Abstract
Objective
We investigated the associations between reproductive and menstrual risk factors for breast cancer and mammographic density, a strong risk factor for breast cancer, in a predominantly ethnic minority and immigrant sample.
Methods
We interviewed women (42% African American, 22% African Caribbean, 22% White, 9% Hispanic Caribbean, 5% other) without a history of breast cancer during their mammography appointment (n = 191, mean age = 50). We used a computer-assisted method to measure the area and percentage of dense breast tissue from cranio-caudal mammograms. We used multivariable linear regression analyses to estimate the associations between reproductive and menstrual risk factors and mammographic density.
Results
Age was inversely associated with percent density and dense area, and body mass index (BMI) was inversely associated with percent density. Adjusting for age, BMI, ethnicity and menopausal status, later age at menarche (e.g., β = −7.37, 95% CI: −12.29, −2.46 for age ≥13 years vs. ≤ 11 years), and any use of hormonal birth control (HBC) methods (β = −5.10, 95% CI: −9.37, −0.84) were associated with reduced dense area. Ethnicity and nativity (foreign- vs. US-born) were not directly associated with density despite variations in the distribution of several risk factors across ethnic and nativity groups.
Conclusions
The mean level of mammographic density did not differ across ethnic and nativity groups, but several risk factors for breast cancer were associated with density in ethnic minority and immigrant women.
Keywords: Breast cancer, Mammographic density, Risk factors, Ethnicity, Nativity status
Introduction
Mammographic density represents the extent of radiologically dense breast tissue, composed primarily of epithelial and stromal tissue [1]. Higher mammographic density, measured through quantitative estimates of the area and percent of dense tissue (dense area and percent density, respectively) or through categorical estimates (e.g., Breast Imaging Reporting and Data System [BIRAD] categories), has been strongly and independently associated with increased risk of breast cancer [1–5]. Many risk factors for breast cancer also similarly influence mammographic density [1, 3, 6–10], and changes in mammographic density have been shown to lead to changes in breast cancer risk [11]. Despite the growing research on mammographic density and the potential for targeting mammographic density in breast cancer prevention efforts, most studies of mammographic density have not focused on ethnic minority and immigrant women.
Breast cancer incidence varies substantially across ethnic groups in the United States (US) with most ethnic minority women showing a lower lifetime risk than white women [12]. Recent evidence has also revealed ethnic differences in predominant breast tumor features such as tumor grade and hormone-receptor status, with young African American women having the highest prevalence of more aggressive breast cancer tumor types [13, 14]. To date, only a few studies have compared mammographic density patterns among African American and Hispanic women to those in other ethnic groups [9, 15– 18]. These studies have produced conflicting results for comparisons between African American and other ethnic groups [15–18], but have generally reported similar mammographic densities in Hispanic and non-Hispanic white women [16, 18, 19]. Furthermore, although several studies have investigated variations in mammographic density among subgroups of Asian and Pacific Islander women in the US by country of origin or acculturation [20–22], no study has explored the heterogeneity that may exist within the African American and Hispanic populations with respect to breast cancer risk factors and mammographic density.
Women of Caribbean descent in the United States are likely to be grouped with African Americans or Hispanics in most studies. Differences in cultural and socioeconomic factors as well as migration history in the Caribbean population when compared with other African American/Black and Hispanic groups may suggest distinct risk profiles across these ethnic populations [23–29]. Only one study conducted in the U.K. has specifically compared mammographic density patterns in women of Caribbean origin to other ethnic groups and reported that African Caribbean women had lower mammographic density than white women but higher mammographic density than South Asian women living in the U.K. [30]. We undertook a study of mammographic density in relation to menstrual and reproductive risk factors for breast cancer in a multiethnic sample of African American, white, and Caribbean women living in New York City.
Methods
Study setting and population
The New York City Multiethnic Breast Cancer Project is a collaborative study between Columbia University in Manhattan, and Long Island University and Long Island College Hospital (LICH) in Brooklyn. Study participants are 200 women who were recruited during screening mammography appointments, in the Radiology Department of LICH from January 2007 to April 2008; recruitment occurred on two days per week when mammograms were performed. Participants provided a signed medical release form to allow us to retrieve their mammograms and provided information on epidemiologic risk factors and sociodemographic characteristics. In the current analysis, we excluded five participants who reported a prior history of breast cancer and four participants whose mammograms were unavailable and/or of poor image quality (n = 191). The Internal Review Boards at all three participating institutions approved this study, and all participants provided written informed consent.
Epidemiologic risk factors
We collected epidemiologic data through a 30 to 45-minute in-person interview, including information on personal and family medical history, body size, reproductive history, and sociodemographic factors. Body mass index (BMI) was calculated as self-reported weight in kilograms (converted from pounds by multiplying by 0.454) divided by height squared in meters (converted from inches by multiplying by 0.0254). Data on gynecologic surgeries and recent menstrual history were used to categorize menopausal status into postmenopausal (women with 12 or more months between the dates of interview and last menstrual period, including women with bilateral oophorectomy) and pre-/perimenopausal status (women with less than 12 months between the dates of interview and last menstrual period, hereafter referred to as “premenopausal”). If data on the date of last menstrual period and reproductive surgery were not available (11.5% of participants), we classified women younger than 54 years as premenopausal and women 54 years or older as postmenopausal, based on the 90th percentile of age of natural menopause among postmenopausal women in the sample. The number and outcome of each pregnancy, age at each pregnancy, and breastfeeding duration for each live birth was used to develop measures of gravidity (number of pregnancies and categorized into 0, 1–2, 3, ≥4 pregnancies), parity (number of full-term pregnancy defined as pregnancies lasting 28 weeks or longer; categorized into no full-term pregnancies/nulliparous, 1 full-term pregnancy, ≥2 full-term pregnancies), age at first full-term pregnancy (FFTP, in years), and lifetime lactation history (nulliparous, parous and never breastfed, parous and breastfed ≤12 months, parous and breastfed >12 months). Exogenous hormonal exposure was measured by past and current use of hormone replacement therapy (HRT) and hormonal birth control (HBC) methods (e.g., pills, shots, patches, and implants). We developed the following variables based on these data: Ever use of HRT, ever use of HBC, duration of HBC use (never used,<5 years, ≥5 years), and HBC use in relation to parity (use after FFTP, use before FFTP, parous but never use, nulliparous). A positive family history of breast cancer was indicated by any history of breast cancer in participant’s mother, father, or full biological siblings.
Racial and ethnic groups were categorized based on self-reported racial group, Hispanic origin, and participants’ and their parents place of birth. We considered participants to be Caribbean if they reported a Caribbean country as their birthplace or the birthplace of at least one parent. We further divided this group into African Caribbean (from English- and Creole-speaking Caribbean countries, e.g., Jamaica, Haiti, Barbados and Tobago) and Hispanic Caribbean (from Spanish speaking Caribbean countries, e.g., Dominican Republic, Puerto Rico). Other participants were classified as non-Caribbean Hispanic, non-Hispanic non-Caribbean African American (African American), non-Hispanic non-Caribbean white (white) and other ethnicities. We also used birthplace data to categorize participants into two groups of US-born and foreign-born women (nativity). Other sociodemographic variables included age (in years) and highest educational attainment (high school or less education, some college or associate degree, and college degree or higher education).
