Abstract
Kanekar, Shami, Olena V. Bogdanova, Paul R. Olson, Young-Hoon Sung, Kristen E. D'Anci, and Perry F. Renshaw. Hypobaric hypoxia induces depression-like behavior in female Sprague-Dawley rats, but not males. High Alt Med Biol 16:52–60, 2015—Rates of depression and suicide are higher in people living at altitude, and in those with chronic hypoxic disorders like asthma, chronic obstructive pulmonary disorder (COPD), and smoking. Living at altitude exposes people to hypobaric hypoxia, which can lower rat brain serotonin levels, and impair brain bioenergetics in both humans and rats. We therefore examined the effect of hypobaric hypoxia on depression-like behavior in rats. After a week of housing at simulated altitudes of 20,000 ft, 10,000 ft, or sea level, or at local conditions of 4500 ft (Salt Lake City, UT), Sprague Dawley rats were tested for depression-like behavior in the forced swim test (FST). Time spent swimming, climbing, or immobile, and latency to immobility were measured. Female rats housed at altitude display more depression-like behavior in the FST, with significantly more immobility, less swimming, and lower latency to immobility than those at sea level. In contrast, males in all four altitude groups were similar in their FST behavior. Locomotor behavior in the open field test did not change with altitude, thus validating immobility in the FST as depression-like behavior. Hypobaric hypoxia exposure therefore induces depression-like behavior in female rats, but not in males.
Key Words: : altitude, depression, forced swim test, gender, hypobaric hypoxia, rat
Introduction
A lifetime history of major depressive disorder (MDD) affects almost 10% of the population of the United States (Barch, 2013), and many depressed individuals do not find adequate relief with the currently available medication. National mental health surveys indicate that rates of depression and suicide, the most negative outcome of depression, are highest in the intermountain states (Mark, 2007). Recent studies suggest that altitude of residence may contribute to the increased suicide rates, independent of other risk factors such as age, gender, race, psychiatric disorders, and sociocultural factors (Brenner et al., 2011; Haws et al., 2009; Kim et al., 2011). Regional variation in suicide rates in the United States correlate directly with state peak elevation (Haws et al., 2009) and county elevation (Brenner et al., 2011), despite the decrease in overall mortality rates with altitude (Brenner et al., 2011). The link between suicide and altitude of residence is also documented in Austria (Helbich et al., 2013) and South Korea (Kim et al., 2011). Depression is strongly associated with suicide in the developed world (Henriksson et al., 1993), and mortality by suicide is highly linked to unresolved depression (Joiner et al., 2005). A recent study showed that people living at altitude have higher levels of depression than those at sea level (Gamboa, 2011). Extraction of altitude-based data from U.S. national mental health surveys confirm that rates of MDD escalate significantly with elevation of residence (DelMastro et al., 2011).
People living at altitude are exposed to hypobaric hypoxia, the low partial pressure of oxygen (ppO2) at altitude. Young (2013) suggested that the elevated suicide rates observed at altitude could arise from hypobaric hypoxia-induced changes that cause more clinical depression in residents at altitude. This is supported by the observation that people living with chronic hypoxic disorders also exhibit significantly higher rates of both depression and suicide (Katz, 1982). Patients with chronic hypoxic conditions, such as chronic obstructive pulmonary disease (COPD) or asthma, consistently show higher rates of suicide (Goodwin, 2011; 2012; Goodwin et al., 2012) vs. those with chronic diseases without hypoxia (Goodwin et al., 2003; Katz, 1982). Higher depression rates are also found in those with chronic hypoxic conditions such as COPD, coronary heart disease, and stroke, vs. conditions without hypoxia such as diabetes, back pain, and osteoarthritis (Webb et al., 2012). The risk of depression was observed to be almost twice as high in those with COPD vs. in those with diabetes or without chronic health issues (van den Bemt et al., 2009), while prevalence of depression was found to be significantly higher in COPD patients (23%) than in those without COPD (17%) (Schneider et al., 2010). These data together suggest that hypoxia could be the link between the higher rates of depression and suicide in people living at altitude and those suffering from chronic hypoxic health conditions.
Barometric pressure decreases with increasing elevation, reducing the partial pressure of oxygen to cause hypobaric hypoxia. People residing at 4500 ft (the altitude of Salt Lake City, UT) are exposed to 18% inspired oxygen, vs. 21% at sea level. At higher altitudes, oxygen levels drop even lower, with 15% inspired oxygen at 10,000 ft and only 10% at 20,000 ft. Several studies have examined the effects of altitude in the extreme range (>18,000 ft) as consequences of mountaineering (Moore et al., 1998; Wilson et al., 2009), but as more people move to reside at higher elevations, altitude-related issues are found to occur at more moderate altitudes (Brenner et al., 2011; Maa, 2010). A recent study highlighted this: evaluation of brain bioenergetic markers in healthy individuals residing at 4500 ft (Salt Lake City, UT) or at 20 ft (Belmont, MA) found reduced frontal lobe creatine levels in residents at 4500 ft vs. those at sea level (Renshaw, 2012), implying that living at altitude can impair brain function even at moderate altitudes.
Living at high altitude reduces blood oxygen saturation, detrimentally affecting cardiovascular, respiratory, and neurological function (Bartsch and Swenson, 2013; Moore et al., 1998; Netzer et al., 2013). High altitude adversely affects cognitive (Wilson et al., 2009) and motor function (Moore et al., 1998), and negatively impacts mood and behavior (Bahrke and Shukitt-Hale, 1993). With the high basal oxygen needs of the brain, neurological symptoms including headaches, sleep disorders, mood disorders, and drug addiction are much more prevalent at altitude (Maa, 2010). Hypobaric hypoxia may contribute to the emergence of these issues, and as more people move to live at or visit regions at or above 2000 ft (Brenner et al., 2011), it becomes more important to address the neurological consequences of living at altitude.
