Abstract
Background and Objectives
Cigarette smoking is common among cancer patients and is associated with negative outcomes. Electronic nicotine delivery systems (“e-cigarettes”) are rapidly growing in popularity and use, but there is limited information on their safety or effectiveness in helping individuals quit smoking.
Data Sources
The authors searched PubMed, Web of Science, and additional sources for published empirical data on safety and use of electronic cigarettes as an aid to quit smoking.
Review Methods
We conducted a structured search of the current literature up to and including November 2013.
Results
E-cigarettes currently vary widely in their contents and are sometimes inconsistent with labeling. Compared to tobacco cigarettes, available evidence suggests that e-cigarettes are often substantially lower in toxic content, cytotoxicity, associated adverse effects, and secondhand toxicity exposure. Data on the use of e-cigarettes for quitting smoking is suggestive, but ultimately inconclusive.
Conclusions
Clinicians are advised to be aware that the use of e-cigarettes, especially among cigarette smokers, is growing rapidly. These devices are unregulated, of unknown safety, and of uncertain benefit in quitting smoking.
Implications for Practice
In the absence of further data or regulation, oncologists are advised to discuss the known and unknown safety and efficacy information on e-cigarettes with interested patients, and to encourage patients to first try FDA-approved pharmacotherapies for smoking cessation.
Keywords: e-cigarettes, smoking, electronic nicotine delivery systems, personal vaporizers, vaping, vapers, toxicity, carcinogens, nicotine
Between 2005–2009, cigarette smoking accounted for over 48% of all cancer-related deaths.1 At least 75% of head and neck cancers are caused by tobacco and alcohol use, with tobacco associated with greater risk than alcohol.2,3 Evidence suggests that not only does smoking cause cancer, continued smoking after cancer diagnosis increases risk of developing other smoking-related illnesses (e.g., coronary heart disease), second primary tumors, cancer recurrence, and mortality.4–8 The majority of head and neck cancer patients attempt to quit smoking, but a substantial portion relapse back to smoking.9,10 Given patients’ substantial smoking histories and difficulties quitting, it is perhaps unsurprising that our research team is increasingly receiving anecdotal reports of patients using electronic nicotine delivery systems (referred to here as “e-cigarettes” for purpose of simplicity and brevity).
E-cigarettes first became commercialized in China in 2003 and entered the United States market in 2007.11–13 E-cigarettes are electronic devices that heat a liquid to produce a vapor inhaled by the user (“vaper”). Notably, vaporization does not involve combustion or tobacco smoke (see Figure 1). A wide variety of brands and modifications exist. Many of these devices resemble cigarettes or cigars, but others appear more similar to pens, screwdrivers, or the tips from a hookah. The heating of the liquid inside an e-cigarette (“e-liquid”) is sometimes initiated by inhalation from the user, but in other models is initiated by the pressing of a button. Most e-liquids contain nicotine from tobacco leaves in a mixture of glycerin or propylene glycol. Some e-liquids contain flavorings advertised as menthol, mint, cherry, coffee, chocolate, or as tasting similar to different varieties of tobacco. These e-liquids may be contained in disposable e-cigarettes themselves, in replaceable cartridges, or in refill liquids. On April 24, 2014, the Food and Drug Administration (FDA) released a proposed rule deeming e-cigarettes subject to FDA regulation, which, if enacted, will require e-cigarette manufactures to report product and ingredient listings, only market new e-cigarettes after FDA review, only make reduced risk claims if FDA confirms there is scientific evidence, and include health warnings with e-cigarette packaging. This rule will be available for a 75-day public comment period before enactment. Providers and patients should be aware that e-cigarettes are not approved as cessation devices and there is currently no federal oversight of e-cigarettes.
Figure 1.
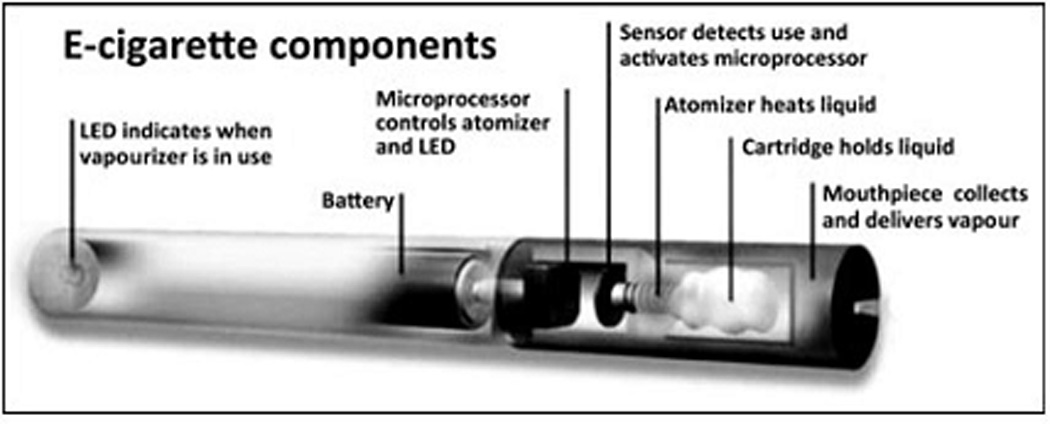
Typical components of an electronic cigarette. Source: Reprinted from Foulds et al. 2011 with permission from John Wiley and Sons, © 2011 Blackwell Publishing Ltd.
Although federal regulation of e-cigarettes have not yet been introduced in the United States, it is instructive to note regulations that have emerged elsewhere. In Canada, electronic nicotine delivery systems cannot be imported, marketed, or sold without being approved as a new drug. As a result, e-cigarettes that contain nicotine are currently illegal, but e-cigarettes without nicotine are legal as long as they do not include a health claim.14 In the European Union, health ministers had attempted to move towards pharmaceutical regulations for e-cigarettes regardless of nicotine content. However, in October of 2013, less stringent regulations were enacted: e-cigarettes should contain no more than 30 mg/ml of nicotine, should carry health warnings, and should not be sold to anyone under 18. Further, manufacturers and importers should supply authorities with a list of ingredients and e-cigarettes are subject to the same advertising restrictions as tobacco products.
The lack of federal oversight in the United States has not deterred growth in the sale and use of electronic cigarettes. Surveys and other evidence indicate rapid, exponential growth, driven by increasingly aggressive marketing and declining price. Estimates of the U.S. population who have ever used e-cigarettes in 2010 ranged from 2 to 3%.15 A survey conducted in the beginning of 2012, before major televised e-cigarette advertisements, reported that about 8% had tried e-cigarettes with a 1% rate of current use; among current smokers, 32% had tried e-cigarettes and 6% used currently.12 Most vapers report smoking cessation as the primary reason for use.16–18{Control, 2013 #172;Corey, 2013 #457} Recently, traditional tobacco manufacturers entered the market, which is likely to accelerate e-cigarette usage. Given increased use among the general population and anecdotal reports of use by cancer patients, we recognized a need for providers to have a summary of the current, relevant literature on these products. To achieve this goal, we conducted a systematic review of the literature regarding the safety of e-cigarettes and their use as aides to quit tobacco cigarettes.
