Abstract
Purpose
Hypoxic conditions cause fibroblasts to differentiate to a smooth muscle cell actin (α -SMA) positive cells (myofibroblasts), which is a hallmark of venous neointimal hyperplasia (VNH) associated with hemodialysis vascular access. The purpose of the present study was to determine if BOEC may reduce fibroblast to myofibroblast conversion under hypoxic conditions and what are the potential mechanisms.
Methods
An experimental model was used in which fibroblasts and BOEC were subjected to hypoxia under contact and transwell conditions to determine if BOEC reduce the conversion of fibroblasts to myofibroblasts under hypoxic conditions. Gene expression under different conditions was performed. In addition, functional assays including cell proliferation and migration were determined.
Results
This study demonstrates that contact needs to occur between BOEC and fibroblast for the reduction in hypoxia driven conversion of fibroblast to α-SMA. This is associated with a decrease in several pro-angiogenic genes including vascular endothelial growth factor (VEGF-A), platelet derived growth factor (PDGF), fibroblast growth factor (FGF-1), and matrix metalloproteinase-2 (MMP-2) in fibroblasts in contact with BOEC when compared to fibroblasts alone. In addition, migration is significantly reduced while proliferation remains unchanged.
Conclusion
This study helps provide rationale for using BOEC delivered to the adventitia of the outflow vein of hemodialysis vascular access to reduce VNH.
Keywords: Blood outgrowth endothelial cells, vascular endothelial growth factor-A, fibroblasts, hypoxia
Introduction
In the next decade, it is estimated that the number of patients with end-stage renal disease (ESRD) who require chronic hemodialysis dialysis will double [1]. Today, in the United States, there are more than 400,000 patients who are on hemodialysis and they require a well functioning hemodialysis vascular access for clearance of uremic toxins. The preferred vascular access is the arteriovenous fistula (AVF) however, its durability is limited by stenosis formation which is in part responsible for poor patency rates of AVFs which are estimated to be 62% at one year [2]. In order to maintain optimal function of hemodialysis AVFs and grafts, more than one billion dollars are spent annually [1]. Therefore, novel therapies that could be developed to reduce AVF stenosis would be of tremendous benefit to patients with ESRD.
Patients with ESRD have an imbalance between endothelial and smooth muscle progenitor cells which is hypothesized to result in the increased incidence of cardiovascular complications accompanied with poor clinical outcomes [3],[4]. Blood outgrowth endothelial cells (BOEC) are a subset of circulating progenitor cells that possess an endothelial cell phenotype and have the ability to reduce vascular injury [5]. A recent study from our laboratory demonstrated that BOEC transplanted to the adventitia of the outflow vein at the time of creation of arteriovenous polytetrafluoroethylene grafts have reduced venous stenosis formation accompanied with a decrease in hypoxia inducible factor-1 alpha (HIF-1α) mean intima to media ratio when compared to controls [6]. Moreover, recent studies from our laboratory demonstrate that hypoxia mediated conversion of fibroblast to myofibroblast may play a role in VNH and reduced mean HIF-1α [7].
We demonstrated that inhibiting VEGF-A using a short hairpin RNA inhibitor delivered to the adventitia of the outflow vein in a murine model of chronic kidney disease with an AVF results in positive vascular remodeling [8]. In this study, we hypothesized that the vasa vasorum of the outflow vein of the AVF may become disrupted after surgical placement, which contributes to local hypoxia within the adventitia. Moreover, the localized hypoxia activates fibroblasts that convert to myofibroblasts and subsequently triggers a cascade of events resulting in VNH. Finally, reducing mRNA for VEGF-A in fibroblasts which are subjected to hypoxia results in a reduction of α-SMA positive cells with resultant decrease in MMP-2, cellular proliferation and migration.
In the present manuscript, we hypothesized that BOEC may reduce the differentiation of fibroblast to myofibroblasts under hypoxic conditions. Experiments outlined in the present manuscript were performed to determine the mechanism using a contact and transwell experimental design. We observed that a reduction in fibroblast to myofibroblast conversion occurs which is accompanied with and reduction is several pro-angiogenic genes including VEGF-A, fibroblast growth factor-1 (FGF-1), platelet derived growth factor (PDGF), and matrix metalloproteinase -2 (MMP-2). Each of these cytokines has been found to be associated with experimental animal models of hemodialysis vascular access failure or hemodialysis vascular access specimens removed from patients [9-14]. We speculate that adventitial delivery of BOEC to the AVF or graft at the time of creation could reduce VNH thus offering a novel therapeutic option.
Methods and materials
Isolation of BOEC
Porcine BOEC were isolated according to the protocol used by our laboratory and described previously [15]. Briefly, under aseptic conditions, we removed one hundredmL of blood from the femoral artery and expanded it ex vivo as previously described, with some modifications [16]. Peripheral blood mononuclear cells were isolated using density gradient centrifugation with Ficoll-Paque Plus (Amersham Biosciences Corporation, Piscataway, NJ). The cell culture was maintained at 37 °C, 5% CO2, and 20% O2. After 2 days, the media was changed and the non-adherent cells were removed. After satisfactory initial growth, the media was subsequently changed every 2-3 days. Typically, colonies of cells appeared that exhibited typical endothelial morphology including a cobblestone pattern occurred between day fifteen to eighteen. Colonies were passaged and expanded for an additional two weeks and the adherent cells were harvested by trypsinization. The late outgrowth BOEC were characterized by a number of methods including staining and morphology as described previously [6]. All BOEC used in the experiments were from early passages with a maximal passage of 5.
Isolation of fibroblasts
Primary fibroblasts were isolated from the jugular vein of pigs as described elsewhere [17].
