Abstract
Although mild therapeutic hypothermia is an effective neuroprotective strategy for cardiac arrest/resuscitated patients, and asphyxic newborns, recent randomized controlled trials (RCTs) have equally shown good neurological outcome between targeted temperature management at 33°C versus 36°C, and have not shown consistent benefits in patients with traumatic brain injury (TBI). We aimed to determine the effect of therapeutic hypothermia, while avoiding some limitations of earlier studies, which included patient selection based on Glasgow coma scale (GCS), delayed initiation of cooling, short duration of cooling, inter-center variation in patient care, and relatively rapid rewarming. We conducted a multicenter RCT in patients with severe TBI (GCS 4–8). Patients were randomly assigned (2:1 allocation ratio) to either therapeutic hypothermia (32–34°C, n=98) or fever control (35.5–37°C, n=50). Patients with therapeutic hypothermia were cooled as soon as possible for ≥72 h and rewarmed at a rate of <1°C/day. All patients received tight hemodynamic monitoring under intensive neurological care. The Glasgow Outcome Scale was assessed at 6 months by physicians who were blinded to the treatment allocation. The overall rates of poor neurological outcomes were 53% and 48% in the therapeutic hypothermia and fever control groups, respectively. There were no significant differences in the likelihood of poor neurological outcome (relative risk [RR] 1.24, 95% confidence interval [CI] 0.62–2.48, p=0.597) or mortality (RR 1.82, 95% CI 0.82–4.03, p=0.180) between the two groups. We concluded that tight hemodynamic management and slow rewarming, together with prolonged therapeutic hypothermia (32–34°C) for severe TBI, did not improve the neurological outcomes or risk of mortality compared with strict temperature control (35.5–37°C).
Key words: : cardiac output, cerebral perfusion, intracranial pressure, therapeutic hypothermia
Introduction
Mild therapeutic hypothermia (MTH) improves neurological outcomes and can prevent severe brain damage after cardiac arrest.1,2 Beneficial effects of MTH have also been demonstrated in several randomized controlled trials (RCTs) in newborn infants with perinatal asphyxia,3–6 whereas a recent RCT could not show significant effects of hypothermia between targeted temperature management (36°C) and TH (33°C) with overall good neurological outcome.7 The efficacy of MTH has also been studied in patients with severe traumatic brain injury (TBI).8–12 However, the outcomes of these RCTs were conflicting, possibly because of the significant pathophysiological complexity and variability of this disease. Additionally, two well-designed RCTs in adults and children did not show favorable outcomes with the use of MTH in patients with severe TBI.13,14 Although the overall results of the National Acute Brain Injury Study: Hypothermia (NABISH) study were negative,13 subsequent analyses identified several factors that might improve the effects of MTH for treating patients with TBI in clinical settings. These factors included the time to reach the target temperature,13 fluid management,15 intercenter variations in the management of MTH,16 and the rewarming process.17 Several systematic reviews suggested that MTH should be maintained for>48 h, and that the rate of rewarming should be very slow.18,19
The present study, the Brain-Hypothermia (B-HYPO) Study, was designed to take into account these earlier discussions and recommendations. Although several trials were published after starting our study,20–22 the effects of MTH in patients with severe TBI remained controversial in terms of neurological outcomes.
We performed this study in emergency and critical care centers in Japan to compare the neurological outcomes between TH (32–34°C) and fever control (35.5–37°C) for patients with TBI, and to clarify the clinical efficacy of MTH.
Methods
Patients, randomization, and blinding
The study covered the period from December 2002 to September 2008. It was designed as a multicenter RCT with prospective analyses and blinded assessment of neurological outcomes. The protocol and consent procedures were approved by the Institutional Review Board of each participating hospital. The study was registered with the University Hospital Medical Information Network (UMIN-CTR, No. C000000231) in Japan and the National Institutes of Health (Clinical Trials.gov Identifier NCT00134472) in the United States of America. The randomization list was automatically generated by the UMIN computer system to allocate patients in a 2:1 ratio to receive TH (32–34°C) or fever control (35.5–37°C). The temperature of the control group was set to 35.5–37°C for ethical reasons, because MTH was reported to be effective in adult cardiac arrest/resuscitated patients at that time.1,2 We also attempted to try quick initiation of temperature managements in which cooling was allowed to be 35.5°C before the randomization in both groups, to prolong duration of hypothermia (>72 h), to reduce rewarming speed (<1.0°C/day) and to randomize for two parameters (Glasgow Coma Scale [GCS] scores 4–5 and 6–8; ages 15–45 years and 46–69 years) in each participating hospital.
Inclusion criteria were age 15–69 years for both sexes, GCS score of 4–8, and ability to initiate cooling within 2 h after the onset of TBI. Patients with any of the following were excluded: good motor response (GCS motor response=6), systolic blood pressure <90 mm Hg after fluid and vasopressor resuscitation, platelet count <50,000 /mm3, severe pre-existing medical conditions (e.g., liver, kidney, or heart failure, or severe arrhythmia), acute myocardial infarction, pregnancy, severe alcohol intoxication that prevented assessment of consciousness, penetrating brain injury, epidural hematoma without brain parenchymal injury, or core body temperature <30°C. Eligible patients were enrolled and their core temperature was lowered to the desired temperature as quickly as possible. Head computed tomography (CT) scans were performed and evaluated on admission and after rewarming in all patients.
After obtaining written informed consent from the patient's legally authorized representative, the patient was randomized to either TH or fever control based on the randomization list. An Internet-based enrolment system managed by the UMIN enabled instant randomization for the patient upon admission at each participating site. Patient private information was secured. If informed consent could not be obtained within 2 h of admission, the consent policy was waived.