Methodology/Search Strategy
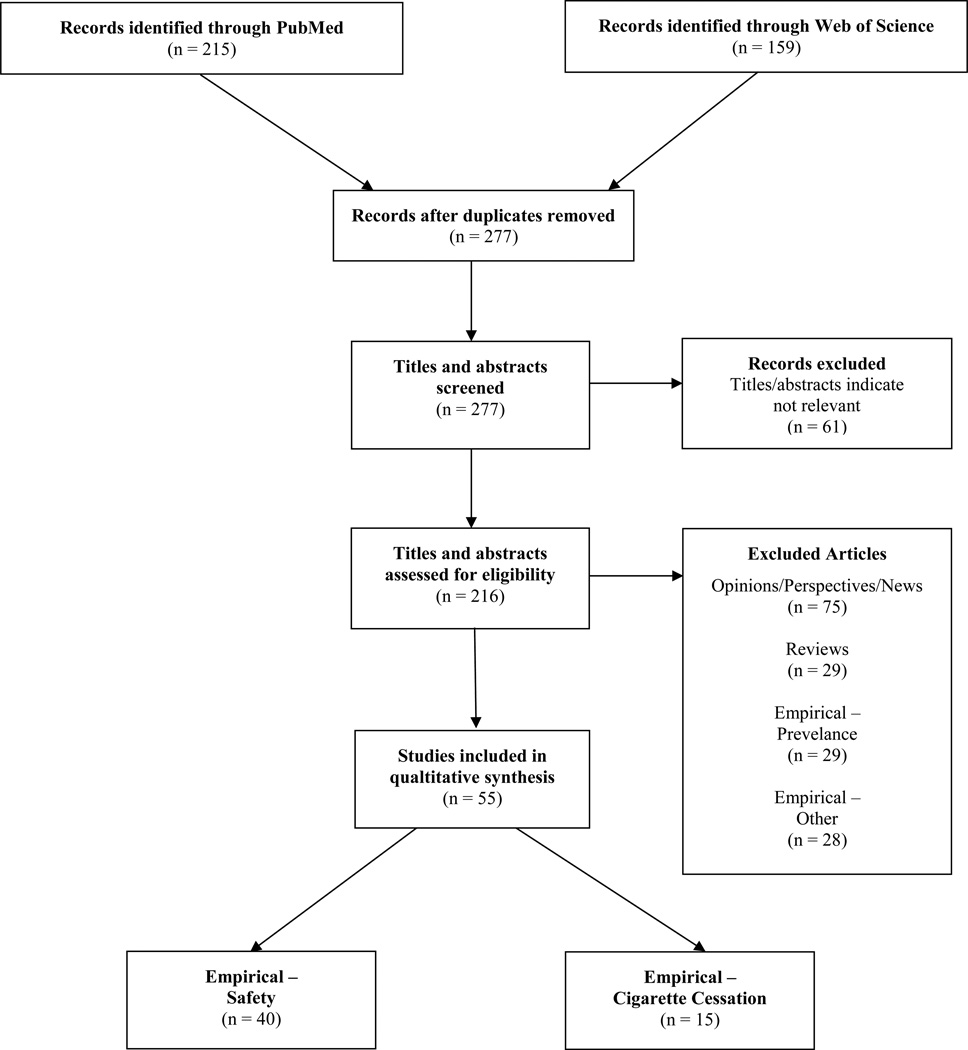
For this review, the literature was searched in PubMed (titles or abstracts) and Web of Science (titles or topics). The most recent search was conducted on November 20, 2013. Search terms used included “electronic cigarette,” “electronic cigarettes,” “e-cig*,” and “electronic nicotine delivery”. The initial search identified 277 original articles. Of these, 61 were not relevant, 75 were opinion/commentary articles, 29 were review articles, 29 provided empirical data regarding e-cigarette prevalence, and 28 provided empirical data not directly relevant, such as media exposure or effects of e-cigarettes on withdrawal symptoms or cognition. The remaining 55, that we review here, were articles with empirical data related to safety (n=40) and tobacco cigarette cessation (n=15) (Figure 2). In addition, recent reviews assisted in structuring this review and providing background.19–21
Figure 2.
Flow diagram of literature search, screening, and selection.
Discussion
Safety Data
Because e-cigarettes are developed by a variety of manufacturers and are unregulated, the contents of e-cigarettes vary widely and in some cases are not consistent with labeling.22–24 Thus, it is not possible to make general statements regarding e-cigarette safety. Further, assessment of the health hazard associated with continued exposure to e-cigarettes over many years is not possible currently, as very few people have used e-cigarettes for an extended period of time. Moreover, the goal of safety assessments is controversial. Some argue that assessments should examine if e-cigarettes pose any risk to health, while others claim that the only relevant question is if they pose less risk than cigarette smoking.25–28 Despite these limits and controversies, there are some initial studies of e-cigarettes that provide useful information.
E-cigarettes are primarily used as a nicotine-delivery system. Nicotine is known to be the major addictive chemical in tobacco and to have acute toxicity at high doses.1 Although nicotine is linked to deregulation of essential biological processes, such as angiogenesis, which may promote the growth of pre-existing tumors,29 it is unclear if nicotine is carcinogenic in humans. Notably, a secondary analysis of the Lung Health Study found that use of Nicotine Replacement Therapies (NRT) was not associated with lung cancer.30 On the other hand, long-term smokeless tobacco use increases the risk of oral cavity, esophageal, and pancreatic cancers, but not to the same degree as smoking and not at all for many other cancers associated with smoking. This potentially suggests that substances other than nicotine contribute to the cancer process. However, another explanation is that the reduced risk is partially or completely due to route of exposure.1 Further, the carcinogen N-nitrosonornicotine (NNN) has been detected among some former smokers using NRT.31,32 Nonetheless, NNN is but one of over 250 chemicals known to be toxic or carcinogenic in tobacco smoke.33 Thus, the 2014 Surgeon’s General’s Report notes the need for quantifying the level of risk from long-term use of NRT and other non-combusted sources of nicotine, especially if long-term use of these nicotine sources becomes more prevalent, as appears to be occurring with e-cigarettes.1
Nicotine content varies by electronic cigarette manufacturer, and actual content is sometimes higher or lower than labeled.21–23,34,35 Under clinical laboratory conditions, e-cigarette users are not always exposed to measurable levels of nicotine.36,37 Automatic smoking machines indicate that, similar to light and ultralight cigarettes, deeper inhalation is needed for e-cigarettes than conventional cigarettes, and the need for deep inhalation increases as vaping progresses.38–40 Although the reduced exposure to nicotine may have some benefits, it is also a cause for concern, because lower nicotine products may result in compensatory behaviors.41 Indeed, more experienced vapers using their own devices in the laboratory have been found to have elevated rates of plasma nicotine, topography indicates experienced vapers have longer puff duration than cigarette smokers vaping for the first time, saliva samples from vapers were found to have levels of nicotine metabolite that are more similar to cigarette smokers than users of nicotine replacement therapies, and survey data seems to indicate that vapers who are former smokers consume more nicotine from e-cigarettes than they previously did from tobacco cigarettes.42–45 These data suggest that vapers may compensate for any initial low levels of nicotine or that the absence of smoke irritants allows vapers to inhale more nicotine compared to smoking. This high usage may be of concern, especially if e-cigarette vapor contains significant amounts of toxic chemicals besides nicotine.
To date, studies have found detectable levels of toxic chemicals in e-cigarettes, but generally at levels much lower than in tobacco smoke. For example, levels of toxicants have been reported to be 9–450 times lower than levels previously reported in tobacco smoke.46 Nonetheless, these do include carcinogens previously found in tobacco smoke, such as formaldehyde, acetaldehyde, NNN, and 4(N-Nitrosomethyamino)-1-(3-pyridyl)-1-butanone (NNK). Most e-cigarettes contain propylene glycol, which at higher temperatures can oxidize and form formaldehyde, acetaldehyde, and methylglyoxal.47 Some, but not all, of the e-cigarette brands produced vapor with levels of formaldehyde and acetaldehyde significantly greater than contained in a medicinal nicotine inhaler. NNK and NNN were not found to be present in the nicotine inhaler, but were found in conventional cigarette smoke at concentrations 40 and 380 times greater than e-cigarette vapor, respectively.46 Similarly, fine and ultrafine particulate matter emissions of an Italian brand of e-cigarette were 10 or 5 times lower than a conventional cigarette.48 Other chemicals identified in e-cigarettes include acrolein, volatile organic compounds (VOC’s), and, in some rare cases, amino-tadalafil and rimonabant.47,49–51 Finally, one study found concentrations of metals in e-cigarette aerosol that were higher than cigarette smoke, which the authors suggested demonstrated a need for improved quality control.52 See Table 1 for a summary of studies of e-cigarette content.
