Abstract
In kidney recipients, the immunosuppressant sirolimus has been associated with a decreased incidence of de novo posttransplant malignancies (including prostate cancer). But the effect of sirolimus on the prostate-specific antigen (PSA) blood level, an important prostate cancer screening tool, remains unknown. We studied male kidney recipients >50 years old (transplanted from January 1994 to December 2006) without clinical evidence for prostate cancer. Pre- and posttransplant PSA levels were analyzed for 97 recipients (n = 19 on sirolimus, n = 78 on tacrolimus [control group]). Pretransplant PSA was similar for sirolimus versus tacrolimus recipients (mean, 1.8 versus 1.7 ng/mL, p = 0.89), but posttransplant PSA was significantly lower for recipients on sirolimus (mean, 0.9 versus 1.9 ng/mL, respectively, p < 0.001). The mean difference between pretransplant and posttransplant PSA was −0.9 ng/mL (50.0%, p = 0.006) for the sirolimus group versus +0.2 ng/mL (+11.8%, p = 0.24) for the tacrolimus group. By multivariate analysis, only pretransplant PSA and immunosuppression with sirolimus independently impacted posttransplant PSA. Our data strongly suggest that sirolimus is associated with a significant PSA decrease in kidney recipients. Future studies must investigate the clinical implications of our findings for the use of PSA for prostate cancer screening in male kidney recipients on sirolimus.
Keywords: Gender, kidney transplantation, posttransplant monitoring, posttransplant cancer, posttransplant malignancies, prevention, prostate cancer, recipient age, sirolimus, tacrolimus, tumor
Introduction
Sirolimus, a mammalian target of rapamycin (mTOR) inhibitor, was originally discovered and described because of its antifungal and antineoplastic properties (1,2). The drug was not further developed, however, until its immunosuppressive properties were fully appreciated (3,4). Sirolimus was eventually introduced into routine clinical kidney transplantation over one decade ago, and is currently part of the standard immunosuppressive armamentarium at many transplant centers worldwide (5,6).
As clinical experience with this drug has increased, it became evident that sirolimus may be associated with a reduced incidence of de novo malignancies (including prostate cancer) in renal recipients (7,8). In nontransplant patients, sirolimus is currently tested in preclinical and clinical studies for treatment of solid organ tumors, including prostate cancer. For instance, in a clinical trial that involved androgen-independent prostate cancer patients, sirolimus was associated with tumor regression in approximately 25% of patients—with variable effects on prostate-specific antigen (PSA) levels (9). Similarly, in both in vitro and in vivo models of prostate cancer, sirolimus was associated with decreased PSA production in only some cases (10,11).
While the antineoplastic role of sirolimus and its effect on PSA levels in established prostate cancer are the subject of active investigation and much debate, the impact of sirolimus on PSA levels in male renal recipients without cancer remains unknown. PSA levels are an important clinical screening tool for early detection of prostate cancer in male patients. As the pool of older male renal recipients is expanding and these recipients are exposed to increasing cumulative lifetime doses of immunosuppression, a better understanding of the potential impact of sirolimus on PSA levels in that particular population would be important (12).
The goal of our study was to determine the effect of posttransplant administration of sirolimus on male renal recipients’ PSA levels. Male renal recipients that received tacrolimus for maintenance immunosuppression served as controls.
Materials and Methods
Patients
After approval by the Institutional Review Board (Protocol #200715786-1), we performed a retrospective chart review of all male kidney allograft recipients that had been transplanted at the University of California, Davis, Medical Center, between May 1994 and December 2006. Inclusion criteria for the study were age ≥50 years at evaluation for kidney transplantation; absence of history of, and current clinical evidence for, prostate cancer and a documented pre- and posttransplant PSA level. Patients <50 years were not included in the study as our evaluation protocol for renal transplantation in that age category does not include a serum PSA level determination.
Posttransplant, all renal recipients were initially placed on an immunosuppressive regimen that included tacrolimus, mycophenolate mofetil and, until August 2004, maintenance steroids. Individual recipients were then converted from tacrolimus to sirolimus (Rapamune®), most frequently because of sirolimus’ less nephrotoxic side-effect profile. The therapeutic sirolimus target level range was 3–10 ng/mL.
Data extracted from the patients’ medical records included age, race, body mass index (BMI), ethnicity (African American vs. non-African American), pre- and posttransplant serum PSA and serum creatinine levels.