Transwell culture and contact experiment of BOEC and Fibroblast
Fibroblasts and BOEC cells were isolated from pig as described above and used for both transwell and contact experiment. For both experiments, 400,000 BOEC and 100,000 fibroblasts were used. Transwell experiments were performed with BOEC alone in the upper chamber and fibroblast alone in the lower chamber. Contact experiments were performed with BOEC's in contact with fibroblasts or BOEC and fibroblasts alone. Transwell experiments allow for passage of secreted molecules and do not allow for contact to occur. The cells were made hypoxic as described previously using a hypoxia chamber at 1% O2 [7,8,18]. BOEC's were cultured in EBM basal medium without growth factors (Lonza Cologne Gmbh, Walkersville, Maryland) and fibroblasts were cultured with DMEM (Invitrogen, 5% FBS, Low glucose). In addition, we performed separate experiments using human umbilical vein endothelial cells (400,000) alone or in contact with pig fibroblasts (100,000) as outlined above. For the contact experiments, at each time point, the cells were washed with PBS and trypsinized. The cell suspension was incubated with pig CD31 (Abcam, USA) in PBS with 1% BSA for 30 min at 4°C. The cell suspension was incubated with an anti-mouse magnetic antibody (Milteyni Biotech, USA) in PBS with 1%BSA CD31-positive cell population and BOEC cells were isolated using magnetic cell sorting and the flow through, fibroblasts, was collected for further analysis.
Western Blot of α-SMA
We assessed the differentiation of fibroblasts to myofibroblasts by performing Western blot analysis for α-SMA. The cultured cells were processed and detected using rabbit polyclonal antibody as described previously [11].
Protein expression using proteomic array
We assessed the potential mechanism of the proteins, which was responsible for decreasing α-SMA expression in the fibroblasts in contact with BOEC when compared to fibroblasts alone under hypoxia. We used a human angiogenic proteomic array (R & D Biosystems, Inc., Minneapolis, MN, Cat number: ARY007) in both the cellular and conditioned media according to manufacturer's directions.
RNA isolation
The tissue was stored in RNA stabilizing reagent (Qiagen, Gaithersburg, MD) as per the manufactures guidelines. To isolate the RNA, the specimens were homogenized and total RNA isolated using RNeasy mini kit (Qiagen) [19,20].
Real time polymerase chain reaction (RT-PCR) analysis
Expression for the gene of interest was determined using RT-PCR analysis as described previously [19]. Please see supplemental methods for full details.
Thymidine Incorporation Assay
BOEC alone, fibroblast alone, fibroblasts in contact with BOEC were seeded in 24-well plates and cultured for 24h to 72h in DMEM medium. After 20h, 1 mCi of (3H) thymidine was added to each well and incubated for 4h. The cells were washed with chilled PBS, fixed with 100% cold methanol and collected for measurement of trichloroacetic acidprecipitable for radioactivity. Experiments were repeated at least three times for each time point.
Gelatin Zymography
Zymography was performed on concentrated conditioned media from the cultured cells of their respective time point as described previously (Misra et al., 2010).
Cell Migration Assay
The fibroblasts were loaded with calcein M dye with 0.1% pluronic acid to observe differential migration of fibroblast. The BOEC (∼400,000) and pig vein calcein M loaded fibroblast (∼100,000) were mixed together in serum free medium and loaded onto upper chamber of fluoroblock 8-micron trans-wells (Qiagen, USA), pre-coated with low growth factor matrigel. The complete media was supplemented under the transwell and incubated for 6h at 37°C. After 6h, transwells were washed with PBS and cell number was analyzed by relative fluorescence units using a microplate reader.
Statistical methods
Data are expressed as mean ± SEM. Multiple comparisons were performed with twoway ANOVA followed by Student t-test with post hoc Bonferroni's correction. Significant difference from control value was indicated by *P < 0.05, **P < 0.01, #P < 0.001, or ##P < 0.0001. JMP version 9 (SAS Institute Inc., Cary, N.C.) was used for statistical analyses.
Results
Alpha-smooth muscle actin is decreased in fibroblasts in contact with BOEC when compared to fibroblasts alone under hypoxic conditions
We wanted to determine the mechanism by which BOEC reduce the conversion of fibroblast to myofibroblast (α-SMA positive cells) under hypoxic conditions. This was determined by performing a transwell and a contact experiment to determine the changes in α-SMA using Western blot analysis and confocal microscopy (Fig. 1). Transwell and contact experiments were performed in order to determine if the mechanism by which BOEC decreased the conversion of fibroblasts to myofibroblast was via a paracrine effect. There was a significant decrease in the average α-SMA expression of fibroblasts when in contact with BOEC, compared to fibroblasts alone throughout all time points (24h: average reduction: 28%, fibroblast alone vs. fibroblast cultured with BOEC; 48h: average reduction: 91%, fibroblast alone vs. fibroblast cultured with BOEC; 72h: average reduction: 93%, fibroblast alone vs. fibroblast cultured with BOEC; Fig. 1A). However, in the transwell experiments, there was no change in the mean α-SMA expression in fibroblasts with BOEC when compared to fibroblasts alone.
Figure 1.