Table 1.
Articles on e-cigarette/vapor chemical content.
| Year | Authors | Locations | Brands | Findings |
|---|---|---|---|---|
| 2010 | Hadwiger et al. | United States (internet purchases from FDA) | E-Cialis, E-rimonabant, [others unidentified] | E-cig products advertised as containing no nicotine did actually contain nicotine; E-Cialis contained amino-tadalafil. E-rimonabant contained an oxidative impurity of rimonabant. |
| 2011 | Ohta et al. | Japan | [unidentified, examined both commercial devices and a device made specifically for study] | Formaldehyde, acetaldehyde, acrolein, glyoxal, and methylglyoxal detected in vapor; when voltage exceeded 3 V, mist contains carbonyl compounds, possibly due to oxidation of carrier substance |
| 2011 | Trehy et al. | Missouri, United States (internet purchases) | CIXI, Johnson Creek, NJOY, Smoking Everywhere | Nicotine content sometimes differs from label (e.g., labeled 0 mg, actual 21.8 mg; labeled 24 mg, actual 0 mg); Detectable levels of mysomine and anatabine |
| 2012 | Cheah et al. | Singapore | Best Ecig, BoJinQiShi, E-pipe, e-joy, ECHL, E-vaporizer, Eluma, 51, PV 510, Pons, SS, Slim, SH, SA, SE, SC, Vapor, VC, V2CIGS | Nicotine content sometimes differs from label (e.g., labeled 11 mg, actual 2 mg, labeled 6 mg, actual 6–12 mg); Propylene glycol and glycerol found to be present in nicotine-containing liquid |
| 2013 | Cameron et al. | US: Spokane, Washington | BE112, Smart Smoke, Vapour | Actual nicotine content in all samples equivalent to or lower than labeled content |
| 2013 | Etter et al. | US, UK, France, Switzerland, China | Dekang, Ecigexpress, FV, Intellicig, Janty, JC, Sedansa, Tecc, TW, Vapor4Life | Nicotine content similar to labeling; Contents include cis-N-oxide, trans-N-oxide, myosmine, antabine, anabasine |
| 2013 | Goniewicz, Knysak, et al. | Poland, United Kingdom | Colinss, Dekang, DSE, Ecis, Intellcig, Joye, Janty, Mild, Nicore, Premium, Trendy | Formaldehyde, Acetaldehyde, Acrolein, Toluene, NNN, and NNK identified at levels 9–450 times lower than mainstream conventional cigarette smoke |
| 2013 | Goniewicz Kuma, et al. | Poland, United Kingdom, United States | Colinss, Dekang, Ecis, Ecigar.pl, EO, Extreme, Gamucci, Intellicig, Janty, Liberro, Mild, Mini, Nicore, NPro, Premium, Red, SGC, SC, Trendy, Virginia | Nicotine content sometimes consistent with labeling; However, in 9 out of 20 analyzed cartridges and 3 out of 15 refill liquids, nicotine content differed by over 20%; |
| 2013 | Kim and Shin | Korean imports from Chinese manufacturers | [unidentified, “purchased in July and August, 2012 from 11 e-cigarette shops] | Maximum concentrations of Tobacco Specific Nitrosamines of 86.92 µg/L, 10 times more than published by Ruyan E-cigarette Company |
| 2013 | Kirschner et al. | United States (smoke shop and internet) | Ecigexpress, Titan, Provape, Hangsen | Nicotine concentrations differed from labeling; (e.g., labeled 36 mg, actual 50 mg; labeled 100 mg, actual 87 mg, labeled 36 mg, actual 18 mg) |
| 2013 | Williams et al. | California, United States | [unidentified, “from a well-known manufacturer”; “purchased from local retailers or on the Internet”] | Vapor contained particles comprised of tin, silver, iron, nickel, aluminum, and silicate and nanoparticles of tin, chromium, and nickel; 9 of 11 elements were equal or higher to cig smoke |
Pre-clinical Studies
An initial study found an association between e-liquid cytotoxicity and the number and concentration of flavoring chemicals used.53 Subsequent studies found that one of twenty-one e-liquids produced vapor with cytotoxic effects.54 A follow-up study found cytotoxicity in four of twenty samples and noted that higher voltage e-cigarettes resulted in reduced cell viability.55 Cigarette smoke was significantly more cytotoxic, meeting cytotoxicity definition at 12.5% dilution, whereas none of the vapor solutions met that criteria even at 25% dilution.55 In a 2014 conference presentation, researchers described a study of human bronchial epithelial cells carrying mutations in the TP53 and KRAS genes and reported that e-cigarette vapors enhanced the cells’ cancerous behaviors, suggesting that e-cigarette exposure might contribute to lung cancer in individuals at high risk for the disease.56 Further studies on the impact of electronic cigarette exposure on lung carcinogenicity are ongoing.
Acute effects
The use of e-cigarettes appears to have resulted in some positive health changes for former smokers. Smokers (n=13) converting to vaping demonstrated reduced carboxyhemoglobin levels after 2 weeks.57 In a case report study, one smoker was able to relieve chronic idiopathic neutrophilia after switching from tobacco smoking to e-cigarettes.58 E-cigarettes are also linked to negative effects. E-cigarettes, especially those with nicotine, reduce lung function.59 However, this effect is less pronounced for e-cigarettes than for tobacco cigarettes.44 Unlike tobacco cigarettes, e-cigarettes do not appear to significantly affect complete blood count.60,61 Studies reported that although both e-cigarettes and tobacco cigarettes increased pulse, increased inflammatory markers, and impacted measures of myocardial function, these changes were only significant for tobacco cigarettes.62–64 See Table 2 for a summary of studies on acute effects.
Table 2.
Articles on acute physiological effect of e-cigarettes.