Statistical analysis
Student’s t-test and univariate linear regression were used to analyze the effect of patient characteristics, age, ethnicity, BMI, serum creatinine level and immunosuppression (tacrolimus vs. sirolimus) on PSA. Variables that reached a significance level of 0.4 on univariate analysis were included in the multivariate regression model. Statistical analyses were performed with STATA statistical software, version 10.0 (StataCorp., College Station, TX). Goodness of fit was verified with analysis of residual plots.
Results
Patient demographics
We identified 19 recipients who had been converted to sirolimus (‘SRL recipients’), and 78 recipients who were immunosuppressed with tacrolimus only (‘Tac recipients’). All SRL recipients had been converted to that drug >6 months posttransplant. Recipient demographics are depicted in Table 1. The mean age of SRL versus Tac recipients was 59.4 versus 59.5 years (p = 0.21). Tac versus SRL recipients were similar with respect to ethnicity (African American, 5.3% vs. 6.4%, p = 0.88), mean BMI (25.3 vs. 25.6, p = 0.88) and mean posttransplant serum creatinine level (1.7 mg/dL vs. 1.6 mg/dL, respectively, p = 0.22).
Table 1.
Patient demographics, mean serum PSA and mean serum creatinine levels by immunosuppressive regimen
| Sirolimus group (n = 19) | Tacrolimus group (n = 78) | p-Value | |
|---|---|---|---|
| African American n (%) | 1 (5.3%) | 5 (6.4%) | 0.88 |
| Mean age (years) (95% CI) | 60.0 (56.2–62.5) | 57.9 (58.2–60.9) | 0.21 |
| Mean BMI (95% CI) | 25.3 (22.7–27.5) | 25.6 (24.3–26.1) | 0.88 |
| Mean posttransplant serum Cr level (mg/dL) (95% CI) | 1.8 (1.5–2.0) | 1.6 (1.5–1.7) | 0.22 |
| Mean pretransplant PSA (ng/mL) (95% CI) | 1.8 (1.3–2.2) | 1.7 (1.5–1.9) | 0.89 |
| Mean posttransplant PSA (ng/mL) (95% CI) | 0.9 (0.7–1.2) | 1.9 (1.7–2.1) | <0.001 |
CI = confidence interval; BMI = body mass index; PSA = prostate-specific antigen.
Mean posttransplant PSA level was significantly lower for recipients converted to sirolimus versus those remaining on tacrolimus
The mean pretransplant PSA for sirolimus versus tacrolimus recipients was 1.8 versus 1.7 ng/mL, respectively (p = 0.89). These PSA levels were obtained at a mean of 7 months pretransplant for SRL recipients versus 8 months for Tac recipients (p = 0.64).
The mean time interval between pre- and posttransplant PSA measurements for SRL recipients was 43.4 months versus 37.9 months for Tac recipients (p = 0.38).
The mean posttransplant PSA for SRL versus Tac recipients was 0.9 versus 1.9 ng/mL, respectively (p < 0.001). For SRL recipients, the mean change in postoperative PSA versus preoperative PSA was −0.9 ng/mL (50.0%, p = 0.006). In contrast, we observed a mean PSA increase of + 0.2 ng/mL (+11.8%, p = 0.24) for Tac recipients.
Figure 1 depicts changes in PSA for SRL and Tac recipients in dropline graphs. All but two SRL recipients showed a decrease in PSA levels following the conversion to sirolimus (17/19, 89.5%). Of these 17 SRL recipients whose PSA level declined, 9 (47.4%) recipients experienced a change in the serum PSA level of ≥1ng/mL (Table 2). In all, 23 (29.5%) of the Tac recipients experienced a decrease in PSA. Among those whose PSA level declined, only four (5.1%) experienced a decrease ≥1ng/mL. Forty-seven (60.3%) Tac recipients experienced an increase in PSA after transplantation (Table 2).
Figure 1. Pre- versus posttransplant PSA levels in individual male renal recipients ≥50 years of age on posttransplant sirolimus versus tacrolimus.
