Abstract
The enumeration of antigen-specific T cells is increasingly relevant in clinical and research settings. This information is useful for evaluating immune responses to treatment, monitoring the efficacy of anticancer vaccines, and for detecting self-reactive T cells in autoimmune disorders. Quantifying antigen-specific T cells can be accomplished via IFNγ ELISpot assay, the measurement of intracellular cytokine production by flow cytometry, or by lymphocyte proliferation assays in response to antigen. While robust, these technologies are labor-intensive and can take several days to obtain results. New technology has led to more powerful tools for quickly and accurately measuring antigen-specific T cells by flow cytometry via fluorescently-labeled TCR-specific multimers. In this study, we evaluated the use of an assay based on Dextramer reagents for enumerating cytomegalovirus (CMV) antigen-specific T cells (CASTs). Assay performance characteristics were assessed by establishing Dextramers’ sensitivity (median= 0.4; range=0.1–1.4 CASTs µl−1), determining their specificity (100%), evaluating assay robustness with different leukocyte sources and assay reproducibility via interlaboratory and interinstrument investigations. Furthermore, the levels of CASTs in 95 peripheral blood samples from 62 unique blood and marrow transplants recipients correlated well between Dextramers and Tetramers (R2 = 0.9042).
Keywords: Dextramers, cytomegalovirus, antigen-specific T-cell, flow cytometry, immune monitoring
INTRODUCTION
Initial attempts to label T cells directly with monomeric antigen to which they harbor specificity failed because of the low affinity of a single peptide-MHC complex for its corresponding T cell receptor (TCR) (1). Dissociation rates for these interactions are only a few seconds; orders of magnitude lower than antibody–antigen interactions (2,3). Consequently, monomeric MHC-TCR interactions are too unstable to be employed as an effective labeling technique for measuring antigen-specific T cells. Combining multiple MHC molecules into a single polymeric complex (i.e., a multimer) greatly increases the binding stability of the reagent (4). In this regard, fluorescently-labeled synthetic compounds comprised of multiple recombinant MHC Class I molecules, each associated with a specific peptide sequence; provide the required avidity necessary to reliably label CD8+ T cells for flow cytometric analyses.
The initial foray into multimer technology is represented by the classic ‘Tetramer’ (Medical & Biological Laboratories, Nagoya, Japan), which is comprised of a fluorescently-labeled, streptavidin-bridged tetrad of bio-tinylated MHC Class I or Class II monomers. Improvements in multimer technology since then have focused on increasing the number of MHC molecules capable of binding to the cell, as well as the number of fluorochromes attached to the multimer complex, in order to improve their detection characteristics (5). To this end, Dextramer reagents (Immudex, Copenhagen, DK) have been developed, consisting of a dextran polymer backbone to which multiple MHC complexes and fluorochrome molecules are covalently bound. Dextramer reagents incorporate more MHC molecules and more fluorochromes than other conventional MHC multimers, resulting in increased detectable fluorescence intensity and improved avidity, which enables the measurement of low affinity TCRs otherwise undetected by other classes of multimer reagents. Owing to such advances, multimer technology is being increasingly introduced into the clinical laboratory for detecting T cell responses to tumor, bacterial, viral, and auto-antigens. In clinical studies, multimers have also been employed to monitor the reconstitution of the antigen-specific cellular repertoire in patients receiving blood and marrow transplants (BMT). Allogeneic BMT patients are at increased risk of developing cytomegalovirus (CMV) infections post-transplant (6–8). The ability to quantify the proportion of antigen-specific CTL as part of the establishment of a protective immune response has proven to be useful in predicting the recurrence of active disease (9–11).
CMV and Immunosuppression
Human CMV is a herpes virus that infects 50% of Americans between the ages of 6–49 years, with prevalence increasing to over 90% in Americans older than 80 (12,13). While CMV infection is usually asymptomatic in healthy individuals, it can be life-threatening for an immunocompromised patient, as viral reactivation in persons lacking a protective immune response can result in serious clinical sequelae (14–17). Ordinarily, a considerable fraction of the CD8+ memory T cell pool in CMV carriers is dedicated to the surveillance of CMV-specific epitopes, and for BMT patients the rapid recovery of CMV antigen-specific T-cell (CAST) mediated immunity is critical for preventing CMV disease (18). CMV reactivation in the absence of such protection would merit rigorous pre-emptive antiviral therapy; however, these drugs are expensive, toxic, and can induce cytopenias in patients who already have compromised immune cell number and function. Presently, CMV reactivation is determined by antigenemia assay or by PCR; however, logistical challenges related to labor-intensive sample processing, insufficient leukocyte levels (during post-treatment aplasia) for detecting CMVpp65 antigenemia, inconsistent standardization, and low assay sensitivity have been reported shortcomings of these detection modalities (19–21). Consequently, monitoring the number of CASTs in peripheral blood using multimer reagents may become another important supplemental methodology to optimize antiviral therapy and minimize drug exposure.
Monitoring CMV-Antigen Specific T Cells in BMT Recipients
Several studies have examined CMV-specific CD8+ T cell recovery after allo-BMT using multimer reagents, and concluded that a failure to recover CMV-specific CD8+ CTL post-BMT is associated with an increased risk for development of CMV disease. Strikingly, the threshold level of measured CASTs µl−1 of peripheral blood that is determined to confer protection against CMV reactivation has evolved only slightly over the course of these studies, ranging from 2 to 10 CMV-specific T cells µl−1 of blood (22,23). In a large study by Gratama et al., the threshold level of CASTs determined to confer protection from CMV infections within the first 65 days after transplantation was determined to be 7 cells µl−1, and this investigation rigorously demonstrated that quantifying CMV-specific CTL with HLA-peptide multimers is an easy and sensitive tool for identifying BMT recipients at risk for CMV disease (9).
While a variety of recombinant MHC Class I molecules are available, their specificities are restricted, therefore overcoming this limitation will require expanding the compendium of multimer reagents that are used. To this end, we have developed and evaluated an assay based on MHC Dextramer reagents for enumerating CASTs from the peripheral blood of BMT recipients. The Dextramer assay comprises 7 different allele specificities that were collectively validated as a tool for measuring CASTs by establishing the assay sensitivity, measuring the level of specificity, assessing the robustness with different leukocyte sources and reproducibility of measurement in interlaboratory and interinstrument investigations. Finally, we report that the levels of CASTs as measured by Dextramers correlates well with those measured by Tetramers.
METHODS
Study Population
Peripheral blood samples were collected prospectively from allogeneic and autologous BMT recipients at days 30, 100, and 365 post-transplant as part of routine standard-of-care. Peripheral blood was also collected from healthy donors at Roswell Park Cancer Institute (Buffalo, NY) according to either an institute-approved or standard-of-care protocol. HLA-typing of blood samples was performed by Immco Diagnostics (Buffalo, NY). The data analysis was performed under an IRB-approved protocol.
Specimens and Reagents
Phycoerythrin (PE)-conjugated Dextramer reagents and PE-conjugated iTAg™ MHC Tetramer reagents were obtained from Immudex and Beckman Coulter, respectively. An overview of the MHC multimer reagents employed in this study is provided in Table 1. For measuring the proportion of antigen-specific CD8+ T cells in patient blood, separate protocols were executed in accordance with each manufacturer’s instructions. For Tetramer labeling, anti-CD3 PeCy5 (clone UCHT1) and anti-CD8 FITC [clone SFCI21Thy2D3(T8)] were obtained from Beckman Coulter. Red blood cell (RBC) lysis was accomplished with iTAg™ MHC Tet-ramer Lyse/Fix Reagent (Beckman Coulter), and cells were fixed with iTAg™ MHC Tetramer Fix Reagent diluted 1:80 in PBS (MBL). To enumerate the absolute number of CD8+ T cells, a Flow-Count Fluorosphere solution was obtained from Beckman Coulter.
Table 1.
PE-Conjugated Dextramer and Tetramer Reagents Employed in the Study
| Dextramers | iTAg™ MHC Tetramers | Antigen |
|---|---|---|
| HLA-A*01 (VTEHDTLLY) | HLA-A*01 (VTEHDTLLY) | pp50 |
| HLA-A*02 (NLVPMVATV) | HLA-A*02 (NLVPMVATV) | pp65 |
| HLA-A*03 (KLGGALQAK) | IE-1 | |
| HLA-A*24 (QYDPVAALF) | HLA-A*24 (QYDPVAALF) | pp65 |
| HLA-B*07 (RPHERNGFTVL) | pp65 | |
| HLA-B*07 (TPRVTGGGAM) | HLA-B*07 (TPRVTGGGAM) | pp65 |
| HLA-B*08 (ELRRKMMYM) | HLA-B*08 (ELRRKMMYM) | pp65 |
| HLA-B*35 (IPSINVHHY) | HLA-B*35 (IPSINVHHY) | pp65 |
| Negative Control 1 (Irrelevant Control Peptide) | Negative Control (Unspecified Haplotype and Peptide) | N / A* |
| Negative Control 2 (Irrelevant Control Peptide) | N / A* |
N/A=Not Applicable. For Dextramer reagents, nonsense peptides are used. For Tetramer reagents, this information is not available.
For Dextramer labeling, ASR-classified anti-CD3 PerCP (Becton Dickinson; clone SK7) and anti-CD8 FITC (Dako; clone DK25 or BD; clone SK1) were employed. For flow cytometry stability assays, anti-CD3 PerCP (Becton Dickinson; clone SK7), anti-CD8 FITC (Becton Dickinson; clone SK1) and anti-CD4 PB (Dako; clone MT310) were employed. Red blood cell lysis was accomplished with Uti-Lyse Reagent (Dako) or FACS Lysing solution (Becton Dickinson), and cells were fixed with 2% methanol-free formaldehyde in PBS (Polysciences). AccuCount Ultra beads (Spherotech) or Trucount tubes (Becton Dickinson) were used to enumerate the absolute number of CD8+ T cells. LIVE/ DEADVR Fixable Violet (LDV) reagent was purchased from Life Technologies and used to remove dead cells from the analysis of every sample labeled with Dextramers, except for the flow cytometric stability assay and investigations of intraoperator and intralaboratory reproducibility.
