2,7,dichlorofluorescein diacetate) and fluorescence was measured with an excitation at 485 nm and emission at 530 nm (Cathcart et al., 1983). Malondialdehyde (MDA) level in the lung was determined by 2-thiobarbuturic acid (TBA) reaction with the method of Okhawa et al. (1979). Reduced glutathione (GSH) in the lung was determined with dithionitrobenzoic acid (DTNB) reagent and by measuring the absorbance at 412 nm (Kum-Tatt et al., 1974). Superoxide dismutase (SOD) level in the lung was measured with a commercial kit according to the manufacturer's instructions. The protein concentration was estimated in a commercial BCA kit (Beyotime, China), and bovine serum albumin was used as a standard (Lowry et al., 1951).
Inflammatory cytokines in the lung
The tissue homogenate was analyzed with IL-1β and TNF-α enzyme-linked immunosorbent assay (ELISA) kits (NeoBioscience, China) according to the manufacturer's instructions. IL-1β and TNF-α concentrations were determined as pictograms per milliliter based on the appropriate standard curve.
Protein expression study
The lungs were perfused with cold PBS, removed wholly, washed with cold NS, and then homogenized with a commercial kit (KeyGEN, China). The homogenate was centrifuged at 10,000 rpm for 30 min at 4°C, and the supernatant was collected and stored at −80°C.
To determine the NF-kB pathway, cytoplasmic and nuclear fractions were isolated from the lung homogenate by using a commercial kit (Bio-Vision, USA) according to the manufacturer's instructions. NF-kB protein levels in cytoplasmic and nuclear fractions were determined by Western blotting.
The protein (100 μg) was separated in 10% sodium dodecylsulphate-polyacryl-amide gel electrophoresis (Bio-Rad, Hercules, CA) and electroblotted onto the nitrocellulose membrane (Millipore, Temecula, CA). The membrane was blocked with 5% dried skim milk for 2 h and washed with TBST for 10 min×3. The membrane with the tissue homogenate was probed with ICAM-1, VCAM-1, and P-selectin (Santa Cruz Technology, Dallas, TX) primary antibodies in 1:1000 dilution. The membrane with the nuclear sample was probed with p65 (Santa Cruz Technology) primary antibodies in 1:1000 dilution, and the membrane with the cytoplasmic sample was probed with Ikk (Santa Cruz Technology) primary antibodies in 1:1000 dilution overnight. The membrane was washed, incubated with secondary antibodies conjugated with horseradish peroxidase (1:2000; Santa Cruz Technology) for 1 h at room temperature, and thoroughly washed with TBST 3 times. The bands were visualized by SuperSignal West Dura Extended Duration Substrate (Thermo Scientific). Imaging was carried out in a UVP BioChem Imaging System, and densitometry was quantified with LabWorks software.
Statistical analysis
Statistical analysis was performed by SPSS 15.0 software. Data were subjected to ANOVA, followed by Student-Newman-Keuls tests. Differences were considered to be significant for p<0.05. Results are expressed as mean±SD.
Results
Determination of pulmonary edema
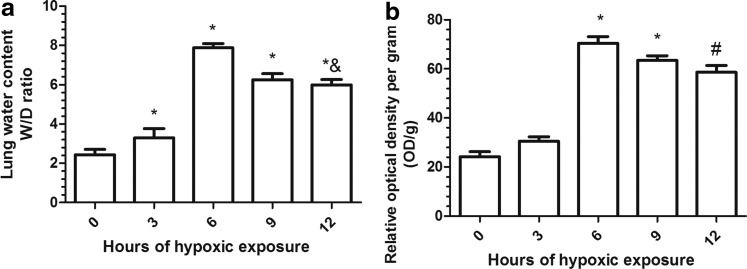
The lung wet-to-dry ratio was significantly affected by hypoxic exposure in the control animals (Fig. 1a). Maximum pulmonary edema was observed at 6 h after hypoxic exposure, and the severity was nearly four-fold that of the control group. With the exposure time extending to 12 h, the wet-to-dry ratio became even lower as compared with the 6 h group (p<0.01).
FIG. 1.
Effect of hypobaric hypoxia on (a) lung water content and (b) pulmonary vascular leakage in mice. Mice were exposed to simulated hypoxia for different time points. The water content was determined using wet-to-dry ratio, and pulmonary vascular leakage was determined using Evans Blue. The maximum water content and pulmonary vascular leakage were obtained at 6 h after hypoxic exposure, indicating that pulmonary edema was induced at this time point. Values are mean±SD (n=10). Significant differences between groups were determined by analysis of variance followed by Student-Newman-Keuls test. #p<0.05, compared with control group (0 h), *p<0.01 compared with control group (0 h), &p<0.05 compared with hypoxia group (6 h).