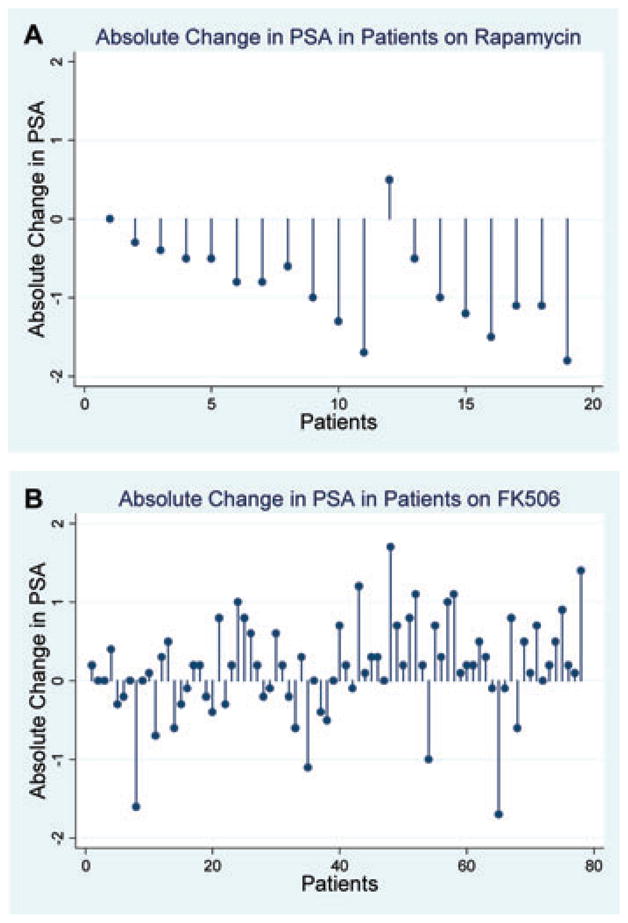
(A) Absolute PSA level changes: pretransplant versus posttransplant (sirolimus group; n = 19). (B) Absolute PSA level changes: pretransplant versus posttransplant (tacrolimus group; n = 78).
Table 2.
Individual pre- versus posttransplant PSA levels by immunosuppressive regimen
| Sirolimus group (n = 19) | Tacrolimus group (n = 78) | |
|---|---|---|
| No change, n (%) | 1 (5.3%) | 8 (10.3%) |
| Increase < 1 ng/mL, n (%) | 1 (5.3%) | 40 (51.3%) |
| Increase ≥ 1 ng/mL, n (%) | 0 (0%) | 7 (9.0%) |
| Decrease < 1 ng/mL, n (%) | 8 (42.1%) | 19 (24.4%) |
| Decrease ≥ 1 ng/mL, n (%) | 9 (47.4%) | 4 (5.1%) |
| Mean change in PSA [ng/mL] (%) | −0.9 (50%)1 | +0.2 (11.76%)2 |
PSA = prostate-specific antigen.
p = 0.006 for pre- versus posttransplant PSA.
p = 0.24 for pre- versus posttransplant PSA.
Pretransplant PSA level and type of immunosuppression are the only significant variables that independently impacted the posttransplant PSA level
For all 97 recipients, we analyzed factors potentially associated with posttransplant PSA. For the variables age, ethnicity, BMI, pretransplant PSA and posttransplant serum creatinine level, we used a univariate linear regression analysis. All tested variables, with the exception of ethnicity and BMI, reached a significance level <0.4 on univariate analyses and were therefore included in the multivariate regression model. By multiple regression analysis, only pretransplant PSA and immunosuppression (tacrolimus as compared to sirolimus) remained significant factors (Table 3).
Table 3.
Postoperative PSA levels: multivariate logistic regression analysis of pre- and postoperative variables
| Odds ratio | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.02 | 1.0–1.04 | 0.09 |
| Pretransplant PSA | 2.18 | 1.90–2.52 | <0.001 |
| Posttransplant creatinine level | 1.17 | 0.92–1.47 | 0.193 |
| Immunosuppression (sirolimus vs. tacrolimus) | 2.62 | 1.96–3.51 | <0.001 |
PSA = prostate-specific antigen; CI = confidence interval.
