SUMMARY
The highly-structured, cis-encoded RNA elements known as riboswitches modify gene expression upon binding a wide range of molecules. The yybP-ykoY motif was one of the most broadly distributed and numerous bacterial riboswitch whose cognate ligand was unknown. Using a combination of in vivo reporter and in vitro expression assays, equilibrium dialysis and northern analysis, we show that the yybP-ykoY motif responds directly to manganese ions in both Escherichia coli and Bacillus subtilis. The identification of the yybP-ykoY motif as a manganese ion sensor suggests the genes that are preceded by this motif, and encode a diverse set of poorly characterized membrane proteins, have roles in metal homeostasis.
Keywords: MntR, Fur, H-NS, translational regulation, cis-acting regulatory RNAs, metal transporters
INTRODUCTION
Transition metal ions are essential trace nutrients in all domains of life. The unique redox properties inherent to these metals make them ideal cofactors for a wide variety of enzymatic reactions (reviewed in (Finney and O'Halloran, 2003)). At excess levels, however, the reactive character of these ions simultaneously poses a potential threat to the cell (reviewed in (Finney and O'Halloran, 2003)). As a result, bacteria have evolved exquisite mechanisms to control transition metal homeostasis (reviewed in (Waldron and Robinson, 2009)). A key component of this homeostasis is the regulated import and export by high affinity transporters. Bacterial cells also possess highly sensitive regulatory strategies to modulate the synthesis of these transporters and other metal binding proteins (reviewed in (Waldron and Robinson, 2009)). For example, in many bacteria, the Fur and MntR transcription factors repress gene expression in response to high levels of iron and manganese, respectively (reviewed in (Waldron and Robinson, 2009)).
The cellular roles of iron and manganese are intertwined. Iron is a critical cofactor of many enzymes. However, when iron levels are low or cells are subject to oxidative stress leading to the detrimental, iron-catalyzed Fenton reaction, the synthesis of manganese-dependent isozymes is increased in E. coli. In addition, manganese is substituted in several iron-containing enzymes allowing continued activity (reviewed by Imlay, 2014). On the other hand, high levels of manganese can be detrimental for the same reason; in this case the manganese is substituted for iron in enzymes in which the substitution cannot be tolerated (Martin et al., 2015).
Given the benefits as well as the potential toxicity of manganese, transport of this transition metal is tightly controlled in E. coli. Expression of the major manganese importer encoded by mntH is repressed by both Fur and MntR and activated by the hydrogen peroxide-responsive transcription activator OxyR (Ikeda et al., 2005; Kehres et al., 2002; Patzer and Hantke, 2001). We recently discovered that E. coli cells also possess a manganese exporter encoded by the mntP gene and found that the levels of both the mntP mRNA and MntP protein increase in the presence of high manganese (Waters et al., 2011). Binding sites for both Fur and MntR were predicted upstream of the promoter for this gene (Chen et al., 2007; Ikeda et al., 2005; Kehres et al., 2002; Stojiljkovic et al., 1994), but it was not clear how these two repressors activate mntP expression. It was also noted that the 5′-untranslated region (5′-UTR) of the mntP gene is unusually long and contains a conserved riboswitch element of the yybP-ykoY family (Barrick et al., 2004).
Riboswitches are cis-acting regulatory RNAs most commonly embedded in the 5′-UTRs of the genes that they regulate (reviewed in (Serganov and Nudler, 2013)). These structured RNA elements are comprised of two components: a highly conserved ligand-binding aptamer domain and a variable expression platform that executes genetic regulation. Upon binding of their cognate ligand(s), most riboswitches modify gene expression through one of two mechanisms: transcription regulation by controlling formation of an intrinsic transcription terminator or translation initiation by controlling the sequestration of ribosome binding sites.
To date, there are more than 25 experimentally validated riboswitch classes, which sense and respond to diverse compounds including enzymatic cofactors, nucleotide derivatives, amino acids, sugars, as well as Mg2+ and F− ions (reviewed in (Breaker, 2011; Serganov and Nudler, 2013)). In addition, the explosion of sequenced bacterial genomes coupled with the highly conserved primary sequence and secondary structure of riboswitch aptamer domains have facilitated the bioinformatic identification of nearly two dozen putative “orphan” riboswitch classes whose ligands have yet to be identified. Of these orphan riboswitches, the yybP-ykoY motif is by far the most numerous (Meyer et al., 2011). The motif was first identified as preceding the yybP and ykoY genes in Bacillus subtilis (Barrick et al., 2004), but over 1,000 unique examples of the yybP-ykoY motif have been found to be broadly distributed across many bacterial phyla (Meyer et al., 2011; Sun et al., 2013). Typically, identification of the ligand sensed by an orphan riboswitch has been inferred from its genetic context (Winkler et al., 2002). However, for the yybP-ykoY riboswitch motif, which precedes genes predicted to encode membrane associated proteins, in particular cation transporters, permeases and poorly understood TerC membrane proteins that contribute to tellurium resistance, did not offer coherent clues as to the compound being sensed by this regulatory RNA (Barrick et al., 2004; Meyer et al., 2011). There are two copies of the yybP-ykoY motif in E. coli; one upstream of mntP and one upstream of alx, which encodes a member of the TerC superfamily (Anantharaman et al., 2012). Since we found mntP to be manganese-inducible, we wondered whether the yybP-ykoY motif responded to manganese.
To elucidate the mechanism of mntP induction by manganese, we assayed lacZ fusions to the mntP promoter and 5′-UTR. Assays of transcriptional fusions showed that Fur and MntR activate the mntP promoter by counteracting the repressive effects of the histone-like H-NS protein. Assays of wild type and mutant translational fusions together with biochemical studies revealed that the 5′-UTR directly binds and responds to manganese. Based on the expression of an alx 5′-UTR-lacZ fusion in E. coli and the ykoY and yybP mRNAs in Bacillus subtilis, we propose that this orphan riboswitch family broadly responds to manganese and discuss the implications of our findings for the large families of membrane proteins whose genes are preceded by the yybP-ykoY motif.
