Abstract
Background
Individuals vary in the degree to which salient threatening stimuli disrupt or distract from goal-directed cognitive processes. Excessive attention to threat or difficulty resolving the interference created by threat cues could contribute to anxious psychopathology; disruptions in frontal brain regions implicated in attentional control or resolution of emotional interference (e.g. anterior cingulate cortex, “ACC”) might play a role. In this study, we explored the hypothesis that trait anxiety would be associated with ACC activity in an attentional control task with varying levels of threat interference.
Methods
During functional magnetic resonance imaging, 20 healthy individuals who varied in trait anxiety levels viewed angry, fearful, and neutral faces superimposed on an indoor or outdoor scene. In a high-threat interference condition, subjects identified the gender of the face (Attend Face). In a low-threat interference condition, they identified the scene type (Attend Scene). Whole-brain analysis was used to compare Attend Face with Attend Scene for angry and fearful (versus neutral) faces. Contrasts were correlated with trait anxiety level.
Results
Behavioral data confirmed that Attend Face produced greater threat interference than Attend Scene. Brain imaging results showed that trait anxiety was inversely associated with bilateral rostral ACC activity for Attend Face relative to Attend Scene for angry faces. A similar relationship was not seen for fearful faces.
Conclusions
The rostral ACC is implicated in assessing the salience of emotional information and controlling attention to resolve emotional interference. The link between higher trait anxiety and decreased ACC activation for angry faces suggests reduced attentional control for signals of interpersonal threat in healthy anxiety-prone individuals.
Keywords: attention, bias, angry, fearful, brain imaging
INTRODUCTION
There is a natural propensity for attention to be captured by threatening stimuli;[1–3] however, individuals differ in the extent to which they selectively attend to threat cues in social environments. For instance, anxious individuals have greater attentional bias for threat signals than nonanxious individuals, particularly for signals of threat conveyed through facial expressions (e.g. angry/fearful faces[4–6]). Selective attention for threat-relevant information is a proposed causal mechanism for anxiety vulnerability[7] or predisposition to developing pathological anxiety.[8] Therefore, a better understanding of the link between attentional bias for threat and anxiety, particularly in relation to individual differences in threat bias sensitivity, may have relevance to the development of anxious psychopathology.
Given the prioritization of threat over other types of information in competition for attention,[2,3] salient emotional cues that signal threat in the environment can divert (e.g. “steal”) attention away from other less biologically significant processes. When the processing of threat cues outcompetes the processing of none-motional cues, “emotional interference” occurs. Indices of emotional interference include behavioral measures such as the time it takes to produce a correct response for a nonemotional, cognitive task which competes with emotional information (e.g. emotional Stroop task[7,9–11]). In these types of cognitive–emotional interaction tasks, increased response latencies can be used as indicators of greater emotional interference.
Over the past decade, a number of brain imaging studies have investigated the neural circuits involved in attentional control and processing threat signals.[2,3,12–17] Data suggest that anxiety-prone individuals may have an attentional bias toward threat signals, and be more vulnerable to emotional interference from such signals, and that this vulnerability may be associated with aberrant activation in frontal regions that are involved in higher order cognitive and/ or attention–emotion functions. For example, Stein et al.[18] demonstrated that anxiety proneness was associated with reduced activation of ventromedial prefrontal areas (e.g. anterior cingulate, subgenual cingulate, medial frontal gyrus) and increased activation of threat-sensitive brain areas such as the amygdala. The finding of reduced frontal–subcortical “balance” in individuals with elevated anxiety sensitivity suggests that individual differences in inefficient engagement of frontal cortex may explain variance in anxious traits.