Similar physiological issues are reported in rodent models of hypobaric hypoxia, with several groups studying respiratory, cardiovascular, and cerebral deficits (Gonzalez and Wood, 2010; Kumar, 2011; Mortola, 2007) involved with exposure to chronic hypobaric hypoxia. The rat has therefore widely been used as an appropriate animal model to study the impact of hypobaric hypoxia on human physiology. Most of these studies however use only male rats, and the few studies that use female rats primarily examine renal and pulmonary adaptation to hypobaric hypoxia (e.g., Martin et al., 1994; Kovaleva et al., 2013). No previous studies have examined the effects of hypoxia on depression-like behavior in an animal model. Only one other group has examined the effect of low barometric pressure on depression-like behavior in rats: this study used male rats and mimicked the intermittent low pressure experienced in typhoons (Mizoguchi et al., 2010). We are the first group to examine the impact of chronic (a week-long) hypobaric hypoxia on mood disorders in an animal model using both male and female rats in four altitude groups ranging from sea level to 20,000 ft, an experimental paradigm more relevant to living at altitude or with chronic hypoxic diseases.
In this study, we examine the impact of hypobaric hypoxia on depression-like behavior in rodents. Rats housed in four altitude groups for a week were examined for depression-like-behavior using the forced swim test (FST) (Detke and Lucki, 1996; Porsolt et al., 1978). The FST is a well-characterized behavioral test used to assess depression-like behavior in rodents (Bogdanova et al., 2013). To validate immobility in the FST as depression-like behavior, we further examined whether hypoxia affects locomotor behavior in the open field test (OFT) (Slattery and Cryan, 2012). Our study shows that exposure to hypobaric hypoxia alone causes depression-like behavior in female Sprague Dawley rats, but not in males.
Materials and Methods
Animals
For all experiments, male and female Sprague Dawley rats, approximately of 125–150 g body weight, were received from Charles River Laboratories (Raleigh, NC). Animals were housed individually and given food and water ad libitum. After a week of acclimatization, animals were randomly assigned to altitude groups, and put into altitude chambers, or at local elevation for one week. All animal procedures were approved by the University of Utah Institutional Animal Care and Use Committee and were performed in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Altitude simulations
Hypobaric hypoxia or hyperbaric chambers were used to reduce or increase pressure from ambient barometric pressure at the University of Utah Research Park (4500 ft) to simulate high altitude conditions or sea level, respectively. The four altitude groups consist of sea level (SL), 4500 ft (4.5K), 10,000 ft (10K), and 20,000 ft (20K). Animals were placed in the hyperbaric chamber set to simulate SL conditions (21% ppO2, 760 mmHg). The 10K group (15% ppO2, 523 mmHg), and 20K group (10% ppO2, 349 mmHg) were housed in hypobaric hypoxia chambers set to the appropriate barometric pressure. Rats in the 4.5K group were housed at local elevation (Salt Lake City, UT: 18% ppO2, 644 mmHg), adjacent to the altitude chambers.
After one week, animals were taken out of altitude chambers and tested in either the FST or the OFT at local conditions (4500 ft). Total food consumption and body weight gain over the week at different altitude settings were also measured. To ensure that females did not face any additional stresses to that experienced by males (Allen et al., 2010), vaginal testing for estrous cycle stage was not conducted.
Forced swim test
After a week of housing in the four altitude groups (n=12 per group), rats were tested for depression-like behavior in the FST (Detke and Lucki, 1996; Porsolt et al., 1978). The FST is conducted in two sessions: the pretest (15 min) to familiarize rats with the swimming paradigm and 24 h later, the test FST (5 min) to assay for depression-like behavior. In the FST, a rat is placed in a clear tank (25 cm in diameter, 65 cm tall) filled with 48 cm deep water at 25°C (Detke and Lucki, 1996), and its behavior is videotaped. Each session is conducted in a clean tank with fresh water. After the swim session, rats are dried off, placed in a warm cage to recover for 15 min, and then returned to their home cage.
The FST was conducted at local conditions of 4500 ft. The pretest was done on day 6 of altitude exposure, and rats were then returned to their home altitude for the 24 h period before the test session. FST sessions were performed in ambient light at the same time of the day/light cycle (1–4 pm), with animals visually blocked from experimenters, for consistent test results (Bogdanova et al., 2013; Slattery and Cryan, 2012). Behavior was scored for both FST sessions, as recommended when animals are subjected to long-term manipulations prior to the FST (Slattery and Cryan, 2012).
Open field test
The open field test (OFT) was used to assay for locomotor behavior on a separate set of rodents (n=10 per group) (Allen et al., 2010; 2012). The OFT was conducted after the 1 week exposure to the four altitude conditions. For the OFT, a rat was placed in a square OFT apparatus (50 cm x 50 cm x 50 cm) divided into 5 cm2 areas in a grid, lit from above for equal illumination of all surfaces. The animal was left in isolation to move around at will for 5 min (Allen et al., 2010; 2012). OFT behavior was recorded and later scored.
Behavioral test analysis
Behavioral tests were scored by two independent scorers, unaware of experimental group. The time sampling technique of Detke (Detke and Lucki, 1996) was used to analyze FST behavior, by scoring the predominant behavior in 5 sec segments of the initial 5 min period of both FST sessions. Behavior was scored for active (swimming and climbing) vs. passive behaviors (immobility) (Detke et al., 1995), and presented as percent of total time. Latency to immobility was calculated as the time in seconds for each animal to achieve the first 10 second period of immobility.
Behavior in the OFT was scored for percent of time spent roaming, rearing, grooming or immobile, and time spent in corners, along walls or in the center of the chamber (Allen et al., 2010; 2012). Data is presented as percent of time spent in each behavior or location.
Statistical analysis
Statistical analysis was performed using Graphpad Prism software (La Jolla, CA) on raw data for food consumption and body weight and on percent data for behavioral tests. Data was analyzed using two-way analysis of variance (ANOVA) to examine the effect of gender and altitude on the dependent variables. When there is a statistically significant interaction, simple main effects analysis was performed between altitude levels for each gender. Bonferroni multiple comparison tests were conducted to determine statistical significance with corrected p-values. Pearson's correlation coefficient was determined within each altitude group for body weight vs. both immobility and latency to immobility in the test FST. Data is presented as mean±standard error of the mean (SEM).