RESULTS
The mntP promoter and 5′-UTR independently contribute to mntP induction by Mn2+
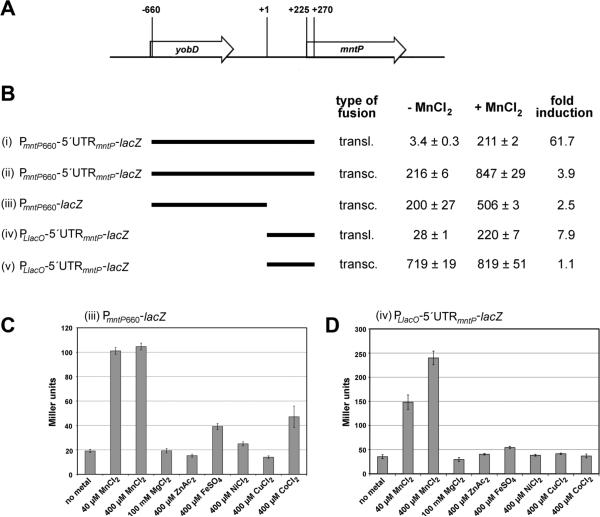
To begin to dissect mntP induction by MnCl2, we first generated PmntP660-5′UTRmntP-lacZ fusions containing the entire mntP promoter region beginning 660 nucleotides (nt) upstream of the transcription start site, the 225 nt mntP 5′-UTR containing the riboswitch homology, and the first 15 amino acids of the MntP open reading frame (ORF) fused to lacZ (Figure 1A). The strain with a PmntP660-5′UTRmntP-lacZ translational fusion (i) (for which translation is dependent on the mntP ribosome binding site) showed a profound increase in activity (61.7-fold) with 400 μM MnCl2 in LB medium (Figure 1B). Cells bearing a PmntP660-5′UTRmntP-lacZ transcriptional fusion (ii) (for which translation is dependent on the lacZ ribosome binding site) also showed MnCl2-dependent induction but to a lower extent (3.9-fold) and had significantly higher basal activity without MnCl2. These data demonstrate MnCl2-dependent regulation of mntP at both the transcriptional and translational levels.
Figure 1. Transcription and translation of mntP are induced by MnCl2.
(A) Diagram of the yobD-mntP region. The numbering is as follows: +1 is the transcription start site, +225 corresponds to the AUG start codon, +270 shows the position of the fusion to the 15th amino acid.
(B) β-galactosidase activity for strains carrying chromosomal fusions with indicated yobD-mntP regions grown in LB medium and incubated without and with 400 μM MnCl2 for 1 h. Transcriptional fusions (ii), (iii) and (v) rely on the lacZ ribosome-binding site and translational fusions (i) and (iv) rely on the mntP ribosome-binding site.
(C-D) β-galactosidase activity for strains carrying the PmntP660-lacZ transcriptional (C) and the PLlacO-5′UTRmntP-lacZ translational (D) fusions grown in M9 glucose medium and incubated without or with indicated metals for 1 h.
For all β-galactosidase assays (B-D), the results are given in Miller units as the mean ± SDM of three independent samples.
The individual contributions of the mntP promoter and 5′-UTR were assessed with three additional fusions: a PmntP660-lacZ promoter fusion (iii) comprising the 660 nt upstream of the transcription start site fused to the lacZ transcript (which lacks the mntP 5′-UTR) and PLlacO-5′UTRmntP-lacZ transcriptional (iv) and translational (v) 5′-UTR fusions consisting of the 225 nt 5′-UTR and the first 15 amino acids of MntP under the control of a heterologous promoter, PLlacO (Figure 1B). Exposure to MnCl2 induced the PmntP660-lacZ promoter fusion (iii) (2.5-fold). The PLlacO-5′UTRmntP-lacZ translational fusion (iv) also showed MnCl2-dependent induction (7.9-fold), but the PLlacO-5′UTRmntP-lacZ transcriptional fusion (v) did not. Thus the promoter and 5′-UTR of mntP independently contribute to MnCl2-dependent regulation, and the 5′-UTR affects translation initiation rather than transcription termination.
Both the mntP promoter and 5′-UTR respond specifically to Mn2+
To test whether the mntP promoter and 5′-UTR respond to metals other than Mn2+, we also examined expression of the fusions in cells exposed to either Mg2+ or several divalent transition metals in minimal medium (Figures 1C and 1D) or the metalloid tellurium in LB medium (data not shown). The PmntP660-lacZ promoter fusion (iii) was strongly induced by both 40 and 400 μM MnCl2 and partially induced by 400 μM FeSO4 and CoCl2, though it is unlikely cells encounter 400 μM Co2+ under physiological conditions. The PLlacO-5′UTRmntP-lacZ translational fusion (iv) showed a concentration dependent induction with MnCl2, but not with any of the other transition metals or K2TeO3. These data indicate that in vivo, the mntP promoter is strongly regulated by Mn2+ and partially by Fe2+ while the mntP riboswitch specifically responds to Mn2+.
MntR and Fur activate the mntP promoter by antagonizing H-NS
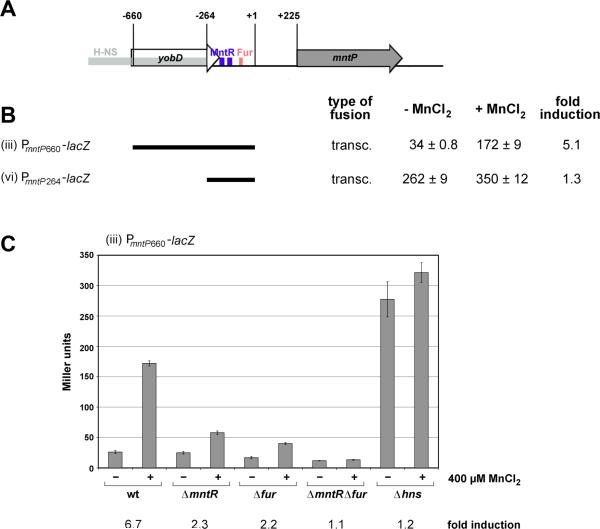
To define the regions of the mntP promoter required for the induction by MnCl2, we generated a shorter promoter fusion, PmntP264-lacZ (vi), comprising the 264 nt upstream of the transcription start site fused to lacZ. This fusion still possesses two MntR consensus binding sites centered at 147 and 187 nt upstream of the transcription start site (Ikeda et al., 2005; Kehres et al., 2002; Yamamoto et al., 2011) as well as a Fur binding site centered at 75 nt upstream of the transcription start site (Stojiljkovic et al., 1994) (Figure 2A). Surprisingly, the shorter promoter fusion showed higher basal expression and very low inducibility by MnCl2 (1.3-fold) in minimal medium (Figure 2B). The region upstream of the MntR binding sites has been observed to bind to the H-NS nucleoid-binding protein in E. coli and Salmonella enterica (Navarre et al., 2006; Oshima et al., 2006). We thus hypothesized that H-NS might bind upstream of mntP and that MntR and Fur might antagonize this H-NS-mediated repression, analogous to what has been observed for H-NS repression and Fur-dependent activation of the ftnA gene (Nandal et al., 2010). We tested this hypothesis by assaying [.beta]-galactosidase activity from the PmntP660-lacZ transcriptional fusion (iii) in wild-type, Δhns, ΔmntR, Δfur and ΔmntR Δfur mutant cells. We found that the activity of the PmntP660-lacZ transcriptional fusion was constitutively high in both the absence and presence of MnCl2 in the Δhns strain, suggesting that H-NS does indeed negatively affect mntP expression (Figure 2C). In contrast, MnCl2-dependent induction was significantly reduced in the ΔmntR and Δfur backgrounds, and was completely abolished in the ΔmntR Δfur double mutant. Thus the two repressors, Mn2+-binding MntR and Fur, which has been shown to be loaded with Mn2+ when the metal is present at high concentrations (Martin et al., 2015), activate transcription by antagonizing the repressive effects of H-NS at the mntP promoter.