Mammographic density assessment
We used mammograms taken on the day of study interview data for 84% (n = 160) of participants. We used mammograms from a different date if the interview was partially completed on a later date, or mammograms taken on the date of interview were of poor image quality or not available for shipment to Columbia University. The median number of days between the dates of mammogram and interview for these 31 participants was 14 days. We assessed mammographic density using the left craniocaudal view mammograms in all but 4 participants who only had right breast mammograms available. There is a high correlation between density in right and left breast as reported in previous research [31]. We digitized all films using a Kodak Lumisys Film Digitizer (Kodak LS85) and measured mammographic density using Cumulus, a thresholding computer software. A single expert reader, blinded to all other study data, outlined the total breast area and dense area on the digitized image of the mammogram, and the software measured the sizes of these areas by identifying the number of pixels within the outlined areas. Percent density was calculated as dense area divided by total breast area, expressed as a percentage, and total dense area was calculated by converting the number of pixels into cm2. We read one film per participant, in batches of approximately 50 films. We read a 10% randomly selected sample of films twice and obtained Pearson correlation coefficients of 0.99 for breast area and 0.9 for dense area for the repeated readings.
Statistical analyses
We performed analysis of variance and chi-square tests to determine whether the distribution of risk factors differed between ethnic and nativity groups. We used linear regression models to examine the associations between measured risk factors and percent density and dense area as continuous variables. We first examined unadjusted associations and age- and BMI-adjusted associations between each risk factor and each density measure. The multivariable models included race/ethnicity, menstrual and reproductive factors, and any confounding variable that changed the estimate of these factors by at least 10%. We also explored whether ethnicity and/or nativity modified the association between each risk factor and density by including an interaction term between each of these variables (i.e., ethnicity and nativity) and each risk factor (e.g., parity, family history, BMI) in separate model; a statistical additive interaction was indicated if the P-value for the interaction term was less than 0.05. Participants with a missing value on any variables were excluded from the analyses. All variables had missing data less than 3%, with the exception of duration of HBC use which had a 4.7% missing data.
Results
The study sample included 191 women with an average age of 50.0 years (standard deviation [SD] ± 5.7, range: 39.8–60.9) at the time of the interview and an average BMI of 29.8 kg/m2 (SD ± 6.7, range: 17.6, 61.5). About two-thirds of participants were either pre- or perimenopausal, and only 16 women reported having ever used HRT. Among the 135 parous participants, the average age at FFTP was 23.0 (SD ± 7.0, range: 13–45) and 56% had breastfed their children. Participants represented 6 ethnic groups: African American (42%), African Caribbean (22%), white (22%), Hispanic Caribbean (9%), Hispanic non-Caribbean (3%), and other (2%). Due to small sample size in some groups, we limited our analyses of ethnic differences in density to the three largest ethnic groups of African American (n = 81), African Caribbean (n = 42), and white (n = 41). Over one-third of the participants were born in a foreign country, and 88% of African Caribbean women were foreign-born. The distribution of breast cancer risk factors varied by ethnicity with African American and African Caribbean women having a higher BMI, higher gravidity, higher parity and earlier age at .FFTP, when compared with white women (Table 1). Other ethnic differences included a lower prevalence of breastfeeding and lower educational attainment among African American women, and a higher prevalence of first HBC use before age 21 among white women. There were also differences by nativity with foreign-born women being more likely to have breastfed but less likely to have ever used HBC and used HBC before FFTP when compared with US-born women (Table 1).
Table 1.
Sample characteristics of New York City Multiethnic Breast Cancer Project (n = 191); 2007–2008
| Overall sample (N = 191) Mean(SD)/ percent (n) |
African American (N = 81) Mean (SD)/ percent (n) |
African Caribbean (N = 42) Mean (SD)/ percent (n) |
White (N = 41) Mean (SD)/ percent (n) |
US-born (N = 122) Mean (SD)/ percent (n) |
Foreign-born (N = 68) Mean (SD)/ percent (n) |
|
|---|---|---|---|---|---|---|
| Age at interview (year) | 50.0 (5.7) | 50.2 (5.6) | 49.5 (5.5) | 50.1 (5.9) | 50.1 (5.9) | 49.8 (5.4) |
| Body mass index (kg/m2) | 29.8(6.7) | 31.5 (6.6)a | 30.1 (6.8) | 25.5 (4.8) | 30.3 (6.9) | 28.7 (6.3) |
| Race/ethnicity | ||||||
| African American | 42.4 (81) | n/a | n/a | n/a | 61.5 (75)b | 7.4 (5) |
| African Caribbean | 22.0 (42) | 4.1 (5) | 54.4 (37) | |||
| White | 21.5 (41) | 26.2 (32) | 13.2 (9) | |||
| Hispanic Caribbean | 9.4 (18) | 6.6 (8) | 14.7 (10) | |||
| Hispanic non-Caribbean | 3.1 (6) | <1 (1) | 7.4 (5) | |||
| Other | 1.6 (3) | <1 (1) | 2.9 (2) | |||
| Nativity | ||||||
| US-born | 64.2 (122) | 93.8 (75)a | 11.9 (5) | 78.0 (32) | N/A | N/A |
| Foreign-born | 35.8 (68) | 6.3 (5) | 88.1 (37) | 22.0 (9) | ||
| Positive family history of breast cancer | 13.2 (25) | 11.1 (9) | 7.3 (3) | 9.8 (4) | 12.3 (15) | 14.9 (10) |
| Age at menarche (year) | 12.4 (1.8) | 12.5 (1.7) | 12.7 (2.0) | 12.8 (1.6) | 12.3 (1.7) | 12.6 (2.0) |
| Gravidity | 2.8 (1.9) | 2.8 (2.0)a | 3.2 (2.0) | 2.1 (1.9) | 2.6 (1.9) | 3.0 (1.9) |
| Parity | 1.6 (1.5) | 1.6 (1.6) | 1.7 (1.5) | 1.0 (1.1) | 1.4 (1.4)b | 1.9 (1.6) |
| Age at first full-term pregnancy (in parous women, year) |
23.0 (7.0) | 21.3 (6.9)a | 21.7 (5.9) | 29.1 (6.2) | 22.7 (6.8) | 23.3 (7.3) |
| Ever breast fed | 39.5 (75) | 27.5 (22)a | 61.9 (26) | 39.0 (16) | 28.9 (35)b | 58.8 (40) |
| Breastfeeding duration | ||||||
| Nulliparous | 29.1 (55) | 29.1 (23)a | 26.2 (11) | 43.9 (18) | 31.7 (38)b | 23.5 (16) |