With respect to relative OD, there was a significant increase in vascular permeability in the lungs of the mice exposed to the hypoxic environment as compared with the control group. The maximum value occurred when the mice were exposed to hypoxia for 6 h. Vascular permeability continued to fall with the exposure time increasing (Fig. 1b).
The subsequent work was performed by exposing the mice for 6 h only, since maximum increase pulmonary vascular leakage was observed at 6 h after hypoxic exposure.
EEPO protection
Histopathology
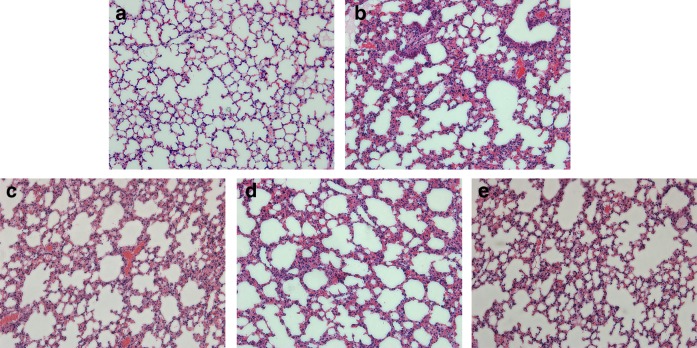
After hypoxic exposure, more protein-rich fluid and more leukocyte infiltration were observed in the alveoli as compared with the control group. Severe congestion and hemorrhage were also observed in the hypoxia groups (Fig. 2b). However, there was less alveolar flooding and less neutrophil infiltration in 100, 200, and 400 mg/kg dose groups as compared with the hypoxia group (Fig. 2c–e).
FIG. 2.
Effect of EEPO preconditioning on lung histology. (a) Control group, and (b) Hypoxia group, showing thickening of the alveolar septa and the presence of scattered red blood cells in alveolar spaces. The low (c), medium (d), and high (e) levels of EEPO administration show that there was less damage to the alveolar septa as compared with hypoxia group. Data are representative of three animals in each group (40X, HE).
The edema scores in high EEPO group was significantly decreased as compared with the hypoxia group (p<0.05). Both neutrophil infiltration score and hemorrhage score in high EEPO groups were significantly reduced when compared with the hypoxia group (p<0.05) (Table 1).
Table 1.
Histologic Scores of Lung Sections1
| Con | Hyp | L | M | H | |
|---|---|---|---|---|---|
| Edema | 0 | 1.87±0.42 | 1.33±0.36 | 0.92±0.612 | 0.72±0.252 |
| Neutrophil | 0 | 3.81±0.76 | 3.09±0.51 | 1.62±0.542 | 1.32±0.462 |
| Hemorrhage | 0 | 1.24±0.21 | 0.48±0.292 | 0.59±0.47 | 0.45±0.182 |
Values are mean±SD (n=4). Significant differences between groups were determined by analysis of variance followed by Student-Newman-Keuls test; 2p<0.05 compared with hypoxia group.
Control group (Con); hypoxia group (Hyp); low-dose EEPO group (L); medium-does EEPO group (M), and high-dose EEPO group (H).
Pulmonary edema
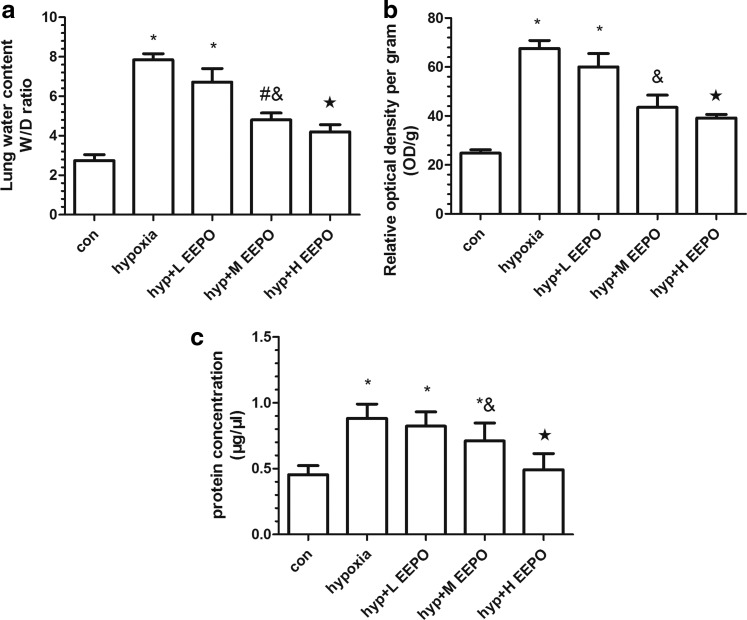
The degree of pulmonary edema was determined by measuring the lung water content as represented by the wet-to-dry ratio, transvascular leakage of Evans Blue dye, and total protein extravasation in BALF. The wet-to-dry weight ratio in mice exposed to hypobaric hypoxia was nearly four-fold that of normoxic control animals (Fig. 3a). However, EEPO administration before exposure reduced the wet-to-dry ratio in a dose-dependent manner as compared with the hypoxia group. There was no significant change in the lung wet-to-dry weight ratio between the medium-dose and high-dose EEPO group.