Figure 2. MntR and Fur antagonize H-NS binding.
(A) Diagram of yobD-mntP chromosomal region with positions of H-NS, MntR and Fur binding sites indicated. Numbering is as in Figure 1.
(B) β-galactosidase activity for strains carrying transcriptional fusions with indicated yobD-mntP regions grown in M9 glucose medium and incubated without and with 400 μM MnCl2 for 1 h.
(C) β-galactosidase activity for strains grown as in (B) carrying the PmntP660-mntP-lacZ transcriptional fusion and indicated ΔmntR, Δfur and Δhns deletions.
For all β-galactosidase assays (B-C), the results are given in Miller units as the mean ± SDM of three independent samples.
Mutations of conserved residues in the mntP aptamer abolish regulation by Mn2+ in vivo and in vitro
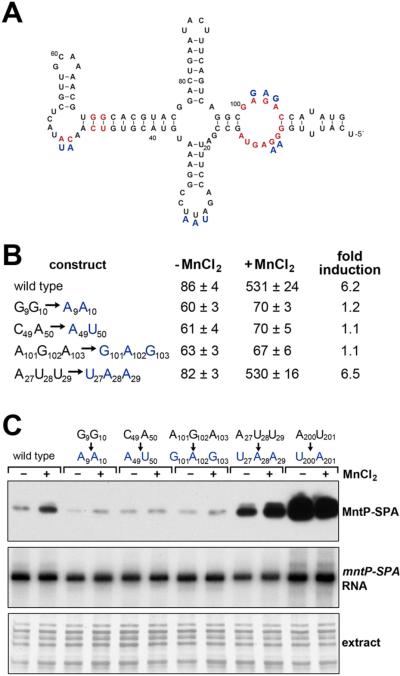
Next we sought to characterize the Mn2+-responsive mntP 5′-UTR. We first concentrated on the highly conserved part of the mntP 5′-UTR (nt 1-110) that corresponds to the yybP-ykoY aptamer motif (Figure S1A). The remainder of the 5′-UTR (nt 111-225) is more variable and is predicted to serve as the expression platform. Figure 3A depicts the secondary structure of the first 110 nt of the mntP 5′-UTR generated using the yybP-ykoY consensus structure (Barrick et al., 2004) and supported by enzymatic and lead structure probing (Figure S2). To test the contributions of specific nucleotides in this aptamer domain to MnCl2 responsiveness, we generated multiple PLlacO-5′UTRmntP-lacZ translational fusions (iv) carrying mutations of three sets of residues that were greater than 97% conserved in the yybP-ykoY consensus structure (G9G10, C49A50, and A101G102A103) (Meyer et al., 2011). We also mutated another set of residues that were only conserved among closely related enteric bacteria (A27U28U29). MnCl2-dependent induction of fusion gene expression was almost completely abolished by mutations in the three sets of highly conserved residues, while wild type induction was observed for the fusion carrying mutations in the less conserved residues (Figure 3B).
Figure 3. mntP riboswitch responds to MnCl2 in vivo and in vitro.
(A) Predicted secondary structure of the mntP aptamer based on conservation and structure probing. Nucleotides in red are conserved in >97% of the yybP-ykoY family members (Meyer et al., 2011). Blue letters correspond to mutants assayed in (B-C). See also Figure S1A and Figure S2.
(B) β-galactosidase activity for strains carrying PLlacO-5′UTRmntP-lacZ translational fusions with mutations of indicated residues grown in M9 glucose medium and incubated without and with 400 μM MnCl2 for 1 h. The results are given in Miller units as the mean ± SDM of three independent samples.
(C) Reconstitution of mntP responsiveness to manganese in vitro with a purified E. coli in vitro translation system. Wild type or mutant RNA (1 μg) encompassing the mntP 5′-UTR and mntP ORF with a C-terminal SPA tag was incubated in the presence of no metal or 400 μM MnCl2 for 2 h and then subjected to western blot analysis. The levels of the mntP-SPA mRNA were determined by primer extension analysis. The proteins were detected by silver staining of the gel after the transfer of the lower molecular weight proteins.
We also tested the synthesis of a MntP-SPA fusion protein in an in vitro translation assay using in vitro synthesized RNA templates encompassing the wild type aptamer, the mutant aptamers described above, and a constitutively active mutant aptamer described below. Consistent with our in vivo assays, the addition of MnCl2 induced synthesis of the MntP-SPA fusion protein with the wild type and A27U28U29 mutant templates, while mutations in the highly conserved nucleotides G9G10 and C49A50 and A101G102A103 eliminated induction by MnCl2 (Figure 3C). In contrast, when we used the mutant with substitutions of A200U201, which displayed constitutively high levels of lacZ activity even without MnCl2 (see below), we observed high levels of MntP-SPA in both the absence and presence of MnCl2. Importantly, these in vitro assays show that, in a minimal expression system, the addition of MnCl2 alone was sufficient to promote translation of the mntP transcript. Together, the results of the in vivo and in vitro expression assays support the conclusion that the mntP riboswitch directly senses and responds to Mn2+.
The mntP 5′-UTR binds Mn2+
To test whether the mntP aptamer directly binds Mn2+, we carried out equilibrium dialysis followed by atomic absorption spectroscopy (Table 1 and Table S1). In these assays, 100 μM in vitro transcribed wild type or C49A50 mutant aptamer (nt 1-110) RNA, 50 μM of the double stranded DNA template used to synthesize the wild type RNA or 100 μM of an unrelated cyclic dinucleotide (c-di-GMP) binding aptamer (Kulshina et al., 2009), all in buffer containing 20 mM MgCl2, were placed in dialysis chambers opposite a second chamber with only the buffer. After a range of MnCl2 concentrations was introduced into the buffer-only side, the samples were equilibrated for 48 h. The solution in each of the dialysis chambers was then subjected to atomic absorption spectroscopy to quantitate Mn2+ levels. If the RNA or DNA specifically bound to Mn2+, the metal would be enriched in the chamber containing the nucleic acids.