Some models of anxiety vulnerability propose that attentional bias for threat is due to excessive interruption in cognitive processes caused by reduced inhibitory control over threatening distracters.[19,20] Brain imaging studies support this proposal by showing aberrant activation in frontal regions in association with emotional interference in anxiety vulnerable subjects.[21–23] Among implicated regions, the anterior cingulate cortex (ACC) is posited to play a key role in assessing the salience of stimuli (e.g. relevance detection) and resolving emotional interference.[9,12,14,24] ACC engagement appears to be critical in maintaining an appropriate balance between sensitivity to potential threat and carrying out task-relevant cognitive goals.[15]
If ACC activation supports optimal performance in conflict resolution and anxiety modulates how much ACC engagement is needed for attentional control,[19–22] it is reasonable to expect that individual differences in trait anxiety will impact ACC activation in the context of emotional interference. In support, a recent study showed that low-trait relative to high-trait healthy anxious subjects had increased rostral ACC (rACC) activation in response to a modified emotional Stroop task, an effect that was particularly driven by emotional, rather than nonemotional, interference.[23] The authors proposed that the rACC activation in nonanxious subjects effectively reduced interference from task-irrelevant threatening distracters by suppressing the processing of threat stimuli. They also interpreted the lack of rACC engagement in high anxious subjects as evidence of insufficient rACC engagement for conflict resolution.
Impoverished frontal activation in psychiatrically “healthy” high anxious subjects has also been seen in other studies. Reduced ACC activation in the presence of threatening face distracters has been observed in individuals with high-trait anxiety, and is modified by attentional demands of the cognitive task.[21,22] The ACC is hypoactive when distracters are infrequent and a neutral task-relevant goal is easy to execute (low perceptual load). Under high load conditions, ACC hypoactivity is not evident. ACC activity thus appears to be influenced by an interaction between anxiety-related attentional bias and task characteristics such as perceptual load, which influence the availability of attentional resources to respond to distracters.[21,22] This is consistent with a biased competition model of attention,[25] which suggests that when stimuli compete for attention, the “attentional capture” of emotional distracters will be influenced by task demand such that attentional resources are sufficiently available (e.g. left over) to process task-irrelevant stimuli.[2]
Collectively, the data described above demonstrate the following: (1) the presence of emotionally salient distracters leads to competition for attentional resources and thus causes “emotional interference” with ongoing task demands; (2) this interference effect is greatest when the distracter cues signal the presence of danger; (3) although a number of brain regions are likely involved, the ACC appears to be critically important in mediating emotional interference; and (4) successful control of emotional interference engages the ACC and is modulated by individual differences in trait anxiety. Existing evidence suggests that high anxiety is associated with impoverished frontal activity, perhaps reflecting a reduced capacity for inhibitory control of attention to distracters that carry potential threat information.
To further explore the effects of attentional control on brain activation patterns during cognitive–emotional interaction, we used a modified version of the shifting emotion attention task (SEAT)[16] which manipulated attention allocation by instructing subjects to focus either on scenes or on faces, when both were fully superimposed within the same visual field. We specifically sought to examine the relationship between trait anxiety and ACC activation in response to high emotional (threat-related) interference relative to low interference.
To manipulate attention, subjects were instructed to either attend to faces, with a cognitive task of identifying gender (Attend Face), or to ignore faces and attend to the scene, with a cognitive task of determining whether it was indoors or outdoors (Attend Scene). Based on prior work,[16] we expected that attending to faces, relative to attending to scenes, would produce greater behavioral evidence of emotional interference with the cognitive task, particularly when the face conveyed a signal of threat. We additionally expected that this emotional interference would be associated with greater ACC activation. We included angry, fearful, and neutral faces, balanced across the two attention conditions.
Our primary objective was to examine the hypothesis that ACC recruitment would be influenced by trait anxiety and its interaction with high- versus low-interference conditions. Low-trait anxious individuals are expected to exhibit ACC activation to modulate attention effectively when attending to faces (high interference) versus scenes (low interference). Whereas, high-trait anxious individuals should show more interference, indicated by ACC hypoactivation, in response to high- versus low-interference conditions (threat faces minus scenes).
METHODS
SUBJECTS
Prior to participation, subjects provided written informed consent as approved by the local Institutional Review Board. Individuals were recruited via advertisement in the community and paid for their participation. There were 20 right-handed individuals (50% male) with a mean age 22.8±75.4 years who were physically, neurologically, and psychiatrically healthy, as confirmed by a physician-conducted medical examination and psychiatric evaluation that included the Structured Clinical Interview for DSM-IV.[26] Subjects completed the trait version of the Spielberger State-Trait Anxiety Inventory[27] and trait anxiety ranged from 23 to 41 with a mean of 28.3±74.7. None of the subjects were taking psychoactive medications at the time of scanning.