Results
Food consumption and body weight
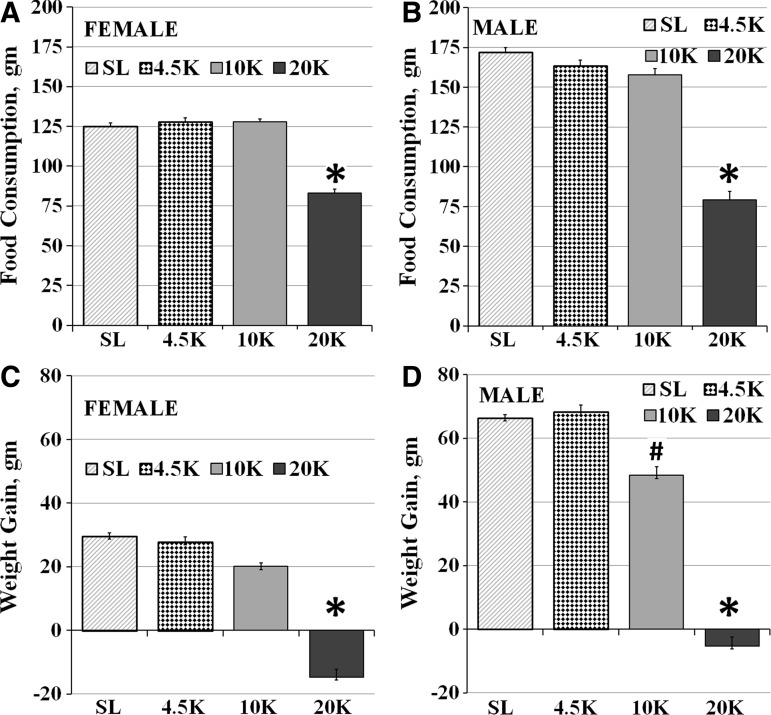
Prior to exposure to simulated altitude or local conditions, female and male rats in all four altitude groups were similar in body weight (p>0.05, one way ANOVA for both females and males). Two-way ANOVA showed significant effects of both altitude (p<0.0001) and gender (p<0.0001), and of their interaction on both food consumption (F (3, 88)=10.6, p<0.0001) and weight gain (F (3, 88)=8.4, p<0.0001, n=12 for all groups). Simple main effects analysis showed that female rats were similar in food consumed in a week at SL (125±3 g), 4.5K (128±4 g), and 10K (128±3 g), but those at 20K consumed significantly less food (83±4g, F (3, 44)=32, p<0.0001, Fig. 1A). Male rats were also similar in food consumed at SL (172±4 g), 4.5K (162±5 g), and 10K (158±6 g), but food consumption was significantly lower at 20K (79±5 g, F (3, 44)=66, p<0.0001, Fig. 1B).
FIG. 1.
The effects of hypobaric hypoxia on food consumption and body weight gain: (A) Food consumption was similar in females after one week at SL, 4.5K, and 10K, but was significantly lower at 20K. (B) In males, food consumption also did not vary between the SL, 4.5K, and 10K groups, but was significantly lower in the 20K groups. (C) Females are similar in the SL, 4.5K and 10K groups in weight gained over a week, but exhibit a significant loss in weight after a week at 20K. (D) Males exhibit lower weight gain in the 10K group vs. SL, and a significant loss in weight after one week of housing at 20K. (Mean±SEM, #p<0.05, *p<0.001 vs. SL).
A similar pattern was seen in weight gain in both females and males. Females gained weight similarly after a week at SL (29±2g), 4.5K (28±3 g), and 10K (20±2 g). In males, weight gained was similar at SL (66±2 g) and 4.5K (68±3 g), but significantly lower at 10K (48±4 g). Both females (−15±4 g, F (3, 44)=58, p<0.0001, Fig. 1C) and males (−5±3 g, F (3, 44)=121, p<0.0001, Fig. 1D) lost weight after a week at 20K, as previously shown at high altitude (Singh et al., 1997).
Within each altitude group, Pearson's correlation analysis showed no significant association between body weight and immobility or latency to immobility in the test FST (p>0.05 for all groups). Thus, while FST behavior is known to vary with rodent weight (Bogdanova et al., 2013), this does not factor in with the weight range of animals used in this study.
Forced swim test
Behavior in the pretest FST
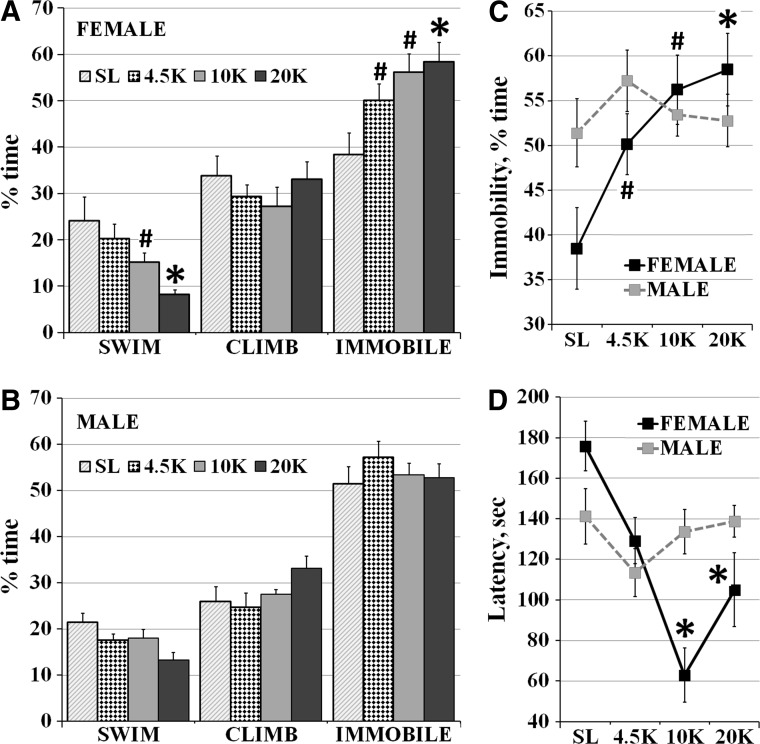
Two-way ANOVA showed a significant effect of altitude (p=0.013) on immobility in the pretest FST, and also of the interaction between altitude and gender (F (3, 88)=2.8, p=0.04). Females were most active at SL, spending only 39±5% time immobile. Immobility increased incrementally with altitude to reach 58±4% at 20K (Fig. 2A, C, F (3, 44)=4.4, p=0.007). Males did not vary in behavior in the pretest FST between altitude groups (Fig. 2B, C).