| Parous, never breastfed | 31.7 (60) | 44.3 (35) | 11.9 (5) | 17.1 (7) | 40.0 (48) | 17.6 (12) |
| Parous, breastfed 1–12 months | 20.6 (39) | 16.5 (13) | 35.7 (15) | 7.3 (3) | 14.2 (17) | 32.4 (22) |
| Parous, breastfed >12 months | 18.5 (35) | 10.1 (8) | 26.2 (11) | 31.7 (13) | 14.2 (17) | 26.5 (18) |
| Ever hormonal birth control (HBC) use | 68.6 (131) | 71.6 (58) | 57.1 (24) | 70.7 (29) | 76.2 (93)b | 54.4 (37) |
| Duration of HBC use (month) | 66.4 (67.6) | 66.8 (69.7) | 47.6 (46.8) | 83.4 (68.8) | 71.3 (70.1) | 55.9 (60.9) |
| Age at first HBC use (in users) | ||||||
| ≤20 years | 58.5 (76) | 56.1 (32)a | 45.8 (11) | 82.8 (24) | 65.2 (60)b | 43.2 (16) |
| >20 years | 41.5 (54) | 43.9 (25) | 54.2 (13) | 17.2 (5) | 34.8 (32) | 56.8 (21) |
| Time since last HBC use (in users) | ||||||
| >30 years | 24.8 (31) | 27.3 (15) | 20.8 (5) | 25.9 (7) | 27.3 (24) | 19.4 (7) |
| >20 years to ≤30 years | 44.0 (55) | 49.1 (27) | 45.8 (11) | 29.7 (8) | 43.2 (38) | 44.4 (16) |
| >10 years to ≤20 years | 20.0 (25) | 16.4 (9) | 25.0 (6) | 25.9 (7) | 18.2 (16) | 25.0 (9) |
| Current user or ≤10 years | 11.2 (14) | 7.3 (4) | 8.4 (2) | 18.5 (5) | 11.3 (10) | 11.1 (4) |
| HBC use in relation to parity (in parous women) | ||||||
| Parous but never used | 26.9 (36) | 25.0 (14)a | 35.5 (11) | 17.4 (4) | 19.5 (16)b | 38.5 (20) |
| Used before first full-term pregnancy | 41.0 (55) | 41.1 (23) | 25.8 (8) | 78.3 (18) | 48.8 (40) | 28.9 (15) |
| Used after first full-term pregnancy | 32.1 (43) | 33.9 (19) | 38.7 (12) | 4.3 (1) | 31.7 (26) | 32.7 (17) |
| Menopausal status | ||||||
| Pre-/peri-menopausal | 64.9 (124) | 59.3 (48) | 73.8 (31) | 73.2 (30) | 63.1 (77) | 69.1 (47) |
| Postmenopausal | 35.1 (67) | 40.7 (33) | 26.2 (11) | 26.8 (11) | 36.9 (45) | 30.9 (21) |
| Education | ||||||
| ≤High school | 29.1 (55) | 35.0 (28)a | 23.8 (10) | 10.0 (4) | 27.5 (33) | 32.4 (22) |
| Some college/associate deg | 33.9 (64) | 38.8 (31) | 50.0 (21) | 17.5 (7) | 32.5 (39) | 35.3 (24) |
| ≥College degree | 37.0 (70) | 26.3 (21) | 26.2 (11) | 72.5 (29) | 40.0 (48) | 32.4 (22) |
SD, standard deviation; HBC, hormonal birth control methods
p < 0.05 for comparison across three ethnic groups
p < 0.05 for US-born versus foreign-born
Table 2 shows the univariable linear regression coefficient and 95% confidence intervals (CI) for each menstrual and reproductive factor and the two measures of density (percent density and dense area). Increasing age and postmenopausal status were significantly associated with both reduced percent density and dense area. A family history of breast cancer and later age at FFTP were associated with higher percent density whereas increasing BMI, lower levels of education and HBC use after FFTP were associated with lower percent density. Later age at menarche, HBC use, duration and use after FFTP were also associated with smaller dense area.
Table 2.
Unadjusted linear regression estimates of percent density and dense area (cm2), New York City Multiethnic Breast Cancer Project (n = 191), 2007–2008
| Percent Density β |
95% CI | Dense Area β |
95% CI | |
|---|---|---|---|---|
| Age (year) | −0.67 | −0.95, −0.40 | −0.85 | −1.19, −0.50 |
| Body mass index (kg/m2) | −0.65 | −0.87, −0.42 | −0.30 | −0.61, 0.01 |
| Race/ethnicity | ||||
| African Caribbean | 1.01 | −3.25, 5.26 | 1.03 | −4.43, 6.50 |
| White | 3.90 | −0.39, 8.19 | 0.35 | −5.16, 5.85 |
| Other | −1.41 | −6.38, 3.56 | −4.04 | −10.42, 2.35 |
| African American | Reference | Reference | ||
| Nativity | ||||
| Foreign-born | 0.91 | −2.50, 4.32 | 1.23 | −3.13, 5.58 |
| US-born | Reference | Reference | ||
| Family history of breast cancer | 4.88 | 0.09, 9.67 | 3.93 | −2.08, 9.94 |
| Age at menarche | ||||
| ≥13 | 0.88 | −3.09, 4.86 | −4.17 | −9.20, 0.87 |
| 12 | 1.19 | −3.25, 5.62 | −2.80 | −8.43, 2.82 |
| ≤11 | Reference | Reference | ||
| Gravidity | ||||
| ≥4 | 0.55 | −4.72, 5.81 | −1.36 | −8.05, 5.33 |
| 3 | −1.39 | −7.13, 4.36 | −4.76 | −12.06, 2.55 |
| 1,2 | 1.11 | −4.14, 6.36 | −0.35 | −7.03, 6.32 |
| 0 | Reference | Reference | ||
| Parity | ||||
| ≥2 full-term child | −2.97 | −6.85, 0.92 | −5.20 | −10.14, −0.26 |
| 1 full-term child | 0.91 | −3.41, 5.23 | −0.72 | −6.21, 4.78 |
| Nulliparous | Reference | |||
| Age at first full-term pregnancy (adjusted for parity) | 0.31 | 0.04, 0.59 | 0.21 | −0.15, 0.56 |
| Breastfeeding duration | ||||
| Parous, breastfed >12 months | 0.33 | −4.55, 5.20 | −3.38 | −9.60, 2.83 |
| Parous breastfed 1–12 months | −1.81 | −6.53, 2.91 | −3.47 | −9.48, 2.55 |
| Parous, never breastfed | −2.11 | −6.32, 2.10 | −3.29 | −8.66, 2.07 |
| Nulliparous | Reference | Reference | ||
| Ever Hormonal birth control use | −2.53 | −6.02, 0.96 | −6.35 | −10.74, −1.96 |
| Duration of hormonal birth control use | ||||
| ≥5 years | −1.99 | −6.26, 2.27 | −6.63 | −11.94, −1.31 |
| <5 years | −3.14 | −7.09, 0.80 | −6.60 | −11.52, −1.69 |
| Never used | Reference | Reference | ||
| HBC use in relation to parity | ||||
| Used after first full-term pregnancy | −7.71 | −12.08, −3.33 | −10.00 | −15.63, −4.37 |
| Used before first full-term pregnancy | 1.45 | −2.65, 5.55 | −1.66 | −6.94, 3.62 |
| Parous but never used | 1.62 | −2.98, −6.23 | 1.78 | −4.15, 7.71 |
| Nulliparous | Reference | Reference | ||
| Menopausal status | ||||
| Postmenopausal versus pre-/perimenopausal | −6.99 | −10.26, −3.73 | −8.78 | −12.95, −4.61 |
| Education | ||||
| ≤High school | −4.50 | −8.50, −0.50 | −2.04 | −7.25, 3.17 |
| Some college or associate degree | −4.69 | −8.52, −0.85 | −1.22 | −6.22, 3.78 |
| Bachelor, masters or doctoral degree | Reference | Reference |
CI, confidence interval; HBC, hormonal birth control
As displayed in Table 3, adjustment for age and BMI reduced the magnitude of most associations for percent density and no other associations reached statistical significance. We further examined whether the associations of age and BMI with percent density were influenced by adjustment for ethnicity, menopausal status, and parity. Although menopausal status was not significantly associated with percent density after adjustment for age, it affected the parameter estimate for the association between age and percent density by over 10% and was therefore included in the final multivariable models.