Treatments
Core body temperature was measured by a thermistor coupled to an internal jugular venous catheter. If the catheter could not be inserted, body temperature was measured in another site that was selected in the following order: pulmonary artery, bladder, rectum, and tympanic membrane. In addition to critical neurological care,23 an arterial catheter, a pulmonary arterial catheter, and an intracranial pressure (ICP) monitoring probe were inserted to maintain hemodynamic status and ICP at the following levels: mean arterial pressure (MAP)>80 mmHg, cardiac index (CI)>2.5 L/min/m2, systemic vascular resistance index (SVRI) 800–1200 dynes/sec/cm5, ICP<20 mm Hg, and cerebral perfusion pressure (CPP)>60 mm Hg. The partial pressures of arterial oxygen (PaO2) and carbon dioxide (PaCO2) were maintained at>100 mm Hg and 30–40 mm Hg, respectively. If ICP was>20 mm Hg, any treatment recommended by the Japanese guidelines could be applied, including mannitol/glycerol and/or a bolus infusion of barbiturates,23 but not continuous infusion of barbiturates, because of the enormous suppressive effects on cardiac function. Hyperventilation was allowed, but excessive hypocapnia<30 mm Hg was prohibited. ICP could also be reduced by decompressive craniectomy. Anticonvulsants were allowed as deemed necessary.
Cooling blankets, rapid cold fluid infusion (up to 1000 mL saline, human plasma products, or dextrose-free plasma expanders), and/or cold gastric lavage could be used during the induction phase in both groups. The aim was to achieve the target temperature within 6 h after the onset of TBI. The desired temperature was to be maintained for ≥72 h, mainly using surface cooling blankets, in each group. The patient was rewarmed at a rate of <1°C/day and core body temperature was maintained at <38°C for 7 days after the onset of TBI.
Our sedation protocol specified either midazolam (0.2–0.4 mg/kg/h) and non-narcotic analgesics for patients treated between December 2002 and April 2005, or neuroleptic analgesia (NLA) (25 μg/kg/h droperidol and 1 μg/kg/h fentanyl) for patients treated between May 2005 and May 2008. Vecronium (0.05 mg/kg/h) or pancuronium (0.05 mg/kg/h) could be used in each sedation protocol during the induction and maintenance phases as deemed necessary. In terms of hemodynamic outcomes, NLA may be superior to midazolam, because NLA can dilate peripheral blood vessels, increase cardiac output, and facilitate quicker cooling.24 Sedatives and analgesics were usually tapered off, once the patients had been rewarmed to 36°C. The muscle relaxant was stopped when shivering had disappeared, which usually occurred during the maintenance phase. Muscle relaxants were restarted as deemed necessary.
Data collection and study outcomes
All data, except head CT data, were transmitted to the UMIN center via an Internet-based system. Injury severity score (ISS) and head CT classification were also assessed. In this study, ISS was also calculated as an abbreviated injury score (AIS).25 Head CT was classified as follows: diffuse injury grade I, all diffuse injuries without CT findings; diffuse injury grade II, high or mixed density lesions with a volume of <25 mL; diffuse injury grade III, high or mixed density lesions with a volume of <25 mL and compressed or absent basal cisterns; diffuse injury grade IV, high or mixed density lesions with a volume of <25 mL and a midline shift of >5 mm; and evacuated/nonevacuated mass, high or mixed density lesions with a volume of >25 mL with or without surgical evacuation.26 Hemodynamic and laboratory data were recorded on Days 0, 1, and 3, as well as 1 day after rewarming (defined as the day on which the core body temperature reached 36°C). Representative values were recorded for each day.
The primary outcome, Glasgow Outcome Scale (GOS) at 6 months, was assessed by a neurosurgeon, a neurologist, or an emergency physician who was unaware of the patient's treatment method.27 After discharge from the original hospital, the patients were evaluated by telephone. Severe disability (SD), persistent vegetative state (PVS), or death (D) were defined as poor neurological outcomes, whereas moderate disability (MD) or good recovery (GR) were defined as good neurological outcomes. The RCT stopped at the interim because of a shortage of TBI patients and the trial's futility.
Statistical analysis
The planned sample size of 300 patients allocated in a 2:1 ratio to receive TH or fever control would detect a 20% difference (90% power) in the percentage of patients with a good neurological outcome because the percentage of good neurological outcomes in severe TBI patients without MTH was 31% by an inquiry taken from the participating hospital in Japan before our RCT and there was a 20% difference between TH and control groups in the cardiac arrest /resuscitated patients.1,2 From these facts, the sample sizes were planned to be 200 and 100 for TH and fever control groups, respectively.
Poor neurological outcomes and mortality were compared between the two groups. Patients were stratified into predefined subgroups according to initial GCS (4–5 or 6–8) and age (≤45 or >45 years old) based on the results of the first NABISH study.13 Continuous variables were statistically analyzed using Student's t test and the Mann–Whitney U test, as appropriate. Categorical variables were statistically analyzed using χ2 tests and Fisher's exact probability test, where appropriate. The threshold of significance was set at p<0.05.