FIG. 2.
The effects of hypobaric hypoxia on behavior in the Pretest FST: (A) Females at 4.5K, 10K and 20K spent significantly more time immobile and those at 10K and 20K spent less time swimming than those at SL. (B) Males in all four groups were similar in their behavior in the pretest FST. (C) Immobility in the FST increased incrementally in females housed at altitude, and was significantly higher in all three high altitude groups vs. SL. (D) Latency to immobility dropped significantly in females housed at 10K and 20K, but did not change significantly in males. (Mean±SEM, #p<0.05, *p<0.001 vs. SL).
Of active behaviors, a significant effect of altitude alone (p=0.0001) was seen with swimming, but none of the interaction with gender (F (3, 88)=1.22, p=0.3). In females, swimming decreased with altitude from 24±5% at SL to 8±1% at 20K (F (3, 44)=4.7, p=0.006, Fig. 2A). In males, swimming behavior ranged from 21±2% at SL to 13.4±1% at 20K (F (3, 44)=3.6, p=0.02, Fig. 2B). Climbing did not change across altitude groups in females or males.
For latency to immobility, a significant effect was seen of both altitude alone (p=0.004) and of the interaction of altitude and gender (F (3, 88)=4.5, p=0.005). In females, latency to immobility decreased significantly with altitude: from 176±17 sec at SL, to 105±18 sec at 20K (F (3, 44)=6.4, p=0.001), while no change was seen across altitude groups in males (Fig. 2D).
Bonferroni post-hoc analysis showed that immobility in the pretest FST was significantly higher in females at 4.5K (p<0.05), 10K (p<0.05), and 20K (p<0.001) vs. SL, while swimming was significantly lower at 10K (p<0.05) and 20K (p<0.001) vs. SL. Females at 10K (p<0.001) and 20K (p<0.001) had significantly lower latency to immobility than those at SL. Males were not significantly different between the four altitude groups.
Behavior in the test FST
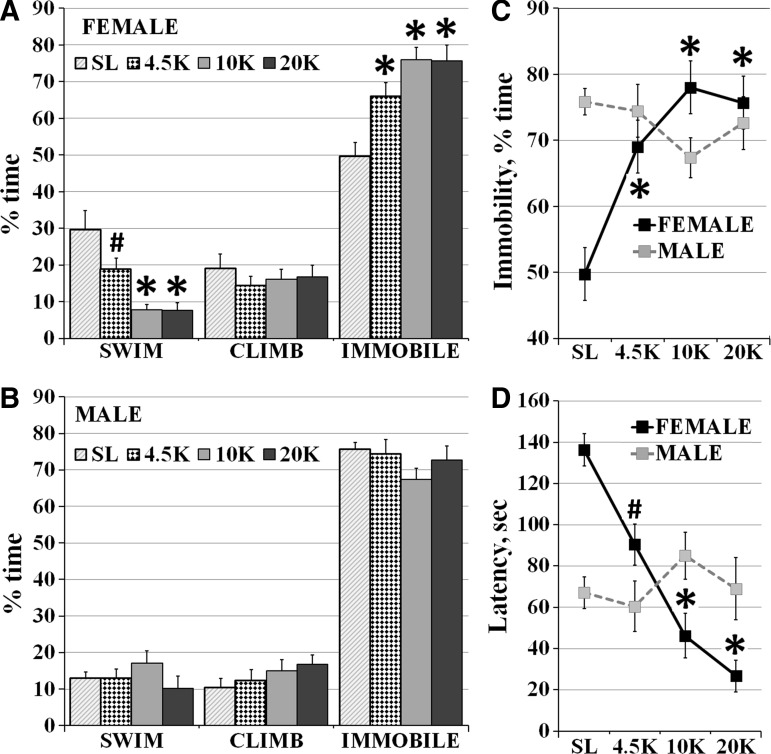
Two-way ANOVA for immobility showed a significant effect of altitude (p=0.014) and of the interaction between altitude and gender (F (3, 88)=9.5, p<0.0001). Females housed at SL were the most active: with only 50±3% immobility, which increased to 69±4% at 4.5K, 76±4% at 10K, and 76±4% at 20K (F (3, 44)=10.5, p<0.0001, Fig. 3A and C). Male rats however, were similar in their test FST behavior across altitude groups (Fig. 3B, C, and D).
FIG. 3.
The effects of hypobaric hypoxia on behavior in the Test FST: (A) Females at altitude exhibited significantly more immobility and less swimming behavior in the test FST vs. SL. (B) Males in the four groups were similar in the behavior in the test FST. (C) Immobility in the test FST was signigicantly higher in females at 4.5K, 10K and 20K than at SL, while immobility did not change across groups in males. (D) Female rats showed incrementally lower latency to immobility with housing at increased altitudes, while males did not vary in latency to immobility across groups. (Mean±SEM, #p<0.05, *p<0.001, vs. SL).
For swimming, a significant effect was seen of altitude (p=0.0005) and of its interaction with gender (F (3, 88)=7.2, p=0.0002). In females, swimming decreased significantly with altitude: from 29±3% at SL to 17.9±3% at 4.5K, 7.8±1% at 10K, and 7.5±2% at 20K (F (3, 44)=11.8, p<0.0001, Fig. 3A). No change was seen in climbing behavior in females or males.
For latency to immobility, a significant effect was also seen of both altitude (p<0.0001) and the interaction between altitude and gender (F (3, 88)=12.8, p<0.0001). In females, latency to immobility decreased with altitude: from 136±8 sec at SL, to 90±9 sec at 4.5K, 51±10 sec at 10K, and 27±8 sec at 20K (F(3, 44)=28, p<0.0001, Fig. 3C). No difference in latency to immobility was seen across groups in males.
Bonferroni post-hoc tests showed that immobility in females was significantly higher at 4.5K (p<0.001), 10K (p<0.001), and 20K (p<0.001) vs. SL. Females at altitude also had significantly less swimming behavior in the test FST vs. SL: 4.5K (p<0.05), 10K (p<0.001), and 20K (p<0.001). Females at 10K and 20K also exhibited lower latency to immobility in the test FST vs. SL (p<0.001 each).