EXPERIMENTAL TASK
We used modified composite face/scene images comprised of 20 angry, 20 fearful, and 20 neutral facial expressions[28] superimposed on 10 scenes of buildings (five indoor and five outdoor). There were 60 unique grayscale pictures in total.
During fMRI, participants were presented the composite images and were asked to either: (1) determine if the “building” image is an indoor or outdoor scene (Attend Scene); or (2) determine if the face on the image is male or female (Attend Face). A third condition was also included (like/dislike) to examine additional and unrelated hypotheses; these results will be reported elsewhere. Images were each presented three times (E-Prime, Inc., Sharpsburg, PA); once for each condition, with condition type in random order. There were four runs and 55 trials per run. Trials comprised a centered fixation crosshair for 3~8 sec, judgment cue for 750+250 ms blank screen, and composite image for 1,500 ms. Prior to experimental trials, subjects completed a practice session with images not used in the experiment. The entire task took approximately 26 min.
MRI ACQUISITION
All scanning was performed with blood-oxygen-level-dependent (BOLD) sensitive whole-brain fMRI on a 3.0-Tesla GE Signa System (General Electric, Milwaukee, WI) using a standard radiofrequency coil. A total of 760 -weighted reverse spiral gradient-recall echo volumes, with BOLD contrast (echo time = 30 ms, repetition time = 2,000 ms, 64 × 64 matrix, flip angle = 90 degree, field of view = 22 cm, 40 contiguous 3-mm axial slices per volume), were acquired during a single session. A high-resolution T1 scan (3D-SPGR; 256 × 160 matrix, field of view = 24 cm; slice thickness = 1.2 mm) was also acquired for anatomical localization.
DATA ANALYSIS
Behavioral analyses
For on-line accuracy and reaction time (RT), a 3 (Face Type: Angry, Fearful, Neutral) × 2 (Task: Attend Face, Attend Scene) repeated measures analysis of variance was performed and Huynh–Feldt corrections were used. Follow-up simple effects analyses with two-tailed t-tests, alpha level .05, were conducted to examine main effects and interactions. In light of the contrasts used for whole-brain evaluation, we were particularly interested in comparing Attend Face versus Attend Scene and the influence of stimuli content within each of these tasks. This post hoc analysis was conducted with planned comparisons.
fMRI Analysis
Data were preprocessed and analyzed using conventional statistical parametric mapping (SPM2; Wellcome Department of Cognitive Neurology, London; www.fil.ion.ucl.ac.uk/spm). The scans were spatially realigned to correct for head motion, warped (nonlinear) to an EPI template in Montreal Neurologic Institute (MNI) space, resampled to 3 × 3 × 3 mm3 voxels, and smoothed with a 5 × 5 × 5-mm kernel in3 the first level and again with a 5 5 5-mm3 kernel in the second level to balance within-subject specificity and between-subject variance in neuroanatomy.
To evaluate the influence of threat on ACC activation within high and low interference, the following contrasts were constructed for angry faces and for fearful faces, respectively: [(Attend Faceangry–Attend Faceneutral)–(Attend Sceneangry–Attend neutral)] and Scene[(Attend Facefearful–Attend Faceneutral)–(Attend Scenefearful Attend Sceneneutral)]. Based on prior literature, we had a strong a priori hypothesis that the effects of trait anxiety modulation on the context of threat interference would be localized to the ACC. To examine our prediction of anxiety modulating ACC, linear contrasts were calculated for each subject and corresponding parameter estimates were entered into a whole-brain simple regression analysis with trait anxiety as an independent variable, controlling for sex. Significance was initially set at a conservative threshold of voxel Puncorrected <.001, cluster size ≥10 contiguous voxels.
To complement and confirm whole-brain findings, a volume of interest (VOI) centered at the peak (5 mm radius) was constructed for any ACC results from the above regression analysis. This VOI was constructed for two reasons: (1) to correct for multiple comparisons across a small volume (SVC) based on family-wise error; and (2) to extract parameter estimates (β weights, arbitrary units) of activation to illustrate the direction and scatter plot of correlation between trait anxiety level and ACC activation to show the variance in brain activation (β weights) and trait anxiety level across individuals.