FIG. 3.
The protective effect of EEPO against hypobaric hypoxia-induced pulmonary damage at different doses. Pulmonary damage was evaluated by (a) wet-to-dry ratio, (b) pulmonary vascular leakage, and (c) total protein concentration in BALF. The mice were exposed to simulated hypobaric hypoxia at an altitude of 7000 meters for 6 h. EEPO was administered orally to mice 7 days before exposure to hypobaric hypoxia. Values are mean±SD (n=10). Significant differences between groups were determined by analysis of variance followed by t-test. #p<0.05 compared with control group, &p<0.05 compared with hypoxia group, *p<0.01 compared with control group, and ★p<0.01 compared with hypoxia group.
Vascular leakage was determined by measuring the extravasated Evans Blue in the lungs of mice exposed to hypoxia. It was found that there was a significant increase in vascular permeability in the lungs of mice exposed to hypobaric hypoxia compared with the control mice (p<0.01) (Fig. 3b). Evans Blue values indicated that prophylactic administration of EEPO reduced lung vascular permeability in a dose-dependent manner.
The mean total protein content in BALF was significantly higher in animals exposed to hypoxia as compared with the control animals (p<0.01). EEPO preconditioning resulted in a decline in the total protein content in BALF relative to hypoxia (Fig. 3c). In addition, the protective effect was more pronounced in the high-level EEPO group than that in the other two EEPO groups in terms of plasma protein leakage into the air space of the lung (p<0.01).
Biochemical parameters in the lung
ROS and lipid peroxidation levels
There was a nearly three-fold increase in ROS generation in the lungs of mice exposed to hypoxia as compared with the control animals. EEPO significantly reduced ROS generation in the lungs of mice exposed to hypoxia as compared with the control group. To determine whether increased ROS levels led to an increase in membrane peroxidation, MDA level was determined, knowing that it is an important biomarker for oxidation and decomposition of polyunsaturated fatty acids. It was found that MDA level decreased significantly in the lungs of EEPO groups (p<0.01) as compared with the EEPO-free groups (Table 2).
Table 2.
Effect of Hypobaric Hypoxia on ROS, MDA, GSH, and SOD Levels in Lungs of Mice1
| Con | Hyp | L | M | H | |
|---|---|---|---|---|---|
| ROS(nmol/mg pro) | 14.08±1.13 | 38.98±2.37* | 25.67±1.59*& | 21.23±2.61#& | 19.25±1.70#★ |
| MDA(nmol/g pro) | 3.71±0.89 | 9.42±1.21* | 8.36±1.02* | 6.14±0.73#& | 5.27±0.69#★ |
| GSH(μmol/gm) | 4.27±0.82 | 2.62±0.61# | 2.85±0.72# | 3.14±0.57# | 3.64±0.68#& |
| SOD (U/mg) | 0.87±0.03 | 0.44±0.07# | 0.58±0.05#& | 0.69±0.06#& | 0.76±0.05#& |
Values are mean±SD (n=10). Significant differences between groups were determined by analysis of variance followed by Student-Newman-Keuls test.
p<0.05 compared with control group, &p<0.05 compared with hypoxia group, *p<0.01 compared with control group, and ★p<0.01 compared with hypoxia group.
The abbreviations are the same as in Table 1.
GSH and SOD levels
Reduced glutathione and SOD are endogenous antioxidants that directly scavenge ROS and protect against damage from free radicals. The GSH level was reduced in the hypoxia group as compared with the level before hypoxia exposure (p<0.05). As expected, SOD level in the lungs of mice exposed to hypoxia was decreased as compared with the control group (p<0.05). However, prophylactic administration of EEPO enhanced the SOD level in the lung during hypobaric hypoxia exposure (p<0.05) (Table 2).