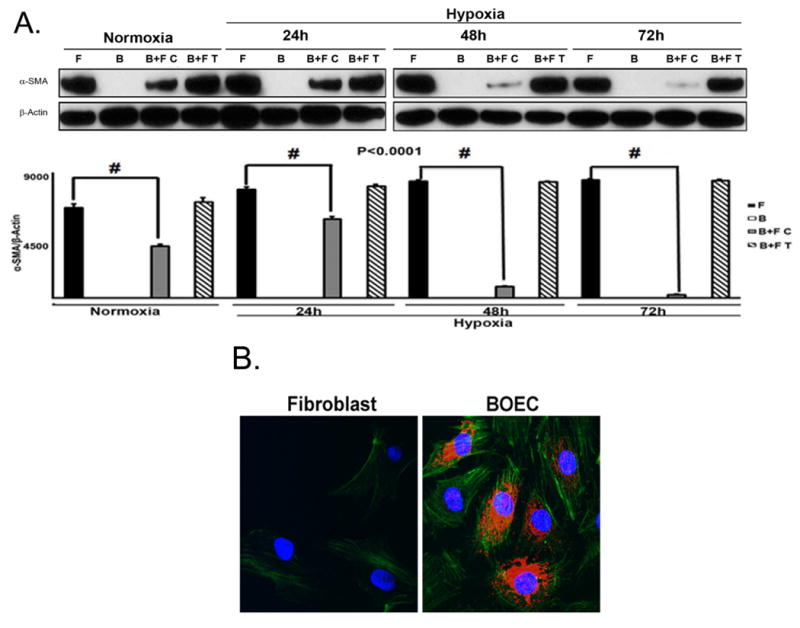
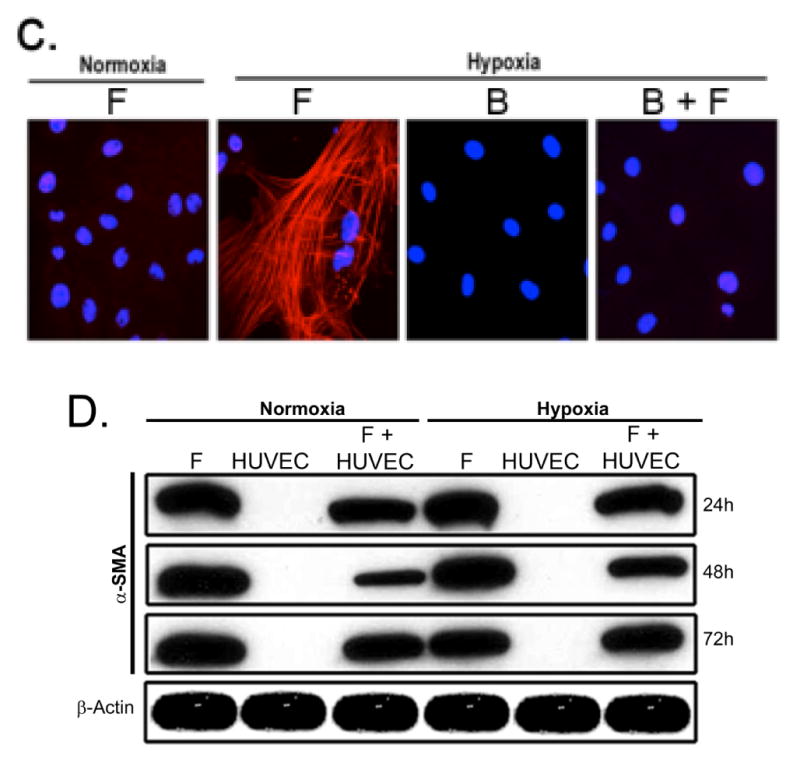
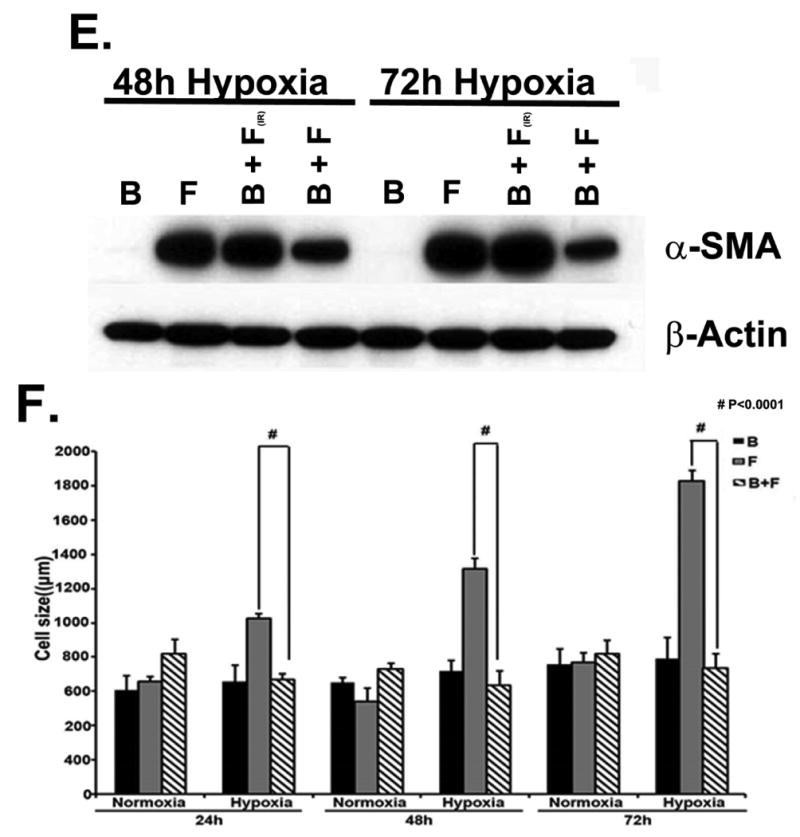
A upper panel is fibroblast in contact with BOEC under hypoxic conditions have reduced α-SMA expression when compared to fibroblast alone. A lower panel is pooled Western blot data for contact (C) and transwell (T) experiments at 24, 48, and 72 hours of hypoxia for fibroblast alone (F), BOEC alone (B), BOEC plus fibroblast in contact (B + F C), and BOEC plus fibroblast Transwell (B + F T) (P<0.0001). B is confocal microscopy showing that α-SMA expression (red). BOEC are phallodin positive (green) and von Willebrand factor (red). C is confocal microscopy of fibroblast alone versus BOEC in contact with fibroblast and exposed to hypoxia at 24-72 hours. Red is α-SMA. D is Western blot of fibroblast, HUVEC, and HUVEC in contact with fibroblast at 24-72 hours of normoxia and hypoxia. E is Western blot of BOEC (B), fibroblast (F), fibroblast in contact with irradiated BOEC (B + F(IR)), and fibroblasts in contact with BOEC (F + B) at 48 and 72 hours of hypoxia. F is average cell size of fibroblast alone (F), BOEC (B), and fibroblast in contact with BOEC (B + F) at different lengths of hypoxia. Two-way ANOVA followed by Student t-test with post hoc Bonferroni's correction was performed. Significant difference from control value was indicated by #P < 0.0001.