Outside the ACC a priori region of interest, to further explore other activations across the entire brain, we also set a more liberal threshold of Puncorrected<.005, cluster size ≥10 contiguous voxels for completeness.
RESULTS
BEHAVIORAL DATA
RT and accuracy (percent correct identification of face gender or scene location) data are presented in Table 1. For RT, there were main effects of Emotion [F(2, 38) 11.14, P<.001] and Task [F(1, 19) 6.81, P<.02] with a nonsignificant trend toward an interaction [F(2, 38) 2.66, P =.08], suggesting that the emotional content of the faces and direction of attention to the faces both produced RT interference. We dissected this interference effect with simple effects analysis for each task.
TABLE 1.
Mean reaction time and accuracy scores with (standard deviations) across all subjects
| Image type | Attend face | Attend scene | t |
|---|---|---|---|
| Reaction time in milliseconds | |||
| Angry | 161.42 (213.92) | 1,054.70 (225.67) | 3.08* |
| Fearful | 1,103.16 (219.87) | 1,067.68 (228.01) | |
| Neutral | 1,074.00 (170.85) | 1,015.31 (226.34) | |
| Accuracy | |||
| Angry | 0.72 (0.09) | 0.91 (0.07) | 7.63*** |
| Fearful | 0.73 (0.10) | 0.90 (0.06) | 7.37*** |
| Neutral | 0.84 (0.09) | 0.93 (0.06) | 3.31** |
Two-tailed paired t-test:
P<.01
P<.004
P<.001.
For Attend Face only, subjects had slower RT for angry versus fearful [t(19) 3.10, P<.01] and neutral faces [t(19) 4.76, P<.001] with no difference between fearful versus neutral [t(19) 1.35, P =.19]. Results for Attend Scene showed that subjects were slower to identify scenes with superimposed angry versus neutral faces [t(19) 2.12, P<.05] and superimposed fearful versus neutral faces [t(19) 2.52, P<.02]; there was no difference between superimposed angry versus fearful faces [t(19) .50, P =.62].
Post hoc analyses for Attend Face versus Attend Scene for each face type showed that subjects were slower to identify gender than scene type (i.e. indoor/ outdoor) for images with superimposed angry faces [t(19) 3.08, P<.01]. There was a nonsignificant trend for superimposed neutral faces [t(19) 2.05, P =.06], and no difference for fearful faces [t(19) 1.15, P =.26].
Accuracy collapsed across task and face type was high and well above chance (78.5±75.9%), indicating subjects understood task instructions. As with RT, there was also evidence of interference effects in accuracy data, with main effects of Emotion [F(2, 38) 14.41, P<.001] and Task [F(1, 19) 79.55, P<.001], moderated by a significant Emotion × Task interaction [F(2, 38) 6.61, P<.004]. To explore the interaction, we performed simple effects analyses for each task. When attending to faces, subjects were less accurate for both angry [t(19) 4.62, P<.001] and fearful faces [t(19) 4.88, P<.001] relative to neutral faces, with no difference between anger and fear [t(19) .46, P =.46]. Facial expressions had no impact on accuracy when subjects were attending to scenes (P>.1). Neither RT nor accuracy were significantly correlated with trait anxiety level (P>.1).