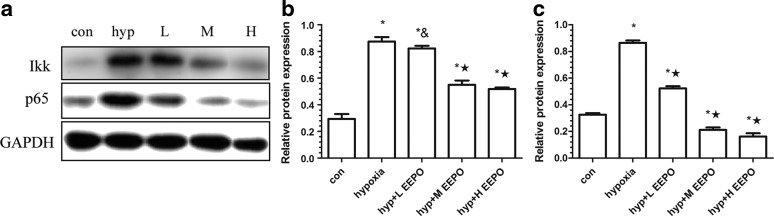
Expression of the NF-kB pathway
To confirm the involvement of the NF-kB pathway in the development of pulmonary vascular leakage and inflammatory reaction in the lung, Western Blot was used to determine the Ikk expression in the cytoplasm and p65 expression in the nucleus, knowing that Ikk plays a key role in the NF-kB pathway. It was observed that p65 and Ikk were increased under hypoxia. The expression of p65 and Ikk was increased upon exposure to hypoxia relative to the control animals (p<0.01). However, EEPO preconditioning resulted in a significant decrease in p65 and Ikk protein expression (p<0.01 or p<0.05) (Fig. 4).
FIG. 4.
The expression of Ikk and p65 proteins in the lungs of mice exposed to simulated hypobaric hypoxia at an altitude of 7000 m for 6 h. (a) Western blot analysis of Ikk and p65; (b) and (c) are Ikk and p65 relative expression. Con, control; H, hypoxia+H EEPO; hyp, hypoxia; L, hypoxia+L EEPO; M, hypoxia+M EEPO; #p<0.05 compared with control group, &p<0.05 compared with hypoxia group, *p<0.01 compared with control group, and ★p<0.01 compared with hypoxia group.
Inflammation in the lung
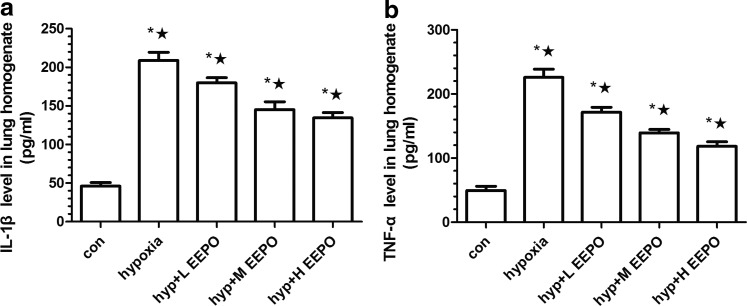
To confirm whether pro-inflammation was upregulated in the lungs after hypoxic exposure, the expression of inflammatory cytokine IL-1β and TNF-α in the lung was detected by ELISA. It was found that IL-1β secretion increased by nearly five-fold after hypoxic exposure (Fig. 5a). Hypoxia exposure also increased TNF-α by more than four-fold as compared with the control group (Fig. 5b). EEPO preconditioning before exposure to hypobaric hypoxia attenuated the abnormal increase of IL-1β and TNF-α (p<0.01), and this preventive effect was most pronounced in the high-dose EEPO group (p<0.01).
FIG. 5.
The expression of inflammatory cytokines (a) IL-1β and (b)TNF-α in mice exposed to simulated hypobaric hypoxia at an altitude of 7000 meters for 6 h. Values are mean±SD (n=10). *p<0.01 compared with control group and ★p<0.01 compared with hypoxia group.