Sample Processing
For each donor sample that was assayed, 4 separate tubes were generated; consisting of a ‘Dextramer’ or ‘Tetramer’ tube for each allele that was tested, a ‘Negative Control’ tube, an ‘FMO’ (‘Fluorescence Minus One’; containing anti-CD3, anti-CD8, and LDV reagents only) tube, and a ‘CD8 Count’ tube. For Tetramer labeling only, LDV was not included in any tube. For flow cytometric stability studies and investigations of intraoperator and intralaboratory reproducibility only ‘Dextramer’ and ‘Negative Control’ tubes were utilized.
Tetramer analysis
Cells were labeled with Tetramers according to the manufacturer’s recommendations. Briefly, 200 µl of HLA-typed whole blood from each donor were incubated in 12 × 75 mm flow tubes with the recommended amounts of fluorochrome-conjugated antibodies and 10 µl each of either TCR-specific Tetramers (‘Tetramer’ tube), Negative Control Tetramer (‘Negative Control’ tube), or without Tetramer (‘FMO’ tube) at room temperature for 30 min. After the incubation period had elapsed, RBCs were lysed for 10 min. After lysis, labeled leukocytes were pelleted by centrifugation at 400g for 5 min. Supernatants were removed by aspiration, and cell pellets were then resuspended and washed with 3 ml of phosphate buffered saline (PBS). Washed cells were centrifuged as above and supernatant-depleted, resuspended cell pellets were fixed using 500 µl iTAg™ MHC Tetramer Fix Reagent diluted to 1 : 80 in PBS.
Dextramer analysis
Cells were labeled with Dextramers according to the manufacturer’s recommendations. Briefly, 200 µl of HLA-typed whole blood from each donor were incubated in 12 × 75 mm flow tubes with 10 µl each of either TCR-specific Dextramers (‘Dextramer’ tube), Negative Control Dextramer(s), (‘Negative Control’ tube), or without Dextramer (‘FMO’ tube) at room temperature for 10 min, prior to labeling cells with the recommended amounts of fluorochrome-conjugated antibodies and LDV for 30 min at 4°C. After the incubation periods had elapsed, RBCs were lysed with either Uti-Lyse or FACS Lysing solution. After lysis, labeled leukocytes were pelleted by centrifugation at 400g for 5 min. Supernatants were removed by aspiration, and cell pellets were subsequently resuspended and washed with 2–3 ml of PBS. Washed cells were centrifuged as above and supernatant-depleted, resuspended cell pellets were fixed using 500 µl 2% formaldehyde in PBS.
Separately, and for each donor, parallel 100 µl samples of blood were incubated in 12 × 75 mm flow tubes (hereafter termed: ‘CD8 Count’ tubes) for both the Dextramer and Tetramer assays, using recommended amounts of fluorochrome-conjugated antibodies and LDV (for the Dextramer CD8 Count tube only) for 30 min at 4°C. After labeling, a ‘Lyse-no-Wash’ procedure was performed, where RBCs were lysed as above without the washing steps. To enumerate CD8+ cells, 100 µl homogenized Spherotech AccuCount beads were added to the Dextramer ‘CD8 Count’ tube or Trucount tubes were used for the analysis. For Tetramers, 100 µl of Flow-Count Fluorospheres were added to the Tetramer ‘CD8 Count’ tube. A reverse pipetting technique was employed for the addition of enumeration beads.
Flow Cytometric Acquisition
Fixed cell samples were acquired by flow cytometry within 1 h of labeling using either FACSCanto II (Becton Dickinson), FACSCalibur (Becton Dickinson), FC 500 (Beckman Coulter), or CyAn (Dako) flow cytometers. For the ‘Dextramer’, ‘Tetramer’, ‘FMO,’ and ‘Negative Control’ tubes, a forward scatter threshold was applied during the acquisition of samples to eliminate electronic noise and small particles from the flow cytometric data. An irregular region was placed on a bivariate histogram of PerCP CD3-A versus FITC CD8-A, and used to circumscribe the CD3+, CD8+ cell population; for which at least 20,000 region-inclusive events were collected at a medium flow rate (60 µl min−1 for FACSCanto II;. 35 µl min−1 for FACSCalibur;. 30 µl min−1 for FC 500). For the ‘CD8 Count’ tubes, a threshold was set and applied based on PerCP CD3+ intensity, such that only CD3+ T cells and brightly-fluorescent enumeration beads were collected. In this manner, lysed cellular debris could be reduced from the data file, whilst ensuring that fluorescent enumeration beads were not lost; as would have occurred by the application of a FSC threshold. Thereafter, a rectangular region was used to capture the fluorescent enumeration bead population on a bivariate histogram of PerCP CD3-A versus SSC-A, for which at least 10,000 region-inclusive events were collected at a medium flow rate. For the flow cytometric stability assay, a Dako CyAn instrument was used to acquire unfixed, labeled peripheral blood mononuclear cells (PBMCs). Therein, an elliptical region was employed to circumscribe the lymphocyte population on a bivariate histogram of FSC-A versus SSC-A, for which at least 30,000 region-inclusive events were collected at a rate of 1000–1500 cells s−1.
Data Analysis
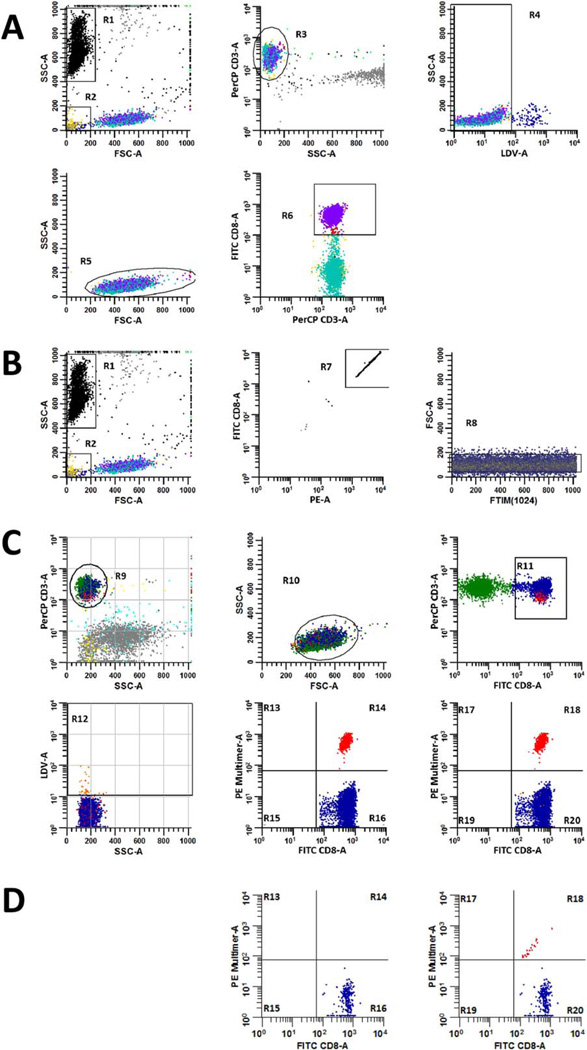
ListMode data were analyzed using WinList v6 software (Verity Software House) or Summit v4.3 (Beckman Coulter). To quantify the absolute number of CD3+/ CD8+ T cells per ll of sample, data from the ‘CD8 Count’ tube for each donor were analyzed separately from all other tubes. Therein, the acquired number of CD3+/ CD8+ events were compared to the acquired number of enumeration bead events using Formula 1, where the ‘Number of Bead Events Added to 100 ml Blood’=(Manufacturer-reported Density of Beads ml−1)*(0.1 ml). The measurement from Formula 1 was then applied in combination with the measured event percentage for positive multimer labeling in both ‘Dextramer’ and ‘Tetramer’ tubes, to determine the absolute number of antigen-specific CD8+ T cells per µl of sample. The full gating strategy is outlined in Figures 1A and 1B.
| Formula 1 |
Fig. 1.
Gating strategies for measuring the absolute number of CD3+/ CD8+ cells µl−1 and the percentage of multimer positive events in peripheral blood samples. The absolute number of CD3+/CD8+ T cells µl−1 in each sample was calculated according to Formula 1 using data derived from the ‘CD8 Count’ tube (A and B). To accomplish this, the number of CD3+/CD8+ events acquired by the flow cytometer (A) was compared to the number of enumeration beads that were also acquired, which were used to determine the fraction of the original sample volume that was collected (B). The acquired number of CD8+ T cells was determined by excluding enumeration beads (R1) and debris (R2) events from the analysis, employing a plot of FSC-A versus SSC-A to resolve these populations. CD3+ events (R3) were identified on a gated plot (NOT R1 and NOT R2) of SSC-A versus PerCP CD3-A, and then a gated plot (NOT R1 and NOT R2 and R3) of LDV-A versus SSC-A was used to identify viable T cells (R4). A gated plot (NOT R1 and NOT R2 and R3 and R4) of FSC-A versus SSC-A was used to discriminate viable, CD3+ T cells that harbored scatter characteristics of lymphocytes (R5). Finally, a gated plot (NOT R1 and NOT R2 and R3 and R4 and R5) of PerCP CD3-A versus FITC CD8-A was used to quantify the number of CD3+/CD8+ T cells (R6) that were acquired from the sample. Thereafter, bead events (R1) were identified on a plot of FSC-A versus SSC-A and were further discriminated from non-bead events by virtue of their high fluorescence intensity (R7) on an R1-gated plot of PE-A versus FITC CD8-A. Single bead events were refined and identified (R8) on a gated plot (R1 and R7) of event chronology (FTIM(1024)) versus FSC-A to assess the compositional homogeneity of acquired beads. The fraction of CD8+ cells that were CAST positive was established in ‘Dextramer’, ‘Tetramer’, ‘Negative Control,’ or ‘FMO’ tubes according to the gating strategy defined in (C). To measure these percentages, CD3+ events were identified on a plot of SSC-A versus PerCP CD3-A (R9), which were then further refined on an R9-gated plot of FSC-A versus SSC-A. CD3+ events having lymphocyte scatter characteristics (R10), were then displayed on a gated plot (R9 and R10) of FITC CD8-A versus PerCP CD3-A, where CD3+/CD8+ T cells were identified (R11). Dead cells (R12) were excluded from the analysis via a gated plot (R9 and R10 and R11) of SSC-A versus LDV-A. Finally, gated plots (R9 and R10 and R11) of FITC CD8-A versus PE Multimer-A that either excluded (R13–R16) or included (R17–R20) dead cells were used to measure the percentage of multimer positive (R14 or R18) events amongst total gated CD3+/CD8+ events. When samples are of good quality or when CAST frequencies are high, the effect of dead cell exclusion may not be readily apparent; however, dead cell exclusion is particularly beneficial when sample viability is sub-optimal or when CAST frequencies are low. Bivariate histograms of FITC CD8-A versus PE-Multimer-A from a separate, multimer-negative sample are gated as described in (C) to demonstrate how dead cell exclusion reduces false positive detection in such cases (D).