| Year | Authors | Location | N | Study Design | Conditions | Brands | Findings |
|---|---|---|---|---|---|---|---|
| 2010 | Vansickel et al. | United States | 32 | Within-subjects design with cigarette smokers | Own brand (OB) cigarette, 18 mg e-cig, 16 mg e-cig, or unlit cig | NPRO (18mg), Hydro (16 mg) | Symptom suppression greater for OB than either e-cig; OB, but not e-cigs, increased plasma nicotine and heart rate |
| 2012 | Czogala et al. | Poland | 42 | Case series | Before and after cigarette and e-cig use | [unidentified] | E-cigs increased diastolic pressure & pulse,; other parameters only showed sig. increases after cig |
| 2012 | Farsalinos et al. | Greece | 42 | 2 case series with 22 ex-smokers who use e-Cig and 20 Smokers (S) | Use of 11mg e-cig for 7 minutes (eCig only) r a regular tobacco cigarette (S only) | [unidentified] | Cigaffected several measures of ventricular function (all p<.05); Using e-cig resulted only in rise in MV-A wave, p<.05 |
| 2012 | Flouris et al. | Greece | 30 | 2 case series with 15 Smokers (S), 15 Never Smokers (NS) | Control, active (S only) / passive (NS only) smoking, and active (S only) / passive (NS) vaping | Nobacco Giant | Complete blood count not significantly altered during control and e-cig sessions, p>.05; cigarette smoking increased white blood cell, lymphocyte, granulocyte counts, p<.05 |
| 2012 | Vardavas et al. | Greece | 30 | Case series | Use of active (11 mg nicotine) or inactive e-cig ad lib for 5 minutes | Nobacco, black line, MLB-MED filter | Active e-cig decreased fraction of exhaled nitrous oxide, increased respiratory impedance, resistance, and overall peripheral airway resistance (all p<.03). |
| 2013 | Flouris et al. | Greece | 30 | Mixed within-subjects between subj.: 15 smokers (S), 15 Never Smokers (NS) | Control, active (S only) / passive (NS only) smoking, and active (S only) / passive (NS) vaping | Nobacco Giant | Activevaping/smoking generated similar effects on cotinine levels (e.g., vaping 60.6±34 vs. smoking 61.3±7); E-cig reduced lung function 3% vs. smoking 7% |
| 2013 | Tzatzarakis et al. | Greece | 20 | 2 case series with Smokers (S), n=10, and Never Smokers (NS), n=10 | Control, active (S only) / passive (NS only) smoking, and active (S only) / passive (NS) vaping | [unidentified] | Active and passive vaping did not sig. increase inflammatory markers, p>.05; Active and passive smoking increased tumour necrosis factor alpha (TNFa), p<.05 |
| 2013 | Van Staden et al. | South Africa | 13 | Case series | Before and after e-cig use for 2 weeks by regular cigarette smokers | Twisp | Decrease in cotinine, carboxyhaemoglobin reduced, increased O2saturation |
Various adverse events for e-cigarettes have been reported. The FDA reported that they received 47 reports about adverse events related to e-cigarette use: eight were classified as serious, including pneumonia and chest pain; thirty-nine were classified as minor, including headache and cough.65 By examining reports by vapers in online forums, researchers classified negative effects from e-cigarettes as occurring primarily in the mouth, throat, and respiratory, neurological, sensory, and digestive systems.66 Case studies have reported e-cigarette use resulting in lipid pneumonia and paroxysmal atrial fibrillation, both of which resolved after cessation of e-cigarette use.67,68 Poison center visits related to e-cigarette exposure were primarily from accidental ingestion resulting from leaky cartridges or other causes, but also from dermal or ocular exposure, as well as standard inhalation.69–71 Three reported suicide attempts involving e-liquid ingestion were ultimately non-fatal, but one individual did commit suicide via e-liquid injection.72,73
Secondhand vaping
Toxic chemicals in e-cigarette vapor are not only inhaled by e-cigarette users; they are also released into the indoor environment. An early study reported no risk of harm to health via the inhalation route of environmental e-cigarette vapor,74 but later studies found that use of e-cigarettes leads to emissions of aerosols, VOC’s, flavoring substances, and nicotine, allowing for “passive vaping”.48,75–77 Particle number concentrations of e-cigarette-generated aerosols were similar or even slightly higher than tobacco cigarettes; high nicotine content e-cigarettes appeared to produce greater particle number concentrations.78 Presently, it is unclear if these particles represent a source of toxicity. Even if some level of toxicity is present, it seems unlikely to be as harmful as second-hand smoke, but the studies reviewed have yet to definitively answer this question. See Table 3 for a summary of passive exposure studies.
Table 3.
Articles on passive exposure to e-cigarettes (second hand vaping).
| Year | Authors | Location | Brands | Flavorings | Nicotine (mg/mL) |
Findings |
|---|---|---|---|---|---|---|
| 2012 | McAuley et al. | United States | [unidentified, 4 “popular e-liquid brands”] | Tobacco | 24 and 26 | No vapor sample exceeded defined risk limits, tobacco smoke approached, but did not exceed, defined risk limits |
| 2012 | Ingebrethsen et al. | United States | [unidentified, 1 rechargeable and 1 disposable] | [none reported] | [not reported] | Undiluted e-cig vapors found to have particle diameters of average mass in the 250–450 nm range and particle number concentration in the 109particles/cm3range |
| 2013 | Schripp et al. | Germany | [unidentified, tank and cotton delivery systems] | Apple and Tobacco | 0, 18 | Increases in volatile organic compounds and (ultra)fine particles identified in passive vapor; exhaled particles are smaller than inhaled |
| 2013 | Zhang et al. | United States | Bloog MaxX Fusion, Propylene (PG) or Vegetable (VG) vehicle | [none reported] | 16 | Peak particle counts at 180 nm (VG) and 120 nm (PG). Small particles were eliminated and large particles were reduced in both size and number by organic vapor removal |
| 2014 | Fuoco et al. | Italy | [unidentified, two rechargeable models (tank and atomizer) and one disposable] | Selene, Strawberry, Menthol, Camel | 0, 8–9, 12–18 | Particle number distributions of e-cig vapor were similar (4.39±0.42 part. cm−3) to tobacco cigarette smoke (3.14±0.61 part. cm−3) |
Efficacy Data on Smoking Cessation
Similar to safety data, there is considerable controversy about how to interpret cessation data. Beyond case studies and qualitative reports,57,79–82 two types of empirical evidence are reviewed here regarding the impact of e-cigarette usage on tobacco smoking: population-based studies and prospective trials. Population-based studies are generally survey-based observational studies that compare vapers to cigarette smokers. These are difficult to interpret for a variety of reasons. Most notably, individuals who purchase e-cigarettes differ significantly from those who do not. For example, vapers, in comparison to non-vaping cigarette smokers, are often younger and more affluent.83,84 Another question concerns whether reduction (as opposed to cessation) of cigarette smoking should be considered as a positive outcome; cigarette smoking reduction is of questionable health benefit.85 Further, self-report of cigarette smoking rate is notoriously unreliable when measured in cigarettes per day, as done in the majority of studies reviewed here.86,87 Even the few prospective trials have methodological limitations such as small sample size and no appropriate control group.
Survey studies
Surveys reveal that the majority of e-cigarette users are current or former smokers who believe e-cigarettes can help them quit smoking and are less harmful than tobacco cigarettes.15–17,20,88 A 2010 survey e-mailed 4,884 consecutive first-time purchasers of e-cigarettes in the United States (US); of the 216 smokers who responded, about 31% were not smoking 6 months after their purchase.89 A 2011 survey of current or recent former smokers (N=1836) weighted to estimate US prevalence found that use of e-cigarettes was significantly associated with an unsuccessful quit attempt.90 Similarly, another survey of current/former smokers in the US, the United Kingdom, Canada, and Australia found a significant reduction in cigarette smoking, but cessation rates did not differ significantly between e-cigarette users and non-users.83 Another study examining tobacco quitline callers from six U.S. states reported that about one-third of the sample reported ever using e-cigarettes; they were significantly less likely to have quit cigarettes at 7-month follow-up.91 A study of former smokers in Europe (N=111) who successfully substituted e-cigarettes for tobacco cigarettes for at least one month reported that 42% quit in the first month; the majority (74%) used liquid with high nicotine concentration.27 These survey studies are difficult to interpret because causality cannot be determined.