Table 1.
mntP aptamer binding to MnCl2 during equilibrium dialysis
| μM manganese | |||
|---|---|---|---|
| Nucleic acid | Buffer side | Nucleic acid side | Percent enrichment |
| wild type mntP RNA | 1.2 ± 0.05 | 29.4 ± 0.7 | 96% |
| C49A50 to A49U50 mutant RNA | 8.0 ± 0.5 | 21.6 ± 0.6 | 73% |
| DNA template | 12.3 ± 0.1 | 17.4 ± 0.7 | 59% |
Strikingly, for the wild type aptamer, 93-100% of the Mn2+ added to the buffer side of the dialysis chamber was associated with the RNA after the incubation. In contrast, only 60-73% of the Mn2+ was associated with the C49A50 mutant. The partial binding by the C49A50 mutant might be explained by the existence of multiple Mn2+ binding sites, similar to the multiple Mg2+ binding “cores” reported for M-box riboswitches (Wakeman et al., 2009). The presence of only 59-60% of the Mn2+ in the chamber with DNA or c-di-GMP aptamer (approximately half, as expected in the absence of binding) suggests that nonspecific association of Mn2+ with nucleic acids is limited and does not explain the near 100% retention of Mn2+ by the wild type aptamer. These data confirm that the aptamer can directly bind Mn2+.
Other members of the yybP-ykoY riboswitch family also respond to Mn2+
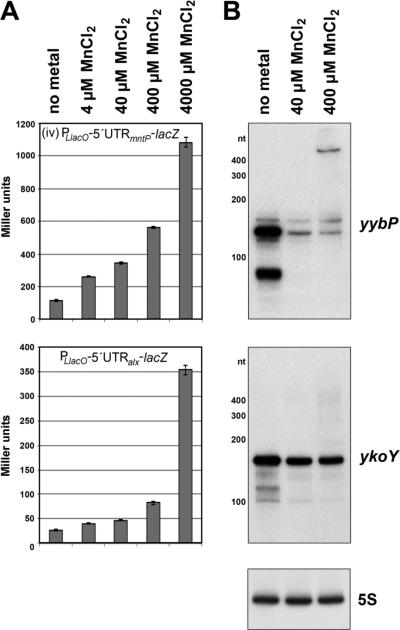
Given our findings that the mntP aptamer was responsive to and directly bound Mn2+, we examined the MnCl2 responsiveness of other members of this broadly conserved family of orphan riboswitches. A paralog of the yybP-ykoY aptamer is found in the 5′-UTR of the E. coli alx gene (Barrick et al., 2004), which is predicted to code for an inner membrane protein of the TerC superfamily. The alx gene previously was found to be induced under alkaline conditions (Bingham et al., 1990). Fusions to lacZ and structure probing of wild type and mutant derivatives indicated that the alx 5′-UTR changes conformation in response to high pH, resulting in increased translation of the Alx protein (Nechooshtan et al., 2014; Nechooshtan et al., 2009). To assess the manganese responsiveness of the alx 5′-UTR, we constructed a PLlacO-5′UTRalx-lacZ translational fusion carrying the 5′-UTR and first 10 codons of alx fused to codon 10 of lacZ, similar to our PLlacO-driven mntP-translational fusion. When exponentially growing cells harboring this construct were incubated with 4, 40, 400 or 4,000 μM MnCl2, we observed an increase in [.beta]-galactosidase expression (Figure 4A) suggesting that, similar to the mntP 5′-UTR, the yybP-ykoY riboswitch preceding the alx ORF is responsive to Mn2+.
Figure 4. E. coli and B. subtilis yybP-ykoY paralogs respond to MnCl2 in vivo.
(A) β-galactosidase activity for E. coli strains carrying either a PLlacO-5′UTRmntP-lacZ or a PLlacO-5′UTRalx-lacZ translational fusion. Cells were grown in LB medium without or with 4, 40, 400 or 4,000 μM MnCl2. The results are given in Miller units as the mean ± SDM of three independent samples.
(B) Northern blot analysis of the B. subtilis yybP and ykoY transcripts.
Total RNA was isolated from wild type B. subtilis stationary phase cells incubated with 40 or 400 μMMnCl2 for 1 h. A blot with the RNA was probed with oligonucleotides specific to either the yybP or ykoY aptamer domain or 5S RNA. In the absence of added MnCl2, bands corresponding to the sizes of the expected termination products of ~140 nt and ~160 nt for yybP and ykoY, respectively, are observed. Upon the addition of MnCl2, higher molecular weight read-through products are detected with concomitant decreases in the lower molecular weight band. See also Figure S3A.
Interestingly, while induction of the PLlacO-5′UTRmntP-lacZ translational fusion was proportional to the MnCl2 concentration, the dose response curve for the PLlacO-5′UTRalx-lacZ translational fusion was different. The alx fusion showed only 2-3 fold induction by 4, 40 and 400 μM MnCl2, but a robust 10-fold induction by 4,000 μM MnCl2. Consistent with this in vivo result, the alx aptamer also showed less MnCl2 binding than the mntP aptamer in equilibrium dialysis experiments (Table S1).
For the yybP and ykoY genes, the latter of which also codes for a member of the TerC superfamily in the Gram-positive B. subtilis (Barrick et al., 2004), effector binding to the aptamers was predicted to prevent formation of Rho-independent transcription terminators (Figure S3C-D). To test this prediction, we carried out northern analysis to examine the yybP and ykoY transcripts present in total RNA isolated from stationary phase cells left untreated or supplemented with 40 and 400 μM MnCl2 for 1 h. Using labeled oligonucleotides complementary to the yybP and ykoY aptamers, we observed prominent low molecular weight bands (~90 and ~140 nt for yybP and ~160 nt for ykoY) corresponding to the aptamer domains in untreated cells (Figure 4B). The intensities of these bands decreased upon the addition of MnCl2 with the concurrent emergence of higher molecular weight species. In the presence of 400 μM MnCl2, a distinct band of the predicted size was observed for the ~600 nt yybP gene (Figure 4B), and a smear of higher molecular weight bands was detected upon overexposure for the >1,000 nt ykoY gene (Figure S3A). A previous microarray analysis of B. subtilis cells exposed to 2.5 μM MnCl2 for 30 min reported 4- and 6-fold increases in yybP and ykoY respectively, though the abundance of each of these full-length transcripts was very low (Guedon et al., 2003). All of these observations indicate that the yybP and ykoY aptamers also respond to Mn2+ resulting in transcriptional read-through of the terminators resident in the expression platforms of these 5′-UTRs.