To determine the fraction of CD8+ cells that were measured as CASTs in each sample; a separate gating strategy was used for the analysis of ‘Dextramer’, ‘Tetramer’, ‘FMO,’ and ‘Negative Control’ tubes, which is outlined in Figure 1C. The absolute number of CAST positive events per µl was determined by multiplying the value obtained for the ‘Absolute Number of CD3+/ CD8+ Events µl−1’ (Formula 1) by the ‘Fraction of CAST Positive CD8+ Events’ (Formula 2).
| Formula 2 |
For the flow cytometric stability assay, the percent of CAST-positive events amongst the CD3+, CD4−, CD8+ population was determined with Summit v4.3 software (Beckman Coulter), and the fraction of CAST positive CD8+ events was ascertained using Formula 2. The full gating strategy is defined in the ‘Supporting Information and Methods’ section.
Linearity and Assay Sensitivity
The linearity and also the sensitivity of the Dextramer assay were determined by constructing a titration curve; comprised of blood samples known to contain CASTs, which were diluted in quarter-log decrements with whole blood samples that were shown not to contain measurable CASTs when assayed using Dextramer reagents. Samples from donors that bound Dextramer reagents from 7 different HLA specificities were assayed in triplicate across 3 logs of dilution.
Comparison of Negative Control Assay
Sixty three samples taken from 25 individuals were evaluated in parallel using both Negative Control 1 and Negative Control 2 Dextramers. The Negative Control 1 Dextramer reagent displays HLA-A*0201 molecules, which contain a negative control peptide harboring the amino acid sequence: “GLAGDVSAV.” Anchor amino acids at position 2 and 9 match the requirements for binding to the HLA-A*0201 allele. In contrast, the Negative Control 2 Dextramer reagent displays HLA-B*0801 molecules, which contain a negative control peptide harboring the amino acid sequence: “AAKGRGAAL.” Anchor amino acids match the requirements for binding to the HLA-B*0801 allele. To determine the effect of dead cell exclusion on the measurement of background, the evaluation of cell viability was removed from the WinList gating strategy by including both LDV negative and LDV positive events in the analysis. Subsequently, the measurement of Dextramer positive events in these analyses were compared to analyses where dead cells had been excluded.
Analytic Specificity Assay
Seronegativity of patient serum samples was established by immunochemiluminometric assay (ViroMed). Blood donors were serotyped to determine their HLA profile and then assayed for CASTs using appropriately-matched Dextramer reagents.
Analysis of CASTs in Whole Blood Versus CPT
Peripheral blood samples from 9 BMT recipients were collected in parallel into glass 10 ml sodium heparin (green top) Vacutainer tubes (Becton Dickinson), and also into glass 8 ml sodium heparin Vacutainer Cell Preparation Tubes (CPT; Becton Dickinson). Blood collected into CPT tubes were centrifuged at 1500g for 30 min at room temperature as per the manufacturer’s instructions to generate a mononuclear cell (MNC) preparation. After centrifugation, MNCs were harvested from the residual RBC- and granulocyte-depleted plasma and washed two times with 15 ml PBS. Thereafter, MNC preparations were enumerated using a Coulter AcT 10 analyzer and 1 × 106 leukocytes from ‘CPT’ tubes were processed alongside 200 µl peripheral blood from ‘green top’ tubes in triplicate or quadruplicate, as described previously for flow cytometric analysis.
Fluorescence Intensity Integrity Assay
The stability of Dextramer reagents was tested using an immunosorbance-based Fluorescence Intensity Integrity assay where separate lots of fluorochrome-conjugated Dextramers comprising 7 distinct HLA haplotypes were evaluated. Between experiments, Dextramer reagents were stored in their respective production vials in a darkened, temperature-logged cold room (2–8°C). During use, reagents were handled at room temperature as described in the manufacturer’s assay protocol. For this investigation, triplicate samples were evaluated on days 1, 41, 110, 183, and 365 post-manufacturing. In brief, the assay was based upon the ability of plate-bound anti-β2-microglobulin antibody to capture fluorescently-labeled Dextramer. Only Dextramer with intact MHC molecules (comprised of an assembled MHC heavy chain, β2 microglobulin subunit, and functional fluorescent fluorochromes) can give rise to appropriate signals in the assay. Using fluorescence data from these measurements, a ‘Test Value’ (TV) is obtained, which reports the proportion of Dextramer reagent that remains bound to the capture antibody after a 4 h incubation period at 25°C has elapsed (T4 Hours/T0 Hours) The TV for assayed Dextramer reagents is then compared to the TV derived from a known reference standard, and the calculated TVDextramer/TVReference proportion provides a metric for evaluating the structural integrity of Dextramer reagents. Additional details related to the Fluorescence Intensity Integrity stability assay are provided in the ‘Supporting Information and Methods’ section.
The performance of the Fluorescence Intensity Integrity assay was corroborated by testing the same Dextramer reagents in parallel using a flow cytometric assay; where CASTs were measured at different time points from PBMCs that had been cryogenically stored in an archive. Specific details related to the application of this flow cytometry assay are provided in the ‘Supporting Information and Methods’ section. For evaluation of each test article by flow cytometry, 1 × 106 thawed HLA-matched PBMCs were resuspended in 50 µl of PBS and were then labeled as described above.
Reliability Between Instruments
Peripheral blood samples from 10 to 15 BMT recipients as indicated were assessed for the presence of CASTs using HLA-matched Dextramers, employing parallel replicates of 10 ‘Dextramer’ tubes and single measurements of ‘Negative Control’ and ‘CD8 Count’ tubes for generating data on either a FACSCanto II, FACSCalibur, or an FC 500 flow cytometer. Dextramers representing 8 HLA alleles were employed in the study.
Interlaboratory Reproducibility
Peripheral blood samples known to contain CASTs from 3 donors were diluted using CAST-negative donor blood to generate three different CAST densities for each donor. Each sample was analyzed for the presence of CASTs using HLA-matched Dextramers in two separate laboratories at the Immudex and Dako facilities. HLA-A*01 (VTEHDTLLY), HLA-A*02 (NLVPMVATV), and HLA-B*08 (ELRRKMMYM) Dextramers were employed in the study.
Statistical Analyses
The paired Student’s t-test was used to evaluate differences in the measurement of CASTs from the blood of seropositive patients when using Negative Control 1 versus Negative Control 2 reagents; in assessing the effect of excluding dead cells from flow cytometric analyses; and in comparing the measurements of CASTs made from whole blood or CPT tubes. ANOVA was applied to evaluate whether variability exists in the measurement of CASTs when using different flow cytometers. In all cases, two-sided P-values <0.05 were considered statistically significant. For determining the sensitivity of the Dextramer assay, mean and standard deviation values were derived from each of the triplicate measurements of CASTs µl−1 in each dilution series, which were then used to calculate a coefficient of variation (CV) for each data point.
Acceptance Criteria
Acceptance criteria were established to define satisfactory performance characteristics of the assays reported herein. For directly comparing the measured levels of CASTs µl−1 (i.e., when assayed at various sample dilutions; when using either Dextramers or Tetramers; when measured on different flow cytometers; when determined by different laboratories; or when detected in whole blood versus CPT preparations), a calculated Coefficient of Determination (R2) value of at least 0.900 was required.
For the measurement of Test Values at any given time point, the predefined acceptance criterion was established to be a deviation of less that 20% from the T= 0 Day measurements. For assessing variability amongst decuplet CASTs µl−1 values derived from the same instrument, standard deviations of less than 2% were determined to be acceptable. The acceptance criteria defined for the investigations of analytical specificity required that the maximum measured CAST µl−1 values were less than the lowest limit of sensitivity that was determined for each specific allele. For comparing the measured levels of CASTs µl−1 when assayed by different operators, the acceptance criterion was established to be a coefficient of variation value of less than 20%.