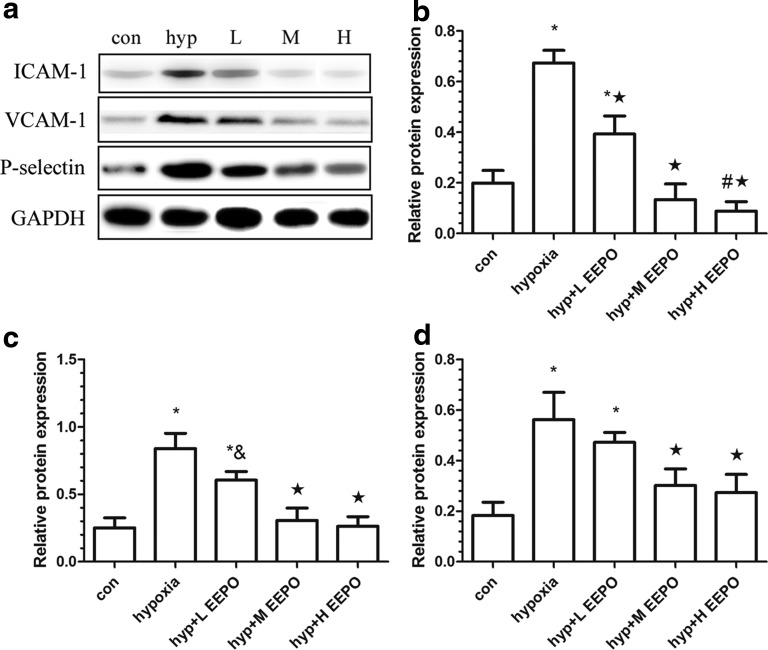
The expression of cell adhesion molecules (ICAM-1, VCAM-1, and P-selectin) in the lungs of each group was determined. It was found that ICAM-1 was nearly undetectable in the control group. However, there was a significant increase in ICAM-1 expression in the hypoxia group (p<0.01). ICAM-1 expression decreased in varying degrees in the three EEPO groups (Fig. 6b). At the same time, VCAM-1 level increased by about four-fold in the lungs of mice exposed to hypoxia as compared with the control animals (Fig. 6c). Similarly, exposure of mice to hypoxia also resulted in a significant increase in lung P-selectin levels (Fig. 6c), and EEPO administration decreased the expression of ICAM-1, VCAM-1, and P-selectin markedly as compared with the hypoxia group (p<0.01).
FIG. 6.
Western blot analysis of ICAM-1, VCAM-1, and P-selectin; (a) is the results of Western blot; (b), (c), and (d) are ICAM-1, VCAM-1, and P-selectin relative expression after hypoxia exposure. Con, control; hyp, hypoxia; L, H, hypoxia+H EEPO; hypoxia+L EEPO; M, hypoxia+M EEPO; #p<0.05 compared with control group, &p<0.05 compared with hypoxia group, *p<0.01 compared with control group, and ★p<0.01 compared with hypoxia group.
Discussion
The purpose of this study was to find suitable hypobaric hypoxia conditions that cause acute pulmonary edema in mice and investigate whether prophylactic treatment with EEPO was able to attenuate pulmonary edema caused by hypoxia-induced lung injury, as well as to explore possible molecular mechanisms. The results of our study showed that after 6 hours exposure at simulated 7000 m altitude, experimental mice were observed to have noticeable pulmonary edema. In this condition, EEPO as a preventive measure appears to reduce pulmonary vascular permeability and inflammatory response. Meanwhile, it can alleviate the activation of NF-kB pathway and the secretion of downstream adhesion molecules, so the pulmonary edema caused by hypoxia can be eased.
When mice were exposed to a simulated 7000 m altitude in a hypobaric chamber for 6 h, pulmonary water contents, vascular permeability, and pulmonary edema were demonstrably more severe than those of other groups. Additionally, the severe shock made histological changes to the mouse lungs obvious in a short period of time. Furthermore, typical acute pathological injuries to the lungs saw observable benefits from the effect of EEPO.
Previous studies have confirmed that PO has antioxidant, anti-inflammatory, and many other pharmacological activities (Uddin et al., 2012). Five flavonoids were found in EEPO by capillary electrophoresis and electrochemical detection methods (Xu et al., 2006). Hypoxia can increase the ROS in mitochondria, which is the major material of oxidative stress (Swamy et al., 2010). Rahman (2003) also reported that hypoxia may result in an imbalance between antioxidant and pro-oxidant levels, which may aggravate lung inflammation. In the lung, oxidative stress induced by hypoxia was associated with vascular leakage and may contribute to alveolar–capillary barrier dysfunction (Kaner et al., 2000; Mura et al., 2004; Schoene, 2004). When the mice were supplemented with EEPO, ROS and MDA levels in the lung tissue decreased significantly and GSH and SOD levels increased. These results suggested that the antioxidant EEPO played an important part in oxidation resistance. It was a further purpose of the study to determine whether the antioxidant effects were ascribable to the flavonoids.
