Abstract
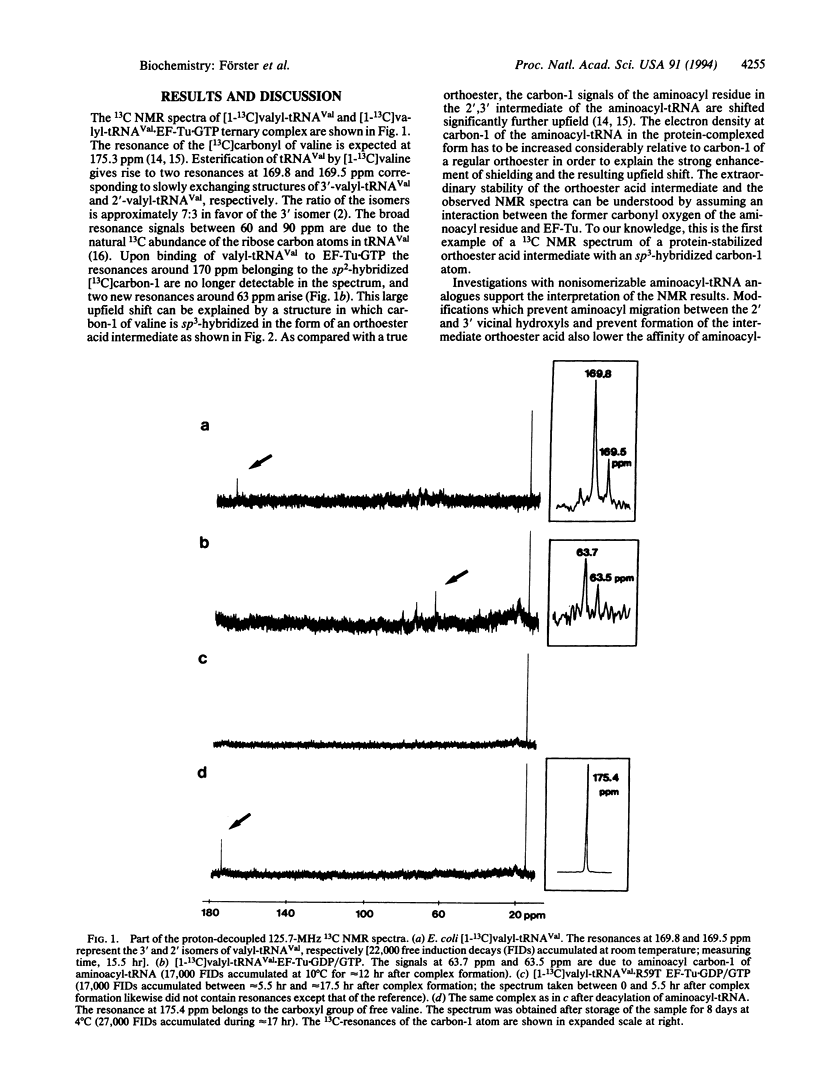
tRNA(Val) from Escherichia coli was aminoacylated with [1-13C]valine and its complex with Thermus thermophilus elongation factor EF-Tu.GTP was analyzed by 13C NMR spectroscopy. The results suggest that the aminoacyl residue of the valyl-tRNA in ternary complex with bacterial EF-Tu and GTP is not attached to tRNA by a regular ester bond to either a 2'- or 3'-hydroxyl group; instead, an intermediate orthoester acid structure with covalent linkage to both vicinal hydroxyls of the terminal adenosine-76 is formed. Mutation of arginine-59 located in the effector region of EF-Tu, a conserved residue in protein elongation factors and the alpha subunits of heterotrimeric guanine nucleotide-binding regulatory proteins (G proteins), abolishes the stabilization of the orthoester acid structure of aminoacyl-tRNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmadian M. R., Kreutzer R., Sprinzl M. Overproduction of the Thermus thermophilus elongation factor Tu in Escherichia coli. Biochimie. 1991 Jul-Aug;73(7-8):1037–1043. doi: 10.1016/0300-9084(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Berchtold H., Reshetnikova L., Reiser C. O., Schirmer N. K., Sprinzl M., Hilgenfeld R. Crystal structure of active elongation factor Tu reveals major domain rearrangements. Nature. 1993 Sep 9;365(6442):126–132. doi: 10.1038/365126a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Chinali G., Wolf H., Parmeggiani A. Effect of kirromycin on elongation factor Tu. Location of the catalytic center for ribosome-elongation-factor-Tu GTPase activity on the elongation factor. Eur J Biochem. 1977 May 2;75(1):55–65. doi: 10.1111/j.1432-1033.1977.tb11503.x. [DOI] [PubMed] [Google Scholar]
- Hecht S. M., Tan K. H., Chinault A. C., Arcari P. Isomeric aminoacyl-tRNAs are both bound by elongation factor Tu. Proc Natl Acad Sci U S A. 1977 Feb;74(2):437–441. doi: 10.1073/pnas.74.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonák J., Petersen T. E., Clark B. F., Rychlík I. N-Tosyl-L-phenylalanylchloromethane reacts with cysteine 81 in the molecule of elongation factor Tu from Escherichia coli. FEBS Lett. 1982 Dec 27;150(2):485–488. doi: 10.1016/0014-5793(82)80795-3. [DOI] [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Limmer S., Reiser C. O., Schirmer N. K., Grillenbeck N. W., Sprinzl M. Nucleotide binding and GTP hydrolysis by elongation factor Tu from Thermus thermophilus as monitored by proton NMR. Biochemistry. 1992 Mar 24;31(11):2970–2977. doi: 10.1021/bi00126a018. [DOI] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989 Nov 9;342(6246):142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Chen C. M. Inactivation of T u factor-guanosine triphosphate recognition and ribosome-binding ability by terminal oxidation-reduction of yeast phenylalanine transfer ribonucleic acid. J Biol Chem. 1972 Apr 10;247(7):2049–2058. [PubMed] [Google Scholar]