Open field test
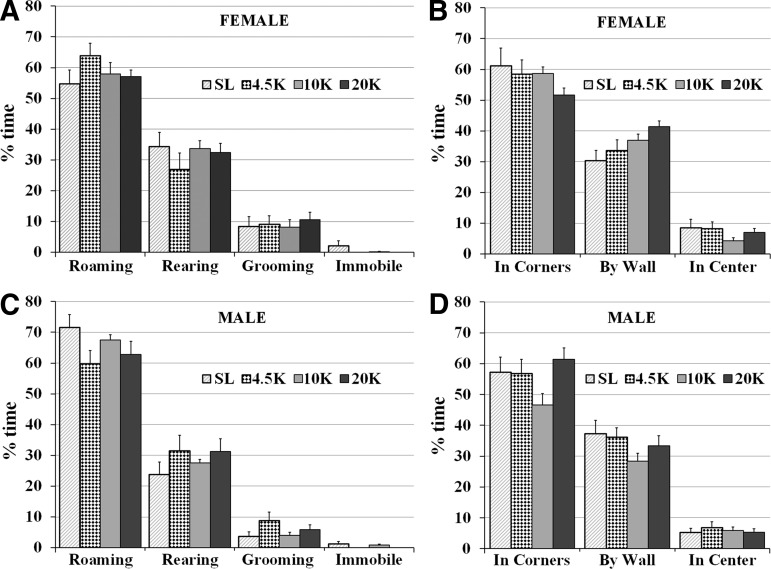
The OFT was conducted on a separate set of rodents after a week at altitude. Female rats were similar in their OFT behavior across groups: time spent roaming, rearing, grooming, or immobile (Fig. 4A), or in corners, by walls, or in the center of chamber (Fig. 4B) were similar between groups (p>0.05 for all).
FIG. 4.
The effects of hypobaric hypoxia on behavior in the OFT: (A) Female rats were similar in their behavior in the OFT: roaming, rearing, grooming, and immobility were not significantly different between altitude groups. (B) Females were also comparable in time spent in different locations in the chamber: time spent in corners, by wells, or in the center were similar in all four altitude groups. (C) Males also showed similar behavior in the OFT: roaming, rearing, grooming, and immobility were not significantly different between altitude groups. (D) Males in all four altitude groups also spent time in corners, by walls, or in the center of the chamber in similar way. (Mean±SEM, p>0.05 for all groups).
Male rats also exhibit no difference in OFT behavior across groups, with time spent in different behaviors (Fig. 4C), or locations (Fig. 4D) similar between groups (p>0.05 for all). Immobility in the OFT did not vary in females (F (3, 36)=0.92, p=0.44) or males (F (3, 36)=1.6, p=0.19) with altitude.
Discussion
The FST is a well-characterized, highly reliable assay for depression-like behavior in rodents (Bogdanova et al., 2013; Detke and Lucki, 1996; Porsolt et al., 1978). The FST is based on the observation that rats exposed to an inescapable stress (being placed in a tank of water), initially attempt to escape by swimming or climbing, and then take on an immobile posture, often referred to as behavioral despair or the lack of ability to cope with stressful stimuli (Bogdanova et al., 2013; Slattery and Cryan, 2012). After acclimatization to the forced swim paradigm in the pretest, rats quickly resume the posture of immobility in response to the inescapable stress in the test FST session. Higher levels of immobility and a shorter latency to immobility are therefore considered signs of increased depression-like behavior in the FST (Detke and Lucki, 1996; Porsolt, 1979; Slattery and Cryan, 2012). The OFT was conducted on animals to validate immobility in the FST as depression-like behavior (Slattery and Cryan, 2012). Locomotor behavior in the OFT, and immobility in particular, did not vary in rats with altitude of housing. Hypobaric hypoxia therefore does not reduce activity in the OFT in this model, validating immobility in the FST as depression-like behavior (Slattery and Cryan, 2012).
In our studies, hypobaric hypoxia causes depression-like behavior in female rats in the FST in a dose-dependent manner: immobility increases and latency to immobility decreases with increasing altitude. These studies imply that irrespective of other risk factors of living at altitude, hypobaric hypoxia alone could increase depression levels in residents at altitude. Increased depression levels at altitude could potentially contribute to the higher suicide rates linked to residence at altitude.
A previous study (Mizoguchi et al., 2011) showed that lowering barometric pressure to 20 hPa below atmospheric pressure (745 mmHg, 600 ft altitude) significantly aggravates depression-like behavior in rats in a repeated forced swim stress model. Rats subjected to daily repeated swim stress for 12 days become more immobile over the course of the test period. When swim stress was combined with intermittent low pressure exposure, rats were significantly more immobile during the test period, and higher immobility persisted for longer (14 days) after withdrawal of the swim stress in the low pressure group. Unlike the present study, the low pressure exposure was intermittent and short (to mimic air pressure reductions during typhoons), even so, this manipulation still significantly increased rodent depression-like behavior. Together this and the present study show that depression-like behavior in rodents can be caused solely by exposure to low atmospheric pressure or hypobaric hypoxia.
In our studies, female rats exhibit incrementally higher levels of depression-like behavior with exposure to lower oxygen saturation, while males do not differ between groups. FST behavior in males in all four altitude groups however corresponds to that seen in females in the higher altitude groups (10,000 ft, 20,000 ft). These studies were conducted after animals acclimatized at 4500 ft for a week. While the data suggest that males do not respond to exposure to increasing levels of hypobaric hypoxia, it is also possible that male FST behavior reaches a high threshold of depression-like behavior (approx. 75% immobility and latency to immobility of 60–80 sec) after a week of acclimatization at 4500 ft, which is not altered after a week in the different altitude conditions. Either way, it is clear that female and male rats respond differently to exposure to hypobaric hypoxia. Gender-based differences in depression-like behavior and response to stress have been noted in both rats (Dalla et al., 2011; Simpson and Kelly, 2012) and humans (Fernandez-Guasti et al., 2012; Kret and De Gelder, 2012; Ngun et al., 2011). Gender-based differences have been previously documented in rodents in the FST in both depression-like behavior and antidepressant response (Allen et al., 2010; 2012; Bogdanova et al., 2013).