Discussion
PSA levels are an important screening tool for prostate cancer in the general population (13). The ability to conduct effective screening for prostate cancer is even more relevant for renal recipients for several reasons. First, posttransplant immunosuppression per se has been consistently demonstrated to be associated with an increased incidence of de novo skin and solid organ malignancies—even in the current era (7,8,14). Moreover, the currently increasing transplant rates of presensitized and ABO-incompatible recipients that undergo pretransplant immunomodulation, and often also require more intensive posttransplant immunosuppression, may increase the propensity for, and development of, de novo malignancies in renal recipients even more. Second, due to expanding renal transplant recipient selection criteria, increasingly older patients with advanced chronic kidney disease are accepted onto the transplant waiting list and also receive eventually a renal transplant (12). This is a highly relevant change in practice, as, as for many other solid organ tumors, age is a significant risk factor for prostate cancer, too (15). Currently, nearly two-thirds of all males receiving kidney transplants are older than 50 years, and this proportion is steadily increasing (16). Third, due to advances in immunosuppression, renal graft half-lives have significantly increased over the past decade, thus exposing the recipients of these longer-lasting renal allografts to increasingly longer periods of cumulative lifetime immunosuppressive medication doses (17). In addition, prevention, management and outcomes of posttransplant cardiovascular and infectious complications have all significantly improved over the past two decades and contribute thereby to longer posttransplant recipient survival and subsequently increased cancer risk as well.
As a result, a 3- to 5-fold increased incidence of malignancies has been reported for renal transplant recipients when compared to the general population (14,18). For prostate cancer, some reports have suggested an increased standardized incidence ratio (SIR) (as high as 3.6) in renal transplant recipients, particularly for those on a calcineurin inhibitor (14,18,19). In contrast, a recent large national transplant registry analysis (unadjusted for type of immunosuppression) suggested no such SIR increase (20). In any case, recent studies in renal recipients that developed prostate cancer suggested earlier occurrence and more aggressive disease with a higher rate of locally advanced disease and lymph node invasion (19,21). Effective and early diagnosis of prostate cancer is therefore paramount in renal recipients, both because of the possibly increased incidence and the potentially more aggressive course of the disease (14,18,19,21).
We studied PSA levels in renal recipients on sirolimus. Each patient’s pretransplant PSA level served as an individual control. Because pre- and posttransplant PSA levels are influenced by the degree of overall renal function, and posttransplant PSA levels may be impacted by additional factors (e.g. transplant-related medications and treatment protocol changes over time), we added a control group: kidney recipients on tacrolimus. Of note, our study and control groups were demographically homogeneous. By study design, both cohorts contained only patients with normal PSA levels at the time of their renal transplant evaluation, because otherwise, they would not have gone on to be wait-listed and eventually transplanted. Accordingly, in spite of individually variable amounts of residual native renal function at the time of transplant evaluation, pretransplant PSA levels were not significantly different in both groups, with the median pretransplant PSA levels in both groups being in the ‘low’ range (22).
Although these pretransplant levels were in the low range, we were still able to observe a significant reduction in the serum PSA levels posttransplant in recipients on sirolimus. This reduction in nearly all SRL recipients suggests a strong effect of sirolimus on PSA levels. In contrast, tacrolimus had an unpredictable impact on serum PSA levels, suggesting that calcineurin inhibitors (or at least tacrolimus) may not affect PSA levels in a consistent fashion. The latter finding is also consistent with the results of prior studies that involved recipients on calcineurin inhibitors (23–25). Thus, our results suggest that the decrease in the PSA levels occurred because of the sirolimus administration, and cannot be attributed to the preceding tacrolimus therapy.
Prior studies did demonstrate a 30% decrease in PSA levels within 1 week after renal transplantation (tacrolimus and cyclosporine immunosuppression) (26). This finding was attributed to the rapid clearance of serum PSA by the kidney graft (27). In other studies, though, comparison of pre- versus posttransplant PSA levels obtained at later posttransplant time points showed no difference in serum PSA levels for recipients on cyclosporine (23–25). In our study, we determined posttransplant PSA levels at a time of stable renal allograft function, outside the immediate posttransplant period. It is therefore unlikely that any potential differential changes in early PSA clearance kinetics impacted our study results. Moreover, there was no indirect evidence that posttransplant PSA clearance kinetics were different for the sirolimus versus the tacrolimus group, as mean posttransplant serum creatinine levels were similar for both groups.
Sirolimus binds to an immunophilin, FKBP12, which mediates its immunosuppressive effect (28). The sirolimus-FKBP12 complex then binds to the mTOR, a serine threonine kinase downstream of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway (29). Inhibition of mTOR by sirolimus results in prevention of cellular proliferation, including T-cell growth, thereby resulting in immunosuppression and antitumor activity. It is therefore not surprising that a recent UNOS database review suggested a significantly reduced incidence of de novo malignancies (including prostate cancer) in renal recipients on maintenance immunosuppression with mTOR inhibitors (7).