Change in ribosome accessibility leads to the activation of mntP translation
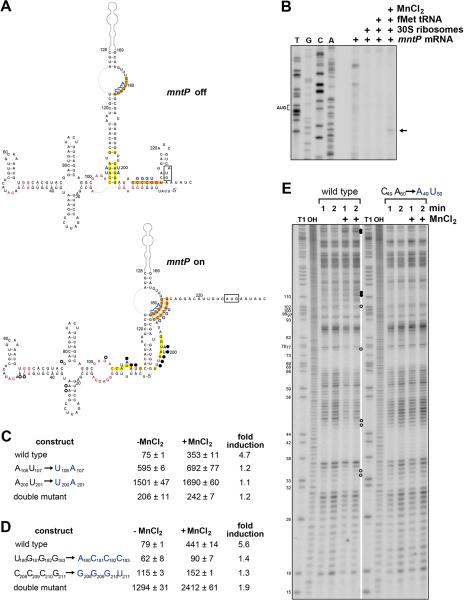
After characterizing the aptamer region, we next turned to the expression platform and the mechanism of the Mn2+-dependent activation of mntP translation. Given that secondary structural changes to the alx, yybP and ykoY riboswitches upon ligand binding are predicted to affect a stem-loop at the 3′ end of the aptamer domain (Figure S3B-D) and the observed conservation of base pairing in the mntP leader (Figure S1A), we predicted a secondary structure in which the annotated mntP start codon is sequestered by base pairing in the absence but not the presence of Mn2+ (Figure 5A). Toeprinting experiments (Figure 5B) confirmed that 30S ribosomes bind at the annotated AUG only in the presence of 400 μM MnCl2, further supporting the conclusion that mntP translation is directly affected by Mn2+ binding. Additionally, mutation of two possible upstream alternate start codons, which are preceded by GA-rich sequences and show ribosome binding in ribosome profiling experiments (Li et al., 2012), did not affect expression of PLlacO-5′UTRmntP-lacZ translation fusion (Figure S1B), suggesting Mn2+ binding does not affect a stand-by ribosome or translation of a leader peptide.
Figure 5. Accessibility of mntP ribosome binding site changes upon manganese binding.
(A) Predicted secondary structure of the complete mntP 5′-UTR from E. coli. The start codon is boxed, nucleotides conserved in >97% of the yybP-ykoY family members are in red (Meyer et al., 2011), regions predicted to base pair in the absence of Mn2+ are highlighted in yellow, while regions predicted to base pair in the presence of Mn2+ are highlighted in orange, and blue letters correspond to mutants assayed in (C-D). Nucleotides whose levels increase in a Mn2+-dependent manner in (E) are indicated with filled circles, nucleotides whose levels decrease are indicated with open circles. See also Figure S4.
(B) Toeprinting assay to examine ribosome binding in the presence and absence of fMettRNA and MnCl2.
(C) β-galactosidase activity for strains carrying PLlacO-5′UTRmntP-lacZ translational fusions with mutations of potential base pairing region highlighted in yellow in (A).
(D) β-galactosidase activity for strains carrying PLlacO-5′UTRmntP-lacZ translational fusions with mutations of potential base pairing region highlighted in orange in (A). For β-galactosidase assays in (C-D), cells grown in M9 glucose medium were incubated with or without 400 μM MnCl2 for 1 h, and the results are given in Miller units as the mean ± SDM of three independent samples.
(E) Lead probing of the mntP 5′-UTR structure. Filled circles indicate bands that increase for wild type but not mutant mntP in the presence of MnCl2, and open circles indicate those bands that decrease. Lanes labeled T1 and OH are the nucleotide size markers derived from the same labeled mntP RNA after incubation with RNase T1 or hydroxyl anions, respectively. Nucleotide positions relative to the transcription start site are labeled to the left of the gel. See also Figure S4.
To test our model, we mutated residues in the long conserved stem overlapping the 3′end of the aptamer (A106U107 to U106A107 and A200U201 to U200A201) in the context of the PLlacO-5′UTRmntP-lacZ translational fusion. This stem is also present in the alx, yybP, and ykoY riboswitches and is predicted to be altered in the “off” versus “on” state (Figure S3B-D). Consistent with a role for this stem in repressing mntP translation, expression was constitutively high in the single mutants and restored to lower levels in the double mutant (Figure 5C). Possible interference of the U106A107 mutations in Mn2+ binding might explain the loss of Mn2+ induction in the double mutant. We also mutated, separately and in combination, conserved G-rich (U180G181G182G183 to A180C181C182C183) and C-rich (C208C209C210G211 to G208G209G210U211) sequences predicted to be basepaired in the “on” state (Figure 5D). For the single mutants, induction by MnCl2 was significantly reduced compared to the wild type fusion. Induction was increased in the double mutant, which had constitutively high basal expression. These mutational data are consistent with a Mn2+-induced structural change that prevents formation of a stem-loop structure, which sequesters the ribosome-binding site of mntP, allowing for translation.
We also examined the structures of the 5′-UTRs of the wild type and the C49A50 reduced-binding mutant by lead-mediated cleavage, which preferentially affects flexible RNA regions. These structure-probing reactions were carried out in the presence and absence of MnCl2. Limited Mn2+-dependent changes were observed in the wild type aptamer domain (Figure 5E and Figure S4); the A34, A35, C46, A47, G77 and G102 residues showed somewhat less cleavage in the presence of Mn2+. These differences were not detected for the C49A50 mutant. More extensive changes were observed for the expression platform where nucleotides between A106 and G110 as well as U196 and G202 showed increased MnCl2-dependent lead cleavage for the wild type 5′-UTR but not the C49A50 mutant (Figure 5E). The increased cleavage observed in these regions in the presence of Mn2+ is consistent with the predicted increase in flexibility of these sequences in the “on” secondary structure, which again would facilitate ribosome binding and translation.
DISCUSSION
We have shown that the mntP promoter and 5′-UTR independently respond to Mn2+, leading to increased expression of the MntP manganese exporter at both the transcriptional and translational levels. At the promoter, MntR and Fur antagonize the repressive effects of H-NS binding when cells are exposed to high Mn2+. Within the 5′-UTR, the yybP-ykoY riboswitch directly binds Mn2+, resulting in a conformation that liberates the ribosome-binding site. This finding of dual regulation raises questions about the advantages of positively regulating both transcription and translation in response to the same ligand. We suggest that the tight control highlights how precise regulation of intracellular Mn2+ levels is critical to cells.
Additionally, we have also shown that other homologs of the yybP-ykoY riboswitch also confer Mn2+-dependent gene regulation through a transcription termination mechanism. These experiments along with a parallel structural study (Price et al., 2014), are the first demonstration of a ligand for this orphan riboswitch class. The Mn2+-sensing capability of the yybP-ykoY motif, together with the newly-discovered NiCo motif which is responsive to Co2+ and Ni2+ (Furukawa et al., 2015) and another possible Mn2+ binding riboswitch repressing the S. enterica mntH gene (Shi et al., 2014), reveal that riboswitches can directly sense and respond to transition metals. We suggest that other less broadly-conserved RNA motifs may be found to recognize still other transition metals.
yybP-ykoY riboswitches exhibit unique properties
A number of features distinguish the yybP-ykoY family from the majority of riboswitch classes studied to date. First, in contrast to most characterized riboswitches, only minimal structural changes to the aptamer are observed upon Mn2+ binding as assessed by both lead and inline probing. For the latter, only one change, increased cleavage at G33, was detected in the aptamer domain (Figure S4C and D). It is possible that the Mg2+ present in the buffers masks some of the effects of Mn2+ binding given that these two ions can occupy the same binding sites in RNA tertiary structures (Ramesh et al., 2011), but increased cleavage at G33 and A34 were the only inline probing changes observed in a buffer lacking Mg2+ (Figure S4E). Alternatively, the yybP-ykoY aptamer may adopt a preformed tertiary structure in the absence of Mn2+. This would be reminiscent of lysine-responsive L-box riboswitches which display minor changes in spontaneous cleavage in response to lysine and have the same three dimensional crystal structure in the absence and presence of lysine (Garst et al., 2008; Serganov et al., 2008; Sudarsan et al., 2003). Interestingly, the NiCo riboswitch also is largely preformed in the absence of its ligand (Furukawa et al., 2015).