Of active behaviors in the FST, swimming was significantly reduced in females with altitude, while climbing remained unchanged. Serotonergic antidepressants increase swimming in the FST, while noradrenergic antidepressants increase climbing (Detke et al., 1995). Exposure to hypoxia, both chronic and intermittent, alters monoamine metabolism in the rodent brain (Kumar, 2011). Chronic exposure to hypobaric hypoxia (2 weeks at 25,000 ft) significantly reduced serotonin in frontal cortex and brainstem in both male and female rats (Ray et al., 2011), while acute hypoxia (24 h at 22,960 ft) depleted serotonin in the male rat striatum and hypothalamus by 34% (Prioux-Guyonneau et al., 1982). Tryptophan hydroxylase, the rate-limiting enzyme in serotonin synthesis, requires molecular oxygen for its activity (Fitzpatrick, 1999), and is extremely vulnerable to hypoxia (Davis et al., 1973; Vaccari et al., 1978), potentially lowering brain serotonin synthesis in hypoxia. Hypoxia alters activity of both synthetic and catabolic enzymes in the noradrenergic pathway (Gozal et al., 2005; Vaccari et al., 1978), potentially changing brain dopamine and norepinephrine levels as well (Kumar, 2011).
While most of these data are either from male rats (Prioux-Guyonneau et al., 1982) or not analyzed by gender (Ray et al., 2011; Vaccari et al., 1978), and vary with period of hypoxia exposure, data from our preliminary studies imply that both female and male rats exhibit lower levels of serotonin but unchanged dopamine levels in the striatum after a week of housing at 20,000 ft vs. at local conditions of 4,500 ft (Kanekar, unpublished observations). If hypobaric hypoxia causes an imbalance in brain monoamines in humans residing at altitude, this could contribute to the increased depression rates seen in populations at altitude.
Katz (1982) proposed that the low oxygen saturation of tryptophan hydroxylase could have clinical implications, with even mild hypoxia decreasing serotonin synthesis, reducing appetite and motivation, and disrupting sleep patterns. Chronic hypoxia in patients with respiratory or cardiac dysfunction could similarly precipitate depression (Katz, 1982). Residents at altitude are also prone to loss of appetite, disrupted sleep cycles (Maa, 2010) and increased depression (DelMastro et al., 2011; Gamboa, 2011), implying that low brain serotonin levels may be an issue here. Low brain serotonin is also linked to suicidal ideation and attempts (Joiner et al., 2005). Hypoxia-induced low brain serotonin has also been suggested to contribute to the high suicide rates seen at altitude and in other hypoxic conditions such as COPD, asthma, or cardiac disease, or smokers (Young, 2013).
The published data on the impact of hypobaric hypoxia on brain monoamines cited above was obtained from studies on sleep deprivation, or cardiac and respiratory function at altitude (Kumar, 2011; Prioux-Guyonneau, 1982; Ray et al., 2011), and varies with the level and period of exposure of hypoxia, and the chronic or intermittent nature of exposure (Kumar, 2011). Studies are currently in progress to describe hypobaric hypoxia-induced changes in monoamines in brain regions involved in depression in our animal model.
Hypobaric hypoxia has also been found to be detrimental to brain bioenergetics. Two recent studies used in vivo 1H-proton-magnetic resonance spectroscopy (1H-MRS) scans to evaluate brain bioenergetic markers in humans and in rats exposed to hypobaric hypoxia. In 40 age- and gender-matched healthy individuals, those residing at 4500 ft (Salt Lake City, UT) were found to exhibit reduced total creatine levels (creatine+phosphocreatine) in the anterior cingulate cortex compared to those at sea level (20 ft, Belmont, MA, or Charleston, SC), implying that living at altitude alone can produce brain hypometabolism in healthy humans (Renshaw, 2012). In vivo 1H-MRS scans of rodents prior to and after 1 week at an altitude simulation of 10,000 ft identified several changes in metabolic markers, including a similar forebrain deficit in total creatine after exposure to altitude (Bogdanova et al., 2014). Similarly, in vivo 1H-MRS scans of Sprague Dawley rats prior to and after a shorter exposure to extreme hypobaric hypoxia (48 h at 21,976 ft) showed altered brain metabolites (including total creatine) in the hippocampus after hypoxia exposure, some of which persisted for 2 weeks after altitude exposure (Koundal et al., 2014). Positron emission tomography (PET) and MRS scan studies find that MDD is linked with brain neuronal hypometabolism, which is resolved after successful treatment, but remains unchanged in nonresponders to treatment (Iosifescu and Renshaw, 2003; Mayberg, 2003). The low oxygen saturation at altitude could aggravate MDD-linked brain hypometabolism, thereby increasing mood instability and causing higher rates of depression and suicide at altitude.
This study has a few limitations. Animals were delivered from sea level and spent a week at local conditions (4500 ft) to acclimatize prior to altitude exposure, which could affect study results. A step-wise increase in altitude was found to temper the physiological impact of high rate ascent in humans at altitude (Beidleman et al., 2009), implying that our results may be more moderate than expected. Also, all behavioral experiments were done at local conditions of 4500 ft. In addition, both females and males ate less and lost weight after a week at 20,000 ft, therefore nutritional deprivation and weight loss could potentially impact depression-like behavior in this group. However, if we analyze data from the sea level, 4500 ft and 10,000 ft groups alone, the main point of our study would remain the same: hypobaric hypoxia can induce depression-like behavior. Despite these limitations, a significant effect of altitude was seen on depression-like behavior in female rats in this study.
While this study provides evidence that hypobaric hypoxia alone can cause depression-like behavior in rats, this is clearly a snapshot of the effects of hypobaric hypoxia at a single time point. Several studies have examined adaptation to living at altitude, and have found that hypoxia-induced imbalances can be resolved with adaptation to living at altitude (Moore et al., 1998; Wilson et al., 2009). However, adaptation to hypobaric conditions clearly varies between individuals (Moore et al., 1998), and may be inadequate in those who have concurrent brain hypometabolism, such as in those with MDD, other mood disorders, or drug addiction (Haws et al., 2009; Iosifescu and Renshaw, 2003), or those living with chronic hypoxic disorders (Haws et al., 2009; Katz, 1982). Further studies examining the role of hypoxia in depression will therefore benefit not only those with altitude-related depression, but potentially other high risk MDD patients, such as those with co-morbid psychiatric disorders, drug addiction, or with other chronic hypoxic conditions such as asthma, sleep apnea, or COPD.