But the effect of sirolimus on PSA levels (particularly in renal recipients) remains unknown. In in vitro studies with LNCAP prostate cancer cell lines, treatment with sirolimus caused an increase in PSA production (10). Similarly, only 2 of 12 nontransplant patients that were given sirolimus for treatment of prostate cancer in a clinical trial showed a decrease in PSA levels (9). Also, in both in vitro and in vivo models of prostate cancer, sirolimus yielded mixed results with regard to its effect on PSA production (10,11,30). Proliferation of prostate epithelial cells is regulated by the androgen receptor, a ligand-induced nuclear steroid receptor. The androgen receptor regulates the transcription of several target genes, including the PSA gene. Long-term treatment of prostate cancer cell lines in vitro with sirolimus was noted to cause activation of the androgen receptor, resulting in tumor recurrence and an increase in PSA (both in preclinical and clinical studies in nontransplant patients) (31). In contrast to the inconclusive findings reported in the aforementioned in vitro, in vivo and clinical prostate cancer studies, our data suggest that in male renal recipients without established prostate cancer, PSA levels do not increase with sirolimus treatment. Rather, PSA levels decreased nearly uniformly by on average 50% for those on sirolimus. Thus, in men without prostate cancer as compared to men with prostate cancer, sirolimus appears to suppress PSA secretion with greater efficiency and perhaps more predictability. Future research must confirm our findings and determine whether this effect is limited to renal recipients and to those with low PSA levels prior to initiation of sirolimus.
Theoretically, our findings could be explained, at least in part, by the previously reported lower testosterone levels in male kidney and heart recipients on sirolimus (32–35). In these studies, the reduction of the testosterone level by as much as 30% in recipients on sirolimus (vs. a calcineurin inhibitor) draws into question whether the effect of sirolimus on PSA levels is in fact an indirect effect mediated by the reduction of testosterone levels to the lower limits of normal (32–36). As testosterone level measurements are not part of our routine pretransplant evaluation or posttransplant monitoring protocol, we were not able to explore our data for a potential sirolimus-testosterone-PSA interaction. In a systematic review of the literature, however, Shabsigh et al. noted no consistent association between serum testosterone levels and PSA (37). Also, in hypogonadal men, exogenous testosterone resulted in a PSA rise only in men ages 61–80, but not in young men (38). Moreover, the effect of testosterone on PSA levels appears to be transient, as PSA was noted to increase during the first 6 months of testosterone therapy, but not in the subsequent years (39). Clearly, prospective studies in male renal recipients on sirolimus are necessary in order to explore the potential association of sirolimus, testosterone levels and PSA levels in that particular patient population.
What are the clinical implications of our findings? Our results suggest that in male renal recipients >50 years old, one should expect declining PSA levels after posttransplant initiation of sirolimus. The clinical significance of a nondecreasing PSA level in that setting is unclear. Future studies will need to investigate whether this latter patient group might in fact have (pre-)neoplastic prostatic changes and might warrant additional diagnostic attention. Similarly, further research is also necessary to determine the significance of the decreasing PSA levels that occurred in the majority of our patients on sirolimus. It might also theoretically be possible that sirolimus precludes the use of PSA as a screening tool for prostate cancer if this drug were to selectively lower the PSA level, irrespective of the presence or absence of any (pre-)malignant prostatic parenchymal changes. Clearly, additional prospective research is necessary in order to provide the clinician with guidelines for the screening for prostate cancer in male renal recipients on sirolimus. Furthermore, the limited data from our study do not allow us to draw any inferences with regard to the potential role of sirolimus as a chemopreventive agent for neoplastic prostatic disease in renal recipients.
Importantly, our study has several limitations. First, it is retrospective. We attempted to control for potentially confounding variables by performing a multivariate regression analysis. Other variables, however, such as undiagnosed urinary tract infection and occult prostatic disease (including inflammation, infection, hyperplasia or cancer) may still have impacted PSA levels and overall analysis results. Second, among our patients, there was variation in pre- and posttransplant timing of PSA level determinations. Even though on average, timing of PSA sampling was not significantly different between SRL and Tac recipients, it was not prospectively standardized. In future prospective studies, PSA levels should be measured at predetermined pre- and posttransplant time points. Third, endorectal ultrasonography and transrectal prostatic biopsies were not performed to assess whether a decrease in the PSA level was truly associated with any measurable prostatic parenchymal changes that may or may not portent a reduced likelihood of developing prostate cancer. Fourth, as discussed previously, we were not able to assess the impact of sirolimus administration on testosterone levels (and thereby potentially on PSA levels, too). Future prospective studies analyzing testosterone and serum PSA levels in male renal recipients on sirolimus with and without prostate cancer are warranted. Finally, therapeutic sirolimus blood target level ranges have evolved over time at our institution. Also, we did not routinely obtain sirolimus levels in our recipients at the time of their posttransplant PSA level check. Our study does therefore not allow us to draw conclusions regarding a potential linear or other interaction between the sirolimus level and the PSA level.