RESULTS
Determinations of Assay Linearity and Sensitivity
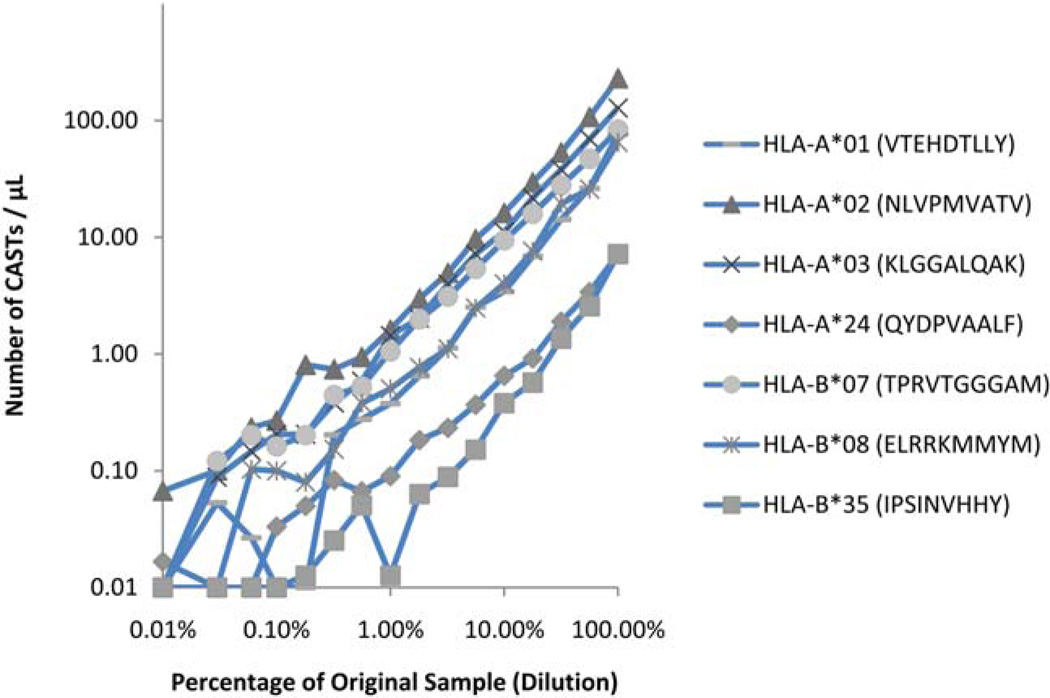
To determine the linear range of the Dextramer assay, peripheral blood samples containing CASTs at different concentrations were evaluated in titration series. Assayed samples contained CASTs at frequencies ranging from 229.9 to 7.2 cells µl−1, and were serially diluted in unrelated peripheral blood from a CMV-seronegative donor 100.25-fold, up to 15 times. Depending on the HLA-haplotype, a dilution effect could no longer be observed at 0.2–0.5% of the blood sample. The data from Figure 2 indicate that for each Dextramer tested, the measured quantity of CASTs at each dilution was consistent with the mathematically-expected value across a wide dynamic range. Linear regression analysis demonstrates that these values were indeed closely correlated as indicated in Table 2, where only the data which exceeded the assay’s limit of sensitivity were analyzed. Assay sensitivity was determined for each tested Dextramer using calculated CV data, and was determined to be the measurement of CAST density that preceded the measurement where the CV exceeded 20%; correlating with assay sensitivity values of 0.1–1.4 CASTs µl−1 (median= 0.4 CASTs µl−1) as indicated in Table 3. These values correspond to actual acquired event counts ranging from 11 to 57 (median= 20) Dextramer positive, CD8+ T cells in clustered distributions that were collected in each file.
Fig. 2.
Determining the linearity of the Dextramer assay. Titration series were generated from blood samples known to contain CMV-reactive T cells, in order to determine the lowest quantity of CASTs that could be accurately measured when employing Dextramer reagents. Seven different Dextramers were used to quantify the number of CASTs µl−1 at 15 distinct quarter-log dilutions of blood from CAST-positive samples. Whole blood from a validated CAST-negative donor was used to dilute CAST-positive blood samples across three logs of titration. A titratable effect was observed in all cases. Dependent upon the specificity of the Dextramer, the titration point at which a dilution effect could no longer be observed was consistently measured to be ~0.2–0.5% of the undiluted sample. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 2.
Linear Regression Analysis of Dextramer Titration Study
| Dextramer Allele | Coefficient of Determination (R2) |
Y Intercept | Slope of Linear Regression |
Residual Sum of Squares |
|---|---|---|---|---|
| HLA-A*01 | 0.9513 | −3.05 | 71.51 | 246.98 |
| HLA-A*02 | 0.9882 | −4.55 | 222.33 | 583.50 |
| HLA-A*03 | 0.9994 | −0.71 | 127.34 | 9.01 |
| HLA-A*24 | 0.9897 | −0.10 | 6.98 | 0.45 |
| HLA-B*07 | 0.9997 | 0.63 | 83.27 | 2.05 |
| HLA-B*08 | 0.9761 | −1.45 | 62.23 | 89.28 |
| HLA-B*35 | 0.9621 | −0.86 | 7.59 | 1.20 |
Table 3.
Determination of the Assay’s Sensitivity when Measuring CASTs with Dextramer Reagentsa
| HLA-A*01 (VTEHDTLLY) |
HLA-A*02 (NLVPMVATV) |
HLA-A*03 (KLGGALQAK) |
HLA-A*24 (QYDPVAALF) |
HLA-B*07 (TPRVTGGGAM) |
HLA-B*08 (ELRRKMMYM) |
HLA-B*35 (IPSINVHHY) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %of Original Sample |
Mean CASTs µl−1 |
Mean % CASTPos |
%CV | Mean CASTs µl−1 |
Mean % CASTPos |
%CV | Mean CASTs µl−1 |
Mean % CASTPos |
%CV | Mean CASTs µr−1 |
Mean % CASTPos |
%cv | Mean CASTs µl−1 |
Mean % CASTPos |
%CV | Mean CASTs µl−1 |
Mean % CASTPos |
%cv | Mean CASTs µl−1 |
Mean % CASTPos |
%cv |
| 100.00 | 76.6 | 10.2 | 4 | 229.9 | 22.8 | 0 | 128.0 | 14.5 | 1 | 7.2 | 1.4 | 4 | 83.8 | 6.9 | 2 | 65.1 | 8.7 | 2 | 7.2 | 1.9 | 10 |
| 56.23 | 26.3 | 3.5 | 7 | 107.3 | 10.6 | 2 | 69.4 | 7.9 | 2 | 3.4 | 0.8 | 9 | 47.0 | 3.9 | 4 | 25.9 | 3.4 | 3 | 2.6 | 0.7 | 1 |
| 31.62 | 14.3 | 1.9 | 12 | 53.3 | 5.3 | 2 | 38.1 | 4.3 | 3 | 1.9 | 0.4 | 6 | 27.8 | 2.3 | 6 | 19.5 | 2.6 | 10 | 1.4 | 0.4 | 8 |
| 17.78 | 6.9 | 0.9 | 2 | 29.5 | 2.9 | 2 | 21.7 | 2.5 | 3 | 0.9 | 0.2 | 17 | 15.9 | 1.3 | 2 | 7.6 | 1.0 | 5 | 0.6 | 0.2 | 12 |
| 10.00 | 3.4 | 0.5 | 6 | 16.2 | 1.6 | 2 | 11.1 | 1.3 | 13 | 0.7 | 0.1 | 13 | 9.4 | 0.8 | 4 | 4.0 | 0.5 | 719 | 0.4 | 0.1 | 10 |
| 5.60 | 2.5 | 0.3 | 17 | 9.6 | 1.0 | 5 | 7.2 | 0.8 | 9 | 0.4 | 0.1 | 16 | 5.4 | 0.4 | 6 | 2.5 | 0.3 | 0.2 | 0.0 | 43 | |
| 3.20 | 1.1 | 0.2 | 12 | 5.0 | 0.5 | 6 | 4.0 | 0.5 | 1 | 0.2 | 0.0 | 12 | 3.1 | 0.3 | 4 | 1.1 | 0.2 | 5 | 0.1 | 0.0 | 25 |
| 1.80 | 0.7 | 0.3 | 13 | 3.0 | 0.3 | 7 | 2.0 | 0.2 | 17 | 0.2 | 0.0 | 16 | 2.0 | 0.2 | 15 | 0.8 | 0.1 | 7 | 0.1 | 0.0 | 35 |
| 1.00 | 0.4 | 0.1 | 20 | 1.6 | 0.2 | 0 6 | 1.4 | 0.2 | 9 | 0.1 | 0.0 | 11 | 1.1 | 0.1 | 18 | 0.5 | 0.1 | 9.0 | 0.0 | 0.0 | 173 |
| 0.56 | 0.3 | 0.0 | 15 | 0.9 | 0.1 | 6 | 0.6 | 0.1 | 23 | 0.1 | 0.0 | 115 | 0.5 | 0.0 | 13 | 0.4 | 0.1 | 0 | 0.1 | 0.0 | 43 |
| 0.32 | 0.2 | 0.0 | 23 | 0.7 | 0.1 | 16 | 0.4 | 0.0 | 35 | 0.1 | 0.0 | 35 | 0.4 | 0.0 | 42 | 0.2 | 0.0 | 49 | 0.0 | 0.0 | 87 |
| 0.18 | 0.0 | 0.0 | - | 0.8 | 0.1 | 22 | 0.2 | 0.0 | 49 | 0.1 | 0.0 | 100 | 0.2 | 0.0 | 35 | 0.1 | 0.0 | 0 | 0.0 | 0.0 | 173 |
| 0.10 | 0.0 | 0.0 | - | 0.3 | 0.0 | 22 | 0.2 | 0.0 | 49 | 0.0 | 0.0 | 173 | 0.2 | 0.0 | 43 | 0.1 | 0.0 | 87 | 0.0 | 0.0 | - |
| 0.06 | 0.0 | 0.0 | 173 | 0.2 | 0.0 | 25 | 0.1 | 0.0 | 35 | 0.0 | 0.0 | - | 0.2 | 0.0 | 35 | 0.1 | 0.0 | 39 | 0.0 | 0.0 | - |
| 0.03 | 0.1 | 0.0 | 87 | 0.1 | 0.0 | 100 | 0.1 | 0.0 | 0 | 0.0 | 0.0 | - | 0.1 | 0.0 | 0 | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| 0.00 | 0.0 | 0.0 | - | 0.1 | 0.0 | 87 | 0.0 | 0.0 | - | 0.0 | 0.0 | 173 | 0.0 | 0.0 | - | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
The assay’s limit of sensitivity was determined to be the titration point prior to the measurement at which the %CV for replicate measurements of CASTs µl−1 exceeded 20% (indicated by horizontal lines in each column).
Comparison of Negative Control Reagents
The most appropriate negative control Dextramers are those which can bind to assayed TCRs by virtue of their HLA type, but fail to do so owing to the presence of an irrelevant peptide in the Dextramers’ MHC binding grooves. Accordingly, two separate negative control Dextramers containing a nonsense peptide sequence were selected and examined in 63 samples. The data from Table 4 indicate that when using the Negative Control 1 Dextramer, an average of 0.11 ±0.17 ‘positive’ events were detected per µl, whereas that number was reduced to 0.05 ± 0.09 ‘positive’ events per µl when using the Negative Control 2 Dextramer (P = 0.0017).
Table 4.