Normally, fibroblasts are α-SMA negative whereas BOEC are Von Willebrand (vWF) positive (Fig. 1B). Using confocal microscopy, we observed a time-dependent hypoxia induced decrease in average α-SMA expression in fibroblasts in contact with BOEC when compared to fibroblasts alone (Fig. 1C). We also stained fibroblasts in contact with BOEC and alone for α-SMA and phylloidin under hypoxic conditions and demonstrated a reduction in α-SMA/phylloidin (+) expression in fibroblasts in contact with BOEC when compared to fibroblasts alone (Suppl. Fig. 1). We then determined if other endothelial cells such as human umbilical vein endothelial cells (HUVEC) would have the same effect. Similarly, HUVEC cells were in contact with pig fibroblasts and determined the α-SMA expression by comparison to fibroblasts alone under hypoxic conditions. Under these conditions, there was no difference in the α-SMA expression in pig fibroblasts in contact with HUVEC when compared to fibroblasts alone by both transwell and in contact with conditions (Fig. 1D). In order to determine if the effect was perpetuated by viable BOEC's, we irradiated BOEC and then cultured them in contact with fibroblasts and compared them to non-irradiated BOEC in contact with fibroblasts, fibroblasts alone, and BOEC alone. They were subjected to 48 and 72 hours of hypoxic conditions. We observed a decrease in average α-SMA expression under hypoxic conditions in fibroblasts in contact with non-irradiated BOEC when compared to fibroblasts in contact irradiated BOEC (Fig. 1E).
Cell size is reduced in fibroblasts in contact with BOEC when compared to fibroblasts alone
The size of the fibroblast, which was in contact with BOEC when compared to fibroblast alone at different times of hypoxic conditions, was determined. This demonstrated a significant decrease in the average size of the fibroblast in contact with BOEC when compared to fibroblast alone (24h: average reduction: 28%, fibroblast alone vs. fibroblast in contact with BOEC, respectively, P<0.0001; 48h: average reduction: 91%, fibroblast alone vs. fibroblast in contact with BOEC, respectively, P<0.0001; 72h: average reduction: 93%, fibroblast alone vs. fibroblast in contact with BOEC, respectively, P<0.0001; Fig. 1F).
Identification of potential mechanism using a proteomic array
In order to determine the mechanism that maybe responsible for the decreased expression of α-SMA expression, an angiogenic protein array was used along with Ingenuity System software for pathway analysis. This demonstrated that there was a decrease in several proteins including vascular endothelial growth factor-A (VEGF-A), fibroblast growth factor-1 (FGF-1), platelet derived growth factor (PDGF), matrix metalloproteinase-2 (MMP-2), and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1).
Gene expression of VEGF-A, PDGF, FGF-1, MMP-2, and TIMP-1 are decreased in fibroblasts in contact with BOEC when compared to fibroblasts alone under hypoxic conditions
Next, we determined the gene expression of VEGF-A (Fig. 2A), PDGF (Fig. 2B), FGF-1 (Fig. 2C), MMP-2 (Fig. 2D), and TIMP-1 (Fig. 2E) in fibroblasts in contact with BOEC when compared to fibroblasts alone under hypoxic conditions using RT-PCR analysis. All of these genes have been implicated in VNH formation associated with hemodialysis vascular access failure. We observed that in fibroblasts in contact with BOEC (B + F) when compared to fibroblasts alone (F), there was a significant decrease in the mean VEGF-A expression at 24 (average reduction: 61%, P<0.0001) and 72 hours (average reduction: 72%, P<0.0001). In addition, we observed a significant decrease in the average gene expression of PDGF, FGF-1, MMP-2, and TIMP-1 in fibroblasts in contact with BOEC when compared to fibroblasts alone at 24 hours (PDGF: average reduction: 95%, P<0.0001; FGF-1: average reduction: 93%, P<0.0001; MMP-2: average reduction: 95%, P<0.0001; and TIMP-1: average reduction: 34%, P<0.01) and 72 hours (PDGF: average reduction: 98%, P<0.0001; FGF-1: average reduction: 87%, P<0.0001; MMP-2: average reduction: 90%, P<0.0001; TIMP-1: and average reduction: 21%, P<0.001).
Figure 2.
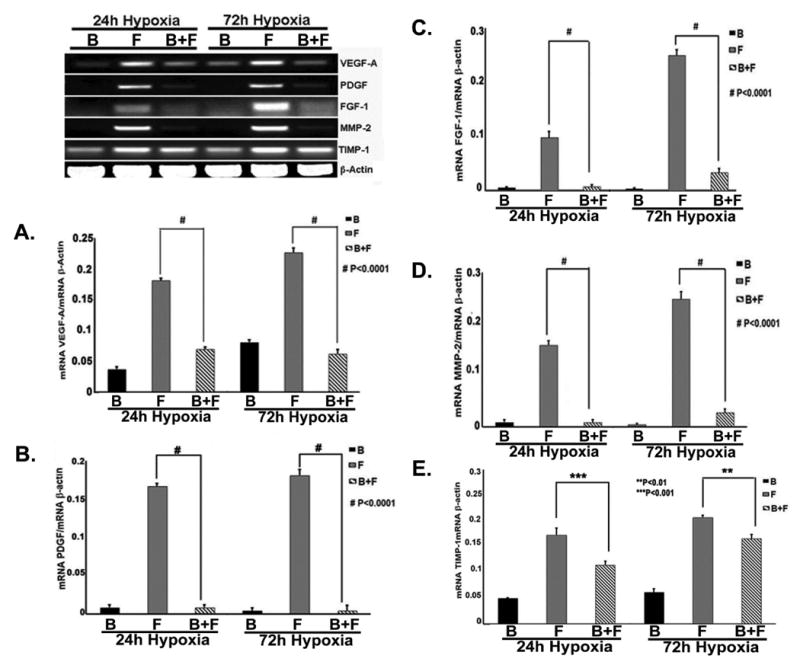
Gene expression of VEGF-A (A), PDGF (B), FGF-1 (C), MMP-2 (D), and TIMP-1 (E) in fibroblast in contact with BOEC (B + F) when compared to fibroblast alone (F) and BOEC alone (B) under hypoxic conditions. Gene expression of VEGF-A (A), PDGF (B), FGF-1 (C), MMP-2 (D), and TIMP-1 (E) is decreased in fibroblast in contact with to fibroblast alone at 24 and 72 hours of hypoxia. Two-way ANOVA followed by Student t-test with post hoc Bonferroni's correction was performed. Significant difference from control value was indicated by **P<0.01, ***P<0.001, or #P < 0.0001.