Table 3.
Multivariable linear regression estimates of percent density, New York City Multiethnic Breast Cancer Project, 2007–2008
| Age- and BMI-adjusted models (n =191)a β |
95% CI | Final model (n = 187)b β |
95% CI | |
|---|---|---|---|---|
| Age (year) | n/a | −0.53 | −0.90,−0.16 | |
| Body mass index (kg/m2) | n/a | −0.59 | −0.82, −0.36 | |
| Race/ethnicity | ||||
| African Caribbean | −0.32 | −4.13, 3.49 | −0.49 | −4.34, 3.36 |
| White | 0.25 | −3.87, 4.38 | 0.06 | −4.11, 4.23 |
| Other | −2.18 | −6.69, 2.32 | −2.16 | −6.67, 2.35 |
| African American | Reference | Reference | ||
| Nativity | ||||
| Foreign-born vs. US-born | −0.25 | −3.31, 2.82 | ||
| Family history of breast cancer | 3.28 | −1.09, 7.65 | ||
| Age at menarche | ||||
| ≥13 | −1.34 | −4.93, 2.25 | ||
| 12 | −0.31 | −4.28, 3.65 | ||
| ≤11 | Reference | |||
| Gravidity | ||||
| ≥4 | 1.88 | −2.81, 6.56 | ||
| 3 | −0.17 | −5.25, 4.90 | ||
| 1,2 | 1.78 | −2.87, 6.42 | ||
| 0 | Reference | |||
| Parity | ||||
| ≥2 full-term child | −1.56 | −5.07, 1.95 | ||
| 1 full-term child | 1.01 | −2.86, 4.88 | ||
| Nulliparous | Reference | |||
| Age at first full-term pregnancy (adjusted for parity) | 0.05 | −0.21, 0.31 | ||
| Breastfeeding duration | ||||
| Parous, breastfed >12 months | −0.46 | −4.88, 3.96 | ||
| Parous breastfed 1–12 months | −1.09 | −5.35, 3.17 | ||
| Parous, never breastfed | −0.18 | −4.02, 3.66 | ||
| Nulliparous | Reference | |||
| HBC use | ||||
| Ever vs. never | −1.40 | −4.56, 1.76 | ||
| Duration of HBC use | ||||
| ≥5 years | −1.49 | −5.33, 2.36 | ||
| <5 years | −1.39 | −4.94, 2.16 | ||
| Never used | Reference | |||
| HBC use in relation to parity | ||||
| Used after first full-term pregnancy | −4.12 | −8.29, 0.04 | ||
| Used before first full-term pregnancy | 0.82 | −2.97, 4.61 | ||
| Parous but never used | 1.47 | −2.84, 5.78 | ||
| Nulliparous | Reference | |||
| Menopausal status | ||||
| Postmenopausal vs. Pre-/perimenopausal | −1.68 | −6.03, 2.67 | −1.56 | −6.01, 2.88 |
| Education | ||||
| ≤High school | −2.20 | −5.92, 1.52 | ||
| Some college or associate degree | −2.82 | −6.40, 0.76 | ||
| Bachelor, masters or doctoral degree | Reference |
CI, confidence interval; HBC, hormonal birth control
Sample size may vary due to missing data
Final model includes race/ethnicity, variables with statistically significant associations with density and confounding variables (see “Statistical analyses” )
Similar to results from models of percent density, adjusting for differences in age and BMI attenuated the associations between each risk factor and dense area (Table 4). In age- and BMI-adjusted models, we observed smaller dense area in women with later age at menarche (β = −6.10, 95% CI: −10.94, −1.27 and β =− 4.23, 95% CI: −9.58, 1.12 for age at menarche ≥13 and 12 vs. ≤11, respectively) and for women with previous HBC use (β = −5.19, 95% CI: −9.46, −0.93). Women who had used HBC for less than five years did not have a significantly different dense area when compared with women who used HBC for more than five years (β = −5.65, 95% CI: −10.80, −0.50 and β = −5.20, 95% CI: −9.96, −0.45, respectively, relative to non-users). Parous women who had used HBC after their FFTP had significantly lower dense area when compared with nulliparous women (β = −7.57, 95% CI: −13.23, −1.90) while parous women who used HBC before FFTP and parous women who never used HBC did not have significantly different dense area than nulliparous women (β = −2.03, 95% CI: −7.19, 3.13 and β = 1.05, 95% CI: −4.82, 6.92, respectively).
Table 4.
Multivariable linear regression estimates of dense area (cm2), New York City Multiethnic Breast Cancer Project, 2007–2008
| Age- and BMI-adjusted models (n =191)a β |
95% CI | Final modelb (n = 186) β |
95% CI | |
|---|---|---|---|---|
| Age (year) | n/a | −0.54 | 1.04, −0.03 | |
| Body mass index (kg/m2) | n/a | −0.30 | −0.61, 0.00 | |