Secretion of inflammatory cytokines plays an important role in the acute inflammation process. IL-1β and TNF-α produced by macrophages, mast cells, and vascular endothelial cells initiate the inflammatory response directly (Bastarache et al., 2011) with respect to adherence of neutrophils and maintenance of sustained activation (Dolinay et al., 2012) and promotion of mast cell degranulation, all of which can lead to lung injury (Soehnlein et al., 2008). Pretreating with the EEPO significantly attenuated the upregulation of IL-1β and TNF-α level in lung homogenate of mice exposed to hypoxia. These indicated that EEPO may have beneficial effects in reducing lung inflammation and edema. Nevertheless, some human studies measuring cytokines in plasma did not find evidence for inflammation with the onset of high-altitude pulmonary edema (Kleger et al., 1996; Bärtsch et al., 2000). For instance, Swenson and his colleagues (2002) demonstrated that pulmonary hypertension led to pulmonary edema in early phase of HAPE, during which no significant change was observed in leucocytes, cytokines, and eicosanoids, suggesting that inflammation may not be necessary for pulmonary vascular leakage in early HAPE in humans. In our opinion, this difference may be caused by species, for the natural structure of the mouse lung is more easily destroyed, which probably is the reason why pulmonary edema can be observed at 6 hours.
It is reported that oxidant stress increases the vascular endothelial permeability and expression of redoxsensitive transcription factor nuclear factor kB (NFkB) (Lum et al., 2001), which is an obligatory mediator of the inflammatory response. In the present study, we observed a marked increase in Ikk and p65 levels in the lungs of the hypoxia exposure group. EEPO could significantly attenuate the quantity of p65 entering the nucleus and promote the phosphorylation of Ikk under hypoxia, suggesting that the anti-inflammatory effect of EEPO in hypoxic conditions may inactivate the NF-kB pathway. To further prove this postulation, we explored the expression of downstream adhesion molecules, which can be regulated by NF-kB (Reiterer et al., 2004) and cytokines (Dolinay et al., 2012) to help migration of leukocytes. It was reported that soluble selectins participated in the regulation of the inflammatory process (Matsumoto et al., 2001). It was found that the expression of ICAM-1 and VCAM-1 as neutrophils accumulating elements was reduced in EEPO groups as compared with the control group, as was the case with P-selectins. This further supports the speculation that the anti-inflammatory effect of EEPO may work by suppressing excitation of the NF-kB pathway.
There are some limitations in our study. This research did not measure pulmonary artery pressure in mice, so we could not observe the change of hypoxic pulmonary vasoconstriction. Some studies reported that ROS and HIF-α signaling pathway could result in ET-1 and VEGF overexpression in hypoxia (Irwin et al., 2009). In addition, ET-1 receptor antagonist proved to be efficacious against pulmonary hypertension (Modesti et al., 2006), and many molecules, such as hypoxia inducible factor-1 (HIF-1), vascular endothelial growth factor (VEGF), and endothelin-1 (ET-1), participated in the mechanism of pulmonary edema induced by hypobaric hypoxia. In future research, we will focus on the role of EEPO in influencing the function of alveolar epithelial and vascular endothelial cells.
In this study, we utilized several methods to demonstrate that EEPO could attenuate hypoxia-induced pulmonary edema in our mouse model. As the results indicated, EEPO could protect the lung by alleviating oxidative stress in hypobaric hypoxia, reducing the expression of cytokines and adhesion molecules, inhibiting NF-kB pathway activation. In the course of the experiment, however, we observed that the oxidative stress and inflammation could also be observed after hypobaric hypoxia exposure in EEPO groups, seemingly suggesting that EEPO could not prevent or reverse pulmonary injury completely. At the same time, histopathology indicated that the pulmonary damage in the hypoxia exposure group was more severe than that in the normal group. Apparently, EEPO could attenuate pulmonary injury but could not prevent hypoxia-induced pulmonary edema in mice completely.
Conclusion
Our results confirmed the protective effect of EEPO on the lung of mice exposed to hypoxia and demonstrated that prophylactic administration of EEPO could lessen vascular permeability and relieve pulmonary edema. The underlying mechanism of this protective effect of EEPO may be through, at least partly, attenuating inflammatory pulmonary reactions in mice by decreasing oxidative stress under hypoxia.
Acknowledgments
This study was supported by researchers from the Department of Military Hygiene of the Second Military Medical University. The authors wish to acknowledge and thank Professor Changquan Ling for his critical assistance with Portulaca Oleracea. Finally, I want to thank my American editor and friend, Dr Jeff Gorbski, for his assistance with the language of the article.
Author Disclosure Statement
No competing financial interests exist.