Another unusual feature of the yybP-ykoY motif is the overlap of the expression platform with the aptamer domain. Riboswitches usually have a “modular” structure with distinct aptamer and expression platform domains (Breaker, 2011). However, examination of the sequences of multiple yybP-ykoY family members reveals that in every instance in which this motif is predicted to affect transcription termination, a portion of the terminator stem corresponding to the expression platform is part of the highly conserved P4 helix of the aptamer (Barrick et al., 2004). This unique arrangement is also observed for many of the Class I c-di-GMP riboswitch family members that utilize Rho-independent transcription terminators (Weinberg et al., 2007). Unlike the majority of riboswitches which are genetic “off” switches, the yybP-ykoY riboswitches and the Class I c-di-GMP switches are all postulated to be genetic “on” switches (Barrick et al., 2004; Weinberg et al., 2007), as we have observed for mntP and alx.
We also note that the predicted first and third stem-loop structures of the yybP-ykoY motif can be quite varied. Although we found activation of E. coli mntP to be specific to Mn2+, it is possible that members of this family respond to different concentrations of Mn2+ as appears to be the case for mntP and alx, different oxidation states of manganese, or even other cations. Members of this riboswitch family also may respond to environmental signals in addition to Mn2+ similar to the multi-layered regulation reported for the mgtA Mg2+-responsive riboswitch (Park et al., 2010). Alkaline conditions have been reported to elevate the levels of the alx, yybP and ykoY transcripts and alx translation (Bingham et al., 1990; Nechooshtan et al., 2014; Nechooshtan et al., 2009; Wiegert et al., 2001). We also observed alkaline pH induction of Palx-5′UTRalx-lacZ transcriptional and translational fusions but not the PLlacO-5′UTRalx-lacZ translational fusion (data not shown). How Mn2+ and high pH signals overlap and affect expression of these genes will be an interesting topic for future research. In the case of alx, we suggest that Mn2+ binding prevents transcription termination, which leads to the accumulation of the short transcript identified as the SraL small RNA (Argaman et al., 2001), independent of the effects of high pH on transcription and translation.
Genes preceded by yybP-ykoY riboswitches are likely to have roles in metal homeostasis
Given the prevalence of the yybP-ykoY family of riboswitches, we sought to better understand their functional associations using the wealth of complete genome sequences from 2755 organisms (Table S2). We identified 1338 genomes with at least one significantly scoring yybP-ykoY motif and found a mean of 1.5 motifs per genome, revealing that a notable fraction of the genomes coded for two or more yybP-ykoY riboswitches. The motif is observed in most major bacterial clades but none of the archaeal clades, suggesting that it first emerged in the bacterial common ancestor. Among the organisms having this riboswitch, we found enrichment for soil and aquatic bacteria. Moreover, even in clades where the motif is present, it is virtually absent in endo-parasitic and symbiotic bacteria. This distribution is consistent with a role for this riboswitch in responding to potentially toxic environmental metals.
Systematic classification of the genes associated with yybP-ykoY riboswitches confirmed that the motif is unique among riboswitch-regulated genes in having an almost exclusive association with genes encoding transmembrane or membrane-associated proteins, particularly transporters (Figures 6 and S5). Tallies of the downstream genes revealed that terC is the gene most frequently preceded by yybP-ykoY riboswitches. Earlier sequence analysis showed that TerC proteins have multiple conserved transmembrane segments, some of which are marked by a distinctive pattern of embedded acidic residues (Anantharaman et al., 2012). Thus the proteins could interact with cations within the membrane and function as metal transporters. The second hub is a P-type ATPase belonging to a distinct clade related to Ca2+-efflux pumps (Chan et al., 2010). Interestingly, the genes encoding TerC and P-type ATPases almost never co-occur in the same genome downstream of paralogous yybP-ykoY motifs. This pattern of near complete mutual exclusivity between riboswitch-regulated TerC and the P-type ATPase genes suggests that they likely perform an equivalent function in divalent metal efflux in the genomes that code for them. Genomes with the P-type ATPase gene preceded by the riboswitch only infrequently showed the presence of a second paralogous yybP-ykoY motif, suggesting the ATPase might have a broader functional spectrum than TerC proteins. In contrast, riboswitch-proximal genes of the MntP family, the UPF0016 family and several other less prevalent families coding for different known or predicted metal-transporters often co-occur with riboswitch-regulated TerC encoding genes. Given that the MntP and UPF0016 families show mutual exclusivity with respect to each other in the genomes in which they are found, UPF0016 proteins are likely a new class of manganese transporters; a proposal that is further supported by comparable patterns of charged residues associated with the transmembrane segments in both of these families.
Figure 6. Network of genes immediately downstream of the yybP-ykoY riboswitch are predicted to have roles in protecting against metal toxicity.
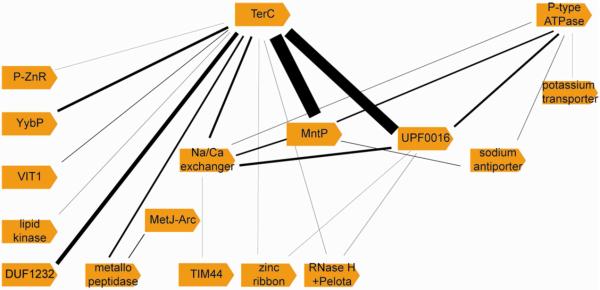
The thickness of the edge (black) connecting genes co-occurring in the same genome downstream of a paralogous yybP-ykoY motif is scaled as per the frequency of such a co-occurrence in our dataset (see Table S2). P-ZnR denotes proteins with a peptidase-associated zinc-ribbon and MetJ-Arc denotes proteins with MetJ-Arc DNA-binding domains. Figure S5 is an expanded version of this figure, which includes other genes encoded in the operons along with the genes preceded by yybP-ykoY motifs.
The overwhelming association of genes encoded downstream of yybP-ykoY riboswitches with membrane proteins known or predicted to participate in metal transport supports our conclusion that this genetic element is linked to regulation of transition metal efflux. However, the frequent presence of multiple, distinct metal transporters linked to paralogous copies of the riboswitch in the same genome suggests that there might be some functional diversification within the yybP-ykoY family.