Results
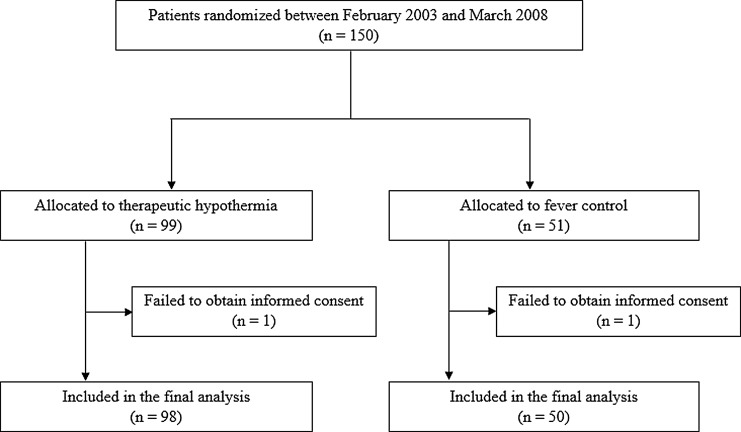
The RCT enrollment began in 2002 and was stopped in 2008 before completion of sample size (300 cases) goals, because of concern about shortage of TBI patients (95 cases) and very small differences in neurological outcome between the two groups at the interim analysis in 2005. After this analysis, the sedative protocol was changed from midazolam to NLA as the initial plan. Figure 1 shows the disposition of the 150 patients enrolled and randomized in this study. Ninety-nine patients were randomized to the TH group and 51 to the fever control group. After enrolment, informed consent could not be obtained from two patients (one patient per group). Therefore, intention-to-treat analyses were performed in 148 patients. One patient in the fever control group was found to be >70 years old after randomization. Six patients in the TH group and one patient in the fever control group had unstable vital signs before temperature management. Neurological outcomes could not be assessed at 6 months in four patients in the TH group and in two patients in the fever control group, respectively. Four patients in the fever control group received hypothermic treatment during their clinical course because of uncontrollable ICP. In these patients, hypothermic treatment was only performed after several attempts to reduce ICP, including decompressive craniectomy.
FIG. 1.
Patient dispositions. Overall, 99 patients were enrolled in the therapeutic hypothermia group and 51 patients were enrolled in the fever control group. Hemodynamic status, laboratory parameters, and the primary outcome were assessed in 148 patients.
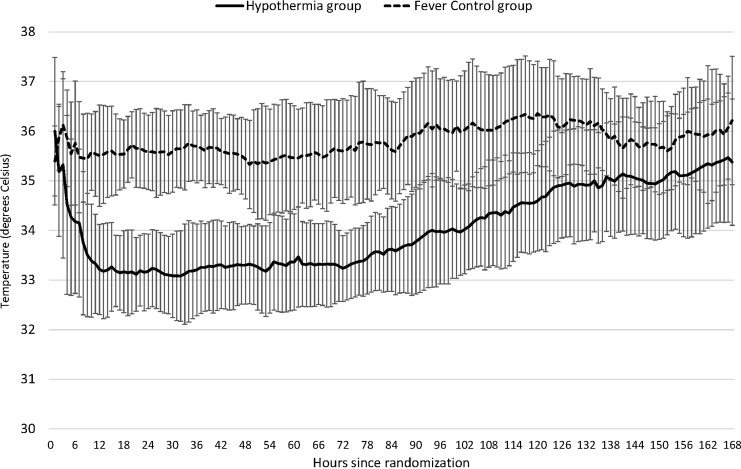
Table 1 shows the patients' characteristics. There were no significant differences between the two groups for age, sex, blood pressure, heart rate, GCS on admission, nonreactive pupil or pupils, head CT scan scores, neurosurgical operation rate, ISS, AIS score for the head, or AIS score ≥4 for other organs, except for ISS which was significantly higher in the TH group. Decompressive craniectomy applied to six patients in the TH group and to five in the fever control group. Table 2 shows the times from the onset of TBI to admission, randomization, initiation of cooling, and reaching the desired core body temperature (35.5°C or 34°C) in the TH group. The durations of the maintenance and rewarming periods in the TH group are also shown. The median times from the onset of TBI to the desired core body temperatures of 35.5°C and 34°C in the TH group were 5.2 h and 8.1 h, respectively. The durations of the cooling and rewarming periods for the hypothermia group were 75.5 h and 76.0 h, respectively. Figure 2 shows temperature curves in both groups.
Table 1.
Patient Characteristics
| Variable | Hypothermia (32.0–34.0°C) n=98 | Fever control (35.5–37.0°C) n=50 | p value |
|---|---|---|---|
| Age (years) | 39±19 | 39±18 | 0.940 |
| Male | 69 (70) | 34 (68) | 0.763 |
| Systolic blood pressure (mmHg) | 144±34 | 151±39 | 0.222 |
| Diastric blood pressure (mmHg) | 81±20 | 83±20 | 0.464 |
| Heart rate (beats/min) | 92±28 | 88±24 | 0.431 |
| Glasgow Coma Scale | 5.8±1.4 | 5.9±1.3 | 0.513 |
| 4–5 | 41 (42) | 16 (32) | 0.245 |
| 6–8 | 57 (58) | 34 (68) | |
| Unreactive pupil or pupils | 47 (48) | 23 (46) | 0.821 |
| Scores on head CT scans | |||
| Diffuse injury grade I | 1 (1) | 1 (2) | 0.600 |
| Diffuse injury grade II | 30 (31) | 16 (32) | |
| Diffuse injury grade III | 15 (15) | 9 (18) | |
| Diffuse injury grade IV | 3 (3) | 1 (2) | |
| Evacuated mass | 43 (44) | 21 (42) | |
| Nonevacuated mass | 5 (5) | 0 (0) | |
| Unknown | 1 (1) | 2 (4) | |
| Surgical operation for TBI | 61 (62) | 31 (62) | 0.977 |
| Injury severity score | 27±9 | 24±7 | 0.037 |
| AIS score for head | 4.3±0.7 | 4.2±0.7 | 0.560 |
| AIS score≥4 for other organs | 10 (10) | 3 (6) | 0.393 |
Values are n (%) or mean±standard deviation.