References
- Awad NE. (1994). Lipid content and antimicrobial activity of phenolic constituents of cultivated Portulaca oleracea L. Bull Fac Pharm 32:137–142 [Google Scholar]
- Bastarache JA, Sebag SC, Grove BS, and Ware LB. (2011). Interferon-γ and tumor necrosis factor-α act synergistically to upregulate tissue factor in alveolar epithelial cells. Exp Lung Res 37:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bärtsch P, Eichenberger U, Ballmer PE, Gibbs JS, Schirlo C, Oelz O, and Mayatepek E. (2000). Urinary leukotriene E4 levels are not increased prior to high-altitude pulmonary edema. Chest 117,1393–1398 [DOI] [PubMed] [Google Scholar]
- Cathcart R, Schwiers E, and Ames BN. (1983). Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 134:111–116 [DOI] [PubMed] [Google Scholar]
- Chandel NS, Trzyna WC, McClintock DS, and Schumacker PT. (2000). Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol 165:1013–1021 [DOI] [PubMed] [Google Scholar]
- Chen CJ, Wang WY, Wang XL, Dong LW, Yue YT, Xin HL, Ling CQ, and Li M. (2009). Antihypoxic activity of the ethanol extract from Portulaca oleracea in mice. J Ethnopharmacol 124:246–250 [DOI] [PubMed] [Google Scholar]
- Chiu KH, Lee WL, Chang CC, Chen SC, Chang YC, Ho MN, Hsu JF, and Liao PC. (2010). A label-free differential proteomic analysis of mouse bronchoalveolar lavage fluid exposed to ultrafine carbon black. Anal Chim Acta 673:160–166 [DOI] [PubMed] [Google Scholar]
- Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, and Choi AM. (2012). Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 185:1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplain H, Vollenweider L, Delabays A, Nicod P, Bärtsh P, and Scherrer U. (1999). Augmented sympathetic activation during short-term hypoxia and high-altitude exposure in subjects susceptible to high-altitude pulmonary edema. Circulation 99:1713–1718 [DOI] [PubMed] [Google Scholar]
- Guzy RD, and Schumacker RT. (2006). Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp Physio 91:807–819 [DOI] [PubMed] [Google Scholar]
- Ingram TE, Pinder AG, Bailey DM, Fraser AG, and James PE. (2010). Low-dose sodium nitrite vasodilates hypoxic human pulmonary vasculature by a means that is not dependent on a simultaneous elevation in plasma nitrite. Am J Physiol Heart Circ Physiol 298:331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DC, McCord JM, Nozik-Grayck E, Beckly G, Foreman B, Sullivan T, White MT, Crossno J, Jr, Bailey D, Flores SC, Majka S, Klemm S, and van Patot MC. (2009). A potential role for reactive oxygen species and the HIF-1alpha-VEGF pathway in hypoxia-induced pulmonary vascular leak. Free Radic Biol Med 47:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, and Crystal RG. (2000). Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 22:657–664 [DOI] [PubMed] [Google Scholar]
- Kleger GR, Bärtsch P, Vock P, Heilig B, Roberts LJ, and Ballmer PE. (1996). Evidence against an increase in capillary permeability in subjects exposed to high altitude. J Appl Physiol 81:1917–1923 [DOI] [PubMed] [Google Scholar]
- Kolosova IA, Mirzapoiazova T, Moreno-Vinasco L, Sammani S, Garcia JG, and Verin AD. (2008). Protective effect of purinergic agonist ATPgammaS against acute lung injury. Am J Physiol Lung Cell Mol Physiol 294:L319–324 [DOI] [PubMed] [Google Scholar]
- Kum-Tatt L, and Tan IK. (1974). A new colorimetric method for the determination of glutathione in erythrocytes. Clin Chim Acta 53:153–161 [DOI] [PubMed] [Google Scholar]
- Kunsch C, and Medford RM. (1999). Oxidative stress as a regulator of gene expression in the vasculature. Circ Res 85:753–766 [DOI] [PubMed] [Google Scholar]
- Lee AS, Kim JS, Lee YJ, Kang DG, and Lee HS. (2012). Anti-TNF-α activity of Portulaca oleracea in vascular endothelial cells. Int J Mol Sci 13:5628–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Howe P, Zhou YF, Xu ZQ, Hocart C, and Zhan R. (2000). Fatty acids and beta-carotene in Australian purslane (portulaca oleracea) varieties. J Chromatogr A 893:207–213 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, and Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Lum H, and Roebuck KA. (2001). Oxidant stress and endothelial dysfunction. Am J Physiol Cell Physiol 280:719–741 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura H, Ueki Y, Tominaga T, and Miyake S. (2001). Correction of hyperglycaemia reduces insulin resistance and serum soluble E-selectin levels in patients with Type 2 diabetes mellitus. Diabet Med 18:224–228 [DOI] [PubMed] [Google Scholar]