Clinical trials
We identified five prospective studies. The first four studies were conducted among current smokers uninterested in quitting. In the first study (N=20), e-cigarette experimentation was associated with reduced cigarette smoking after one week.92 Similarly, in a second study involving smokers who were provided e-cigarettes for 24 weeks (N=40), about 23% reported 30-day cigarette abstinence and about a third reduced cigarette consumption by at least 50%.93 Results remained similar 18 months later, not including 17 who were lost to follow-up.94 Notably, neither of the studies mentioned so far included control groups. A third study compared groups based on e-liquid nicotine content. One group (n=100) received e-cigarettes with no nicotine, while a second group (n=100) received 7.2 mg, and a third group (n=100) received 7.2 mg for the first 6 weeks and then 5.4 mg for the remainder of the study. There were no significant differences between groups. Collapsing across groups, 11% and 9% of participants reported tobacco smoking abstinence at week 12 and week 52 respectively.95 Similar results were found among schizophrenic smokers, with sustained abstinence in 2 of the 14 participants, with another 7 reporting a 50% reduction or more.96
Finally, the most recent study randomly assigned 657 smokers interested in quitting to three groups: e-cigarettes with nicotine (n=289), e-cigarettes without nicotine (n=73), and nicotine transdermal patch (n=295), a FDA-approved pharmacotherapy.97,98 Participants were given free supplies and instructed to use their e-cigarette or patch for one week before until 12 weeks after their quit day. The primary outcome, self-reported abstinence over the whole follow-up period (allowing ≤ 5 cigarettes total and verified by carbon monoxide), was assessed six months after the quit date. Rates of abstinence were not significantly different, but was highest in the nicotine e-cigarettes group (n=21, 7.3%), followed by the nicotine patch group (n=17, 5.8%), and nicotine-free e-cigarettes group (n=3, 4.1%). The authors attributed the lack of significant findings to lower rates of abstinence achievement observed during the study than assumed in statistical power calculations. Indeed, the rates appeared to be quite low in comparison to other studies.99 See Table 4 for a summary of smoking cessation studies. Overall, these studies show that use of e-cigarettes appears to be associated with cessation or reduction of cigarette smoking for some individuals, but studies have yet to demonstrate that e-cigarettes are superior to an appropriate control condition.
Table 4.
Empirical articles on use of e-cigarettes for cigarette cessation.
| Year | Authors | Locations | N | Study Design | Conditions | Brands | Findings |
|---|---|---|---|---|---|---|---|
| 2011 | Caponnetto et al. | Italy | 2 | Case series | 7.2mg nicotine e-cigarette | [unidentified] | All participants quit smoking (CO = 2–4 ppm) |
| 2011 | Caponnetto et al. | Italy | 3 | Case series (6 months) | 7.2mg nicotine e-cigarette (2/3 participants) | [unidentified] | All participants quit smoking (CO = 2–5 ppm) |
| 2011 | Polosa et al. | Italy | 40 | 6 month prospective pilot study | 7.4 mg nicotine e-cigarette | Categoria | 23% quit smoking |
| 2011 | Siegel et al. | United States | 216 | 6 month follow-up survey | [Not Applicable] | Blu | 31% quit smoking |
| 2013 | Bullen et al. | New Zealand | 657 | 6 month 3 arm, Randomized Controlled Trial | 16 mg e-cigarette, 21 mg patch, 0 mg e-cigarette | Elusion | No differences between conditions |
| 2013 | Caponnetto et al. | Italy | 300 | 52 week, 3 arm, Randomized Controlled Trial | 7.2 mg e-cigarette, 5.4 mg e-cigarette, 0 mg e-cigarette | Categoria | No significant differences; All reduced use |
| 2013 | Caponnetto et al. | Italy | 14 | 52 week case series | 7.4 mg nicotine e-cigarette | Categoria | 14% quit |
| 2013 | Farsalinos & Romagna | Greece | 1 | Case report | [Not Applicable] | [unidentified] | Participant able to quit; increased leukocyte count |
| 2014 | Wagener et al. | United States | 20 | 1 week case series | ad libitum use of brand of choosing | ProSmoke (14/18mg), Blu (13–15 or 9–12 mg), SmokeTip (12–16 mg) | 44% reduction in cigarettes per day following ad libitum use |
Implications for Practice
Safety data indicates that e-cigarettes contain detectable levels of toxic substances, but generally at lower levels than tobacco cigarettes. Acute effects are generally similar to effects of tobacco cigarettes, but at a lower severity. Negative effects reported, e.g., poisonings, appear to result primarily from accidental exposure.69,70,100,101 Secondhand vaping can occur, but current evidence is still ambiguous regarding related toxicity. Prospective trials appear to suggest e-cigarettes can help some individuals quit or reduce smoking; however evidence has yet to demonstrate rates higher than FDA-approved pharmacotherapies. Further, some individuals do not quit smoking, suggesting that e-cigarettes may allow them to maintain their tobacco dependence, perhaps by allowing nicotine use in locations where smoking is not permitted. In summary, available evidence suggest that e-cigarettes warrant further investigation, but cannot currently be unequivocally recommended as a smoking cessation strategy or harm reduction technique.
Healthcare providers, such as oncologists, can play a crucial role in promoting smoking cessation.102 The most common model for delivering this information is summarized in Table 5 as the 5A’s approach: 1) ask the patient about their smoking, 2) advise them to quit if they are smoking, 3) assess their willingness and readiness to make a quit attempt, 4) assist them in their quit attempt by providing resources or referrals, and 5) arrange follow-up consultations to promote long-term cessation. This model is endorsed by the most recent treatment guidelines for tobacco dependence and is enhanced when providers advise patients to use FDA-approved pharmacotherapies (e.g., nicotine patch, nicotine gum, nicotine inhaler, nicotine lozenge, varenicline, bupropion) further improving cessation rates.103–105 However, a large percentage of primary care physicians do not complete steps 3–5 in this model, and the same is true for cancer specialists, as less than 50% of practitioners discuss smoking cessation pharmacotherapies with their patients or provide assistance in helping them make a quit attempt.106–108 These low percentages motivated the American Association for Cancer Research (AACR) to recommend universal assessment and documentation of tobacco use, as well as improved provision of cessation assistance to all cancer patients who have used tobacco or recently quit.109
Table 5.
The 5 A’s for Promoting Smoking Cessation in Primary Care Settings.
| Step | Description | Action |
|---|---|---|
| Ask about cigarette use. | Determine whether a patient is currently using cigarettes at every appointment, and document every response in the patient’s medical record. | Directly ask the patient whether he/she is currently smoking tobacco cigarettes. |
| Advise patient to quit. | Encourage patients using tobacco cigarettes to quit smoking as soon as possible. | Tailor a clear and personalized message to the patient about the benefits of quitting and the risks associated with continued smoking. |
| Assess* readiness to quit. | Determine whether a patient is ready to quit smoking and identify barriers to making a quit attempt. | Directly ask the patient whether he/she is ready to quit smoking. If so, proceed to Assist. If not, probe for source of lack of motivation. |
| Assist* with quit attempt. | Provide assistance to patients who are motivated to quit. | Work with the patient on selecting a quit date. Explore potential triggers and cues which might promote relapse after quitting. Provide information about pharmacotherapy and referrals for psychotherapy. |
| Arrange* follow-up contact. | Maintain contact with the patient to ensure that he/she is successfully maintaining abstinence. | Schedule multiple follow-ups within the first month of the target quit date. Provide positive reinforcement at the follow-up contacts and offer additional services/referrals if patient relapses. If patient is not interested in making a quit attempt, follow up at future appointments |
Notes: Adapted from A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35(2):158–176.
The Assess, Assist, and Arrange steps may be executed via a referral to a tobacco treatment specialist, including a tobacco cessation telephone quitline.