TBI, traumatic brain injury; AIS, Abreviated Injury Score.
Table 2.
Patient Characteristics
| Variable | Group | n | Median (interquartile range) | p value |
|---|---|---|---|---|
| Time from the onset of TBI | ||||
| To admission (h) | Hypothermia | 98 | 0.7 (0.5–1.0) | 0.780 |
| Fever control | 50 | 0.8 (0.5–0.9) | ||
| To randomization (h) | Hypothermia | 91 | 3.8 (2.7–5.1) | 0.906 |
| Fever control | 46 | 3.3 (2.3–4.5) | ||
| To the start of cooling (h) | Hypothermia | 94 | 3.0 (2.3–4.5) | |
| to 35.5°C (h) | Hypothermia | 91 | 5.2 (3.5–7.5) | |
| to 34.0°C (h) | Hypothermia | 92 | 8.1 (5.3–11.8) | |
| Duration of cooling (h) | Hypothermia | 82 | 75.5 (68.9–84.0) | |
| Duration of rewarming (h) | Hypothermia | 80 | 76.0 (51.5–113.5) | |
TBI, traumatic brain injury.
FIG. 2.
Blood temperature. Core temperature was defined as a bladder temperature or blood temperature in the study. Pulmonary blood temperature of therapeutic hypothermia group and fever control group are plotted. A pulmonary arterial catheter was used for tight hemodynamic monitoring in 133 patients and automatically recorded blood temperature was available in 84 patients. The temperature curves display the means, and the I bars indicate±standard deviation.
Table 3 shows the hemodynamic parameters measured on days 0, 1, and 3 of treatment, and 1 day after rewarming. Most of the patients had an arterial catheter (100%), a pulmonary arterial catheter (89%), a jugular venous catheter (85%), and an ICP monitor (90%). Consequently, hemodynamic status was well controlled (Table 3), and none of these parameters differed between the two groups on any measurement day, except for systemic vascular resistance index which was significantly higher in the TH group on day 1. The white blood cell count at 1 day after rewarming and aspartate aminotransferase (AST) on day 3 of treatment were significantly higher in the TH group than in the fever control group, whereas the other laboratory parameters did not differ significantly between the two groups.
Table 3.
Hemodynamic Parameters
| Day 0 | Day 1 | Day 3 | 1 day after rewarming | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interval from onset (h) Median Interquatile range Variable | 5.8 (1.5–9.3) Hypothermia | 5.9 (2.2–8.3) Fever control | p value | 24 (20–29) Hypothermia | 26 (19–31) Fever control | p value | 71 (65–78) Hypothermia | 72 (67–76) Fever control | p value | 178 (147–219) Hypothermia | 121 (111–130) Fever control | p value |
| Core temperature (°C) | 35.3±1.6 | 36.1±0.9 | 0.002 | 33.4±0.8 | 35.7±1.0 | <0.001 | 33.6±1.0 | 35.6±1.0 | <0.001 | 36.5±0.8 | 36.7±0.8 | 0.113 |
| MAP (mmHg) | 89±19 | 95±23 | 0.135 | 89±15 | 89±15 | 0.960 | 92±16 | 94±18 | 0.458 | 98±15 | 99±14 | 0.832 |
| ICP (mmHg) | 21±19 | 29±23 | 0.088 | 22±24 | 22±17 | 0.969 | 22±24 | 20±15 | 0.602 | 18±15 | 22±16 | 0.188 |
| ≤30 | 48 (74) | 25 (71) | 0.795 | 70 (80) | 37 (84) | 0.612 | 70 (82) | 35 (90) | 0.289 | 65 (89) | 29 (81) | 0.226 |
| >30 | 17 (26) | 10 (29) | 17 (20) | 7 (16) | 15 (18) | 4 (10) | 8 (11) | 7 (19) | ||||
| CPP (mmHg) | 69±27 | 76±31 | 0.296 | 67±27 | 64±27 | 0.611 | 69±33 | 71±28 | 0.817 | 80±23 | 77±23 | 0.413 |
| ≤50 | 12 (19) | 6 (18) | 0.946 | 12 (14) | 9 (20) | 0.326 | 16 (19) | 6 (15) | 0.642 | 8 (11%) | 3 (8) | 0.669 |
| >50 | 52 (81) | 27 (82) | 75 (86) | 35 (80) | 69 (81) | 33 (85) | 65 (89) | 33 (92) | ||||
| CVP (mmHg) | 5.2±3.8 | 7.0±3.7 | 0.059 | 6.6±4.0 | 5.9±3.7 | 0.338 | 7.6±4.8 | 7.3±3.5 | 0.688 | 6.5±3.4 | 7.4±4.9 | 0.278 |
| CI (L/min/m2) | 3.3±1.4 | 3.3±1.1 | 0.824 | 3.1±0.9 | 3.5±1.0 | 0.057 | 3.6±1.0 | 3.8±1.1 | 0.426 | 4.4±1.1 | 4.2±1.8 | 0.380 |
| SvO2 (%) | 73±14 | 76±7 | 0.401 | 76±11 | 78±6 | 0.251 | 79±9 | 79±7 | 0.936 | 78±9 | 78±8 | 0.736 |
| SVRI (dynes/s/cm–5) | 807±390 | 750±458 | 0.578 | 822±298 | 709±256 | 0.038 | 712±284 | 666±242 | 0.390 | 622±214 | 634±384 | 0.849 |
Values are n (%) or mean±standard deviation.