- Modesti PA, Vanni S, Morabito M, Modesti A, Marchetta M, Gamberi T, Sofi F, Savia G, Mancia G, Gensini GF, and Parati G. (2006). Role of endothelin-1 in exposure to high altitude: Acute Mountain Sickness and Endothelin-1 (ACME-1) Study. Circulation 114:1410–1416 [DOI] [PubMed] [Google Scholar]
- Mura M, dos Santos CC, Stewart D, and Liu M. (2004). Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol 97:1605–1617 [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, and Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358 [DOI] [PubMed] [Google Scholar]
- Rahman I. (2003). Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. J Biochem Mol Biol 36:95–109 [DOI] [PubMed] [Google Scholar]
- Reiterer G, Toborek M, and Henning B. (2004). Quercetin protects against linolenic acid induced porcine endothelial cell dysfunction. J Nutr 134:771–775 [DOI] [PubMed] [Google Scholar]
- Sakai N., Inada K., Okamoto M., Shizuri Y., Fukuyama Y. (1996). Portuloside A, a monoterpene glucoside from Portulaca oleracea. Phytochemistry 42:1625–1628 [Google Scholar]
- Schoene RB. (2004). Unraveling the mechanism of high altitude pulmonary edema. High Alt Med Biol 5:125–135 [DOI] [PubMed] [Google Scholar]
- Soehnlein O, Oehmcke S, Ma X, Rothfuchs AG, Frithiof R, van Rooijen N, Morgelin M, Herwald H, and Lindbom L. (2008). Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur Respir J 32:405–412 [DOI] [PubMed] [Google Scholar]
- Staub NC. (1974). Pulmonary edema. Physiol Rev 54:678–811 [DOI] [PubMed] [Google Scholar]
- Su X, Bai C, Hong Q, Zhu D, He L, Wu J, Ding F, Fang X, and Matthay MA. (2003). Effect of continuous hemofiltration on hemodynamics, lung inflammation and pulmonary edema in a canine model of acute lung injury. Intensive Care Med 29:2034–2042 [DOI] [PubMed] [Google Scholar]
- Swamy M, Salleh MJ, Sirajudeen KN, Yusof WR, and Xhandran G. (2010). Nitric oxide, citrulline-no cycle enzymes, glutamine synthetase and oxidative stress in anoxia (hypobaric hypoxia) and reperfusion in rat brain. Int J Med Sci 7:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson ER, Maggiorini M, Mongovin S, Gibbs JS, Greve I, Maribaurl H, and Bartsch P. (2002). Pathogenesis of high-altitude pulmonary edema: Inflammation is not an etiologic factor. JAMA 287:2228–2235 [DOI] [PubMed] [Google Scholar]
- Toledano MB, and Leonard WJ. (1991). Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci USA 88:4328–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MK, Juraimi AS, Ali ME, and Ismail MR. (2012). Evaluation of antioxidant properties and mineral composition of purslane (Portulaca oleracea L.) at different growth stages. Int J Mol Sci 13:10257–10267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Gu L, Dong L, Wang X, Ling C, and Li M. (2007). Protective effect of Portulaca oleracea extracts on hypoxic nerve tissue and its mechanism. Asia Pac J Clin Nutr 16Suppl 1:227–233 [PubMed] [Google Scholar]
- Wanyin W, Liwei D, Lin J, Hailiang X, Changquan L, and Min L. (2012). Ethanol extract of Portulaca oleracea L. protects against hypoxia-induced neuro damage through modulating endogenous erythropoietin expression. J Nutr Biochem 23:385–391 [DOI] [PubMed] [Google Scholar]
- Waypa GB, and Schumacker PT. (2008) Oxygen sensing in hypoxic pulmonary vasoconstriction: Using new tools to answer an age-old question. Exp Physiol 93:133–138 [DOI] [PubMed] [Google Scholar]
- Xu XQ, Yu LS, and Chen GN. (2006). Determination of flavonoids in Portulaca oleracea L. by capillary electrophoresis with electrochemical detection. J Pham Biomed Anal 41:493–499 [DOI] [PubMed] [Google Scholar]
- Yoshinari D, Takeyoshi I, Koibuchi Y, Matsumoto K, Kawashima Y, koyama T, Ohwada S, and Morishita Y. (2001). Effects of a dual inhibitor of tumor necrosis factor-α and interleukin-1 on lipopolysaccharide-induced lung injury in rats: Involvement of p38 mitogen-activated protein kinase pathway. Crit Care Med 29:628–634 [DOI] [PubMed] [Google Scholar]