Health and professional associations have begun to weigh in on e-cigarettes. AACR and the American Society of Clinical Oncology are currently preparing a joint policy statement. The American Cancer Society (ACS) had taken on a very cautionary role, releasing a memo of support in 2010 of the effort to halt the sale of e-cigarettes in New York unless approved by the FDA.110 However, more recent statements seem to be more open to potential public health benefits. A statement in 2011 by Dr. Thomas Glynn, Director of Cancer Science and Trends at ACS, stressed the need for “solid, independent data” and stated that while e-cigarettes are not likely to be a “magic bullet” any more than any other quit smoking tool, they “have the potential to make an important contribution to public health by helping some smokers stop.”111 A statement revised in July, 2013 states that ACS “has not taken a position on whether electronic cigarettes should be banned from the US market.”112 The American Lung Association reports that “it is urgent for FDA to begin its regulatory oversight of e-cigarettes, which would include ingredient disclosure by e-cigarette manufacturers to FDA.”113 Finally, in an April 2014 publication, the Tobacco Control and Smoking Cessation Committee of the International Association for the Study of Lung Cancer (IASLC) released a statement on the use of e-cigarettes by cancer patients, stating that cancer patients who used e-cigarettes to quit smoking should be congratulated, but also monitored for adverse effects of e-cigarettes and encouraged to wean themselves off e-cigarettes, perhaps by switching to cessation pharmacotherapy.114
Oncologists should remain vigilant regarding the evolving research and policy issues surrounding e-cigarettes and are directed to sources such as the FDA Center for Tobacco Products’ “This Week in Tobacco” to stay abreast of potential changes in evidence, recommendations, or guidelines. The FDA is expected to release regulatory statements regarding e-cigarettes in the near future. We recognize that the limited available scientific data on safety and efficacy, combined with the current lack of standardization and federal oversight, creates a difficult situation for health care providers. Most evidence suggests that e-cigarettes are less toxic than tobacco cigarettes47–51, but evidence regarding long-term effects of frequent use, and the impact of e-cigarette use on attempts to quit smoking, is still lacking. We advise clinicians to have frank discussions with patients about the known and unknown costs and benefits associated with e-cigarette use. Although use of FDA-approved pharmacotherapies certainly appears to be the more prudent choice at this juncture, we must recognize that many patients report turning to e-cigarettes following dissatisfying experiences with these pharmacotherapies. Unlike nicotine patches, e-cigarettes provide an acute dose of nicotine. Further, use of nicotine gum or lozenge may be difficult for some in this population due to post-surgical oral limitations. The nicotine inhaler is another FDA-approved pharmacotherapy that may prove helpful for some patients, but it provides a relatively low dose of nicotine, which is slowly absorbed through the buccal mucosa without reaching the lung in significant amounts.115,116 As mentioned above, nicotine toxicity is a concern, but given the numerous deleterious effects of continued smoking by head and neck cancer patients, smoking cessation remains the prepotent goal. Therefore, pending the emergence of compelling data, clinicians may consider keeping an open mind regarding patients’ informed decisions to attempt smoking cessation in unconventional ways, including via the short-term use of e-cigarettes.
Vapers often report tobacco cessation as a primary motivation for use of e-cigarettes. As such, it is important that oncologists be aware of these devices. However, unlike the organizational support and guidelines recommending cessation of traditional tobacco products, limited guidelines exist for e-cigarette use in the oncology setting. Therefore, clinicians are advised to follow prior recommendations for primary care physicians to advise patients that the inhalation of the complex mixtures from e-cigarette vapors is not known to be safe, that there is not clear evidence that e-cigarettes help smokers to quit smoking, and that FDA-approved treatments for smoking cessation, proven safe and effective, are available.19 Although research has improved our understanding of e-cigarettes since these initial 2011 recommendations, safety and efficacy remains uncertain. The exponential growth of e-cigarettes in recent years is now producing a similarly exponential growth in research into the area, but at this point there are still more questions than answers. Moving forward, it will be critical to understand if adolescents use e-cigarettes as a gateway to smoking, whether e-cigarette use promotes cessation or maintenance of smoking, and the long-term health consequences of direct or indirect exposure to e-cigarette vapor.
ACKNOWLEDGMENTS
This research was funded by the National Cancer Institute Behavioral Oncology Training Grant (R25CA090314) at Moffitt Cancer Center in Tampa, FL, awarded to Paul Jacobsen, and by grants R01CA134347 and R01CA154596, awarded to Thomas Brandon and Vani Simmons, respectively.
The authors wish to acknowledge the help and support of Asad Shaikh and other staff at the Tobacco Research and Intervention Program.
Thomas Brandon receives research support from Pfizer, Inc. The authors alone are responsible for the content and writing of this paper.
Footnotes
DECLARATION OF INTEREST
The other authors report no conflict of interest.
References
* Additional review article
** Article included in this systematic review
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [PubMed] [Google Scholar]
- 2.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer research. 1988;48(11):3282–3287. [PubMed] [Google Scholar]
- 3.Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27(12):1969–1975. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328(3):159–163. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 7.Kawahara M, Ushijima S, Kamimori T, et al. Second primary tumours in more than 2-year disease-free survivors of small-cell lung cancer in Japan: the role of smoking cessation. Br J Cancer. 1998;78(3):409–412. doi: 10.1038/bjc.1998.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens MH, Gardner JW, Parkin JL, Johnson LP. Head and neck cancer survival and life-style change. Arch Otolaryngol. 1983;109(11):746–749. doi: 10.1001/archotol.1983.00800250040009. [DOI] [PubMed] [Google Scholar]
- 9.Gritz ER, Carr CR, Rapkin DA, Chang C, Beumer J, Ward PH. A smoking cessation intervention for head and neck cancer patients: trial design, patient accrual, and characteristics. Cancer Epidemiol Biomarkers Prev. 1991;1(1):67–73. [PubMed] [Google Scholar]
- 10.Ostroff JS, Jacobsen PB, Moadel AB, et al. Prevalence and predictors of continued tobacco use after treatment of patients with head and neck cancer. Cancer. 1995;75(2):569–576. doi: 10.1002/1097-0142(19950115)75:2<569::aid-cncr2820750221>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Pauly J, Li Q, Barry MB. Tobacco-free electronic cigarettes and cigars deliver nicotine and generate concern. Tob Control. 2007;16(5):357. doi: 10.1136/tc.2006.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu SH, Gamst A, Lee M, Cummins S, Yin L, Zoref L. The Use and Perception of Electronic Cigarettes and Snus among the U.S. Population. PLoS One. 2013;8(10):e79332. doi: 10.1371/journal.pone.0079332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): views of aficionados and clinical/public health perspectives. Int J Clin Pract. 2011;65(10):1037–1042. doi: 10.1111/j.1742-1241.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 14.Saitta D, Ferro GA, Polosa R. Achieving appropriate regulations for electronic cigarettes. Ther Adv Chronic dis. 2014;5(2):50–61. doi: 10.1177/2040622314521271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and Ever Use of Electronic Cigarettes Among U.S. Adults, 2010–2011. Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 17.Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231. doi: 10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: An Internet survey. Drug Alcohol Rev. 2012 doi: 10.1111/j.1465-3362.2012.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardiner P. E-Cigarettes: The Vapor This Time? 2013 This document can be accessed at: http://www.trdrp.org/docs/E-Cigarettes%20The%20Vapor%20This%20Time.pdf. [Google Scholar]