In conclusion, our data strongly suggest that male renal transplant recipients that receive sirolimus-based immunosuppression nearly uniformly experience a significant decrease in PSA levels. Further studies are necessary to investigate the clinical implications of our findings with regard to the use of PSA levels for prostate cancer screening in male renal recipients, and the absent PSA decrease level that was observed in some recipients.
Acknowledgments
The authors would like to thank Deborah Hoang for preparation of the manuscript.
References
- 1.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic: I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 2.Eng CP, Sehgal SN, Vezina C. Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot. 1984;37:1231–1237. doi: 10.7164/antibiotics.37.1231. [DOI] [PubMed] [Google Scholar]
- 3.Martel RR, Klicius J, Galet S. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can J Physiol Pharmacol. 1977;55:48–51. doi: 10.1139/y77-007. [DOI] [PubMed] [Google Scholar]
- 4.Morris RE, Meiser BM. Identification of a new pharmacologic action for an old compound. Med Sci Res. 1989;17:609–610. [Google Scholar]
- 5.Kahan BD, Podbielski J, Napoli K, Katz SM, Meier-Kriesche HU, Van Buren CT. Immunosuppressive effects and safety of a sirolimus/cyclosporine combination regimen for renal transplantation. Transplantation. 1998;66:1040–1046. doi: 10.1097/00007890-199810270-00013. [DOI] [PubMed] [Google Scholar]
- 6.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67:369–391. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–889. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 8.Campistol JM, Eris J, Oberbauer R, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17:581–589. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 9.Sharef S, Jac J, Khan M, Amato R. Rapamycin for androgen-independent prostate cancer (AIPC). J Clin Oncol; ASCO Annual meeting Proceedings Part I; 2006. p. 14584. [Google Scholar]
- 10.Cinar B, De Benedetti A, Freeman MR. Post-transcriptional regulation of the androgen receptor by Mammalian target of sirolimus. Cancer Res. 2005;65:2547–2553. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of sirolimus inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–2831. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 12.Leichtman AB, Cohen D, Keith D, et al. Kidney and pancreas transplantation in the United States, 1997–2006: The HRSA Breakthrough Collaboratives and the 58 DSA Challenge. Am J Transplant. 2008;8:946–957. doi: 10.1111/j.1600-6143.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- 13.Barry M. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N Engl J Med. 2001;344:1373–1377. doi: 10.1056/NEJM200105033441806. [DOI] [PubMed] [Google Scholar]
- 14.Kessler M, Jay N, Molle R, Guillemin F. Excess risk of cancer in renal transplant patients. Transpl Int. 2006;19:908–914. doi: 10.1111/j.1432-2277.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 15.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 16.Huyghe E, Zairi A, Nohra J, Kamar N, Plante P, Rostaing L. Gonadal impact of target of sirolimus inhibitors (sirolimus and everolimus) in male patients: An overview. Transpl Int. 2007;20:305–311. doi: 10.1111/j.1432-2277.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 17.Cecka JM. The OPTN/UNOS renal transplant registry. In: Cecka JM, Terasaki PI, editors. Clinical transplants, 2005. Los Angeles, CA: UCLA Immunogenetics Center; 2006. pp. 1–16. [Google Scholar]
- 18.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 19.Cormier L, Lechevallier E, Barrou B, et al. Diagnosis and treatment of prostate cancers in renal-transplant recipients. Transplantation. 2003;75:237–239. doi: 10.1097/01.TP.0000041785.38998.6C. [DOI] [PubMed] [Google Scholar]
- 20.Vajdic CM, McDonald SP, McCredie MRE, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 21.Kleinclauss F, Gigante M, Neuzillet Y, et al. Prostate cancer in renal transplant recipients. Nephrol Dial Transplant. 2008;23:2374–2380. doi: 10.1093/ndt/gfn008. [DOI] [PubMed] [Google Scholar]