Migration is decreased in fibroblasts in contact with BOEC when compared to Fibroblasts under hypoxic conditions
Since several genes implicated in cellular migration were reduced by RT-PCR analysis, we hypothesized that there would be a decrease in cellular migration in fibroblasts in contact with BOEC when compared to fibroblasts alone. Cellular migration was determined in fibroblasts in contact with BOEC and compared to fibroblasts alone. We observed a significant decrease in the migration of fibroblasts in contact with BOEC when compared to fibroblasts alone at both 6 and 24 hours of hypoxia (6h: average reduction: 28%, fibroblast alone vs. fibroblast in contact with BOEC, respectively, P<0.0001; 24h: average reduction: 91%, fibroblast alone vs. fibroblast in contact with BOEC, respectively, P<0.0001; Fig. 3).
Figure 3.
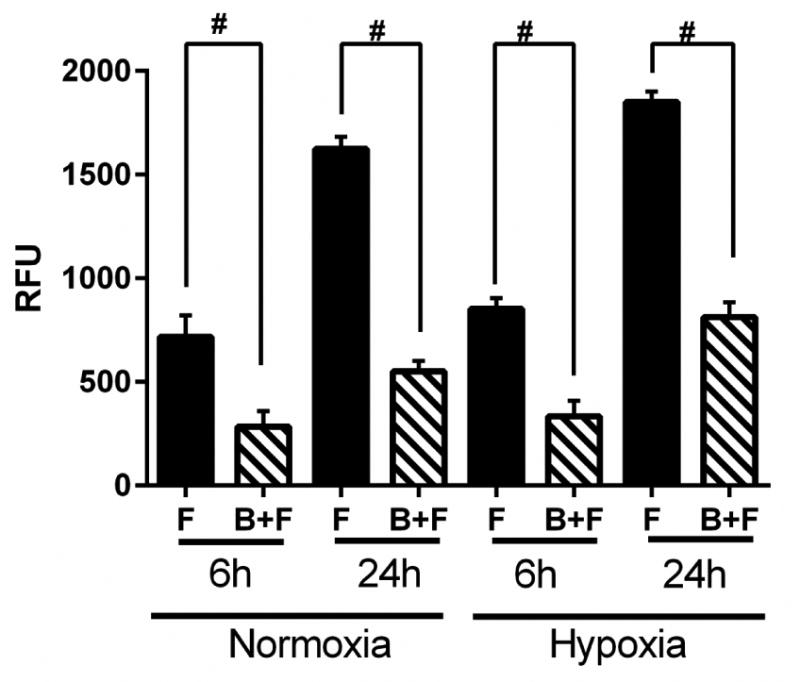
Differential migration of fibroblasts in fibroblasts in contact with BOEC (B + F), fibroblasts (F) alone, and BOEC (B) alone during different lengths of hypoxia. Two-way ANOVA followed by Student t-test with post hoc Bonferroni's correction was performed. Significant difference from control value was indicated by #P < 0.0001.
Proliferation is not decreased in fibroblasts in contact with BOEC when compared to Fibroblasts under hypoxic conditions
Finally, we determined cellular proliferation changes in fibroblasts in contact with BOEC when compared to fibroblasts alone. We observed a significant increase in proliferation under hypoxia of fibroblasts in contact with BOEC when compared to fibroblasts alone at 24 to 72 hours of hypoxia (P<0.0001, Fig. 4).
Figure 4.
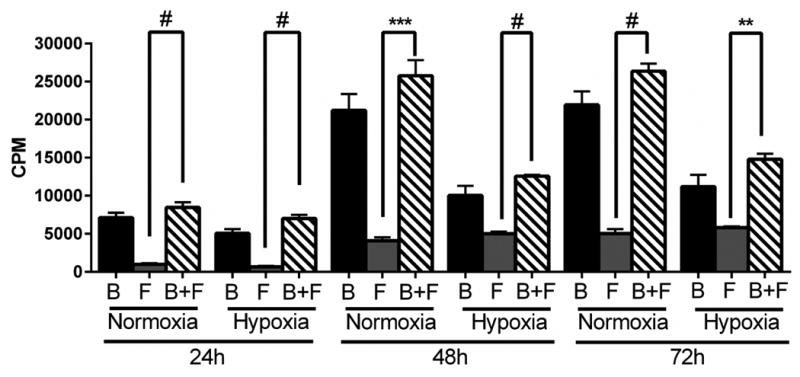
Proliferation of fibroblasts using by [3H]thymidine incorporation in contact with BOEC (B + F), fibroblasts alone (F), and BOEC alone (B) during different lengths of hypoxia. Two-way ANOVA followed by Student t-test with post hoc Bonferroni's correction was performed. Significant difference from control value was indicated by **P<0.01, ***P<0.0001, and #P < 0.0001.
MMP-9 activity is increased in fibroblast in contact with BOEC compared to fibroblast alone during hypoxic conditions
In our experiments with adventitial transplantation of BOEC to AV hemodialysis grafts, a significant increase in MMP-9 activity by zymography was observed and MMP-9 has been shown to cause an increase in cellular proliferation. We determined the MMP-9 expression using zymography in fibroblasts in contact with BOEC and compared to fibroblast alone. We observed an increase in both pro-MMP-9 and active MMP-9 in fibroblasts in contact with BOEC when compared to fibroblast alone (Fig. 5). In addition, no significant change in MMP-2 was observed for the same experiments (Supp. Fig. 2). These results suggest that the increase in MMP-9 activity may be responsible for the increase in proliferation.