- 20.Kuschner WG, Reddy S, Mehrotra N, Paintal HS. Electronic cigarettes and thirdhand tobacco smoke: two emerging health care challenges for the primary care provider. Int J Gen Med. 2011;4:115–120. doi: 10.2147/IJGM.S16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: a systematic review. Tob Control. 2013 doi: 10.1136/tobaccocontrol-2013-051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liq Chromatogr Relat Technol. 2011;34(14):1442–1458. [Google Scholar]
- 23.Cameron JM, Howell DN, White JR, Andrenyak DM, Layton ME, Roll JM. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control. 2013 doi: 10.1136/tobaccocontrol-2012-050604. [DOI] [PubMed] [Google Scholar]
- 24.Cheah NP, Chong NW, Tan J, Morsed FA, Yee SK. Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective. Tob Control. 2012 doi: 10.1136/tobaccocontrol-2012-050483. [DOI] [PubMed] [Google Scholar]
- 25.Odum LE, O'Dell KA, Schepers JS. Electronic cigarettes: do they have a role in smoking cessation? J Pharm Pract. 2012;25(6):611–614. doi: 10.1177/0897190012451909. [DOI] [PubMed] [Google Scholar]
- 26.Flouris AD, Oikonomou DN. Electronic cigarettes: miracle or menace? BMJ. 2010;340:c311. doi: 10.1136/bmj.c311. [DOI] [PubMed] [Google Scholar]
- 27.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of "vapers" who had achieved complete substitution of smoking. Subst Abuse. 2013;7:139–146. doi: 10.4137/SART.S12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benowitz NL, Goniewicz ML. The Regulatory Challenge of Electronic Cigarettes. JAMA. 2013 doi: 10.1001/jama.2013.109501. [DOI] [PubMed] [Google Scholar]
- 29.Cardinale A, Nastrucci C, Cesario A, Russo P. Nicotine: specific role in angiogenesis, proliferation and apoptosis. Crit Rev Toxicol. 2012;42(1):68–89. doi: 10.3109/10408444.2011.623150. [DOI] [PubMed] [Google Scholar]
- 30.Murray RP, Connett JE, Zapawa LM. Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine Tob Res. 2009;11(9):1076–1082. doi: 10.1093/ntr/ntp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepanov I, Carmella SG, Briggs A, et al. Presence of the carcinogen N'-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer research. 2009;69(21):8236–8240. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stepanov I, Carmella SG, Han S, et al. Evidence for endogenous formation of N'-nitrosonornicotine in some long-term nicotine patch users. Nicotine Tob Res. 2009;11(1):99–105. doi: 10.1093/ntr/ntn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services. Report on Carcinogens. 12th edition. Washington, D.C.: U.S. Department of Health and Human Services Public Health Service National Toxicology Program; 2011. [Google Scholar]
- 34.Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013 doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- 35.Kirschner RI, Gerona R, Jacobitz KL. Nicotine content of liquid for electronic cigarettes. Clin Toxicol. 2013;51(7):684–684. [Google Scholar]
- 36.Eissenberg T. Electronic nicotine delivery devices: Ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19(1):87–88. doi: 10.1136/tc.2009.033498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic "cigarettes": nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine Tob Res. 2010;12(9):905–912. doi: 10.1093/ntr/ntq114. [DOI] [PubMed] [Google Scholar]
- 39.Williams M, Talbot P. Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production. Nicotine Tob Res. 2011;13(12):1276–1283. doi: 10.1093/ntr/ntr164. [DOI] [PubMed] [Google Scholar]
- 40.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15(1):158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 41.Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86(2–3):294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15(1):267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219–1220. doi: 10.1183/09031936.00066011. [DOI] [PubMed] [Google Scholar]
- 44.Flouris AD, Chorti MS, Poulianiti KP, et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol. 2013;25(2):91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- 45.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Public Health. 2013;10(6):2500–2514. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2013 doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohta K, Uchiyama S, Inaba Y, Nakagome H, Kunugita N. Determination of Carbonyl Compounds Generated from the Electronic Cigarette Using Coupled Silica Cartridges Impregnated with Hydroquinone and 2,4-Dinitrophenylhydrazine. Bunseki Kagaku. 2011;60(10):791–797. [Google Scholar]
- 48.Pellegrino RM, Tinghino B, Mangiaracina G, et al. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM) Ann Ig. 2012;24(4):279–288. [PubMed] [Google Scholar]
- 49.Hadwiger ME, Trehy ML, Ye W, Moore T, Allgire J, Westenberger B. Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J Chromatogr A. 2010;1217(48):7547–7555. doi: 10.1016/j.chroma.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Kim HJ, Shin HS. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. Journal of Chromatogr A. 2013;1291:48–55. doi: 10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 51.Ayala G, Smith CA. Chemical analysis of electronic cigarette smoke. Abstr Papers of the ACS. 2010;240 [Google Scholar]
- 52.Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34(4):529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, Farsalinos KE. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25(6):354–361. doi: 10.3109/08958378.2013.793439. [DOI] [PubMed] [Google Scholar]
- 55.Farsalinos KE, Romagna G, Allifranchini E, et al. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013;10(10):5146–5162. doi: 10.3390/ijerph10105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park SJ, Walser TC, Perdomo C, et al. Abstract B16: The effect of e-cigarette exposure on airway epithelial cell gene expression and transformation. Clinical Cancer Research. 2014;20(2 Supplement):B16. Also see www.ascopost.com/ViewNews.aspx?nid=11890. [Google Scholar]
- 57.Van Staden SR, Groenewald M, Engelbrecht R, Becker PJ, Hazelhurst LT. Carboxyhaemoglobin levels, health and lifestyle perceptions in smokers converting from tobacco cigarettes to electronic cigarettes. S Afr Med J. 2013;103(11):865–868. doi: 10.7196/samj.6887. [DOI] [PubMed] [Google Scholar]
- 58.Farsalinos KE, Romagna G. Chronic idiopathic neutrophilia in a smoker, relieved after smoking cessation with the use of electronic cigarette: a case report. Clin Med Insights Case Rep. 2013;6:15. doi: 10.4137/CCRep.S11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 60.Flouris AD, Poulianiti KP, Chorti MS, et al. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol. 2012;50(10):3600–3603. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 61.Kouretas D, Poulianiti K, Chorti M, et al. Effects of electronic cigarette and tobacco cigarette smoking on complete blood count. Toxicology Letters. 2012;211:S64–S64. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 62.Tzatzarakis MN, Tsitoglou KI, Chorti MS, et al. Acute and short term impact of active and passive tobacco and electronic cigarette smoking on inflammatory markers. Toxicology Letters. 2013;221:S86–S86. [Google Scholar]
- 63.Czogala J, Cholewinski M, Kutek A, Zielinska-Danch W. Evaluation of changes in hemodynamic parameters after the use of electronic nicotine delivery systems among regular cigarette smokers. Przegl Lek. 2012;69(10):841–845. [PubMed] [Google Scholar]
- 64.Farsalinos K, Tsiapras D, Kyrzopoulos S, et al. Acute effects of using an electronic nicotine-delivery device (e-cigarette) on myocardial function: comparison with the effects of regular cigarettes. European Heart Journal. 2012;33:203–203. doi: 10.1186/1471-2261-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen I-L. FDA Summary of Adverse Events on Electronic Cigarettes. Nicotine Tob Res. 2013;15(2):615–616. doi: 10.1093/ntr/nts145. [DOI] [PubMed] [Google Scholar]
- 66.Hua M, Alfi M, Talbot P. Health-Related Effects Reported by Electronic Cigarette Users in Online Forums. Journal of Medical Internet Research. 2013;15(4) doi: 10.2196/jmir.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monroy AE, Hommel E, Smith ST, Raji M. Paroxysmal Atrial Fibrillation Following Electronic Cigarette Use in an Elderly Woman. Clinical Geriatrics. 2012;20:28–32. [Google Scholar]
- 68.McCauley L, Markin C, Hosmer D. An Unexpected Consequence of Electronic Cigarette Use. Chest. 2012;141(4):1110–1113. doi: 10.1378/chest.11-1334. [DOI] [PubMed] [Google Scholar]
- 69.Ordonez J, Forrester MB, Kleinschmidt K. Electronic cigarette exposures reported to poison centers. Clin Toxicol. 2013;51(7):685–685. [Google Scholar]
- 70.Cantrell L. E-cigarette exposures-nothing to get choked up about. Clin Toxicol. 2013;51(7):684–685. [Google Scholar]