Effect of Excluding Dead Cells from the Flow Cytometric Analysis, on the Amount of Background Signal obtained when using Negative Control Reagents
| Negative Control 1 |
||||
|---|---|---|---|---|
| Total Sample |
Dead Cell Exclusion |
|||
| % CASTs | # CASTs / µl | % CASTs | # CASTs / µl | |
| Mean | 0.04% | 0.11 | 0.01% | 0.03 |
| SD | 0.06% | 0.17 | 0.01% | 0.08 |
| n | 63 | 63 | 63 | 63 |
| Min | 0.00% | 0.00 | 0.00% | 0.00 |
| Max | 0.38% | 0.87 | 0.05% | 0.58 |
| Negative Control 2 |
||||
|---|---|---|---|---|
| Total Sample |
Dead Cell Exclusion |
|||
| % CASTs | # CASTs / µl | % CASTs | # CASTs / µl | |
| Mean | 0.02% | 0.05 | 0.00% | 0.00 |
| SD | 0.04% | 0.09 | 0.00% | 0.01 |
| n | 63 | 63 | 63 | 63 |
| Min | 0.00% | 0.00 | 0.00% | 0.00 |
| Max | 0.22% | 0.38 | 0.02% | 0.06 |
Impact of Dead Cell Exclusion on Multimer Analysis
The presence of dead cells in samples which have been labeled and acquired by flow cytometry can influence the measurement of rare events, as dead cells will nonselectively bind antibodies and other fluorescent reagents, which can lead to false-positive events. The data from Table 4 indicate that by removing dead cells from the analysis through selective gating techniques, background measurements were reduced to 0.03±0.08 (P < 0.0001) and 0.002 ±0.009 (P = 0.0001) ‘positive’ events per µl for Negative Control 1 and Negative Control 2 Dextramers, respectively. These values translate to an overall reduction of background noise from 0.04%± 0.06% to 0.01% ±0.01% (P= 0.0003) ‘positive’ events using Negative Control 1. As 99.7% of normally-distributed data will fall within three standard deviations of the mean, the range for measuring noise with the Negative Control 1 Dextramer is reduced from ±0.18% to ±0.03% by the exclusion of dead cells. Similarly, removing dead cells from samples labeled with the Negative Control 2 Dextramer resulted in a decrease of noise from 0.02%± 0.04% ‘positive’ events to 0.00%± 0.00% (P =0.0003) ‘positive’ events; yielding a decrease in the measured range from ±0.12% to ±0.00%.
Analytical Specificity
To test the specificity of the assay, CMV-specific Dextramer reagents were used to measure the level of CASTs in 51 CMV-seronegative blood specimens from 25 BMT patients. In 35/51 cases, zero CASTs µl−1 were measured, with less than 0.05 CASTs µl−1 being measured in all but one of the remaining samples (Table 5). When stratified according to HLA type, no difference in the mean level of CASTs µl−1 was found for either HLA-A or HLA-B. In all cases, the measured level of Dextramer-positive events was below the detection threshold that was established in Table 5, and indicates that the analytical specificity of Dextramer reagents is measured to be 100% (51/51).
Table 5.
CAST-Reactive, HLA-Matched Dextramer Reagents do not bind to the CD8+ T Cells of CMV Seronegative Donors
| Specimen (HLA Type) |
MHC Dextramer | Result (Cells/µl) |
Specimen (HLA Type) |
MHC Dextramer | Result (Cells/µl) |
|---|---|---|---|---|---|
| A*0101 | HLA-A*01 (VTEHDTLLY) | 0.00 | B*0702 | HLA-B*07 (TPRVTGGGAM) | 0.02 |
| A*0101 | HLA-A*01 (VTEHDTLLY) | 0.01 | B*0702 | HLA-B*07 (RPHERNGFTVL) | 0.00 |
| A*0101 | HLA-A*01 (VTEHDTLLY) | 0.05 | B*0702 | HLA-B*07 (TPRVTGGGAM) | 0.01 |
| A*0101 | HLA-A*01 (VTEHDTLLY) | 0.00 | B*0702 | HLA-B*07 (RPHERNGFTVL) | 0.03 |
| A*0101 | HLA-A*01 (VTEHDTLLY) | 0.00 | B*0702 | HLA-B*07 (TPRVTGGGAM) | 0.00 |
| A*0101 | HLA-A*01 (VTEHDTLLY) | 0.00 | B*0702 | HLA-B*07 (RPHERNGFTVL) | 0.01 |
| A*0101 | HLA-A*01 (VTEHDTLLY) | 0.00 | B*0702 | HLA-B*07 (TPRVTGGGAM) | 0.01 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | B*0801 | HLA-B*08 (ELRRKMMYM) | 0.04 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.05 | B*0801 | HLA-B*08 (ELRRKMMYM) | 0.00 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | B*0801 | HLA-B*08 (ELRRKMMYM) | 0.00 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | B*0801 | HLA-B*08 (ELRRKMMYM) | 0.00 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | B*3501 | HLA-B*35 (IPSINVHHY) | 0.00 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | B*3501 | HLA-B*35 (IPSINVHHY) | 0.02 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | B*3501 | HLA-B*35 (IPSINVHHY) | 0.06 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | B*3501 | HLA-B*35 (IPSINVHHY) | 0.00 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | B*3501 | HLA-B*35 (IPSINVHHY) | 0.00 |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.03 0.04 | B*3501 | HLA-B*35 (IPSINVHHY) | 0.00 |
| A*0201 | HLA-A*02 (NLVPMVATV) | For HLA-B Alleles: Mean | 0.01 | ||
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | CV% | 1.39 | |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 | Min | 0.00 | |
| A*0201 | HLA-A*02 (NLVPMVATV) | 0.00 0.00 | Max | 0.06 | |
| A*0301 | HLA-A*03 (KLGGALQAK) | ||||
| A*0301 | HLA-A*03 (KLGGALQAK) | 0.00 | For All Alleles: Mean | 0.01 | |
| A*0301 | HLA-A*03 (KLGGALQAK) | 0.04 | CV% | 1.76 | |
| A*0301 | HLA-A*03 (KLGGALQAK) | 0.00 | Min | 0.00 | |
| A*0301 | HLA-A*03 (KLGGALQAK) | 0.00 | Max | 0.06 | |
| A*0301 | HLA-A*03 (KLGGALQAK) | 0.00 | |||
| A*2402 | HLA-A*24 (QYDPVAALF) | 0.00 | |||
| A*2402 | HLA-A*24 (QYDPVAALF) | 0.00 | |||
| A*2402 | HLA-A*24 (QYDPVAALF) | 0.00 | |||
| A*2402 | HLA-A*24 (QYDPVAALF) | 0.00 | |||
| A*2402 | HLA-A*24 (QYDPVAALF) | 0.00 | |||
| A*2402 | HLA-A*24 (QYDPVAALF) | 0.01 | |||
| A*2402 | HLA-A*24 (QYDPVAALF) | 0.03 | |||
| For HLA-A Alleles: Mean | 0.01 | ||||
| CV% | 2.06 | ||||
| Min | 0.00 | ||||
| Max | 0.05 | ||||
Comparison of Dextramer Versus Tetramer Assays
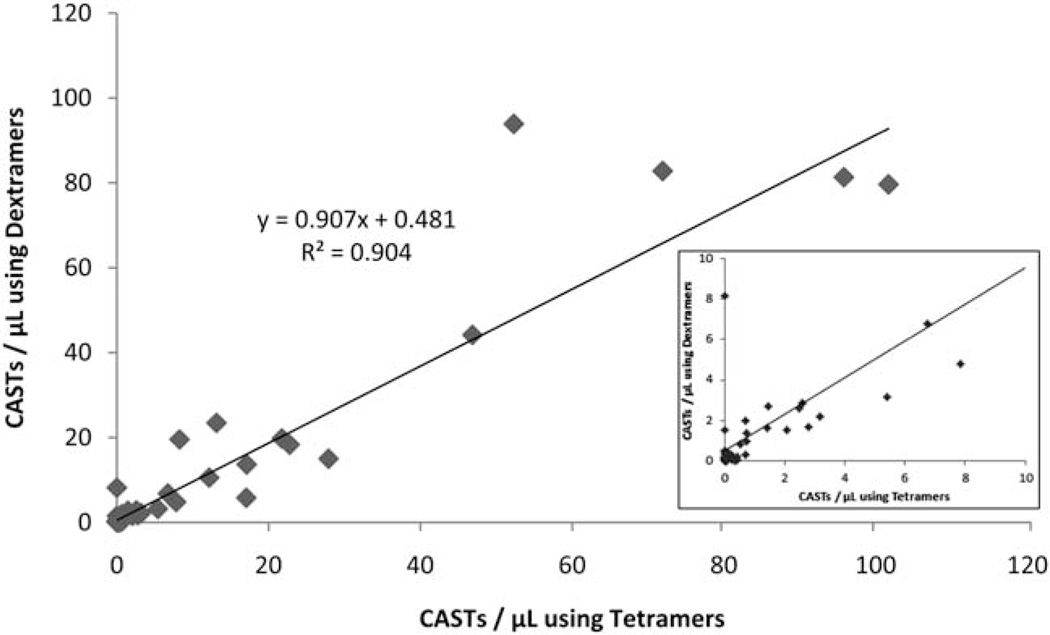
Prior investigations have qualified MHC Tetramers for use in the enumeration of antigen-specific T cells (9). To determine the extent to which the measurement of CASTs with Dextramers approximates the measurement of CASTs with Tetramers, 95 peripheral blood specimens from 62 separate BMT recipients were analyzed for CASTs using both reagents. The pooled data from all HLA alleles reveals a close correlation between the measurement of CASTs µl−1 by both Dextramer and Tetramer reagents (R2 = 0.9042; Fig. 3). When only those measurements of CASTs µl−1, which exceeded the allele-specific functional sensitivities of employed Dextramer reagents were evaluated, the correlation between the two assays was determined to be 0.8758 (n = 34; data not shown).
Fig. 3.
Dextramer reagents are comparable to iTAg™ MHC Tetramer reagents for quantifying the number of CASTs in whole blood. Ninety-five peripheral blood samples were assayed for CASTs in parallel, using both Dextramers and iTAg™ MHC Tetramers. For each patient sample, the absolute number of CASTs µl−1 was calculated using both platforms. Linear regression analysis of the plotted data set reveals a Coefficient of Determination of 0.9042 for all samples, indicating that both Dextramers and Tetramers are equally capable of detecting CASTs in patient samples. Inset: Expanded view of graph, showing the correlation for CASTs measured by Dextramer and Tetramer at values of less than 10 CASTs µl−1.