FUNCTIONAL MRI RESULTS
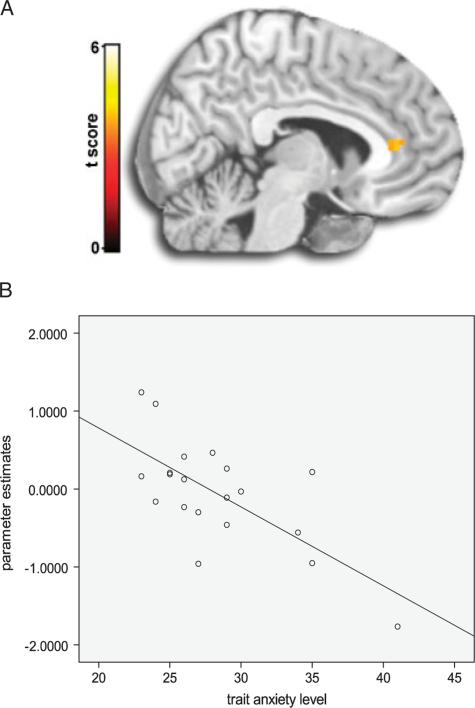
Simple regression results showed that trait anxiety was negatively associated with bilateral ACC activation (left [−6, 36, 9]; k = 19; Z-score 5 3.32; Puncorrected<.001 whole-brain, PSVC<.05); (right [12, 33, 6]; k = 27, Z-score = 3.96; Puncorrected<.001 whole-brain, PSVC<.05) for angry composite images [(Attend Faceangry–Attend Faceneutral)−(Attend Sceneangry–Attend Sceneneutral)]. See Figure 1 for ACC activation based on this whole-brain regression analysis, and the illustrative scatter plot of brain activation (β weights) and trait anxiety level across individual subjects.
Figure 1.
(A)Whole-brain anterior cingulate activation (peak voxel at −6, 36, 9) from negative regression of trait anxiety level for [(Attend Faceangry–Attend Faceneutral)–(Attend Sceneangry–Attend Sceneneutral)]. (B) Illustrative scatter plot of trait anxiety level and anterior cingulate activation (β weights) extracted from a 5-mm radius volume of interest centered at the peak (−6, 36, 9).
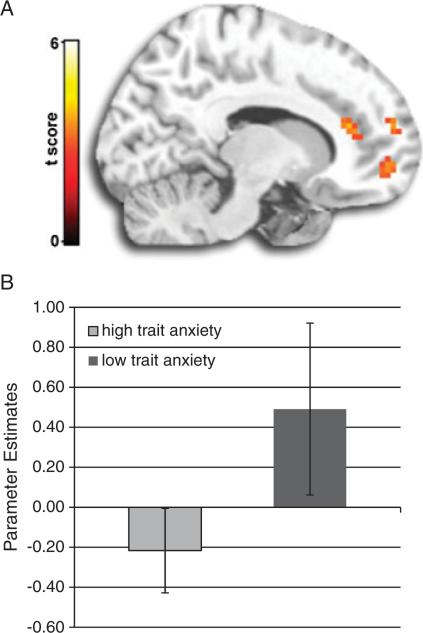
To further illustrate the effect of trait anxiety on ACC activity, we assigned subjects to high (n = 11) and low (n = 9) anxiety groups based on a median split, with a median score of 27 (two subjects scored 27 and they were included in the high anxiety group). The two groups appeared strikingly different in ACC reactivity (Fig. 2; [(12, 33, 15]; k = 16; Z-score 5 3.24; Puncorrected<.005). See Figure 2 for ACC group effects. Finally, when the high- and low-anxiety groups were combined, general ACC activation was not detected, even at a more liberal threshold (Puncorrected<.005). For completeness, Table 2 summarizes these findings.
Figure 2.
(A) Differences in activation to threat interference between low and high anxious groups based on median split score for trait anxiety. Whole-brain anterior cingulate activation (peak voxel at −12, 33, 15) based on groups (low trait>high trait) for [(Attend Faceangry–Attend Faceneutral)–(Attend Sceneangry–Attend Sceneneutral)]. (B) Mean activations (β weights) and standard deviations are associated with a 5-mm radius volume of interest centered at the peak of anterior cingulate activation (−12, 33, 15).
TABLE 2.