In a limited number of instances, the yybP-ykoY motif is associated with a downstream gene encoding either a MetJ/Arc (ribbon-helix-helix) or a Zn-ribbon transcription factor (Aravind and Koonin, 1999). Some of these operons additionally code for membrane proteins immediately downstream of the predicted transcription factor. This arrangement suggests that these mRNAs may also be subject to two-levels of regulation by both the riboswitch and transcription factor as we observed for MntP.
An accompanying article presenting the crystal structure of the yybP-ykoY riboswitch indicates that specificity for Mn2+ is dependent in large part on the adenine corresponding to A50 in the mntP 5′-UTR (Price et al., 2014). This residue is almost completely conserved suggesting manganese specificity is likely to be preserved across all representatives of the family. However, the riboswitch also has a second metal-binding site with lower specificity, which might be more prone to accepting other metals and could influence binding at the specific site. Manganese has a range of oxidation states, which vary with pH and voltage potential, and higher pH favors the prevalence of states such as Mn(III). Given the connection between the yybP-ykoY family and alkaline pH, the possibility that yybP-ykoY-associated gene products act on different states of the metal is intriguing.
Overall, our study points to a large set of poorly understood genes preceded by yybP-ykoY motifs whose further characterization in the context of manganese homeostasis undoubtedly will give insights into the cellular mechanisms used to protect against transition metal toxicity.
EXPERIMENTAL PROCEDURES
Strains
All strains and oligonucleotides used in this study are listed in Table S3. Strain construction and growth conditions are described in Supplemental Experimental Procedures.
β-galactosidase assays
For all β-galactosidase assays, three separate colonies grown overnight in LB or minimal M9 medium supplemented with 0.2% glucose, were diluted to OD600 ~0.01-0.025 in the indicated media and grown to OD600 ~0.2. If required, IPTG was added at a final concentration of 1 mM. Immediately following IPTG addition, cells were either left untreated or incubated with the indicated final metal concentrations. After a 60 min incubation, cells were harvested and assayed for β-galactosidase activity (Miller, 1992).
In vitro translation assays
The RNA templates were first synthesized using the MEGAscript T7 Transcription Kit (Ambion) according to manufacturer's instructions. The transcripts were treated with 8 Units of DNase for 30 min, purified over a G50 spin column, extracted with phenol-chloroform and ethanol precipitated before 1 μg of RNA was mixed with the PURExpress in vitro Protein Synthesis kit containing E. coli ribosomes (New England Biolabs).
Equilibrium dialysis and atomic absorption spectroscopy
Indicated in vitro synthesized RNA or DNA (PCR template used for RNA synthesis) in 75 μl of buffer consisting of 50 mM Tris pH 8.3, 100 mM KCl, and 20 mM MgCl2 was placed into one chamber of a 5 kDa cutoff equilibrium dialysis chamber (Harvard Apparatus). The opposing chamber was loaded with buffer only and subsequently injected with 20 μMMnCl2. The dialysis apparatus was incubated with rocking at room temperature for 48 h, whereupon the samples from both sides of the chamber were collected into separate tubes. All samples were diluted with nitric acid (final 6%) and incubated at 95°C for 16 h to extract Mn2+. The concentration of Mn2+was measured by the 4100ZL graphite furnace atomic absorption spectrometer equipped with AS-70 automatic sampler (Perkin Elmer). The system was calibrated with standards of Mn2+ (AAMN1-1; 1000 +/− 10 μg/ml) obtained from Inorganic Ventures Inc. Measurements for each sample were carried out in triplicate and given as the mean ± standard deviation of the mean (SDM) of the three values.
RNA isolation and northern analysis
RNA was isolated and analyzed by northern analysis as in (Zhang et al., 2013); details are provided in Supplemental Experimental Procedures.
Toeprinting assay
Toeprinting assays were carried out as in (Altuvia et al., 1998); details are provided in Supplemental Experimental Procedures.
RNA radiolabeling and in vitro structure probing
Template DNA for T7 transcription was amplified from the MG1655 genome starting at the +1 of the 5′-UTR of mntP (225 nt upstream MntP ORF) to the 15th amino acid of the MntP ORF by PCR with primers containing the T7 promoter (TAATACGACTCACTATAGG). T7 transcription was conducted using the MegaShortscript T7 Transcription Kit (Ambion) according to manufacturer's instructions. In vitro structural probing with RNase T1 and lead (II) was preformed in 10 μl reactions as in (Beisel et al., 2012).
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Altuvia, N. Baird, A. Banerjee, A. Ferre-d'Amare, S. Gottesman, J. Imlay, B. Rosen, W. Winkler and J. Zhang for helpful discussions, and D.-Y. Lee for the atomic absorbance analysis. This work was supported by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Library of Medicine as well as the Pharmacology Research Associate Program (L.S.W.) and the National Institutes of Health Undergraduate Scholarship Program (M.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
M.D. and M.S. designed and performed the majority of the experiments and wrote the manuscript. T.B.U. helped with experiments to characterize in vitro synthesized RNA and helped write the manuscript, V.A. and L.A. carried out computational analysis of the yybP-ykoY motif and helped write the discussion, L.S.W. carried out experiments, guided the project and wrote the manuscript, and G.S. guided the project and wrote the manuscript.