| Race/ethnicity | ||||
| African Caribbean | −0.05 | −5.25, 5.15 | −0.80 | −5.96, 4.35 |
| White | −1.63 | −7.26, 3.99 | −1.43 | −6.98, 4.12 |
| Other | −4.63 | −10.77, 1.51 | −5.89 | −11.95, 0.16 |
| African American | Reference | Reference | ||
| Nativity | ||||
| Foreign-born vs. US-born | 0.59 | −3.60, 4.79 | ||
| Family history of breast cancer | 3.35 | −2.44, 9.14 | ||
| Age at menarche | ||||
| ≥13 | −6.10 | −10.94, −1.27 | −7.37 | −12.29, −2.46 |
| 12 | −4.23 | −9.58, 1.12 | −3.84 | −9.17, 1.49 |
| ≤11 | Reference | Reference | ||
| Gravidity | ||||
| ≥4 | −0.40 | −6.80, 5.99 | ||
| 3 | −3.99 | −10.91, 2.94 | ||
| 1,2 | 0.35 | −5.99, 6.68 | ||
| 0 | Reference | |||
| Parity | ||||
| ≥2 Full-term child | −4.13 | −8.92, 0.66 | ||
| 1 Full-term child | −1.11 | −6.39, 4.17 | ||
| Nulliparous | Reference | |||
| Age at first birth (adjusted for parity) | 0.04 | −0.32, 0.39 | ||
| Breastfeeding duration | ||||
| Parous, breastfed >12 months | −3.28 | −9.31, 2.74 | ||
| Parous breastfed 1–12 months | −3.34 | −9.14, 2.47 | ||
| Parous, never breastfed | −2.35 | −7.58, 2.88 | ||
| Nulliparous | Reference | |||
| HBC use | ||||
| Ever vs Never | −5.19 | −9.46, −0.93 | −5.10 | −9.37, −0.84 |
| Duration of HBC use | ||||
| ≥5 years | −5.65 | −10.80, −0.50 | ||
| <5 years | −5.20 | −9.96, −0.45 | ||
| Never used | Reference | |||
| HBC use in relation to parity | ||||
| Used after first full-term pregnancy | −7.57 | −13.23, −1.90 | ||
| Used before first full-term pregnancy | −2.03 | −7.19, 3.13 | ||
| Parous but never used | 1.05 | −4.82, 6.92 | ||
| Nulliparous | Reference | |||
| Menopausal status | ||||
| Postmenopausal vs. Pre-/perimenopausal | −2.75 | −8.70, 3.19 | −4.51 | −10.63, 1.61 |
| Education | ||||
| ≤High school | −0.77 | −5.90, 4.36 | ||
| Some college or associate degree | −0.29 | −5.22, 4.64 | ||
| Bachelor, masters or doctoral degree | Reference |
CI, confidence interval; HBC, hormonal birth control
Sample size may vary due to missing data
Final model includes race/ethnicity, variables with statistically significant associations with density and confounding variables (see “Statistical analyses” )
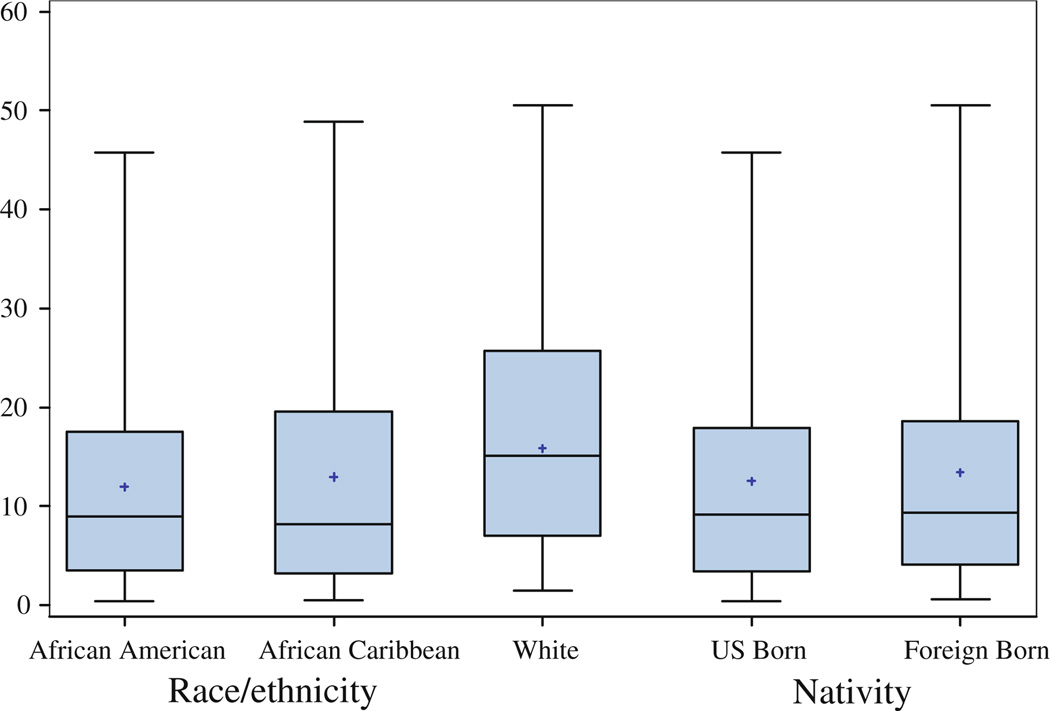
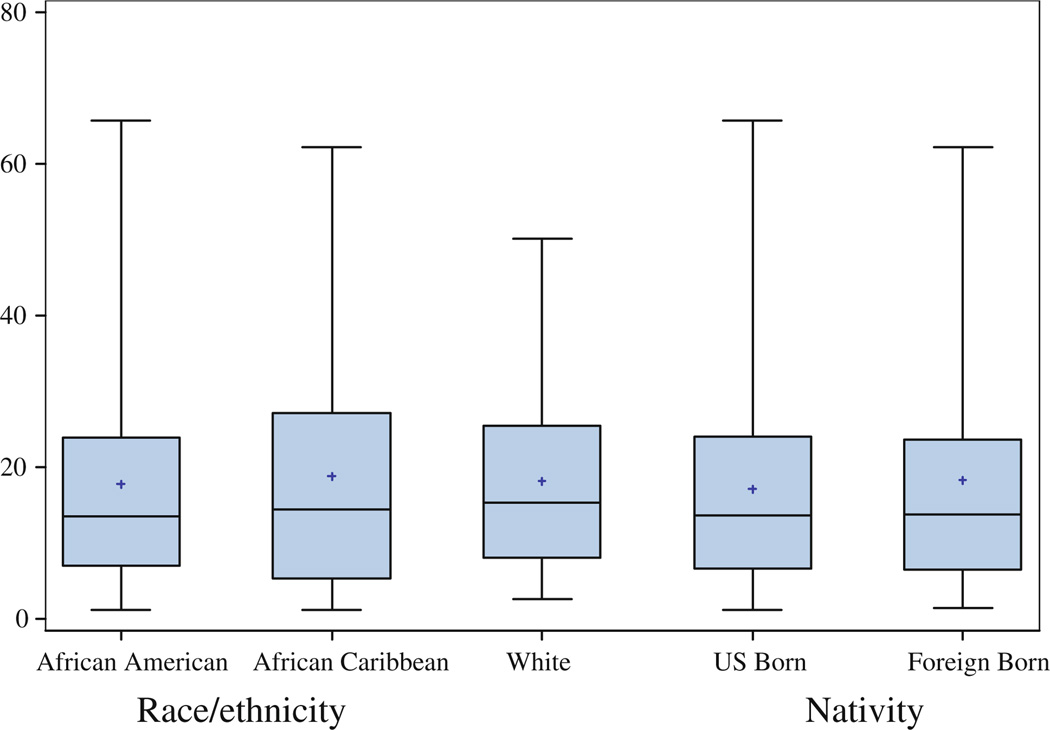
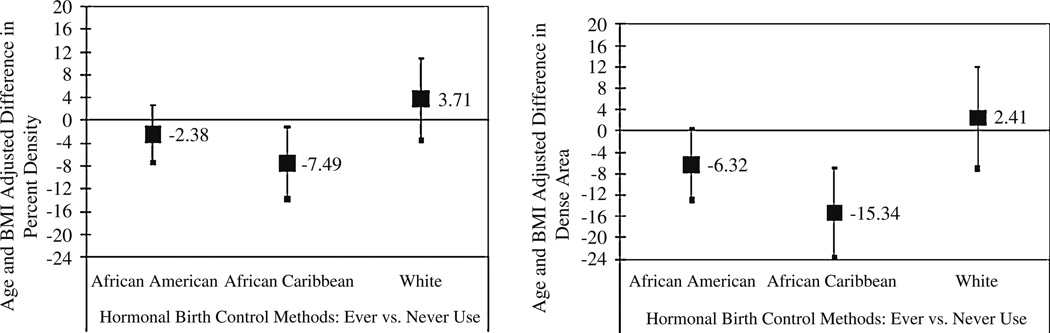
The three ethnic groups and the two nativity groups did not differ significantly in percent density or dense area in unadjusted or adjusted models (See Fig. 1, Fig. 2; Table 3, Table 4; e.g., Table 3, final model for Percent Density with African Americans as the reference group: β = 0.06, 95% CI: −4.11, 4.23 for white women; β = −0.49, 95% CI: −4.34, 3.36 for African Caribbean women; and β = −2.16, 95% CI: −6.67, 2.35 for the other ethnic group). We found evidence for a statistically significant interaction between ethnicity and HBC use for dense area, with inverse significant association for African Caribbean women (β = −15.34, 95% CI: −23.73, −6.95), and inverse borderline significant association for African American women (β = −6.32, 95% CI: −13.03, 0.38) (Fig. 3). The interaction effects of HBC use and ethnicity on percent density (P = 0.07), and of HBC use and nativity on both percent density (P = 0.25) and dense area (P = 0.12) did not reach statistical significance. While we also observed a significant interaction between family history and ethnicity (P = 0.01 and P = 0.05 for percent density and dense area, respectively), the small number of participants with a family history of breast cancer per ethnic group resulted in very large confidence intervals and imprecise estimates (only 3 African Caribbean women and 9 African American women reported a positive family history of breast cancer). Other variables including those in the final model (age, BMI, age at menarche and menopausal status) did not show an interaction effect with ethnicity or nativity on either measure of density.