Measurement of CASTs in Whole Blood Versus CPT
We next investigated whether the Dextramer assay could be used equally well on whole blood samples or PBMCs isolated from whole blood using BD Vacutainer CPT tubes. The data from Table 6 demonstrate that significant differences do not exist amongst cell processing groups as a whole; whether data is reported as a percentage of the total CD8+ population (P =0.3420), or in terms of the total number of CASTs µl−1 (P =0.5307). Linear regression analysis of the CASTs µl−1 values when measured from different cell sources revealed a high degree of correlation (R2 = 0.9721).
Table 6.
Comparison of the Parallel Measurement of CASTs from Whole Blood and from Cell Preparation (CPT) Tubes
| Whole Blood |
CPT |
||||
|---|---|---|---|---|---|
| Dextramer | % | SD | % | SD | n |
| HLA-A*01 (VTEHDTLLY) | 5.06% | 0.00 | 4.90% | 0.00 | 4 |
| HLA-A*01 (VTEHDTLLY) | 3.26% | 0.00 | 2.66% | 0.00 | 3 |
| HLA-A*02 (NLVPMVATV) | 4.67% | 0.00 | 1.96% | 0.00 | 4 |
| HLA-A*24 (QYDPVAALF) | 0.38% | 0.00 | 0.18% | 0.00 | 4 |
| HLA-B*07 (RPHERNGFTVL) | 5.53% | 0.00 | 5.15% | 0.00 | 4 |
| HLA-B*07 (TPRVTGGGAM) | 2.94% | 0.00 | 3.79% | 0.00 | 4 |
| HLA-B*08 (ELRRKMMYM) | 0.72% | 0.00 | 0.72% | 0.00 | 3 |
| HLA-B*08 (ELRRKMMYM) | 0.13% | 0.00 | 0.12% | 0.00 | 4 |
| HLA-B*35 (IPSINVHHY) | 4.66% | 0.00 | 4.89% | 0.00 | 4 |
| Whole Blood |
CPT |
||||
| Dextramer | CASTs / µl | SD | CASTs / µl | SD | n |
| HLA-A*01 (VTEHDTLLY) | 73.19 | 2.14 | 70.90 | 1.71 | 4 |
| HLA-A*01 (VTEHDTLLY) | 34.87 | 2.68 | 28.40 | 0.89 | 3 |
| HLA-A*02 (NLVPMVATV) | 7.89 | 0.57 | 3.30 | 0.16 | 4 |
| HLA-A*24 (QYDPVAALF) | 1.80 | 0.28 | 0.90 | 0.20 | 4 |
| HLA-B*07 (RPHERNGFTVL) | 60.74 | 0.97 | 56.60 | 2.00 | 4 |
| HLA-B*07 (TPRVTGGGAM) | 32.26 | 0.73 | 41.60 | 1.37 | 4 |
| HLA-B*08 (ELRRKMMYM) | 7.73 | 0.56 | 7.70 | 0.13 | 3 |
| HLA-B*08 (ELRRKMMYM) | 0.23 | 0.07 | 0.20 | 0.06 | 4 |
| HLA-B*35 (IPSINVHHY) | 4.65 | 0.17 | 4.90 | 0.13 | 4 |
Stability of Dextramers Over Time
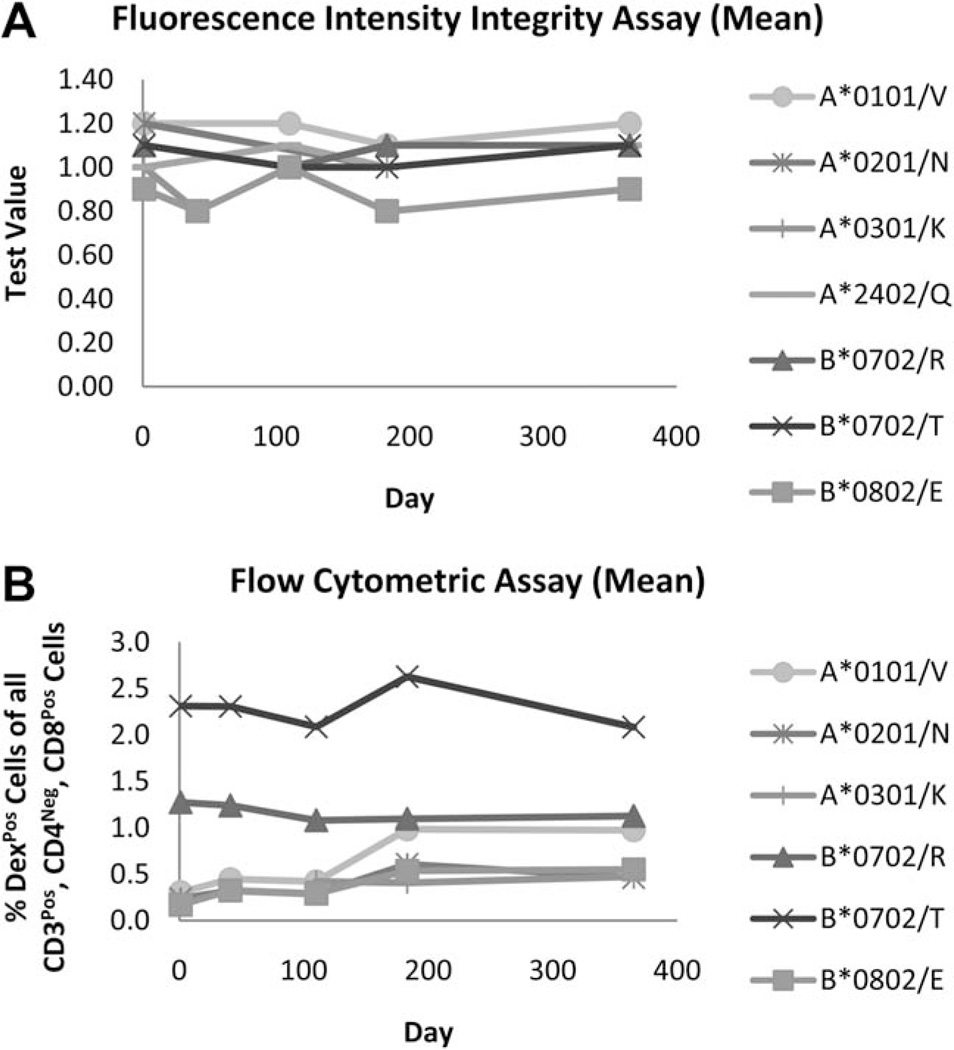
The real-time stability of CMV Dextramer reagents was tested by measuring their structural integrity in a fluorescence-based binding assay, and their functionality was demonstrated by the ability of Dextramers to consistently measure CAST populations from archived PBMC samples by flow cytometry. As shown in Figure 4A, Test Values for each CMV-specific Dextramer reagent deviated by less than 20% (range = 80–111%) of the original value over the course of a year, indicating that Dextramer reagents are compositionally-stable and do not degrade during long-term storage at 2–8°C. The same Dextramer preparations as used for the Fluorescence Intensity Integrity assay were also used to measure CASTs in PBMC samples at corresponding time points using flow cytometry For each Dextramer formulation tested, the measured percentage of CASTs in HLA-matched, archived PBMC samples did not dramatically modulate (range = 0.85–3.24 fold difference) as a function of time (Fig. 4B); confirming that regardless of the allele tested, that CMV-specific Dextramers are functionally-stable for at least one year and yield consistent measurements of antigen-specific T cells at all assayed time points.
Fig. 4.
Dextramer reagents are stable for at least one year. A Fluorescence Intensity Integrity assay was performed to evaluate the stability characteristics of Dextramer reagents over time (A). Seven distinct PE-conjugated Dextramer reagents were synthesized and then assayed on days 1, 41, 110, 183, and 365 post-manufacturing for the ability to bind to an anti-β2-microglobulin-coated plate. Spectrophotometric measurements of PE emission were used to calculate a ‘Test Value’ (TV), (see Formulae 3–5 in the ‘Supporting Information and Methods’ section). The average TV for triplicate samples at each time point is plotted for Dextramer reagents of 7 different haplotypes and peptide specificities. These data demonstrate that for each reagent preparation tested, the amount of compositionally-intact Dextramer reagent is consistent over the course of the assay. The same lots of Dextramer reagents were also evaluated in a flow cytometric assay to demonstrate functional stability (B). These data demonstrate that for any given Dextramer reagent, the measured percentage of CASTs in PBMC samples is consistent over time, further indicating that Dextramer reagents are functionally stable for at least one year.
Reliability Between Instruments
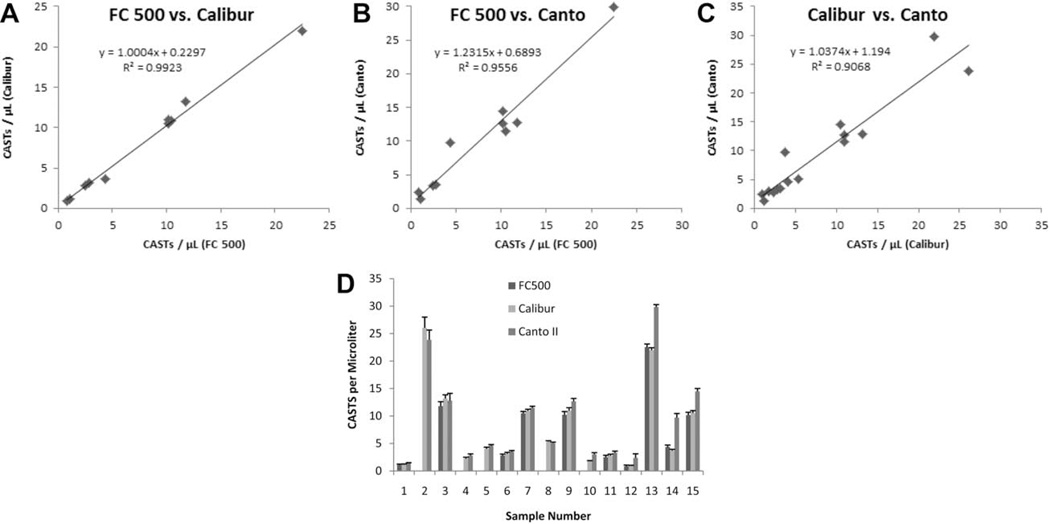
Intrinsic variability exists amongst the performance characteristics of different commercially-available flow cytometers. To determine whether these differences influenced the measurement of CASTs when using Dextramer reagents, peripheral blood samples were assayed for CASTs in parallel on three different flow cytometers maintained at the same institution. Each plotted data point from the scatter plots in Figure 5A and 5C represents the mean of 10 replicates that were acquired for each sample, and reveal that an excellent correlation was obtained for the matched samples regardless of the measured CAST density. Subsequent linear regression analyses yielded R2-values of 0.9923 (FC 500 versus Calibur, n =10), 0.9556 (FC 500 versus Calibur, n = 10), and 0.9068 (Calibur versus Canto II, n = 15), indicating that the three instruments are equally capable of measuring Dextramer-labeled CASTs. ANOVA did not identify statistically-significant interinstrument differences amongst the average values of repeated CAST measurements for each donor (P = 0.8232), or when all independent measurements were analyzed (P =0.1255). Variability amongst the decuplet measurements for each of the 15 samples is presented in Figure 5D, which demonstrates that repeated measurements of CASTs on any instrument are highly reproducible.