REFERENCES
- Altuvia S, Zhang A, Argaman L, Tiwari A, Storz G. The Escherichia coli OxyS regulatory RNA represses fhlA translation by blocking ribosome binding. EMBO J. 1998;17:6069–6075. doi: 10.1093/emboj/17.20.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman V, Iyer LM, Aravind L. Ter-dependent stress response systems: novel pathways related to metal sensing, production of a nucleoside-like metabolite, and DNA-processing. Mol Biosyst. 2012;8:3142–3165. doi: 10.1039/c2mb25239b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Koonin EV. DNA-binding proteins and evolution of transcription regulation in the archaea. Nucleic Acids Res. 1999;27:4658–4670. doi: 10.1093/nar/27.23.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EGH, Margalit H, Altuvia S. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol. 2001;11:941–950. doi: 10.1016/s0960-9822(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, Lee M, Roth A, Sudarsan N, Jona I, et al. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci USA. 2004;101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel CL, Updegrove TB, Janson BJ, Storz G. Multiple factors dictate target selection by Hfq-binding small RNAs. EMBO J. 2012;31:1961–1974. doi: 10.1038/emboj.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham RJ, Hall KS, Slonczewski JL. Alkaline induction of a novel gene locus, alx, in Escherichia coli. J Bacteriol. 1990;172:2184–2186. doi: 10.1128/jb.172.4.2184-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breaker RR. Prospects for riboswitch discovery and analysis. Mol Cell. 2011;43:867–879. doi: 10.1016/j.molcel.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H, Babayan V, Blyumin E, Gandhi C, Hak K, Harake D, Kumar K, Lee P, Li TT, Liu HY, et al. The p-type ATPase superfamily. J Mol Microbiol Biotechnol. 2010;19:5–104. doi: 10.1159/000319588. [DOI] [PubMed] [Google Scholar]
- Chen Z, Lewis KA, Shultzaberger RK, Lyakhov IG, Zheng M, Doan B, Storz G, Schneider TD. Discovery of Fur binding site clusters in Escherichia coli by information theory models. Nucleic Acids Res. 2007;35:6762–6777. doi: 10.1093/nar/gkm631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney LA, O'Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Ramesh A, Zhou Z, Weinberg Z, Vallery T, Winkler WC, Breaker RR. Bacterial riboswitches cooperatively bind Ni2+ or Co2+ ions and control expression of heavy metal transporters. 2015 doi: 10.1016/j.molcel.2015.02.009. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garst AD, Héroux A, Rambo RP, Batey RT. Crystal structure of the lysine riboswitch regulatory mRNA element. J Biol Chem. 2008;283:22347–22351. doi: 10.1074/jbc.C800120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedon E, Moore CM, Que Q, Wang T, Ye RW, Helmann JD. The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sB regulons. Mol Microbiol. 2003;49:1477–1491. doi: 10.1046/j.1365-2958.2003.03648.x. [DOI] [PubMed] [Google Scholar]
- Ikeda JS, Janakiraman A, Kehres DG, Maguire ME, Slauch JM. Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur. J Bacteriol. 2005;187:912–922. doi: 10.1128/JB.187.3.912-922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Janakiraman A, Slauch JM, Maguire ME. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+. J Bacteriol. 2002;184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshina N, Baird NJ, Ferré-D'Amaré AR. Recognition of the bacterial second messenger cyclic diguanylate by its cognate riboswitch. Nat Struct Mol Biol. 2009;16:1212–1217. doi: 10.1038/nsmb.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GW, Oh E, Weissman JS. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature. 2012;484:538–541. doi: 10.1038/nature10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Waters LS, Storz G, Imlay JA. The Escherichia coli protein MntS and exporter MntP collaborate to keep intracellular manganese at sufficient but sub-inhibitory levels. PLoS Genet. 2015 Mar 9; doi: 10.1371/journal.pgen.1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MM, Hammond MC, Salinas Y, Roth A, Sudarsan N, Breaker RR. Challenges of ligand identification for riboswitch candidates. RNA Biol. 2011;8:5–10. doi: 10.4161/rna.8.1.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor Laboratory Press; Plainview, NY: 1992. [Google Scholar]
- Nandal A, Huggins CC, Woodhall MR, McHugh J, Rodríguez-Quiñones F, Quail MA, Guest JR, Andrews SC. Induction of the ferritin gene (ftnA) of Escherichia coli by Fe2+-Fur is mediated by reversal of H-NS silencing and is RyhB independent. Mol Microbiol. 2010;75:637–657. doi: 10.1111/j.1365-2958.2009.06977.x. [DOI] [PubMed] [Google Scholar]
- Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- Nechooshtan G, Elgrably-Weiss M, Altuvia S. Changes in transcriptional pausing modify the folding dynamics of the pH-responsive RNA element. Nucleic Acids Res. 2014;42:622–630. doi: 10.1093/nar/gkt868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechooshtan G, Elgrably-Weiss M, Sheaffer A, Westhof E, Altuvia S. A pH-responsive riboregulator. Genes Dev. 2009;23:2650–2662. doi: 10.1101/gad.552209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T, Ishikawa S, Kurokawa K, Aiba H, Ogasawara N. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 2006;13:141–153. doi: 10.1093/dnares/dsl009. [DOI] [PubMed] [Google Scholar]
- Park SY, Cromie MJ, Lee EJ, Groisman EA. A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell. 2010;142:737–748. doi: 10.1016/j.cell.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer SI, Hantke K. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J Bacteriol. 2001;183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price IR, Gaballa A, Ding F, Helmann JD, Ke A. Mn2+-sensing mechanisms of yybP-ykoY orphan riboswitches. Mol Cell submitted. 2015 doi: 10.1016/j.molcel.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A, Wakeman CA, Winkler WC. Insights into metalloregulation by M-box riboswitch RNAs via structural analysis of manganese-bound complexes. J Mol Biol. 2011;407:556–570. doi: 10.1016/j.jmb.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serganov A, Huang L, Patel DJ. Structural insights into amino acid binding and gene control by a lysine riboswitch. Nature. 2008;455:1263–1267. doi: 10.1038/nature07326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serganov A, Nudler E. A decade of riboswitches. Cell. 2013;152:17–24. doi: 10.1016/j.cell.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhao G, Kong W. Genetic analysis of riboswitch-mediated transcriptional regulation responding to Mn2+ in Salmonella. J Biol Chem. 2014;289:11353–11366. doi: 10.1074/jbc.M113.517516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojiljkovic I, Bäumler AJ, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- Sudarsan N, Wickiser JK, Nakamura S, Ebert MS, Breaker RR. An mRNA structure in bacteria that controls gene expression by binding lysine. Genes Dev. 2003;17:2688–2697. doi: 10.1101/gad.1140003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun EI, Leyn SA, Kazanov MD, Saier MHJ, Novichkov PS, Rodionov DA. Comparative genomics of metabolic capacities of regulons controlled by cis-regulatory RNA motifs in bacteria. BMC Genomics. 2013;14 doi: 10.1186/1471-2164-14-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman CA, Ramesh A, Winkler WC. Multiple metal-binding cores are required for metalloregulation by M-box riboswitch RNAs. J Mol Biol. 2009;392:723–735. doi: 10.1016/j.jmb.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nature Rev. Microbiol. 2009:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- Waters LS, Sandoval M, Storz G. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol. 2011;193:5887–5897. doi: 10.1128/JB.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg Z, Barrick JE, Yao Z, Roth A, Kim JN, Gore J, Wang JX, Lee ER, Block KF, Sudarsan N, et al. Identification of 22 candidate structured RNAs in bacteria using the CMfinder comparative genomics pipeline. Nucleic Acids Res. 2007;35:4809–4819. doi: 10.1093/nar/gkm487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert T, Homuth G, Versteeg S, Schumann W. Alkaline shock induces the Bacillus subtilis sW regulon. Mol Microbiol. 2001;41:59–71. doi: 10.1046/j.1365-2958.2001.02489.x. [DOI] [PubMed] [Google Scholar]
- Winkler WC, Nahvi A, Breaker RR. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002;419:952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ishihama A, Busby SJ, Grainger DC. The Escherichia coli K-12 MntR miniregulon includes dps, which encodes the major stationary-phase DNA-binding protein. J Bacteriol. 2011;193:1477–1480. doi: 10.1128/JB.01230-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Schu DJ, Tjaden BC, Storz G, Gottesman S. Mutations in interaction surfaces differentially impact E. coli Hfq association with small RNAs and their mRNA targets. J Mol Biol. 2013;425:3678–3697. doi: 10.1016/j.jmb.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.