Activation from simple negative regression analysis for trait anxiety level and threat interference [(Attend Facethreat–Attend Faceneutral)–(Attend Scenethreat–Attend Sceneneutral)]
| Face type | Region | MINI coordinates | Cluster | Z-score | ||
|---|---|---|---|---|---|---|
| Angry > Neutral | ||||||
| Middle frontal gyrus | –21 | –6 | 42 | 238 | 4.23 | |
| 21 | 15 | 60 | 34 | 4.04 | ||
| –42 | 51 | 9 | 25 | 3.37 | ||
| –24 | 60 | 6 | 13 | 3.02 | ||
| Inferior parietal lobe | –69 | –18 | 21 | 12 | 4.16 | |
| –57 | –42 | 27 | 35 | 4.02 | ||
| Superior frontal gyrus | 6 | 63 | 21 | 76 | 3.83 | |
| *Anterior cingulate | 12 | 33 | 6 | 27 | 3.96 | |
| –6 | 36 | 9 | 19 | 3.32 | ||
| Insula | –45 | –21 | 15 | 14 | 3.72 | |
| 36 | –33 | 18 | 10 | 3.19 | ||
| Middle occipital gyrus | 27 | –78 | 0 | 10 | 3.72 | |
| Caudate | –3 | 9 | 9 | 23 | 3.63 | |
| Cerebeller tonsil | –42 | –48 | –51 | 71 | 3.32 | |
| Inferior frontal gyrus | –54 | 3 | 21 | 16 | 3.18 | |
| –48 | 30 | –9 | 18 | 3.01 | ||
| Occipital lobe | –9 | –102 | –3 | 10 | 3.12 | |
| Precentral gyrus | 57 | 3 | 39 | 15 | 3.10 | |
| Postcentral gyrus | 21 | –36 | 75 | 10 | 2.78 | |
| Fearful > Neutral | ||||||
| Cerebellar tonsil | –24 | –63 | –51 | 37 | 3.75 | |
| Pons | 15 | –27 | –30 | 16 | 3.41 | |
| Putamen | –24 | –6 | –9 | 10 | 3.35 | |
| Uncus | 27 | 0 | –42 | 22 | 3.26 | |
| Cerebellum posterior lobe | –36 | –81 | –45 | 21 | 3.24 | |
| Inferior temporal gyrus | –48 | –12 | –36 | 17 | 3.16 | |
| Insula | –48 | 9 | 3 | 14 | 2.86 | |
| Inferior parietal lobule | –51 | –42 | 27 | 16 | 3.06 | |
| Superior frontal gyrus | –18 | 33 | 36 | 10 | 2.94 | |
Results at Puncorrected < .001, 10 voxel minimum. All other regions are at Puncorrected <.005, 10 voxel minimum. MNI refers to Montreal Neurological Institute.
In contrast to angry composite images, results for fearful composite images [(Attend Facefear–Attend Faceneutral)–(Attend Scenefear Attend Sceneneutral)] showed no correlations between ACC activation and trait anxiety. Similar to images with superimposed angry faces, there was no main effect of ACC activation when collapsing across anxiety level. Results are summarized in Table 2.
DISCUSSION
In this study, we examined the relationship between trait anxiety level in healthy subjects and anterior cingulate activation in the contrast high- versus low-emotional interference. Stimuli consisted of pictures that compounded angry, fearful, and neutral faces with neutral scenes. Emotional interference was manipulated with instructions to either attend to faces by identifying gender or to ignore faces by identifying indoor or outdoor scenes. To evaluate the influence of threat content, angry faces were contrasted with neutral faces, and fearful faces with neutral faces, within high-and low-interference tasks.
When subjects attended to faces, there was evidence of slower RT and reduced accuracy relative to when they attended to scenes, suggesting that attention to faces created emotional interference. The data suggest that potential threat information carried by facial expressions interfered with efforts to complete the assigned cognitive task of identifying the gender of the face. When attention was volitionally directed away from faces by focusing on the embedded scene, there was less emotional interference as there was less competition for attentional resources. Therefore, the cognitive task could be completed more efficiently. This effect was consistent and most striking when the composite image included a face with an angry expression, suggesting that angry faces produced more interference than fearful or neutral faces, perhaps because they conveyed clearer or more direct threat to the viewer (see below).
Brain imaging results for the interference contrast involving angry faces confirmed our hypothesis that trait anxiety would modulate bilateral rostral ACC (rACC) activation in the context of interference. Higher trait anxiety was associated with reduced rACC activation in a contrast that highlighted the high-interference condition (attend faces) by controlling for a low-interference condition (attend scenes). These differential effects are further illustrated when subjects are dichotomized into high- and low-trait anxiety groups. Results support the proposal that healthy, anxiety vulnerable individuals might have diminished top-down prefrontal control of selective attention for threat signals.[21–23] This anxiety modulation effect on ACC activity was seen in a contrast that highlighted a high-interference condition, indicating emotional interference levels, as well as task demand characteristics,[21,22] may be important modulators of neural activity in studies of anxious individuals, and need to be considered in neuroimaging studies of patients with anxiety disorders. Taken together, accumulating evidence suggests that high cognitive task demands may obscure impoverished frontal control of attentional resources in anxious subjects, and that high emotional interference contexts can highlight this deficit.