Figure 5.
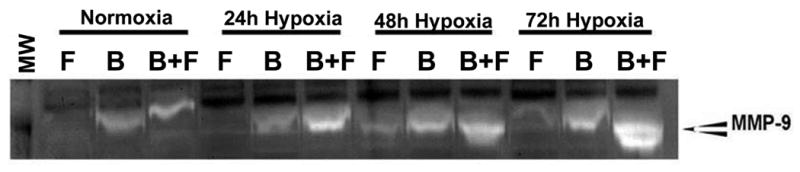
Zymographic analysis of BOEC alone (B), fibroblasts alone (F), and fibroblast in contact with BOEC (B + F) during different lengths of hypoxia. Changes in pro MMP-9 and active MMP-9 expression in fibroblasts cultured with BOEC (B + F) compared to fibroblasts alone (F) under hypoxic conditions.
Discussion
In the present paper, we determined the mechanism through which BOEC may decrease the conversion of fibroblasts to myofibroblasts mediated under hypoxic conditions. Previously, we have described that BOEC transplantation to the adventitia of the outflow vein of porcine AV hemodialysis grafts results in reduction of VNH through a reduction in HIF-1α expression [6]. We wanted to determine how BOEC reduce VNH and we hypothesized that they influence the conversion of fibroblast to myofibroblast through either a paracrine or autocrine mechanism. The present paper demonstrates that contact needs to occur between the BOEC and fibroblast. Under hypoxic conditions, when fibroblasts are in contact with BOEC, there is a significant reduction in the cell migration, cell size, and a decrease in the mean α-SMA expression. In addition, we observed a significant decrease in the expression of several genes including VEGFA, PDGF, FGF-1, MMP-2, and TIMP-1. These findings have many implications for hemodialysis vascular failure as all of these cytokines including VEGF-A, PDGF, FGF-1, MMP-2, and TIMP-1 have been increased in experimental animal models of hemodialysis vascular access failure or specimens removed from patients [9-12].
Previous research has shown that hypoxia can cause an increase in conversion of fibroblast to myofibroblast [7]. Our laboratory has shown that there is an increase in hypoxia inducible factor-1 alpha (HIF-1α) in specimens removed from experimental animal models and clinical specimens removed from patients with failed hemodialysis vascular access [12,21]. Furthermore, adventitial fibroblasts have been shown to contribute to smooth muscle cells comprising VNH associated with hemodialysis graft failure [13,22,23]. We hypothesize that disruption of the vasa vasorum at the time of vascular access placement can cause an increase in local vessel hypoxia that leads to activation of adventitial fibroblast that convert to myofibroblasts. Furthermore, a study performed by our laboratory demonstrated that adventitial transplantation of BOEC to the outflow vein causes a decrease in HIF-1α in the transplanted BOEC vessels when compared to controls [15].
We assessed the mechanism by which BOEC might influence conversion of fibroblast to myofibroblast using a contact experiment between the BOEC and fibroblasts and a transwell experiment. We observed in the transwell experiment, there was no reduction in the conversion of fibroblast to myofibroblast when cultured with BOEC compared to fibroblasts under hypoxic conditions. However, when BOEC were in contact with fibroblast and then the BOEC were separated using magnetic beads, there was a significant reduction in α-SMA expression in fibroblasts in contact with BOEC compared to fibroblasts alone. This experiment implies that contact is needed to occur between the fibroblast and the BOEC for the reduction in α-SMA to occur. Furthermore, we demonstrated that the same results would not occur if fibroblasts were in contact with with HUVEC or irradiated BOEC cells. These observations are consistent with experimental animal models of wound-healing model. The authors found that the BOEC needed to be in contact with the fibroblasts for the beneficial effects for wound healing to occur [24].
In order to determine a potential mechanism responsible for these observations, we performed a protein array that identified several proteins that were decreased including VEGF-A, PDGF, FGF-1, and MMP-2. Interestingly, each of these proteins has been found to be elevated and associated with VNH [9-13,25,26]. We determined the messenger RNA levels for each of these genes using RT-PCR and found that they were all reduced in fibroblast in contact with BOEC compared to fibroblasts alone. These results are consistent with other experiments, which have demonstrated that BOEC under hypoxic conditions will secrete pro-angiogenic genes that have a paracrine effect. The present study demonstrates that using this in vitro model that the there is a decrease pro-angiogenic gene expression in other cells notably fibroblast which is mediated through contact [27]. This decrease in pro-angiogenic genes is most likely responsible for the decrease in α-SMA expression. We have recently shown that silencing VEGF-A in fibroblast and subjecting them to hypoxia decreased α-SMA expression compared to controls [8]. This decrease in α-SMA is consistent with other studies, which have used experimental models of skin healing [24].
Finally, we determined if proliferation and migration of fibroblasts in contact with BOEC was reduced. We observed that migration was significantly reduced in fibroblasts in contact with BOEC when compared to fibroblasts alone. These results are consistent with observations where VEGF-A reduction has been shown to result in a decrease in proliferation [8] . However, proliferation was increased which most likely reflects the increase in MMP-9 activity, which was observed. These results are consistent with our in vivo experiments where we observed increased MMP-9 activity in BOEC transplanted vessels compared to controls [28].
There are several limitations to the present study. One, the results from the research needs to be validated in a human cell line model with BOEC from patients, both healthy and with chronic kidney disease. This would address the benefits of BOEC as cell therapy in patients. In addition, the in vitro experiments do not simulate the in vivo experiments and these need to be conducted with the cells using a xenotransplant model. Finally, the durability of the observations needs to be determined in chronic animals.