MAP, mean arterial pressure; ICP, intracranial pressure; CPP, cerebral perfusion pressure; CVP, central venus pressure; CI, cardiac index; SvO2, mixed venous oxygen saturation; SVRI, systemic vascular resistance index.
Table 4 shows the proportions of patients with poor neurological outcomes (i.e., SD, PVS, or D) and the mortality rates at 6 months after the onset of TBI in both groups. Data are shown for all patients and for subgroups of patients stratified by GCS and age. The overall rate of poor neurological outcomes in the TH and fever control groups were 53% and 48%, respectively. We found no significant difference in the likelihood of poor neurological outcomes, evaluated by GOS (relative risk [RR] 1.24, 95% confidence interval [CI] 0.62–2.48, p=0.597) and mortality (RR 1.82, 95% CI 0.82–4.03, p=0.180), between the patients treated with hypothermia or strict fever control groups. There was no significant difference in the outcome between the two subgroups.
Table 4.
Rates of Poor Neurological Outcomes and Mortality
| Treatment group | Total n | Poor outcomes n (%) | Relative risk (95% CI) | p value | Total n | Mortality | Relative risk (95% CI) | p value |
|---|---|---|---|---|---|---|---|---|
| All patients | 142 | 1.24 (0.62–2.48) | 0.597 | 142 | 1.82 (0.82–4.03) | 0.180 | ||
| Hypothermia | 94 | 50 (53) | 94 | 33 (35) | ||||
| Fever control | 48 | 23 (48) | 48 | 11 (23) | ||||
| Patients with GCS 4–5 | 56 | 0.50 (0.14–1.83) | 0.365 | 56 | 1.00 (0.30–3.31) | 1.000 | ||
| Hypothermia | 40 | 24 (60) | 40 | 15 (38) | ||||
| Fever control | 16 | 12 (75) | 16 | 6 (38) | ||||
| Patients with GCS 6–8 | 86 | 1.77 (0.72–4.38) | 0.263 | 86 | 2.70 (0.89–8.19) | 0.084 | ||
| Hypothermia | 54 | 26 (48) | 54 | 18 (33) | ||||
| Fever control | 32 | 11 (34) | 32 | 5 (16) | ||||
| Patients >45 years old | 63 | 0.89 (0.28–2.82) | 1.000 | 63 | 1.82 (0.61–5.41) | 0.418 | ||
| Hypothermia | 42 | 29 (69) | 42 | 20 (48) | ||||
| Fever control | 21 | 15 (71) | 21 | 7 (33) | ||||
| Patients ≤45 years old | 79 | 1.61 (0.60–4.35) | 0.462 | 79 | 1.92 (0.56–6.58) | 0.392 | ||
| Hypothermia | 52 | 21 (40) | 52 | 13 (25) | ||||
| Fever control | 27 | 8 (30) | 27 | 4 (15) |
CI, confidence interval; GCS, Glasgow coma scale.
Table 5 shows the incidence of protocol deviation and complication rates. The time to the target temperature of 34°C exceeded 6 h in 62% of patients in the TH group. The overall rates of complications were 17% in the TH group and 2% in the fever control group, which were statistically significant.
Table 5.
Deviations from the Study Protocol and Complications
| Variable | n (%) |
|---|---|
| Deviations from the study protocol | |
| Therapeutic hypothermia group | 98 |
| Patients who could not be cooled to | |
| 35.5°C within 6 h of TBI onset | 35 (36) |
| 34.0°C within 6 h of TBI onset | 61 (62) |
| Unexpected hypotension before temperature management | 5 (5) |
| Unexpected hypoxia before temperature management | 1 (1) |
| Untreatable bleeding after cooling | 2 (2) |
| Continuous infusion of barbiturate or propofol | 3 (3) |
| Fever control group | 50 |
| Found to be >70 years old after randomization | 1 (2) |
| Unexpected hypotension after temperature management | 1 (2) |
| Uncontrollable ICP and using hypothermia treatment | 4 (8) |
| Complications | |
| Therapeutic hypothermia group | 98 |
| Thrombocytopaenia | 6 (6) |
| Sepsis | 5 (5) |
| Severe pneumonia | 3 (3) |
| Coagulopathy | 2 (2) |
| Arrythmia | 1 (1) |
| Fever control group | 50 |
| Severe pneumonia | 1 (2) |
TBI, traumatic brain injury; ICP, intracranial pressure
Discussion
We found no significant difference in the likelihood of poor neurological outcomes, evaluated by GOS and mortality, between the patients treated with TH (32–34°C) or strict temperature control (35.5–37°C). Background characteristics and the severity of injury were similar, except for ISS in both groups, indicating appropriate randomization of patients (Table 1). In addition, patient care delivered in the intensive care units of the participating hospitals was comparable, as only one hemodynamic parameter (Table 3) and only a few laboratory parameters differed between the two groups on any of the measurement days. These results might be attributable to our treatment protocol, hemodynamic management, and nursing care, among other factors.
Compared with the NABISH and NABISH II RCTs that also used TH for severe TBI,13,22 the rates of poor neurological outcomes and mortality rate were generally similar in the present study (Table 4).
We also compared outcomes in subgroups of patients stratified by GCS (4–5 and 6–8) and age (≤45 years old or >45 years old) (Table 4). There were no significant differences in the rates of poor neurological outcomes or mortality between the two groups. Among patients with a GCS of 6–8, the mortality rate was higher in the TH group (33%) than in the fever control group (16%, p=0.084), although this difference was not statistically significant. Similar observations were reported in a study of children with severe TBI.14 A probable explanation is that hypothermia itself is a risk factor for mortality in trauma patients.28 Actually, accumulated complications were significantly higher (Table 5) in the TH group (17%) than in the fever control group (2%). Our results may indicate that TH may be harmful or should be applied more carefully in patients with TBI and a GCS of 6–8.