- Ott G., Faulhammer H. G., Sprinzl M. Interaction of elongation factor Tu from Escherichia coli with aminoacyl-tRNA carrying a fluorescent reporter group on the 3' terminus. Eur J Biochem. 1989 Sep 15;184(2):345–352. doi: 10.1111/j.1432-1033.1989.tb15025.x. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Block W., Wittinghofer A., Wolf H., Fischer E. The elongation factor Tu binds aminoacyl-tRNA in the presence of GDP. J Biol Chem. 1982 Oct 10;257(19):11261–11267. [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting I., Almo S. C., Rapp G., Wilson K., Petratos K., Lentfer A., Wittinghofer A., Kabsch W., Pai E. F., Petsko G. A. Time-resolved X-ray crystallographic study of the conformational change in Ha-Ras p21 protein on GTP hydrolysis. Nature. 1990 May 24;345(6273):309–315. doi: 10.1038/345309a0. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Cramer F. Accepting site for aminoacylation of tRNAphe from yeast. Nat New Biol. 1973 Sep 5;245(140):3–5. doi: 10.1038/newbio245003a0. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Cramer F. Site of aminoacylation of tRNAs from Escherichia coli with respect to the 2'- or 3'-hydroxyl group of the terminal adenosine. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3049–3053. doi: 10.1073/pnas.72.8.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Kucharzewski M., Hobbs J. B., Cramer F. Specificity of elongation factor Tu from Escherichia coli with respect to attachment to the amino acid to the 2' or 3'-hydroxyl group of the terminal adenosine of tRNA. Eur J Biochem. 1977 Aug 15;78(1):55–61. doi: 10.1111/j.1432-1033.1977.tb11713.x. [DOI] [PubMed] [Google Scholar]
- Stouten P. F., Sander C., Wittinghofer A., Valencia A. How does the switch II region of G-domains work? FEBS Lett. 1993 Mar 29;320(1):1–6. doi: 10.1016/0014-5793(93)81644-f. [DOI] [PubMed] [Google Scholar]
- Taiji M., Yokoyama S., Miyazawa T. Aminoacyl-tRNA exclusively in the 3'-isomeric form is bound to polypeptide chain elongation factor Tu. J Biochem. 1985 Dec;98(6):1447–1453. doi: 10.1093/oxfordjournals.jbchem.a135413. [DOI] [PubMed] [Google Scholar]
- Taiji M., Yokoyama S., Miyazawa T. Slow transacylation of peptidyladenosine allows analysis of the 2'/3'-isomer specificity of peptidyltransferase. Biochemistry. 1985 Oct 8;24(21):5776–5780. doi: 10.1021/bi00342a013. [DOI] [PubMed] [Google Scholar]
- Taiji M., Yokoyama S., Miyazawa T. Transacylation rates of (aminoacyl)adenosine moiety at the 3'-terminus of aminoacyl transfer ribonucleic acid. Biochemistry. 1983 Jun 21;22(13):3220–3225. doi: 10.1021/bi00282a028. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Dix D. B., Karim A. M. The reaction of ribosomes with elongation factor Tu.GTP complexes. Aminoacyl-tRNA-independent reactions in the elongation cycle determine the accuracy of protein synthesis. J Biol Chem. 1986 Apr 15;261(11):4868–4874. [PubMed] [Google Scholar]
- Tompson J. G., Hayashi F., Paukstelis J. V., Loeppky R. N., Agris P. F. Complete nuclear magnetic resonance signal assignments and initial structural studies of [13C]methyl-enriched transfer ribonucleic acid. Biochemistry. 1979 May 15;18(10):2079–2085. doi: 10.1021/bi00577a037. [DOI] [PubMed] [Google Scholar]
- Wagner T., Cramer F., Sprinzl M. Activity of the 2' and 3' isomers of aminoacyl transfer ribonucleic acid in the in vitro peptide elongation on Escherichia coli ribosomes. Biochemistry. 1982 Mar 30;21(7):1521–1529. doi: 10.1021/bi00536a009. [DOI] [PubMed] [Google Scholar]
- Wagner T., Sprinzl M. Inhibition of ribosomal translocation by peptidyl transfer ribonucleic acid analogues. Biochemistry. 1983 Jan 4;22(1):94–98. doi: 10.1021/bi00270a013. [DOI] [PubMed] [Google Scholar]
- Weijland A., Parmeggiani A. Toward a model for the interaction between elongation factor Tu and the ribosome. Science. 1993 Feb 26;259(5099):1311–1314. doi: 10.1126/science.8446899. [DOI] [PubMed] [Google Scholar]