In summary, our results in this study indicate that BOEC when in contact with fibroblasts reduce conversion of fibroblasts to myofibroblasts, which is mediated in part through a reduction of several pro-angiogenic genes including VEGF-A, PDGF, FGF-1, and MMP-2 with a reduction in migration. There results have implications for hemodialysis vascular access failure as many of the cytokines, which were reduced, are hypothesized to cause VNH.
Supplementary Material
Supplemental Figure 1: Confocal microscopy of fibroblast alone (Top) compared to BOEC in contact with fibroblast (bottom) exposed to hypoxia from 24 to 72 hours. Red is α-SMA, green is phalloidin and yellow is the overlay.
Figure 6.
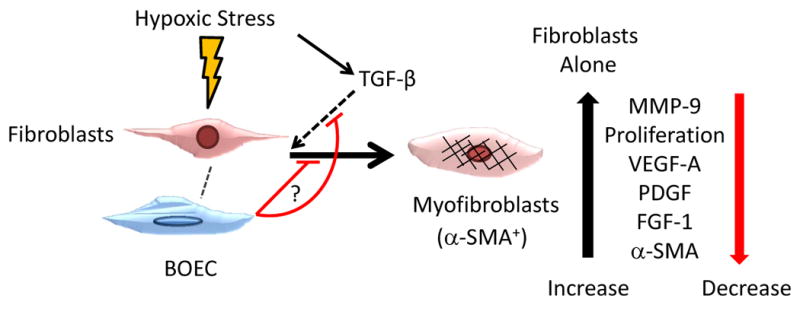
Schematic of proposed interaction by BOEC under hypoxic conditions changes.
Table 1. PCR primers used in current study.
| Gene | Sequence | Amplicon Length | Cycles |
|---|---|---|---|
|
| |||
| VEGF-A | 5′ –ACCATGCCAAGTGGTCCCAGGC– 3′ (sense) | 250 | 35 |
| 5′ –TCCAGACCTTCGTCGTTGCAGC– 3′ (antisense) | |||
|
| |||
| PDGF | 5′ –AGGGCTGTTCCGACAAGCGC– 3′ (sense) | 259 | 35 |
| 5′ –TGGCAGGTCCTCTCGGGAGC– 3′ (antisense) | |||
|
| |||
| FGF-1 | 5′ –GCCATGGCTGAAGGCGAAATCACA– 3′ (sense) | 250 | 35 |
| 5′ –CACCTCCCCCACGCTTTCCG– 3′ (antisense) | |||
|
| |||
| MMP-2 | 5′ –GATGTCGCCCCCAAAACGGAC– 3′ (sense) | 852 | 35 |
| 5′ –GTAGTCCTCGGTGGTGCCGC– 3′(antisense) | |||
|
| |||
| TIMP-1 | 5′ –CCTGGCCTCTGGCATCCTGC– 3′ (sense) | 162 | 35 |
| 5′ –CGCTGGTACGAGGCGGTCTG– 3′(antisense) | |||
Acknowledgments
This work was funded by a HL098967 (SM) from the National Heart, Lung, And Blood Institute.
Footnotes
Disclosures none
References
- 1.Collins AJ, Kasiske B, Herzog C, Chen SC, Everson S, Constantini E, Grimm R, McBean M, Xue J, Chavers B, Matas A, Manning W, Louis T, Pan W, Liu J, Li S, Roberts T, Dalleska F, Snyder J, Ebben J, Frazier E, Sheets D, Johnson R, Dunning S, Berrini D, Guo H, Solid C, Arko C, Daniels F, Wang X, Forrest B, Gilbertson D, St Peter W, Frederick P, Eggers P, Agodoa L. Excerpts from the united states renal data system 2003 annual data report: Atlas of end-stage renal disease in the united states. Am J Kidney Dis. 2003;42:A5–7. [PubMed] [Google Scholar]
- 2.Rooijens PPGM, Tordoir JHM, Stijnen T, Burgmans JPJ, Smet de AAEA, Yo TI. Radiocephalic wrist arteriovenous fistula for hemodialysis: Meta-analysis indicates a high primary failure rate. European Journal of Vascular and Endovascular Surgery. 2004;28:583–589. doi: 10.1016/j.ejvs.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Westerweel PE, Hoefer IE, Blankestijn PJ, de Bree P, Groeneveld D, van Oostrom O, Braam B, Koomans HA, Verhaar MC. End-stage renal disease causes an imbalance between endothelial and smooth muscle progenitor cells. Am J Physiol Renal Physiol. 2007;292:F1132–1140. doi: 10.1152/ajprenal.00163.2006. [DOI] [PubMed] [Google Scholar]
- 4.Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. The Lancet. 2000;356:147–152. doi: 10.1016/S0140-6736(00)02456-9. [DOI] [PubMed] [Google Scholar]
- 5.Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 6.Hughes D, Fu AA, Puggioni A, Glockner JF, Anwer B, McGuire AM, Mukhopadhyay D, Misra S. Adventitial transplantation of blood outgrowth endothelial cells in porcine haemodialysis grafts alleviates hypoxia and decreases neointimal proliferation through a matrix metalloproteinase-9-mediated pathway--a pilot study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:85–96. doi: 10.1093/ndt/gfn433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misra S, Fu AA, Misra KD, Shergill UM, Leof EB, Mukhopadhyay D. Hypoxiainduced phenotypic switch of fibroblasts to myofibroblasts through a matrix metalloproteinase 2/tissue inhibitor of metalloproteinase-mediated pathway: Implications for venous neointimal hyperplasia in hemodialysis access. J Vasc Interv Radiol. 2010;21:896–902. doi: 10.1016/j.jvir.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang B, Janardhanan R, Vohra P, Greene EL, Bhattacharya S, Withers S, Roy B, Nieves Torres E, Mandrekar J, Leof EB, Mukhopadhyay D, Misra S. Adventitial transduction of lentivirus-shrna-vegf-a treatment in arteriovenous fistula reduces venous stenosis formation. Kid Int. 2013 doi: 10.1038/ki.2013.290. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney international. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 10.Misra S, Shergill U, Yang B, Janardhanan R, Misra KD. Increased expression of hif-1alpha, vegf-a and its receptors, mmp-2, timp-1, and adamts-1 at the venous stenosis of arteriovenous fistula in a mouse model with renal insufficiency. J Vasc Interv Radiol. 2010;21:1255–1261. doi: 10.1016/j.jvir.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misra S, Fu AA, Puggioni A, Karimi KM, Mandrekar JN, Glockner JF, Juncos LA, Anwer B, McGuire AM, Mukhopadhyay D. Increased shear stress with up regulation of vegf-a and its receptors and mmp-2, mmp-9, and timp-1 in venous stenosis of hemodialysis grafts. Am J Physiol Heart Circ Physiol. 