In the present study, the fever control group (strict temperature control to 35.5–37°C) had 48% unfavorable outcomes and 23% mortality (Table 4). Consequently, approximately half of the patients with severe TBI could return to their daily life with good outcomes or only moderate disability, a result that may have been attributable to suppression of reactive hyperthermia.
We admitted that GCS and ISS differed slightly between the two groups despite the randomization. Therefore, we conducted a post-hoc multivariate logistic analysis to confirm that the differences in severity of the cases did not have any effect on the outcome. We set the poor outcome (binary) as the object variable, and age, sex, GCS, ISS, evacuated mass lesion, and TH as the explanatory variables. As a result, it was proved that the status of statistical nonsignificance of TH was not affected at all even after adjusted for other factors including GCS and ISS.
One limitation of our study may be that 62% of patients in the TH group could not be cooled to the target temperature within 6 h (Table 5). In the present study (Table 2), cooling started within a median of 3.0 h, and randomization was achieved within a median of 3.8 h after the onset of TBI, respectively. Physicians might take time to perform a trauma survey before cooling, and 62% of patients in the therapeutic hypothermia group underwent a surgical procedure for TBI (Table 1). Nevertheless, patients should be kept cool during the surgical procedures. It took 8.1 h to reach the target of 34°C in the TH group in the present study (Table 2), which was similar to the time taken to reach the target temperature in NABISH study.13 Although it had become 4.4 h in NABISH II in 2011, the neurological outcome was similar between the hypothermia and the control groups. 22 However, this was the fastest time possible using the available surface cooling methods at the time of performing this study. Most of the patients allocated to the TH group were not cooled to the target temperature within 6 h, highlighting the need for additional rapid cooling methods, such as intravenous cooling in a clinical setting.29 It is,therefore, notable that much better results were reported in a large retrospective observational study in Japan, in which quicker cooling was achieved using a cardiopulmonary supporting device after cardiac arrest.30
The second limitation was that we enrolled only 150 of the planned 300 patients, and had used a 2:1 randomization strategy. Accordingly, this study was underpowered. The prevalence of TBI decreased during the course of the study because of the implementation of educational campaigns and because the Japanese government introduced severe penalties for drunk drivers and their passengers with driving licenses.
The third limitation was that the control temperature range was chosen to be 35.5–37°C. When we designed our protocol, MTH was reported to be effective in the cardiac arrest/resuscitated patients.1,2 Therefore, we set the control temperature to 35.5–37°C for ethical reasons. If we had selected it to 36.5–37.5°C, finding a difference in neurological outcome would have had much less significance. In patients with severe TBI, we found no differences in the rates of poor neurological outcomes or mortality between patients who received prolonged MTH (≥72 h), slow rewarming, and neurological intensive care, and those who received strict fever control only. For these reasons, we recommend that further studies be performed to assess the clinical outcomes of very rapid cooling and fever control.
Conclusion
Prolonged TH (≥72 h) for patients with severe TBI together with tight hemodynamic management and slow rewarming (<1.0°C/day) did not improve neurological outcomes or mortality compared with strict fever control. However, the CIs for the primary outcome were wide, and do not exclude either benefit or harm for MTH.
Appendix: Authors' Contributions
T.M. designed the study concept with input from other authors. S.Y. wrote the entire study protocol and built the data input system. Y.O. managed all data and performed statistical analysis. S.N. and N.H. greatly contributed to the study concept and writing of the report. T.M. wrote the first draft of the report and other study group members contributed to subsequent drafts.
Other B-HYPO Study Group members were Mayuki Aibiki, Toru Aruga, Yasufumi Asai, Kenji Dohi, Yutaka Eguchi, Motoki Fujita, Toshio Fukuoka, Kazumi Ikeda, Tomomi Iwashita, Kotaro Kaneda, Tadashi Kaneko, Yoko Kato, Kenya Kawakita, Kosaku Kinoshita, Takao Kitahara, Kazuo Kitazawa, Hitoshi Kobata, Hiroyasu Koizumi, Yasuhiro Kuroda, Seishiro Marukawa, Kazuhisa Mori, Hiroshi Nakamura, Shunsuke Nakamura, Masashi Nakatsukasa, Norifumi Ninomiya, Shigeto Oda, Yasutaka Oda, Kazuo Okuchi, Ryoichi Saito, Atsushi Sugie, Shinichiro Suzaki, Koichiro Suzuki, Michiyasu Suzuki, Yasuhiko Taira, Jun Takezawa, Hiroki Tomita, Hiroyuki Yokota, Shinichi Yoshimura, and Tetsuo Yukioka, These authors contributed to the research and preparation of the report.