Acknowledgments
We thank Mr. Barry Evans for his expertise with the altitude chambers. We extend special thanks to Dr. Wendy Pouliot and Dr. Ed Dudek for their assistance.
Author Disclosure Statement
Dr. Renshaw has received grants from the NIH, the VA, and USTAR, and compensation as a consultant to Kyowa Hakko Kirin and Ridge Diagnostics.
References
- Allen PJ, D'Anci KE, Kanarek RB, and Renshaw PF. (2010). Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology 35:534–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PJ, D'Anci KE, Kanarek RB, and Renshaw PF. (2012). Sex-specific antidepressant effects of dietary creatine with and without sub-acute fluoxetine in rats. Pharmacol Biochem Behav 101:588–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrke MS, and Shukitt-Hale B. (1993). Effects of altitude on mood, behaviour and cognitive functioning. A review. Sports Med 16:97–125 [DOI] [PubMed] [Google Scholar]
- Barch DM. (2013). Introduction to special issue on the neurobiology of depression. Neurobiol Dis 52:1–3 [DOI] [PubMed] [Google Scholar]
- Bartsch P, and Swenson E. (2013). Clinical practice: Acute high-altitude illnesses. N Eng J Med 368:2294–2302 [DOI] [PubMed] [Google Scholar]
- Beidleman BA, Fulco CS, Muza SR, Rock PB, Staab JE, Forte VA, Brothers MD, and Cymerman A. (2009). Effect of six days of staging on physiologic adjustments and acute mountain sickness during ascent to 4300 meters. High Alt Med Biol 10:253–260 [DOI] [PubMed] [Google Scholar]
- Bogdanova OV, Abdullah O, Kanekar S, Bogdanov VB, Prescot AP, and Renshaw PF. (2014). Neurochemical alterations in frontal cortex of the rat after one week of hypobaric hypoxia. Behav Brain Res 263:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova OV, Kanekar S, D'Anci KE, and Renshaw PF. (2013). Factors influencing behavior in the forced swim test. Physiol Behav 118:227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F, and Meli A. (1988). Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharm (Berl) 94:147–160 [DOI] [PubMed] [Google Scholar]
- Brenner B, Cheng D, Clark S, and Camargo CA., Jr. (2011). Positive association between altitude and suicide in 2584 U.S. counties. High Alt Med Biol 12:31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, and Papadopoulou-Daifoti Z. (2011). Sex differences in response to stress and expression of depressive-like behaviours in the rat. Curr Top Behav Neurosci 8:97–118 [DOI] [PubMed] [Google Scholar]
- Davis JN, Carlsson A, MacMillan V, and Siesjo BK. (1973). Brain tryptophan hydroxylation: Dependence on arterial oxygen tension. Science 182:72–74 [DOI] [PubMed] [Google Scholar]
- DelMastro K, Hellem T, Kim N, Kondo D, Sung YH, and Renshaw PF. (2011). Incidence of major depressive episode correlates with elevation of substate region of residence. J Affect Disord 129:376–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, and Lucki I. (1996). Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: The effects of water depth. Behav Brain Res 73:43–46 [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, and Lucki I. (1995). Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharm (Berl) 121:66–72 [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Fiedler JL, Herrera L, and Handa RJ. (2012). Sex, stress, and mood disorders: At the intersection of adrenal and gonadal hormones. Horm Metab Res 44:607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick PF. (1999). Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem 68:355–381 [DOI] [PubMed] [Google Scholar]
- Gamboa JL CR, and Arregui A. (2011). Is depression the link between suicide and high altitude? High Alt Med Biol 12:403–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez NC, and Wood JG. (2010). Alveolar hypoxia-induced systemic inflammation: what low PO(2) does and does not do. Adv Exp Med Biol 662:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD. (2011). Is COPD associated with suicide behavior? J Psychiatr Res 45:1269–1271 [DOI] [PubMed] [Google Scholar]
- Goodwin RD. (2012). Asthma and suicide: Current knowledge and future directions. Curr Psychiatry Rep 14:30–35 [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Demmer RT, Galea S, Lemeshow AR, Ortega AN, and Beautrais A. (2012). Asthma and suicide behaviors: Results from the Third National Health and Nutrition Examination Survey (NHANES III). J Psychiatr Res 46:1002–1007 [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Kroenke K, Hoven CW, and Spitzer RL. (2003). Major depression, physical illness, and suicidal ideation in primary care. Psychosom Med 65:501–505 [DOI] [PubMed] [Google Scholar]
- Gozal E, Shah ZA, Pequignot JM, Pequignot J, Sachleben LR, Czyzyk-Krzeska MF, Li RC, Guo SZ, and Gozal D. (2005). Tyrosine hydroxylase expression and activity in the rat brain: Differential regulation after long-term intermittent or sustained hypoxia. J Appl Physiol 99:642–649 [DOI] [PubMed] [Google Scholar]
- Haws CA, Gray DD, Yurgelun-Todd DA, Moskos M, Meyer LJ, and Renshaw PF. (2009). The possible effect of altitude on regional variation in suicide rates. Med Hypotheses 73:587–590 [DOI] [PubMed] [Google Scholar]
- Helbich M, Bluml V, Leitner M, and Kapusta ND. (2013). Does altitude moderate the impact of lithium on suicide? A spatial analysis of Austria. Geospat Health 7:209–218 [DOI] [PubMed] [Google Scholar]
- Henriksson MM, Aro HM, Marttunen MJ, Heikkinen ME, Isometsa ET, Kuoppasalmi KI, and Lonnqvist JK. (1993). Mental disorders and comorbidity in suicide. Am J Psychiatry 150:935–940 [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, and Renshaw PE. (2003). 31P-magnetic resonance spectroscopy and thyroid hormones in major depressive disorder: Toward a bioenergetic mechanism in depression? Harv Rev Psychiatry 11:51–63 [DOI] [PubMed] [Google Scholar]