The reduced rACC activation in anxious subjects in the high-interference context suggests that anxiety is associated with a difficulty in attentional control for threatening distracters, or an inability to resolve anger-related interference. Higher ACC activation in less anxious subjects may represent enhanced recruitment of resources to efficiently resolve threat-related interference.[29] Together, these results are consistent with the proposal that ACC recruitment is important in maintaining cognitive goals while monitoring for salient emotional signals.[15] Anxiety may be associated with a possible deficit in this brain region that plays a critical role in creating an appropriate balance between sensitivity to potential threats and maintenance of focus on goal-directed cognitive tasks.
Intriguingly, our results were more striking for angry than fearful faces. Angry faces showed more consistent evidence of an interference effect, and the link between rACC activation and trait anxiety was seen in contrasts involving angry but not fearful faces. Others have also noted greater emotional interference from angry faces than from fearful expressions in highly anxious subjects.[30] Angry faces may be perceived as more directly salient or threatening, causing more emotional interference when attention is directed toward them. Although both convey potential threat, with a fear face the source of threat is ambiguous,[31,32] but an angry face suggests immediate threat from the person pictured directed toward the viewer.[30–33] The ACC might be specifically sensitive to individual differences in processing signals of interpersonal aggression, including direct threat to self. However, this difference could also be due, at least in part, to the individual's interpretation of the signal conveyed by fearful faces (e.g. the extent to which threat conveyed is relevant to self) or greater ambiguity communicated by fearful faces, which are sometimes confused with less negative expressions (e.g. surprise;[33,34]). Prior exposure could also play a role, since angry faces are seen more often than fearful ones.[32,35] Further work is needed to determine the source of the differences between angry and fearful faces and whether angry faces convey a direct threat that is particularly salient to anxious individuals.
This study has limitations and thus presented findings warrant cautious interpretation. Our sample size was small for careful analysis of individual differences and replication in a larger sample is needed. Anxiety modulation of the ACC was seen in the absence of a main effect of ACC activation. Though we might have expected to see a general effect of interference on ACC activation, regardless of trait anxiety, it is possible that under conditions of high interference, the impact of individual differences in trait anxiety may be sufficient to undermine main effects. If so, task-related differences in interference levels and individual differences in trait anxiety may impact the likelihood of finding general ACC activation across studies and require more consideration. We did not preselect volunteers on trait anxiety and there was a restricted range of anxiety level in this nonclinical sample. This may have actually weakened the strength of our findings, but replication across a broader range of trait anxious individuals is necessary to determine the real impact of this variable. Because the sample was nonclinical, conclusions cannot be extrapolated to clinical populations and parallel studies on psychiatric patients are needed. Finally, our paradigm was not primarily designed to maximize detection of behavioral evidence of interference. There was not perfect alignment of our behavioral interference data and our neuroimaging results. We used a longer duration for stimuli presentation than is commonly used in behavioral interference studies, in order to optimize neural effects. Further work to more carefully align behavioral and neural data is needed.
Despite limitations, this preliminary study indicates that individual differences in anxiety modulate rACC activation and deficiencies in attentional control contribute to selective attention to threat, a purported key mechanism of anxiety vulnerability.[7] Findings are consistent with mounting data implicating impoverished frontal mechanisms of attentional control for socioemotional threatening signals in anxiety-prone healthy individuals,[21–23] with potential relevance to anxious psychopathology.
Acknowledgments
This study was supported in part by National Institutes of Health grant R24MH075999 (IL) and in part by grant UL1RR024 986 (HK) from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of NCRR or the National Institutes of Health.
Contract grant sponsor: National Institutes of Health; Contract grant number: R24MH075999; Contract grant sponsor: National Center for Research Resources; Contract grant number: UL1RR024 986.