- 71.Waldman W, Sein Anand J. Cardiac arrest during intoxication with nicotine--a case report and a review of literature. Przegl Lek. 2012;69(8):606–608. [PubMed] [Google Scholar]
- 72.Christensen LB, van'tVeen T, Bang J. Three cases of attempted suicide by ingestion of nicotine liquid used in e-cigarettes. Clin Toxicol. 2013;51(4):290–290. [Google Scholar]
- 73.Thornton S, Oller L, Sawyer T. Fatal intravenous injection of electronic cigarette "eLiquid" solution. Clin Toxicol. 2013;51(7):683–683. [Google Scholar]
- 74.McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24(12):850–857. doi: 10.3109/08958378.2012.724728. [DOI] [PubMed] [Google Scholar]
- 75.Ingebrethsen BJ, Cole SK, Alderman SL. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol. 2012;24(14):976–984. doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- 76.Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25–31. doi: 10.1111/j.1600-0668.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Sumner W, Chen DR. In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine Tob Res. 2013;15(2):501–508. doi: 10.1093/ntr/nts165. [DOI] [PubMed] [Google Scholar]
- 78.Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523–529. doi: 10.1016/j.envpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 79.Caponnetto P, Polosa R, Russo C, Leotta C, Campagna D. Successful smoking cessation with electronic cigarettes in smokers with a documented history of recurring relapses: a case series. J Med Case Rep. 2011;5(1):585. doi: 10.1186/1752-1947-5-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barbeau AM, Burda J, Siegel M. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: a qualitative approach. Addict Sci Clin Pract. 2013;8:5. doi: 10.1186/1940-0640-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McQueen A, Tower S, Sumner W. Interviews with "vapers": implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13(9):860–867. doi: 10.1093/ntr/ntr088. [DOI] [PubMed] [Google Scholar]
- 82.Caponnetto P, Polosa R, Auditore R, Russo C, Campagna D. Smoking cessation with E-cigarettes in smokers with a documented history of depression and recurring relapses. Int J Clin Med. 2011;2:281–284. doi: 10.1186/1752-1947-5-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adkison SE, O'Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44(3):207–215. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pokhrel P, Fagan P, Little MA, Kawamoto CT, Herzog TA. Smokers Who Try E-Cigarettes to Quit Smoking: Findings From a Multiethnic Study in Hawaii. Am J Public Health. 2013 doi: 10.2105/AJPH.2013.301453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631–646. doi: 10.1080/14622200701365327. [DOI] [PubMed] [Google Scholar]
- 86.Jena PK, Kishore J, Jahnavi G. Correlates of digit bias in self-reporting of cigarette per day (CPD) frequency: results from Global Adult Tobacco Survey (GATS), India and its implications. Asian Pac J Cancer Prev. 2013;14(6):3865–3869. doi: 10.7314/apjcp.2013.14.6.3865. [DOI] [PubMed] [Google Scholar]
- 87.Klesges RC, Debon M, Ray JW. Are self-reports of smoking rate biased? Evidence from the Second National Health and Nutrition Examination Survey. J Clin Epidemiol. 1995;48(10):1225–1233. doi: 10.1016/0895-4356(95)00020-5. [DOI] [PubMed] [Google Scholar]
- 88.Dawkins L, Turner J, Roberts A, Soar K. 'Vaping' profiles and preferences: an online survey of electronic cigarette users. Addiction. 2013 doi: 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- 89.Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation tool: results from an online survey. Am J Prev Med. 2011;40(4):472–475. doi: 10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 90.Popova L, Ling PM. Alternative tobacco product use and smoking cessation: A national study. Am J Public Health. 2013:e1–e8. doi: 10.2105/AJPH.2012.301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vickerman KA, Carpenter KM, Altman T, Nash CM, Zbikowski SM. Use of electronic eigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt061. [DOI] [PubMed] [Google Scholar]
- 92.Wagener TL, Meier E, Hale JJ, et al. Pilot Investigation of Changes in Readiness and Confidence to Quit Smoking After E-cigarette Experimentation and 1 Week of Use. Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt138. [DOI] [PubMed] [Google Scholar]
- 93.Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Polosa R, Morjaria JB, Caponnetto P, et al. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med. 2013 doi: 10.1007/s11739-013-0977-z. [DOI] [PubMed] [Google Scholar]
- 95.Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as Tobacco Cigarettes Substitute: A Prospective 12-Month Randomized Control Design Study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446–461. doi: 10.3390/ijerph10020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bullen C, Williman J, Howe C, et al. Study protocol for a randomised controlled trial of electronic cigarettes versus nicotine patch for smoking cessation. BMC Public Health. 2013;13:210. doi: 10.1186/1471-2458-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 99.de Bruin-Visser JC, Ackerstaff AH, Rehorst H, Hilgers FJM. Integration of a smoking cessation program in the treatment protocol for patients with head and neck and lung cancer. Eur Arch ORL. 2012;269:659–665. doi: 10.1007/s00405-011-1673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Henningfield JE, Zaatari GS. Electronic nicotine delivery systems: Emerging science foundation for policy. Tob Control. 2010;19(2):89–90. doi: 10.1136/tc.2009.035279. [DOI] [PubMed] [Google Scholar]
- 101.Etter JF, Bullen C, Flouris AD, Laugesen M, Eissenberg T. Electronic nicotine delivery systems: a research agenda. Tob Control. 2011;20(3):243–248. doi: 10.1136/tc.2010.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, Reece GP. Successes and failures of the teachable moment: smoking cessation in cancer patients. Cancer. 2006;106(1):17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 103.Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169(22):2148–2155. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 105.Quinn VP, Hollis JF, Smith KS, et al. Effectiveness of the 5-As tobacco cessation treatments in nine HMOs. J Gen Intern Med. 2009;24(2):149–154. doi: 10.1007/s11606-008-0865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tong EK, Strouse R, Hall J, Kovac M, Schroeder SA. National survey of U.S. health professionals' smoking prevalence, cessation practices, and beliefs. Nicotine Tob Res. 2010;12(7):724–733. doi: 10.1093/ntr/ntq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Emmons KM, Sprunck-Harrild K, Puleo E, de Moor J. Provider advice about smoking cessation and pharmacotherapy among cancer survivors who smoke: practice guidelines are not translating. Transl Behav Med. 2013;3(2):211–217. doi: 10.1007/s13142-013-0202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Warren GW, Marshall JR, Cummings KM, et al. Addressing tobacco use in patients with cancer: a survey of american society of clinical oncology members. J Oncol Pract. 2013;9(5):258–262. doi: 10.1200/JOP.2013.001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Toll BA, Brandon TH, Gritz ER, et al. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19(8):1941–1948. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosenthal A. 1468. Memorandum in support. 2010 http://acscan.org/ovc_images/file/action/states/ny/A1468_Memo.pdf. [Google Scholar]
- 111.Glynn TJ. Electronic Cigarettes – Boon, Bane, Blessing or Boondoggle? [Accessed March 28, 2014]; http://www.cancer.org/cancer/news/expertvoices/post/2011/05/03/electronic-cigarettes-e28093-boon-bane-blessing-or-boondoggle.aspx. [Google Scholar]
- 112.American Cancer Society. [Accessed March 28, 2014]; http://www.cancer.org/cancer/cancercauses/tobaccocancer/questionsaboutsmokingtobaccoandhealth/questions-about-smoking-tobacco-and-health-e-cigarettes. [Google Scholar]
- 113.American Lung Association. [Accessed March 28, 2014]; http://www.lung.org/stop-smoking/tobacco-control-advocacy/federal/e-cigarettes.html. [Google Scholar]
- 114.Cummings KM, Dresler CM, Field JK, et al. E-cigarettes and cancer patients. J Thorac Oncol. 2014;9(4):438–441. doi: 10.1097/JTO.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rose JE, Turner JE, Murugesan T, Behm FM, Laugesen M. Pulmonary delivery of nicotine pyruvate: sensory and pharmacokinetic characteristics. Exp Clin Psychopharmacol. 2010;18(5):385–394. doi: 10.1037/a0020834. [DOI] [PubMed] [Google Scholar]
- 116.Lunell E, Molander L, Ekberg K, Wahren J. Site of nicotine absorption from a vapour inhaler--comparison with cigarette smoking. Eur J Clin Pharmacol. 2000;55(10):737–741. doi: 10.1007/s002280050007. [DOI] [PubMed] [Google Scholar]