- Joiner TE, Jr., Brown JS, and Wingate LR. (2005). The psychology and neurobiology of suicidal behavior. Annu Rev Psychol 56:287–314 [DOI] [PubMed] [Google Scholar]
- Katz IR. (1982). Is there a hypoxic affective syndrome? Psychosomatics 23:846,849–850,852–853 [DOI] [PubMed] [Google Scholar]
- Kim N, Mickelson JB, Brenner BE, Haws CA, Yurgelun-Todd DA, and Renshaw PF. (2011). Altitude, gun ownership, rural areas, and suicide. Am J Psychiatry 168:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundal S, Gandhi S, Kaur T, and Khushu S. (2014). Neurometabolic and structural alterations in rat brain due to acute hypobaric hypoxia: In vivo H MRS at 7 T. NMR Biomed 27:341–347 [DOI] [PubMed] [Google Scholar]
- Kovaleva IO, Artem'eva MM, Medvedev OS, and Medvedeva NA. (2013). Chronic administration of estradiol to ovariectomized female Wistar rats causes development of hypoxic pulmonary hypertension. Eksp Klin Farmakol 76:7–9 [PubMed] [Google Scholar]
- Kret ME, and De Gelder B. (2012). A review on sex differences in processing emotional signals. Neuropsychologia 50:1211–1221 [DOI] [PubMed] [Google Scholar]
- Kumar GK. (2011). Hypoxia. 3. Hypoxia and neurotransmitter synthesis. Am J Physiol Cell Physiol 300:C743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa EH. (2010). Hypobaric hypoxic cerebral insults: The neurological consequences of going higher. NeuroRehabilitation 26:73–84 [DOI] [PubMed] [Google Scholar]
- Mark TL, Shern DL, Bagalman JE, and Cao Z. (2007). Ranking America's mental health: An analysis of depression across the States Mental Health America [Google Scholar]
- Martin I, Basso N, Aquirre F, and Sarchi MI. (1994) Changes in the renin-angiotensin-aldosterone system in 2 kidney-2clip Goldblatt hypertensive rats of both sexes submitted to chronic hypobaric hypoxia. Arch Physiol Biochim Biophys 102:209–214 [DOI] [PubMed] [Google Scholar]
- Mayberg HS. (2003). Positron emission tomography imaging in depression: A neural systems perspective. Neuroimaging Clinics N America 13:805–815 [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Fukaya K, Mori R, Itoh M, Funakubo M, and Sato J. (2011). Lowering barometric pressure aggravates depression-like behavior in rats. Behav Brain Res 218:190–193 [DOI] [PubMed] [Google Scholar]
- Moore LG, Niermeyer S, and Zamudio S. (1998). Human adaptation to high altitude: Regional and life-cycle perspectives. Am J Phys Anthropol Suppl 27:25–64 [DOI] [PubMed] [Google Scholar]
- Mortola JP. (2007). Hypoxia and circadian patterns. Respir Physiol Neurobiol 158:274–279 [DOI] [PubMed] [Google Scholar]
- Netzer N, Strohl K, Faulhaber M, Gatterer H, and Burtscher M. (2013). Hypoxia-related altitude illnesses. J Travel Med 20:247–255 [DOI] [PubMed] [Google Scholar]
- Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, and Vilain E. (2011). The genetics of sex differences in brain and behavior. Front Neuroendocrinol 32:227–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD. (1979). Animal model of depression. Biomedicine 30:139–140 [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, and Jalfre M. (1978). Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391 [DOI] [PubMed] [Google Scholar]
- Prioux-Guyonneau M M-CE, Redjimi-Hafsi F, and Jacquot C. (1982). Changes in brain 5-hydroxytryptamine metabolism induced by hypobaric hypoxia. Gen Pharmacol 13:251–254 [DOI] [PubMed] [Google Scholar]
- Ray K, Dutta A, Panjwani U, Thakur L, Anand JP, and Kumar S. (2011). Hypobaric hypoxia modulates brain biogenic amines and disturbs sleep architecture. Neurochem Int 58:112–118 [DOI] [PubMed] [Google Scholar]
- Renshaw PA. Prescot D, Ongur R, Huber D, and Yurgelun-Todd (2012). Suicide and brain chemical changes with altitude. International Society for Affective Disorders Abstracts [Google Scholar]
- Schneider C, Jick SS, Bothner U, and Meier CR. (2010). COPD and the risk of depression. Chest 137:341–347 [DOI] [PubMed] [Google Scholar]
- Simpson J, and Kelly JP. (2012). An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behav Brain Res 229:289–300 [DOI] [PubMed] [Google Scholar]
- Singh SB, Sharma A, Panjwani U, Yadav DK, Chandra K, Sharma KN, and Selvamurthy W. (1997). Hypobaric hypoxia and hedonic matrix in rats. Jpn J Physiol 47:327–333 [DOI] [PubMed] [Google Scholar]
- Slattery DA, and Cryan JF. (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7:1009–1014 [DOI] [PubMed] [Google Scholar]
- Vaccari A, Brotman S, Cimino J, and Timiras PS. (1978). Adaptive changes induced by high altitude in the development of brain monoamine enzymes. Neurochem Res 3:295–311 [DOI] [PubMed] [Google Scholar]
- van den Bemt L, Schermer T, Bor H, Smink R, van Weel-Baumgarten E, Lucassen P, and van Weel C. (2009). The risk for depression comorbidity in patients with COPD. Chest 135:108–114 [DOI] [PubMed] [Google Scholar]
- Webb RT, Kontopantelis E, Doran T, Qin P, Creed F, and Kapur N. (2012). Suicide risk in primary care patients with major physical diseases: A case-control study. Arch Gen Psychiatry 69:256–264 [DOI] [PubMed] [Google Scholar]
- Wilson MH, Newman S, and Imray CH. (2009). The cerebral effects of ascent to high altitudes. Lancet Neurol 8:175–191 [DOI] [PubMed] [Google Scholar]
- Young SN. (2013). Elevated incidence of suicide in people living at altitude, smokers and patients with chronic obstructive pulmonary disease and asthma: Possible role of hypoxia causing decreased serotonin synthesis. J Psychiatry Neurosci 38:130002. [DOI] [PMC free article] [PubMed] [Google Scholar]