Fig. 1.
Boxplot distribution of percent density by race/ethnicity and nativity status. The boxes show the 25th and 75th percentiles, the whiskers above and below the box show the range of the distribution, the middle horizontal lines show the median and the asterisks show the mean
Fig. 2.
Boxplot distribution of dense area (cm2) by race/ethnicity and nativity status. The boxes show the 25th and 75th percentiles, the whiskers above and below the box show the range of the distribution, the middle horizontal lines show the median and the asterisks show the mean
Fig. 3.
The associations between hormonal birth control use and mammographic density by ethnicity. Filled squares are coefficients. Error bars are 95% confidence intervals
Discussion
We investigated the associations between menstrual and reproductive factors and mammographic density in a multiethnic sample of middle-aged women. We examined both percent density and dense area, which respectively capture the relative and absolute amount of dense breast tissue. Studies that examine both percentage and absolute dense area have largely observed similar overall patterns with breast cancer risk factors. However, there are some differences in the magnitude of the associations as well as the type of risk factors associated with dense area and percent density across studies [32–35]. The reasons for these differences and their significance to breast cancer etiology remain largely unknown. Non-dense fat tissue and hence percent density are highly dependent on body size and weight [36, 37], factors that vary considerably by ethnicity [30, 38, 39]. In contrast, dense area has been less strongly and less consistently associated with body size and weight [19, 32, 40, 41]. Consistent with this research, BMI was strongly associated with percent density in our study, but it did not appear to significantly affect the extent of dense area. Age and postmenopausal status were inversely associated with both dense area and percent density although the associations for menopausal status did not reach statistical significance in multivariable models. We also observed significant associations between later age at menarche and hormonal birth control use and reduced dense area, after adjusting for age, BMI, and menopausal status. Furthermore, we did not observe significant differences in either dense area or percent density by ethnicity or nativity (i.e., direct associations between ethnicity and nativity and density), but found some evidence for ethnic differences in the associations between HBC use and dense area. (i.e., effect modification of association between HBC and dense area by ethnicity).
Menstrual and reproductive factors can influence mammographic density through exposures to endogenous and exogenous hormones, which may contribute to changes in breast tissue proliferation [42]. Research has more consistently reported associations between high mammographic density and nulliparity, later age at first birth and premenopausal status, while relatively less research has examined and/or consistently reported associations between mammographic density and menarcheal age and HBC use, primarily oral forms of HBC. Our finding that later age at menarche is associated with reduced dense area is consistent with the association between later age at menarche and reduced breast cancer risk. However, prior research has mainly found no associations [7, 30, 40, 43, 44] or positive associations [9, 35, 41, 45] between menarcheal age and mammographic density. It is possible that the association between age at menarche and mammographic density is affected by other exposures in later life periods. For example, earlier menarche is related to higher adult BMI [46–48], and adjustment for BMI in our study strengthened the inverse association between menarcheal age and dense area. Variations in the type of covariates considered in different studies as well as differences in study populations and density measures may account for the different findings reported.
We found that HBC use was associated with smaller dense area. Our measure of HBC included non-oral forms including hormonal patches and injections, but only 5.7% of HBC users had exclusively used non-oral forms of HBC (81.7% only used the oral form of HBC). As a result, our findings are likely to be comparable to most published results that have included measures of oral contraceptive or pill use. The effect of oral birth control use on breast cancer risk is believed to be transient and diminish over time with cessation of use [49]. The age at initiation of oral contraceptive use, particularly in relation to parity, may also play a role in the association between hormonal contraceptive use and breast cancer risk [50]. Only three women in our study currently used HBC and 14 (11.2%) had used HBC in the last 10 years. Duration of HBC use also did not appear to influence dense area but smaller dense area was observed particularly in women who initiated HBC use after their FFTP. Because the direction and strength of the association between HBC use and dense area varied by ethnicity in our sample, the differences in the ethnic composition of our study sample and those of other studies may at least partially explain the different results found in our study. Furthermore, the observed associations may reflect differences in the type and content of HBC methods that may exist across ethnic groups. Due to the small sample size in our study, we cannot explore these alternative explanations, including the role of statistical chance. Interestingly, a recent study also reported that ethnicity modified the association between oral contraceptive use and mammographic density among white women and first generation African Caribbean women in the U.K [30]. However, the direction of the associations between oral contraceptive use and density in the Caribbean women in that study was opposite to ours, with African Caribbean women living in the U.K. who had used oral contraceptive having a higher density than never users. This study reported no associations between oral contraceptive use and mammographic density for U.K. white women, which is similar to our results for white women [30].
We expected similar distributions of breast cancer risk factors for African Americans and African Caribbean women due to shared ancestral origin in West Africa (density is highly heritable [51]) as well as the expectation that African Caribbean immigrants may integrate into the African American communities and culture in the United States and therefore may share common environmental and lifestyle risk factors for breast cancer. The two groups were similar to each other and distinct from white women in terms of BMI, gravidity, parity, and age at first full-term pregnancy. The African Caribbean women in this study showed considerable heterogeneity with respect to the country of origin, which represented 8 countries. The best estimates from the GLOBOCAN program of the International Agency for Research in Cancer (IARC) suggests substantial variations in breast cancer risk within the Caribbean region; however, in all the seven Caribbean countries represented in 2002 in the GLOBOCAN program, breast cancer incidence rates were lower than the rates observed among US African American and white women in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registries [52]. We also did not find lower percent density or dense area in foreign-born immigrant women when compared with US-born women. It is possible that nativity affects mammographic density within each ethnic group, but we were not able to assess this, because in our sample only a few African Caribbean women were US-born and very few white and African Americans were foreign-born. It is also possible that ethnic differences in breast cancer risk involve pathways that do not include mammographic density.
Our overall study was limited by data on a single measure of mammographic density, and hence we were unable to examine if reproductive and menstrual factors were associated with changes in density over time. Mammographic density is not a static biologic marker; rather it declines with age, reflecting the aging of the breast tissue [53]. Risk factors that were not associated with density in our cross-sectional analyses, including ethnicity and nativity, may still influence the rate of decline in density over time, and thus affect the exposure to varying levels of density across the lifecourse. Studies with repeated measures of density are needed to better understand longitudinal changes in density and factors associated with these changes.