Collaborating hospitals were Fujita Health University Hospital, Aichi; Nagoya Medical Center, Aichi; Chiba Emergency Medical Center, Chiba; Chiba University Hospital, Chiba; Ehime University Hospital, Ehime; Ohota Nishinouchi Hospital, Fukushima; Gifu University Hospital, Gifu; Sapporo University Hospital, Hokkaido; The Hospital of Hyogo College of Medicine, Hyogo; Iwate Medical University Hospital, Iwate; Kagawa University Hospital, Kagawa; Kitasato University Hospital, Kanagawa; St. Marianna University Hospital, Kanagawa; Aizawa Hospital, Nagano; Shinshu University Hosiptal, Nagano; Nara Medical University Hospital, Nara; Oita University Hospital, Oita; Kawasaki Medical School Hospital, Okayama; Kansai Medical University Takii Hospital, Osaka; Mishima Emergency Critical Care Center, Osaka; Osaka National Hospital, Osaka; Saitama Medical Center, Saitama; Shiga University Hospital, Shiga; Saiseikai Utsunomiya Hospital, Tochigi; Tokushima University Hospital, Tokushima; Japanese Red Cross Musashino Hospital, Tokyo; National Tokyo Medical Center, Tokyo; Nihon University Hospital, Tokyo; Nippon Medical School Hospital, Tokyo; Nippon Medical School Tama Nagayama Hospital, Tokyo; Showa University Hospital, Tokyo; Teikyo University Hospital, Tokyo; Toho University Omori Medical Center, Tokyo; Tokyo Medical University Hachioji Medical Center, Tokyo; Tokyo Medical University Hospital, Tokyo; and Yamaguchi University Hospital, Yamaguchi. All collaborating hospitals are in Japan.
Acknowledgments
This study was supported by grants from Japanese Ministry of Health, Labour and Welfare (H-14-shinkin-005, H-15-shinkin-001, and H-16-shinkin-001), and the Japanese Human Science Association 2002–2004. We thank Takahiro Kiuchi (Director of the UMIN Centre) and Kiyoshi Ichihara (Yamaguchi University Graduate School of Medicine) for their support. We also thank Kees H Polderman (University of Pittsburgh Medical Center) for reviewing our manuscript and for his valuable suggestions.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.The Hypothermia After Cardiac Arrest Study Group (2002). Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 346, 549–556 [DOI] [PubMed] [Google Scholar]
- 2.Bernard S.A., Gray T.W., Buist M.D., Jones B.M., Silvester W., Gutteridge G., and Smith K. (2002). Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 346, 557–563 [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S., Laptook A.R., Ehrenkranz R.A., Tyson J.E., McDonald S.A., Donovan E.F., Fanaroff A.A., Poole W.K., Wright L.L., Higgins R.D., Finer N.N., Carlo W.A., Duara S., Oh W., Cotton C.M., Stevenson D.K., Stoll B.J., Lemons J.A., Guillet R., and Jobe A.H. (2005). Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 [DOI] [PubMed] [Google Scholar]
- 4.Gluckman P.D., Wyatt J.S., Azzopardi D., Ballard R., Edwards A.D., Ferriero D.M., Polin R.A., Robertson C.M., Thoresen M., Whitelaw A., and Gunn A.J. (2005). Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter radomised trial. Lancet 365, 663–670 [DOI] [PubMed] [Google Scholar]
- 5.Eicher D.J., Wagner C.L., Katikaneni L.P., Hulsey T.C., Bass W.T., Kaufman D.A., Horgan M.J., Languani S., Bhatia J.J., Givelichian L.M., Sankaran K., and Yager J.Y. (2005). Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr. Neurol. 32, 11–17 [DOI] [PubMed] [Google Scholar]
- 6.Azzopardi D.V., Strohm B., Edwards A.D., Dyet L., Halliday H.L., Juszczak E., Kapellou O., Levene M., Marlow N., Porter E., Thoresen M., Whitelaw A., Brocklehurst P., and TOBY Study Group. (2009). Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen N., Wetterslev J., Cronberg T., Erlinge D., Gasche Y., Hassager C., Horn J., Hovdenes J., Kjaergaard J., Kuiper M., Pellis T., Stammet P., Wanscher M., Wise M. P., Åneman A., Al-Subaie N., Boesgaard S., Bro-Jeppesen J., Brunetti I., Bugge J. F., Hingston C. D., Juffermans N. P., Koopmans M., Køber L., Langørgen J., Lilja G., Møller J. E., Rundgren M., Rylander C., Smid O., Werer C., Winkel P., and Friberg H., for the TTM Trial Investigators (2013). Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl. J. Med. 369, 2197–2206 [DOI] [PubMed] [Google Scholar]
- 8.Clifton G.L., Allen S., Barrodale P., Plenger P., Berry J., Koch S., Fletcher J., Hayes R.L., and Choi S.C. (1993). A phase II study of moderate hypothermia in severe brain injury. J. Neurotrauma 10, 263–271 [DOI] [PubMed] [Google Scholar]
- 9.Marion D.W., Penrod L.E., Kelsey S.F., Obrist W.D., Kochanek P.M., Palmer A.M., Wisniewski S.R., and DeKosky S.T. (1997). Treatment of traumatic brain injury with moderate hypothermia. N. Engl. J. Med. 336, 540–546 [DOI] [PubMed] [Google Scholar]
- 10.Aibiki M., Maekawa S., and Yokono S. (2000). Moderate hypothermia improves imbalances of thromboxane A2 and prostaglandin I2 production after traumatic brain injury in humans. Crit. Care Med. 28,3902–3906 [DOI] [PubMed] [Google Scholar]