- 22.Thompson I, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4. 0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 23.Ha R, Jindal RM, Milgrom MM, Leapman SB, Filo RS, Pescovitz MD. Prostate-specific antigen values and their clinical significance in renal transplant recipients. South Med J. 1998;91:847–850. doi: 10.1097/00007611-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Morton JJ, Howe SF, Lowell JA, Stratta RJ, Taylor RJ. Influence of end-stage renal disease and renal transplantation on serum prostate-specific antigen. Br J Urol. 1995;75:498–501. doi: 10.1111/j.1464-410x.1995.tb07272.x. [DOI] [PubMed] [Google Scholar]
- 25.Oberbauer R, Banyai S, Schmidt A, Kornek G, Scheithauer W, Mayer G. Serum tumor markers after renal transplantation. Transplantation. 1996;62:1506–1509. doi: 10.1097/00007890-199611270-00021. [DOI] [PubMed] [Google Scholar]
- 26.Kamali K, Zargar MA. Effects of renal transplantation on serum-free and total PSA levels. Transplant Proc. 2007;39:1027–1028. doi: 10.1016/j.transproceed.2007.03.089. [DOI] [PubMed] [Google Scholar]
- 27.Bruun L, Ekberg H, Bjork T, Lilja H, Hoglund P, Christensson A. Rapid elimination by glomerular filtration of free prostate specific antigen and human kallikrein 2 after renal transplantation. J Urol. 2004;171:1432–1435. doi: 10.1097/01.ju.0000118580.19344.68. [DOI] [PubMed] [Google Scholar]
- 28.Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 29.Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol. 2007;47:443–467. doi: 10.1146/annurev.pharmtox.47.120505.105359. [DOI] [PubMed] [Google Scholar]
- 30.Kaper F, Dornhoefer N, Giaccia AJ. Mutations in the PI3K/PTEN/TSC2 pathway contribute to mammalian target of sirolimus activity and increased translation under hypoxic conditions. Cancer Res. 2006;66:1561–1569. doi: 10.1158/0008-5472.CAN-05-3375. [DOI] [PubMed] [Google Scholar]
- 31.Culig Z, Klocker H, Bartsch G, Steiner H, Hobisch A. Androgen receptors in prostate cancer. J Urol. 2003;170:1363–1369. doi: 10.1097/01.ju.0000075099.20662.7f. [DOI] [PubMed] [Google Scholar]
- 32.Fritsche L, Budde K, Dragun D, Einecke G, Diekmann F, Neumayer HH. Testosterone concentrations and sirolimus in male renal transplant patients. Am J Transplant. 2004;4:130–131. doi: 10.1046/j.1600-6135.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaczmarek I, Groetzner J, Adamidis I, Landwehr P, Mueller M, Vogeser M. Sirolimus impairs gonadal function in heart transplant recipients. Am J Transplant. 2004;4:1084–1088. doi: 10.1111/j.1600-6143.2004.00456.x. [DOI] [PubMed] [Google Scholar]
- 34.Tondolo V, Citterio F, Panocchia N, Nanni G, Castagneto M. Sirolimus impairs improvement of the gonadal function after renal transplantation. Am J Transplant. 2005;5:197. doi: 10.1111/j.1600-6143.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- 35.Tondolo V, Citterio F, Panocchia N, et al. Gonadal function and immunosuppressive therapy after renal transplantation. Transplant Proc. 2005;37:1915–1917. doi: 10.1016/j.transproceed.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Klein EA, Platz EA, Thompson IM. Epidemiology, etiology, and prevention of prostate cancer. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9. Vol. 3. Philadelphia: Saunders Elsevier; 2007. pp. 2854–2873. [Google Scholar]
- 37.Shabsigh R, Crawford ED, Nehra A, Slawin KM. Testosterone therapy in hypogonadal men and potentential prostate cancer risk: A systematic review. Int J Impot Res. 2008 doi: 10.1038/ijir.2008.31. published online: 17 July 2008. [DOI] [PubMed] [Google Scholar]
- 38.Guay AT, Perez JB, Fitaihi WA, Vereb M. Testosterone treatment in hypogonadal men: Prostate-specific antigen level and risk of prostate cancer. Endocr Pract. 2000;6:132–138. doi: 10.4158/EP.6.2.132. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2839–2853. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]