Our study strengths include the unique ethnic composition of the study population and thorough assessment of breast cancer risk factors collected at the time of mammography visit. The use of a single mammography location, with uniform mammography calibration and a small number of mammography technicians, and a standardized protocol for mammographic density assessment that included a single digitizer, computer, and expert reader also reduce the potential for errors in the measurement of density. The high mammography rates in New York City women (about 72% in whites, 80% in African American and 75% in Hispanics in the past two years, ages 40 and older) also reduce the limitations on the generalizability of findings arising from our recruitment of a screened population [54].
In conclusion, the mean level of mammographic density did not differ across ethnic and nativity groups, but several risk factors for breast cancer were associated with density in ethnic minority and immigrant women.
Acknowledgments
We would like to thank Diane Levy, Zoe Quandt, Wendy Lewis, Gladys Rivera, Joy White, Jessica Cabildo, and Renata Khanis for assisting with data collection and recruitment activities. Funded by grants from the National Cancer Institute (grant number U54 CA101598) and the National Institute of Environmental Health Sciences Center Support (grant number ES009089).
Contributor Information
Parisa Tehranifar, Department of Epidemiology, Columbia University Mailman School of Public Health, 722 West 168th Street, New York, NY 10032, USA pt140@columbia.edu; Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY, USA.
Diane Reynolds, School of Nursing, Long Island University, Brooklyn Campus, Brooklyn, NY, USA.
Julie Flom, Department of Epidemiology, Columbia University Mailman School of Public Health, 722 West 168th Street, New York, NY 10032, USA.
Loralee Fulton, Othmer Cancer Center, Long Island College Hospital, Brooklyn, NY, USA.
Yuyan Liao, Department of Epidemiology, Columbia University Mailman School of Public Health, 722 West 168th Street, New York, NY 10032, USA.
Elizabeth Kudadjie-Gyamfi, Department of Psychology, Long Island University, Brooklyn Campus, Brooklyn, NY, USA.
Mary Beth Terry, Department of Epidemiology, Columbia University Mailman School of Public Health, 722 West 168th Street, New York, NY 10032, USA; Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY, USA.
References
- 1.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biom Prev. 1998;7:1133–1144. [PubMed] [Google Scholar]
- 2.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87(9):670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 3.Oza AM, Boyd NF. Mammographic parenchymal patterns: a marker of breast cancer risk. Epidemiol Rev. 1993;15(1):196–208. doi: 10.1093/oxfordjournals.epirev.a036105. [DOI] [PubMed] [Google Scholar]
- 4.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 5.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 6.Laya MB, Gallagher JC, Schreiman JS, Larson EB, Watson P, Weinstein L. Effect of postmenopausal hormonal replacement therapy on mammographic density and parenchymal pattern. Radiology. 1995;196(2):433–437. doi: 10.1148/radiology.196.2.7617857. [DOI] [PubMed] [Google Scholar]
- 7.Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15(4):732–739. doi: 10.1158/1055-9965.EPI-05-0798. [DOI] [PubMed] [Google Scholar]
- 8.Nordevang E, Azavedo E, Svane G, Nilsson B, Holm LE. Dietary habits and mammographic patterns in patients with breast cancer. Breast Cancer Res Treat. 1993;26(3):207–215. doi: 10.1007/BF00665798. [DOI] [PubMed] [Google Scholar]
- 9.Butler LM, Gold EB, Greendale GA, Crandall CJ, Modugno F, Oestreicher N, et al. Menstrual and reproductive factors in relation to mammographic density: the Study of Women’s Health Across the Nation (SWAN) Breast Cancer Res Treat. 2008;112(1):165–174. doi: 10.1007/s10549-007-9840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin LJ, Melnichouk O, Guo H, Chiarelli AM, Hislop TG, Yaffe MJ, et al. Family history, mammographic density, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(2):456–463. doi: 10.1158/1055-9965.EPI-09-0881. [DOI] [PubMed] [Google Scholar]
- 11.Kerlikowske K, Ichikawa L, Miglioretti DL, Buist DS, Vacek PM, Smith-Bindman R, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99(5):386–395. doi: 10.1093/jnci/djk066. [DOI] [PubMed] [Google Scholar]
- 12.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 13.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 14.Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251–1259. doi: 10.1093/aje/kwp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Carmen MG, Halpern EF, Kopans DB, Moy B, Moore RH, Goss PE, et al. Mammographic breast density and race. AJR Am J Roentgenol. 2007;188(4):1147–1150. doi: 10.2214/AJR.06.0619. [DOI] [PubMed] [Google Scholar]
- 16.del Carmen MG, HE Hughes KS, Rafferty E, Kopans D, Parisky YR, Sardi A, et al. Racial differences in mammographic breast density. Cancer. 2003;198(3):590–596. doi: 10.1002/cncr.11517. [DOI] [PubMed] [Google Scholar]
- 17.Habel LA, Capra AM, Oestreicher N, Greendale GA, Cauley JA, Bromberger J, et al. Mammographic density in a multiethnic cohort. Menopause. 2007;14(5):891–899. doi: 10.1097/gme.0b013e318032569c. [DOI] [PubMed] [Google Scholar]
- 18.El-Bastawissi AY, White E, Mandelson MT, Taplin S. Variation in mammographic breast density by race. Ann Epidemiol. 2001;11:257–263. doi: 10.1016/s1047-2797(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 19.Caire-Juvera G, Arendell LA, Maskarinec G, Thomson CA, Chen Z. Associations between mammographic density and body composition in Hispanic and non-Hispanic white women by menopause status. Menopause. 2008;15(2):319–325. doi: 10.1097/gme.0b013e3181405b8a. [DOI] [PubMed] [Google Scholar]
- 20.Tseng M, Byrne C, Evers KA, London WT, Daly MB. Acculturation and breast density in foreign-born, US Chinese women. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1301–1305. doi: 10.1158/1055-9965.EPI-06-0159. [DOI] [PubMed] [Google Scholar]
- 21.Maskarinec G, Pagano I, Lurie G, Wilkens LR, Kolonel LN. Mammographic density and breast cancer risk: the multiethnic cohort study. Am J Epidemiol. 2005;162(8):743–752. doi: 10.1093/aje/kwi270. [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G, Pagano I, Chen Z, Nagata C, Gram IT. Ethnic and geographic differences in mammographic density and their association with breast cancer incidence. Breast Cancer Res Treat. 2007;104(1):47–56. doi: 10.1007/s10549-006-9387-5. [DOI] [PubMed] [Google Scholar]
- 23.Gans KM, Burkholder GJ, Upegui DI, Risica PM, Lasater TM, Fortunet R. Comparison of baseline fat-related eating behaviors of Puerto Rican, Dominican, Colombian, and Guatemalan participants who joined a cholesterol education project. J Nutr Educ Behav. 2002;34(4):202–210. doi: 10.1016/s1499-4046(06)60094-8. [DOI] [PubMed] [Google Scholar]