Fig. 5.
The accurate measurement of CASTs with Dextramers is independent of the acquisition instrumentation employed. CASTs in peripheral blood samples from 10 or 15 patients were measured in parallel using 3 separate instruments (A–C). Regardless of instrument manufacturer or the use of analog or digital circuitry, the measured number of Dextramer positive CD8+ T cells µl−1 was consistent at high (>20), medium (>10 and <20) and low (<10) levels of CASTs for every combination of instruments. Linear regression analyses of the pooled data sets reveal R2 values of 0.9923 (FC 500 versus Calibur, n= 10), 0.9556 (FC 500 versus Calibur, n= 10), and 0.9068 (Calibur versus Canto II, n= 15), demonstrating the robustness of the Dextramer reagent in measuring antigen-specific T cells with different instruments. Decuplet measurements of CASTs µl−1, for each of the 10 or 15 samples were highly reproducible regardless of the instrument that was utilized (D). Error bars represent the standard deviation of the mean.
Reliability Between Operators
We next assessed assay variability when different operators analyzed the same samples using the same flow cytometer. The measurement of CMV-specific T cells with the Dextramer assay was found to be highly reproducible amongst individuals, at both low and high levels of CASTs. As summarized in Table 7, measures of central tendency and dispersion were consistent for two separate operators independently, and collectively; regardless of the density of CASTs in measured samples. Subsequent linear regression analysis for all combined data points reveals a Coefficient of Determination between the two operators of R2 = 0.9786 (data not shown).
Table 7.
Assay Reproducibility between Operators
| Low Number of CASTs µl−1 | ||||||
|---|---|---|---|---|---|---|
| CAST+ Cells µl−1 |
% CAST+ of Total CD8+ |
|||||
| Operator | Mean | SD | CV (%) | Mean | SD | CV (%) |
| 1a | 1.45 | 0.18 | 12 | 0.18 | 0.02 | 11 |
| 2a | 1.50 | 0.17 | 11 | 0.19 | 0.02 | 11 |
| 1+2b | 1.47 | 0.18 | 12 | 0.19 | 0.02 | 12 |
| High Number of CASTs µl−1 | ||||||
|---|---|---|---|---|---|---|
| CAST+ Cells µl−1 |
% CAST+ of Total CD8+ |
|||||
| Operator | Mean | SD | CV (%) | Mean | SD | CV (%) |
| 1a | 25.72 | 2.01 | 8 | 4.02 | 0.25 | 6 |
| 2a | 24.48 | 1.26 | 5 | 3.97 | 0.17 | 4 |
| 1+2b | 25.01 | 1.80 | 7 | 4.00 | 0.22 | 5 |
Mean, SD and %CV values for decuplet measurements from an individual operator at each CAST density (n=10).
Mean, SD and %CV values for pooled decuplet measurements from both operators at each CAST density (n=20).
Interlaboratory Reproducibility
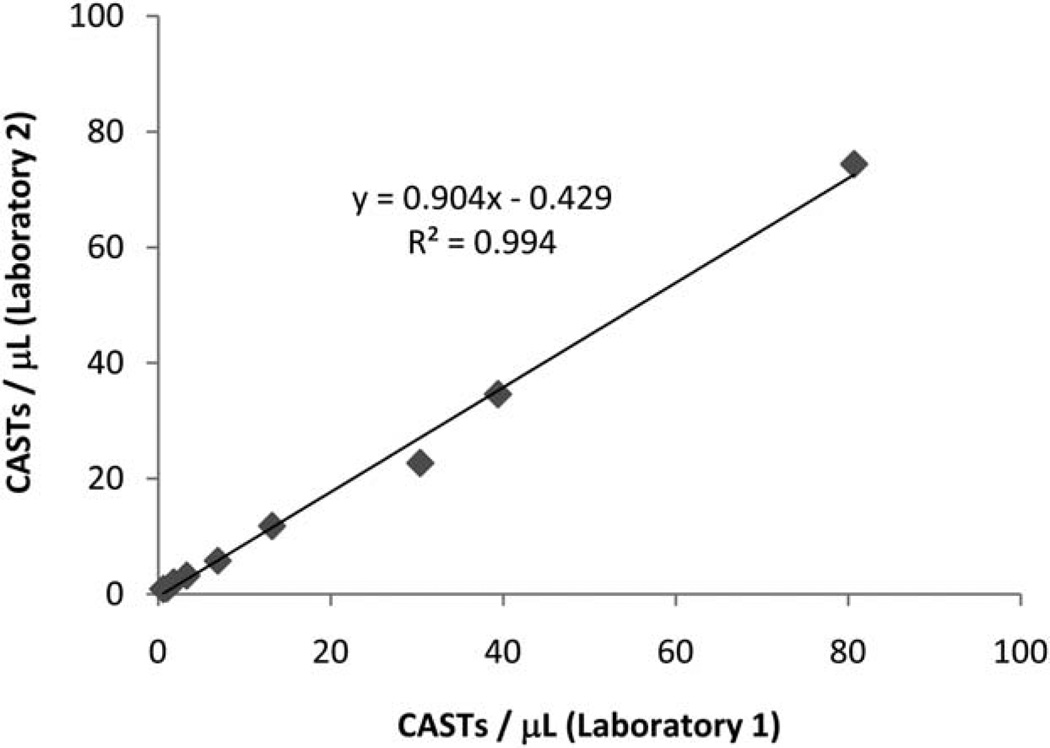
To evaluate assay reproducibility between different laboratories, blood specimens from three distinct CAST-positive donors with different HLA haplotypes were separately diluted with blood from a validated CAST-negative donor to generate simulated blood samples having either zero, or low, or medium, or high levels of CASTs as per Figure 6. These 9 samples were assayed for CASTs using HLA-matched Dextramer reagents by two separate laboratories that had been blinded to the sample compositions. As indicated in Figure 6, linear regression analysis demonstrates that a close correlation (R2 = 0.9947) exists between the measured level of CASTs performed by either of the two laboratories.
Fig. 6.
Low inter-lab variability is obtained when Dextramer reagents are used to measure CASTs. Nine peripheral blood samples were assayed in parallel at two different laboratories to determine the amount of intra-lab variability that existed when measuring CASTs using Dextramers. The absolute number of CMV-specific T cells µl−1 of blood were determined for each sample at both facilities. Linear regression analysis of the plotted data reveals a close correlation (R2 = 0.9947) for these measurements, indicating that Dextramers can be used for reliable enumeration of CASTs in different laboratory environments.
DISCUSSION
A number of investigations have utilized flow cytometry to measure the breadth and kinetics of immune reconstitution post-BMT; and have studied the impact of these metrics on the course of clinical outcome, the development of graft-versus-host disease (GvHD) and in the prevention of opportunistic infection (24–30). In recent years, the measurement of antigen-specific T cells via the use of MHC multimers has refined the study of leukocyte reconstitution post-transplant, and represents an important advancement in the prevention of CMV disease. Recent studies have benefitted from the ability to detect antigen-specific T cells from an expanded group of HLA haplotypes, thus extending these investigations to include larger cohorts of the study population (9). We predict that increased availability of reagents to probe adaptive immune responses will facilitate the development and implementation of improved experimental research, clinical trials, and protocols for the management of infectious disease. In accordance with this, and in the course of this investigation, we have ascertained that Dextramers represent a valuable technology that when compared to existing Tetramer reagents, are equally-capable of enumerating CMV positive cells; however a greater diversity of Dextramer reagents exist, which allows for the measurement of T cells from a larger repertoire of HLA alleles.
Accuracy, Linearity, and Reportable Range
Our data demonstrate that Dextramers can be used to detect CASTs at both high and low levels in peripheral blood. Through the use of titration experiments, we successfully measured CASTs across a broad dynamic range, which was consistent amongst the measured MHC haplotypes. We further verified that the assay sensitivity was higher than the level of noise that was obtained, when measuring CASTs in samples via two separate negative control reagents. The background labeling of CD81 cells with either Negative Control 1 or Negative Control 2 reagents could be further improved by the inclusion of a viability reagent in the assay; which yielded a statistically-significant reduction in both the number and percentage of CASTs that were detected with either control reagent. The specificity of Dextramer reagents was tested and we validated that Dextramers fail to bind T cells from CAST-negative patients. These findings are consistent with results which have previously been obtained with Tetramer reagents (31).