2008;294:H2219–2230. doi: 10.1152/ajpheart.00650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra S, Fu AA, Rajan DK, Juncos LA, McKusick MA, Bjarnason H, Mukhopadhyay D. Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. J Vasc Interv Radiol. 2008;19:252–259. doi: 10.1016/j.jvir.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Misra S, Doherty MG, Woodrum D, Homburger J, Mandrekar JN, Elkouri S, Sabater EA, Bjarnason H, Fu AA, Glockner JF, Greene EL, Mukhopadhyay D. Adventitial remodeling with increased matrix metalloproteinase-2 activity in a porcine arteriovenous polytetrafluoroethylene grafts. Kidney international. 2005;68:2890–2900. doi: 10.1111/j.1523-1755.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- 14.Weiss MF, Scivittaro V, Anderson JM. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis. 2001;37:970–980. doi: 10.1016/s0272-6386(05)80013-7. [DOI] [PubMed] [Google Scholar]
- 15.Hughes D, Fu AA, Puggioni A, Glockner JF, Anwer B, McGuire AM, Mukhopadhyay D, Misra S. Adventitial transplantation of blood outgrowth endothelial cells in porcine haemodialysis grafts alleviates hypoxia and decreases neointimal proliferation through a matrix metalloproteinase-9-mediated pathway--a pilot study. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn433. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das M, Dempsey EC, Reeves JT, Stenmark KR. Selective expansion of fibroblast subpopulations from pulmonary artery adventitia in response to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L976–986. doi: 10.1152/ajplung.00382.2001. [DOI] [PubMed] [Google Scholar]
- 18.Janardhanan R, Yang B, Vohra P, Roy B, Withers S, Bhattacharya S, Mandrekar J, Kong H, Leof EB, Mukhopadhyay D, Misra S. Simvastatin reduces venous stenosis formation in a murine hemodialysis vascular access model. Kidney Int. 2013 doi: 10.1038/ki.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang B, Shergill U, Fu AA, Knudsen B, Misra S. The mouse arteriovenous fistula model. J Vasc Interv Radiol. 2009;20:946–950. doi: 10.1016/j.jvir.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 20.Misra S, Shergill U, Yang B, Janardhanan R, Misra K. Increased expression of hif-1α, vegf-a and its receptors, mmp-2, timp-1, and adamts-1 at the venous stenosis of arteriovenous fistula in a mouse model with renal insufficiency. J Vasc Interv Radiol. 2010 doi: 10.1016/j.jvir.2010.02.043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misra S, Fu A, Pugionni A, Glockner J, Rajan D, McKusick M, Bjarnason H, Mukhopadhyay D. Increased expression of hypoxia inducible factor-1a in a porcine model of chronic renal insufficiency with arteriovenous polytetrafluoroethylene grafts. J Vasc Interv Radiol. 2008;19:260–265. doi: 10.1016/j.jvir.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Terry CM, Blumenthal DK, Kuji T, Masaki T, Kwan BCH, Zhuplatov I, Leypoldt JK, Cheung AK. Cellular and morphological changes during neointimal hyperplasia development in a porcine arteriovenous graft model. Nephrol Dial Transplant. 2007;22:3139–3146. doi: 10.1093/ndt/gfm415. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Krishnamoorthy M, Banerjee R, Zhang J, Rudich S, Holland C, Arend L, Roy-Chaudhury P. Venous stenosis in a pig arteriovenous fistula model anatomy, mechanisms and cellular phenotypes. Nephrol Dial Transplant. 2007;22:3139–3146. doi: 10.1093/ndt/gfm547. [DOI] [PubMed] [Google Scholar]
- 24.Hendrickx B, Verdonck K, Van den Berge S, Dickens S, Eriksson E, Vranckx JJ, Luttun A. Integration of blood outgrowth endothelial cells in dermal fibroblast sheets promotes full thickness wound healing. Stem cells. 2010;28:1165–1177. doi: 10.1002/stem.445. [DOI] [PubMed] [Google Scholar]
- 25.Misra S, Misra KD, Glockner JF. Vascular endothelial growth factor-a, matrix metalloproteinase-1, and macrophage migration inhibition factor changes in the porcine remnant kidney model: Evaluation by magnetic resonance imaging. J Vasc Interv Radiol. 2010;21:1071–1077. doi: 10.1016/j.jvir.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotmans JI, Velema E, Verhagen HJ, Blankensteijn JD, de Kleijn DP, Stroes ES, Pasterkamp G. Matrix metalloproteinase inhibition reduces intimal hyperplasia in a porcine arteriovenous-graft model. J Vasc Surg. 2004;39:432–439. doi: 10.1016/j.jvs.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: Insights from a novel human angiogenesis assay. Journal of the American College of Cardiology. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 28.Legrand C, Polette M, Tournier JM, de Bentzmann S, Huet E, Monteau M, Birembaut P. Upa/plasmin system-mediated mmp-9 activation is implicated in bronchial epithelial cell migration. Experimental cell research. 2001;264:326–336. doi: 10.1006/excr.2000.5125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Confocal microscopy of fibroblast alone (Top) compared to BOEC in contact with fibroblast (bottom) exposed to hypoxia from 24 to 72 hours. Red is α-SMA, green is phalloidin and yellow is the overlay.