- 11.Jiang J., Yu M., and Zhu C. (2000). Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J. Neurosurg. 93, 546–549 [DOI] [PubMed] [Google Scholar]
- 12.Shiozaki T., Hayakata T., Taneda M., Nakajima Y., Hashiguchi N., Fujimi S., Nakamori Y., Tanaka H., Shimazu T., and Sugimoto H. (2001). A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. Mild Hypothermia Study Group in Japan. J. Neurosurg. 94, 50–54 [DOI] [PubMed] [Google Scholar]
- 13.Clifton G.L., Miller E.R., Choi S.C., Levin H.S., McCauley S., Smith K.R, Jr., Muizelaar J.P., Wagner F.C, Jr., Marion D.W., Luerssen T.G., Chesnut R.M., and Schwartz M. (2001). Lack of effect of induction of hypothermia after acute brain injury. N. Engl. J. Med. 344, 556–563 [DOI] [PubMed] [Google Scholar]
- 14.Hutchison J.S., Ward R.E., Lacroix J., Hébert P.C., Barnes M.A., Bohn D.J., Dirks P.B., Doucette S., Fergusson D., Gottesman R., Joffe A.R., Kirpalani H.M., Meyer P.G., Morris K.P., Moher D., Singh R.N., Skippen P.W., and Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group. (2008). Hypothermia therapy after traumatic brain injury in children. N. Engl. J. Med. 358, 2447–2456 [DOI] [PubMed] [Google Scholar]
- 15.Clifton G.L., Miller E.R., Choi S.C., and Levin H.S. (2002). Fluid thresholds and outcome from severe brain injury. Crit. Care Med. 30, 739–745 [DOI] [PubMed] [Google Scholar]
- 16.Clifton G.L., Choi S.C., Miller E.R., Levin H.S., Smith K.R, Jr., Muizelaar J.P., Wagner F.C, Jr., Marion D.W., and Luerssen T.G. (2001). Intercenter variance in clinical trials of head trauma–experience of the National Acute Brain Injury Study: Hypothermia. J. Neurosurg. 95, 751–755 [DOI] [PubMed] [Google Scholar]
- 17.Clifton G.L., Miller E.R., Choi S.C., Levin H.S., McCauley S., Smith K.R, Jr., Muizelaar J.P., Marion D.W., and Luerssen T.G. (2002). Hypothermia on admission in patients with severe brain injury. J. Neurotrauma 19, 293–301 [DOI] [PubMed] [Google Scholar]
- 18.McIntyre L.A., Fergusson D.A., Hébert P.C., Moher D., and Hutchison J.S. (2003). Prolonged therapeutic hypothermia after traumatic brain injury in adults: a systematic review. JAMA 289, 2992–2999 [DOI] [PubMed] [Google Scholar]
- 19.Peterson K., Carson S., and Carney N. (2008). Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J. Neurotrauma 25, 62–71 [DOI] [PubMed] [Google Scholar]
- 20.Jiang J.Y., Xu W., Li W.P., Gao G.Y., Bao Y.H., Liang Y.M., and Luo Q.Z. (2006). Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J. Cereb. Blood Flow Metab. 26, 771–776 [DOI] [PubMed] [Google Scholar]
- 21.Qiu W., Zhang Y., Sheng H., Zhang J., Wang W., Liu W., Chen K., Zhou J., and Xu Z. (2007). Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J. Crit. Care 22, 229–235 [DOI] [PubMed] [Google Scholar]
- 22.Clifton G.L., Valadka A., Zygun D., Coffey C.S., Drever P., Fourwinds S., Janis L.S., Wilde E., Taylor P., Harshman K., Conley A., Puccio A., Levin H.S., McCauley S.R., Bucholz R.D., Smith K.R., Schmidt J.H., Scott J.N., Yonas H., and Okonkwo D.O. (2011). Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomized trial. Lancet Neurol. 10, 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Japan Society of Neurotraumatology (2001). Guidelines for the Management of Severe Head Injury. Igaku-Shoin: Tokyo [Google Scholar]
- 24.Kasaoka S., Oda Y., Yamashita S., Tsuruta R., Fujisawa H., Okabayashi K., and Maekawa T. (2006). Hemodynamic efficacy of neuroleptanesthesia for therapeutic hypothermia in acute brain injury, in: Minimally Invasive Neurosurgery and Multidisciplinary Neurotraumatology. Kanno T., Kato Y. (eds.). Springer-Verlag: Tokyo, pps. 335–338 [Google Scholar]
- 25.Civil I.D., and Schwab C.W. (1988).The Abbreviated Injury Scale, 1985 revision: a condensed chart for clinical use. J. Trauma 28, 87–90 [DOI] [PubMed] [Google Scholar]
- 26.Marshall L.F., Marshall S.B., Klauber M.R., van Berkum Clark M., Eisenberg H.M., Jane J.A., Luerssen T.G., Marmarou A., and Foulkes M.A. (1991). A new classification of head injury based on computerized tomography. J. Neurosurg. 75, S14–S20 [Google Scholar]
- 27.Jennett B., and Bond M. (1975). Assessment of outcome after severe brain damage. Lancet 1, 480–484 [DOI] [PubMed] [Google Scholar]
- 28.Eddy V.A., Morris J.A., Jr., and Cullinane D.C. (2000). Hypothermia, coagulopathy, and acidosis. Surg. Clin. North Am. 80, 845–854 [DOI] [PubMed] [Google Scholar]
- 29.Holzer M., Müllner M., Sterz F., Robak O., Kliegel A., Losert H., Sodeck G., Uray T., Zeiner A., and Laggner A.N. (2006). Efficacy and safety of endovascular cooling after cardiac arrest: cohort study and Bayesian approach. Stroke 37, 1792–1797 [DOI] [PubMed] [Google Scholar]
- 30.Nagao K., Kikushima K., Watanabe K., Tachibana E., Tominaga Y., Tada K., Ishii M., Chiba N., Kasai A., Soga T., Matsuzaki M., Nishikawa K., Tateda Y., Ikeda H., and Yagi T. (2010). Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circ. J. 74, 77–85 [DOI] [PubMed] [Google Scholar]