- 24.John ES, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2905–2913. doi: 10.1158/1055-9965.EPI-05-0483. [DOI] [PubMed] [Google Scholar]
- 25.Stroup-Benham CA, Treviño FM. Reproductive characteristics of Mexican-American, mainland Puerto Rican, and Cuban-American women Data from the Hispanic Health and Nutrition Examination Survey. JAMA. 1991;265(2):222–226. [PubMed] [Google Scholar]
- 26.Marks G, Garcia M, JM S. Health risk behaviors of Hispanics in the United States: findings from HHANES, 1982–84. Am J Public Health. 1990;80(Suppl):20–26. doi: 10.2105/ajph.80.suppl.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernal G, Enchautegui-de-Jesus N. Latinos and Latinas in community psychology: a review of the literature. Am J Commun Psychol. 1994;22(4):531–557. doi: 10.1007/BF02506892. [DOI] [PubMed] [Google Scholar]
- 28.Consedine NS, Magai C, Conway F, Neugut AI. Obesity and awareness of obesity as risk factors for breast cancer in six ethnic groups. Obes Res. 2004;12(10):1680–1689. doi: 10.1038/oby.2004.208. [DOI] [PubMed] [Google Scholar]
- 29.Nazroo J, Jackson J, Karlsen S, Torres M. The Black diaspora and health inequalities in the US and England: does where you go and how you get there make a difference? Sociol Health Illn. 2007;29(6):811–830. doi: 10.1111/j.1467-9566.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 30.McCormack VA, Perry N, Vinnicombe SJ, Silva Idos S. Ethnic variations in mammographic density: a British multiethnic longitudinal study. Am J Epidemiol. 2008;168(4):412–421. doi: 10.1093/aje/kwn169. [DOI] [PubMed] [Google Scholar]
- 31.Byng JW, Boyd NF, Little L, Lockwood G, Fishell E, Jong RA, et al. Symmetry of projection in the quantitative analysis of mammographic images. Eur J Cancer Prev. 1996;5(5):319–327. doi: 10.1097/00008469-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Stone J, Warren RM, Pinney E, Warwick J, Cuzick J. Determinants of percentage and area measures of mammographic density. Am J Epidemiol. 2009;170(12):1571–1578. doi: 10.1093/aje/kwp313. [DOI] [PubMed] [Google Scholar]
- 33.Maskarinec G, Pagano I, Chen Z, Nagata C, Gram IT. Ethnic and geographic differences in mammographic density and their association with breast cancer incidence. Breast Cancer Res Treat. 2007;104(1):47–56. doi: 10.1007/s10549-006-9387-5. [DOI] [PubMed] [Google Scholar]
- 34.Reeves KW, Stone RA, Modugno F, Ness RB, Vogel VG, Weiss-feld JL, et al. Longitudinal association of anthropometry with mammographic breast density in the Study of Women’s Health Across the Nation. Int J Cancer. 2009;124(5):1169–1177. doi: 10.1002/ijc.23996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haars G, van Noord PA, van Gils CH, Grobbee DE, Peeters PH. Measurements of breast density: no ratio for a ratio. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2634–2640. doi: 10.1158/1055-9965.EPI-05-0824. [DOI] [PubMed] [Google Scholar]
- 36.McCormack VA, dos Santos Silva I, De Stavola BL, Perry N, Vinnicombe S, Swerdlow AJ, et al. Life-course body size and perimenopausal mammographic parenchymal patterns in the MRC 1946 British birth cohort. Br J Cancer. 2003;89(5):852–859. doi: 10.1038/sj.bjc.6601207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyd NF, Lockwood GA, Byng JW, Little LE, Yaffe MJ, Tritchler DL. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br J Cancer. 1998;78(9):1233–1238. doi: 10.1038/bjc.1998.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oestreicher N, Capra A, Bromberger J, Butler LM, Crandall CJ, Gold EB, et al. Physical activity and mammographic density in a cohort of midlife women. Med Sci Sports Exerc. 2008;40(3):451–456. doi: 10.1249/MSS.0b013e31815f5b47. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Wu AH, Gauderman WJ, Bernstein L, Ma H, Pike MC, et al. Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol. 2004;159(2):140–147. doi: 10.1093/aje/kwh028. [DOI] [PubMed] [Google Scholar]
- 40.Heng D, Gao F, Jong R, Fishell E, Yaffe M, Martin L, et al. Risk factors for breast cancer associated with mammographic features in Singaporean Chinese women. Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt 1):1751–1758. [PubMed] [Google Scholar]
- 41.Dite GS, Gurrin LC, Byrnes GB, Stone J, Gunasekara A, McCredie MR, et al. Predictors of mammographic density: insights gained from a novel regression analysis of a twin study. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3474–3481. doi: 10.1158/1055-9965.EPI-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density: a hormonally responsive risk factor for breast cancer. J Br Menopause Soc. 2006;12(4):186–193. doi: 10.1258/136218006779160436. [DOI] [PubMed] [Google Scholar]
- 43.Gapstur SM, Lopez P, Colangelo LA, Wolfman J, Van Horn L, Hendrick RE. Associations of breast cancer risk factors with breast density in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1074–1080. [PubMed] [Google Scholar]
- 44.Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11(7):653–662. doi: 10.1023/a:1008926607428. [DOI] [PubMed] [Google Scholar]
- 45.Warren R, Skinner J, Sala E, Denton E, Dowsett M, Folkerd E, et al. Associations among mammographic density, circulating sex hormones, and polymorphisms in sex hormone metabolism genes in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1502–1508. doi: 10.1158/1055-9965.EPI-05-0828. [DOI] [PubMed] [Google Scholar]
- 46.Pierce MB, Leon DA. Age at menarche and adult BMI in the Aberdeen children of the 1950 s cohort study. Am J Clin Nutr. 2005;82(4):733–739. doi: 10.1093/ajcn/82.4.733. [DOI] [PubMed] [Google Scholar]
- 47.van Lenthe FJ, Kemper CG, van Mechelen W. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. Am J Clin Nutr. 1996;64(1):18–24. doi: 10.1093/ajcn/64.1.18. [DOI] [PubMed] [Google Scholar]
- 48.Okasha M, McCarron P, McEwen J, Smith GD. Age at menarche: secular trends and association with adult anthropometric measures. Ann Hum Biol. 2001;28(1):68–78. doi: 10.1080/03014460150201896. [DOI] [PubMed] [Google Scholar]
- 49.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 50.Kahlenborn C, Modugno F, Potter DM, Severs WB. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc. 2006;81(10):1290–1302. doi: 10.4065/81.10.1290. [DOI] [PubMed] [Google Scholar]
- 51.Boyd NF, Martin LJ, Rommens JM, Paterson AD, Minkin S, Yaffe MJ, et al. Mammographic density: a heritable risk factor for breast cancer. Methods Mol Biol. 2009;472:343–360. doi: 10.1007/978-1-60327-492-0_15. [DOI] [PubMed] [Google Scholar]
- 52.Phillips AA, Jacobson JS, Magai C, Consedine N, Horowicz-Mehler NC, Neugut AI. Cancer incidence and mortality in the Caribbean. Cancer Invest. 2007;25(6):476–483. doi: 10.1080/07357900701359841. [DOI] [PubMed] [Google Scholar]
- 53.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6(10):798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 54.New York City Department of Health & Mental Hygiene. Health Department Urges New York City Women to Stand Up to Breast Cancer. 2008 Oct 20; Press Release # 068-08, 2008. [Google Scholar]