Footnotes
The authors disclose the following financial relationships within the past 3 years:
REFERENCES
- 1.Öhman A, Lundqvist D, Esteves F. The face in the crowd revisited: a threat advantage with schematic stimuli. J Pers Soc Psychol. 2001;80:381–396. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- 2.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vuilleumier P. Facial expression and selective attention. Curr Opin Psychiatry. 2002;15:291–300. [Google Scholar]
- 4.Bar-Haim Y, Lamy D, Pergamin L, et al. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Bradley BP, Mogg K, Falla SJ, Hamilton LR. Attentional bias for threatening facial expressions in anxiety: manipulation of stimulus duration. Cogn Emotion. 1998;12:737–753. [Google Scholar]
- 6.Mogg K, Bradley BP. Some methodological issues in assessing attentional biases for threatening faces in anxiety: a replication study using a modified version of the probe detection task. Behav Res Ther. 1999;37:595–604. doi: 10.1016/s0005-7967(98)00158-2. [DOI] [PubMed] [Google Scholar]
- 7.MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Barlow DH. Anxiety and its Disorders: The Nature and Treatment of Anxiety and Panic. 2nd ed. Guilford Press; New York: 2002. [Google Scholar]
- 9.Etkin A, Egner T, Peraza DM, et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 10.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 11.McKenna FP. Effects of unattended emotional stimuli on color-naming performance. Curr Psychol Res Rev. 1986;5:3–9. [Google Scholar]
- 12.Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- 13.Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 14.Whalen PJ, Bush G, McNally RJ, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 15.Whalen PJ, Bush G, Shin LM, Rauch SL. The emotional counting Stroop: a task for assessing emotional interference during brain imaging. Nat Protoc. 2006;1:293–296. doi: 10.1038/nprot.2006.45. [DOI] [PubMed] [Google Scholar]
- 16.Anderson AK, Christoff K, Panitz D, et al. Neural correlates of the automatic processing of threat facial signals. J Neurosci. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 19.Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. J Abnorm Psychol. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- 20.Eysenck MW, Calvo MG. Anxiety and performance: the processing efficiency theory. Cogn Emotion. 1992;6:409–434. [Google Scholar]
- 21.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 22.Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- 23.Krug MK, Carter CS. Adding fear to conflict: a general purpose cognitive control network is modulated by trait anxiety. Cogn Affect Behav Neurosci. 2010;10:357–371. doi: 10.3758/CABN.10.3.357. [DOI] [PubMed] [Google Scholar]
- 24.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 25.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 26.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV Patient Edition (SCID-P) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 27.Spielberger CD, Gorsuch RL, Lushene R. Manual for State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- 28.Gur RC, Schroeder L, Turner T, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16:651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- 29.Kanske P, Kotz SA. Emotion speeds up conflict resolution: a new role for the ventral anterior cingulate cortex? Cereb Cortex. 2010 doi: 10.1093/cercor/bhq157. in press. [DOI] [PubMed] [Google Scholar]
- 30.Fox E, Calder AJ, Mathews A, Yiend J. Anxiety and sensitivity to gaze direction in emotionally expressive faces. Emotion. 2007;7:478–486. doi: 10.1037/1528-3542.7.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewbank MP, Lawrence AD, Passamonti L, et al. Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. NeuroImage. 2009;44:1144–1151. doi: 10.1016/j.neuroimage.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 32.Whalen PJ. Fear, vigilance, and ambiguity: intial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:177–188. [Google Scholar]
- 33.Biehl M, Matsumoto D, Ekman P. Matsumoto and Ekman’s Japanese and Caucasian facial expressions of emotion (JACFEE): reliability data and cross-national differences. J Nonverbal Behav. 1997;21:3–21. [Google Scholar]
- 34.Ekman P. Strong evidence for universals in facial expressions. Psychol Bull. 1994;115:268–287. doi: 10.1037/0033-2909.115.2.268. [DOI] [PubMed] [Google Scholar]
- 35.Bond NW, Siddle DAT. The preparedness account of social phobia: some data and alternative explanations. In: Rapee RM, editor. Current Controversies in the Anxiety Disorders. Guilford Press; New York: 1996. pp. 291–316. [Google Scholar]