In clinical and research settings, leukocyte test articles for CAST analysis are derived from different sources including bone marrow, peripheral whole blood samples, and from peripheral blood mononuclear cell preparations. We found no statistically-significant difference between the measured level of CASTs obtained from matched CMV-seropositive donors in samples from whole blood or CPT tubes, indicating that Dextramers can be employed in protocols utilizing either cell source. Data from matched samples acquired on different instrumentation in the same laboratory, or by different operators in different laboratories further confirmed the robustness of the Dextramer assay, with a high degree of reproducibility obtained from both studies. Collectively, these data establish that Dextramers can selectively bind to CMV-specific T cells with prodigious specificity and fidelity; which when combined with fluorescent counting beads constitute a robust assay for enumerating CASTs. Finally, Dextramer reagents demonstrate durable structural stability and functional binding characteristics that are consistent for at least one calendar year when stored at 2–8°C.
Methods Comparison
In a comparative study, we concluded that Dextramers are as accurate as iTAg ™ Tetramers in detecting CASTs, particularly at low levels where enumerating antigen-specific T cells is most challenging and important for clinical decision making. We also determined that Dextramers are qualitatively similar to the oft-studied Tetramers, in terms of how easily CAST-positive CD8+ T cells can be discriminated from CAST-negative CD8+ T cells, and in our stability study that demonstrates similar durations of shelf-life (32). Accordingly, we propose that Dextramers represent a suitable alternative to the use of Tetramers in the enumeration of CASTs in peripheral blood samples from BMT recipients.
ACKNOWLEDGMENTS
Joseph D. Tario, Jr. is an ISAC Scholar. The authors are grateful for the BMT recipients who provided their blood samples for this study. Liselotte Brix is the Chief Operating Officer of Immudex. Charlotte Halgreen and Kivin Jacobsen are employed by Immudex and generated the data for Table 7, Figure 4, and Figure 6. The other coauthors declare no competing financial interests.
Grant sponsor: National Cancer Institute (NCI) Cancer Center; Grant number: 5P30 CA016056.
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- 1.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Matsui K, Boniface JJ, Reay PA, Schild H, Fazekas de St Groth B, Davis MM. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991;254:1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- 3.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 4.Wooldridge L, Lissina A, Cole DK, van den Berg HA, Price DA, Sewell AK. Tricks with tetramers: How to get the most from multimeric peptide-MHC. Immunology. 2009;126:147–164. doi: 10.1111/j.1365-2567.2008.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker AH, Schumacher TN. MHC multimer technology: current status and future prospects. Curr Opin Immunol. 2005;17:428–433. doi: 10.1016/j.coi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Castagnola E, Cappelli B, Erba D, Rabagliati A, Lanino E, Dini G. Cytomegalovirus infection after bone marrow transplantation in children. Hum Immunol. 2004;65:416–422. doi: 10.1016/j.humimm.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Stocchi R, Ward KN, Fanin R, Baccarani M, Apperley JF. Management of human cytomegalovirus infection and disease after allogeneic bone marrow transplantation. Haematologica. 1999;84:71–79. [PubMed] [Google Scholar]
- 8.Takenaka K, Gondo H, Tanimoto K, Nagafuji K, Fujisaki T, Mizuno S, Miyamoto T, Okamura T, Hayashi S, Eto T, et al. Increased incidence of cytomegalovirus (CMV) infection and CMV-associated disease after allogeneic bone marrow transplantation from unrelated donors. The Fukuoka Bone Marrow Transplantation Group. Bone Marrow Transplant. 1997;19:241–248. doi: 10.1038/sj.bmt.1700637. [DOI] [PubMed] [Google Scholar]
- 9.Gratama JW, Boeckh M, Nakamura R, Cornelissen JJ, Brooimans RA, Zaia JA, Forman SJ, Gaal K, Bray KR, Gasior GH, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: A prospective multicenter study. Blood. 2010;116:1655–1662. doi: 10.1182/blood-2010-03-273508. [DOI] [PubMed] [Google Scholar]
- 10.Abate D, Cesaro S, Cofano S, Fiscon M, Saldan A, Varotto S, Mengoli C, Pillon M, Calore E, Biasolo MA, et al. Diagnostic utility of human cytomegalovirus-specific T-cell response monitoring in predicting viremia in pediatric allogeneic stem-cell transplant patients. Transplantation. 2012;93:536–542. doi: 10.1097/TP.0b013e31824215db. [DOI] [PubMed] [Google Scholar]
- 11.Borchers S, Bremm M, Lehrnbecher T, Dammann E, Pabst B, Wolk B, Esser R, Yildiz M, Eder M, Stadler M, et al. Sequential anti-cytomegalovirus response monitoring may allow prediction of cytomegalovirus reactivation after allogeneic stem cell transplantation. PLoS One. 2012;7:e50248. doi: 10.1371/journal.pone.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: The national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50:1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 14.Ho M. Epidemiology of cytomegalovirus infections. Rev Infect Dis. 1990;12(Suppl 7):S701–S710. doi: 10.1093/clinids/12.supplement_7.s701. [DOI] [PubMed] [Google Scholar]
- 15.Yao J, Bechter C, Wiesneth M, Harter G, Gotz M, Germeroth L, Guillaume P, Hasan F, von Harsdorf S, Mertens T, et al. Multimer staining of cytomegalovirus phosphoprotein 65-specific T cells for diagnosis and therapeutic purposes: A comparative study. Clin Infect Dis. 2008;46:e96–e105. doi: 10.1086/587749. [DOI] [PubMed] [Google Scholar]
- 16.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 17.Gratama JW, Brooimans RA, van der Holt B, Sintnicolaas K, van Doornum G, Niesters HG, Lowenberg B, Cornelissen JJ. Monitoring cytomegalovirus IE-1 and pp65-specific CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation may identify patients at risk for recurrent CMV reactivations. Cytometry Part B Clin Cytom. 2008;74:211–220. doi: 10.1002/cyto.b.20420. [DOI] [PubMed] [Google Scholar]
- 18.de Jongste AH, de Graaf MT, van den Broek PD, Kraan J, Sillevis Smitt PA, Gratama JW. Effector memory and late memory T cells accumulate in the blood of CMV-carrying individuals but not in their cerebrospinal fluid. Cytometry Part B Clin Cytom. 2013;84:218–221. doi: 10.1002/cyto.b.21073. [DOI] [PubMed] [Google Scholar]
- 19.Pang XL, Chui L, Fenton J, LeBlanc B, Preiksaitis JK. Comparison of LightCycler-based PCR, COBAS amplicor CMV monitor, and pp65 antigenemia assays for quantitative measurement of cytomegalovirus viral load in peripheral blood specimens from patients after solid organ transplantation. J Clin Microbiol. 2003;41:3167–3174. doi: 10.1128/JCM.41.7.3167-3174.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentile G, Picardi A, Capobianchi A, Spagnoli A, Cudillo L, Dentamaro T, Tendas A, Cupelli L, Ciotti M, Volpi A, et al. A prospective study comparing quantitative Cytomegalovirus (CMV) polymerase chain reaction in plasma and pp65 antigenemia assay in monitoring patients after allogeneic stem cell transplantation. BMC Infect Dis. 2006;6:167. doi: 10.1186/1471-2334-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boaretti M, Sorrentino A, Zantedeschi C, Forni A, Boschiero L, Fontana R. Quantification of cytomegalovirus DNA by a fully automated real-time PCR for early diagnosis and monitoring of active viral infection in solid organ transplant recipients. J Clin Virol. 2013;56:124–128. doi: 10.1016/j.jcv.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Cwynarski K, Ainsworth J, Cobbold M, Wagner S, Mahendra P, Apperley J, Goldman J, Craddock C, Moss PA. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97:1232–1240. doi: 10.1182/blood.v97.5.1232. [DOI] [PubMed] [Google Scholar]
- 23.Gratama JW, van Esser JW, Lamers CH, Tournay C, Lowenberg B, Bolhuis RL, Cornelissen JJ. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98:1358–1364. doi: 10.1182/blood.v98.5.1358. [DOI] [PubMed] [Google Scholar]
- 24.Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, Bryan SG, Kaur I, Martin S, Wieder ED, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, Ames S, Lerner S, Ebcioglu Z, Nair V, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10:2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staley EM, Tanner SM, Daft JG, Stanus AL, Martin SM, Lorenz RG. Maintenance of host leukocytes in peripheral immune compartments following lethal irradiation and bone marrow reconstitution: Implications for graft versus host disease. Transpl Immunol. 2013;28:112–119. doi: 10.1016/j.trim.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shima T, Miyamoto T, Kikushige Y, Mori Y, Kamezaki K, Takase K, Henzan H, Numata A, Ito Y, Takenaka K, et al. Quantitation of hematogones at the time of engraftment is a useful prognostic indicator in allogeneic hematopoietic stem cell transplantation. Blood. 2013;121:840–848. doi: 10.1182/blood-2012-02-409607. [DOI] [PubMed] [Google Scholar]
- 28.Le Bourgeois A, Lestang E, Guillaume T, Delaunay J, Ayari S, Blin N, Clavert A, Tessoulin B, Dubruille V, Mahe B, et al. Prognostic impact of immune status and hematopoietic recovery before and after fludarabine, IV busulfan, and antithymocyte globulins (FB2 regimen) reduced-intensity conditioning regimen (RIC) allogeneic stem cell transplantation (allo-SCT) Eur J Haematol. 2013;90:177–186. doi: 10.1111/ejh.12049. [DOI] [PubMed] [Google Scholar]
- 29.Darlington PJ, Touil T, Doucet JS, Gaucher D, Zeidan J, Gauchat D, Corsini R, Kim HJ, Duddy M, Jalili F, et al. Diminished Th17 (not Th1) responses underlie multiple sclerosis disease abrogation after hematopoietic stem cell transplantation. Ann Neurol. 2013;73:341–354. doi: 10.1002/ana.23784. [DOI] [PubMed] [Google Scholar]
- 30.de Pagter PJ, Boelens JJ, Jacobi R, Schuurman R, Nanlohy NM, Sanders EA, van Baarle D. Increased proportion of perforin-expressing CD8+T-cells indicates control of herpesvirus reactivation in children after stem cell transplantation. Clin Immunol. 2013;148:92–98. doi: 10.1016/j.clim.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Ravkov EV, Pavlov IY, Hanson KE, Delgado JC. Validation of cytomegalovirus immune competence assays for the characterization of CD8(+) T cell responses posttransplant. Clin Dev Immunol. 2012;2012:451059. doi: 10.1155/2012/451059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. http://www.mblintl.com/support/faq.aspx.