Abstract
Our aim was to analyze the potential role of chemokine receptors CXCR2 and CXCR4 signalling pathways in liver metastatic colorectal cancer (CRC) relapse. CXCR2, CXCR4, and their chemokine ligands were evaluated in liver metastases of colorectal cancer in order to study their correlation with overall and disease-free survival of patients having received, or not received, a neoadjuvant chemotherapy regimen. Quantitative RT-PCR and CXCR2 immunohistochemical staining were carried out using CRC liver metastasis samples. Expression levels of CXCR2, CXCR4, and their ligands were statistically analyzed according to treatment with neoadjuvant chemotherapy and patients’ outcome. CXCR2 and CXCL7 overexpression are correlated to shorter overall and disease-free survival. By multivariate analysis, CXCR2 and CXCL7 expressions are independent factors of overall and disease-free survival. Neoadjuvant chemotherapy increases significantly the expression of CXCR2: treated group 1.89 (0.02–50.92) vs 0.55 (0.07–3.22), P = 0.016. CXCL7 was overexpressed close to significance, 0.40 (0.00–7.85) vs 0.15 (0.01–7.88), P = 0.12. We show the involvement of CXCL7/CXCR2 signalling pathways as a predictive factor of poor outcome in metastatic CRC. 5-Fluorouracil-based chemotherapy regimens increase the expression of these genes in liver metastasis, providing one explanation for aggressiveness of relapsed drug-resistant tumors. Selective blockage of CXCR2/CXCL7 signalling pathways could provide new potential therapeutic opportunities.
Keywords: 5FU-based chemotherapy, colorectal cancer, CXCL7, CXCR2, liver metastasis
Colorectal cancer (CRC) is a major public health problem with 63% of 5-year overall survival for all stages combined.1 Liver metastases are the first metastatic site, affecting approximately half of patients within 5 years of diagnosis.2 Complete surgical resection is the only potential curative treatment of CRC liver metastases, resulting in 5-year survival rates of 36–58%.3 Neoadjuvant and/or adjuvant chemotherapies typically involve cytotoxic drugs, mainly 5-fluorouracil (5FU) and leucovorin associated with irinotecan and/or oxaliplatin, and more recently biotherapies such as bevacizumab, a mAb directed against vascular endothelial growth factor (VEGF) or cetuximab, an anti-epidermal growth factor receptor.4,5 In patients with metastatic CRC, the addition of bevacizumab to fluorouracil-based chemotherapy resulted in statistically significant improvement in survival.6 Nevertheless, a major limitation in the long-term efficacy of chemotherapeutic and biologic agents is related to drug resistance and metastatic relapse.
Several studies have highlighted the role of chemokines in tumor growth, metastatic spread, or resistance to anticancer therapies.7–9 Chemokines are a family of small heparin-binding and secretory proteins subdivided into four families (CXC, C, CC, and CX3C). Previous studies have brought to evidence the important role of the CXCR4/CXCL12 axis in tumor progression and patient survival. CXCR4 promotes tumor progression at different levels of malignancy including tumor growth, angiogenesis, metastatic dissemination, and homing in CXCL12-enriched cellular niches in metastasis target tissues.10–12 CXCR4 expression was shown to be a prognostic marker in various types of cancer, including colon and breast carcinoma or acute myelogenous leukemia.13–15 A high expression level of interleukin-8 (IL-8)/CXCL8 in the tumor has further been associated with poorer survival.16 The expression of CXCR2 is greater in CRC than in tissue of colorectal adenoma, and in adenoma tissue than in normal colonic mucosa.17 Activation of CXCR2 by its ligands, such as CXCL8, in tumor microenvironment could change the behavior of tumor cells and promote CRC growth and metastatic spread.18–20 In gastric cancer, the tumoral expression of CXCR2 was found to be connected to the patient prognosis.21 Few studies have been carried out on neutrophil-activating peptide-2/CXCL7, however, the prognosis of pancreatic cancer seems related to its plasma level of expression.22
Our previous work showed that chronic treatment of the HT29 metastastic colon carcinoma cell line by chemotherapy promoted the emergence of cell clones overexpressing CXCR4, and that CXCR4 played a role in the metastatic dissemination.7 In resected liver metastases from patients with colon cancer, we showed elevated CXCR4 levels in patients treated with neoadjuvant chemotherapy.7
In this study we show that the expression of CXCR2 and CXCL7 are independent prognostic factors of cancer relapse and worse survival in patients treated for liver metastasis of colon cancer. We also show that the expression levels of both CXCR2 and CXCL7 are higher in liver metastases from patients who have received neoadjuvant therapy. Moreover, the correlation between CXCR2/CXCL7 expression and overall or disease-free survival is primarily supported by the subgroup of patients treated by neoadjuvant chemotherapy.
Materials and Methods
Patients and tissue processing
From February 2002 to May 2009, 55 patients who underwent hepatic resection at our institution for colorectal liver metastases gave informed consent to enter this study. All patients had regular follow-up with physical examination and abdominal imaging every 3–6 months by the referring surgeon and/or oncologist.
After hepatic resection, 58 samples of liver metastases were harvested for snap-freezing within the tumor cell and tissue bank of the Regional Reference Cancer Center of Lille (Lille, France). Fifty-two samples were originated from individual patients. Two independent samples were harvested in two patients before and after neoadjuvant chemotherapy. For one patient, two independent samples were harvested on distant liver metastasis. The whole remaining tissue was fixed in 10% formalin and several other fragments were taken from the fixed metastases and embedded in paraffin for conventional histology. Regarding histological examination of the liver metastasis samples, the mean proportion of necrosis was 30.8 ± 25.9%. The mean proportion of tumor cells to stromal tissue was 54.3 ± 19.8%. In patients who received chemotherapy before liver resection, the drugs consisted of capecitabine alone or 5FU and leucovorin/folinic acid either alone or associated with oxaliplatin (FOLFOX), irinotecan (FOLFIRI), or with irinotecan and bevacizumab (FOLFIRI–bevacizumab) (Table2).6 Once the patients had been treated with curative hepatectomy, no further adjuvant chemotherapy was given. Samples of human origin and associated data were obtained from the tumor cell and tissue bank of Regional Reference Cancer Center of Lille that operates under the authorization AC-2013-1847, granted by the French Ministry of Research. Prior to scientific use of samples and data, patients were appropriately informed and asked to consent in writing in compliance with French and European regulations. The project was approved by the Comité Scientifique de la Tumorothèque du C2RC de Lille.
Table 2.
Clinicopathologic and histologic details of metastatic liver samples from hepatectomy (n = 58)
| No. of patients | % | |
|---|---|---|
| Origin of liver samples (n = 58) | ||
| Tumorectomy/segmentectomy | 26 | 44.8 |
| Right hepatectomy | 22 | 38.0 |
| Left hepatectomy | 5 | 8.6 |
| Major hepatectomy | 5 | 8.6 |
| Neoadjuvant chemotherapy (n = 34) | ||
| FOLFOX or capecitabine | 19 | 55.9 |
| FOLFIRI | 2 | 5.9 |
| FOLFIRI and bevacizumab | 13 | 38.2 |
| Neoadjuvant chemotherapy with bevacizumab (n = 58) | ||
| Yes | 13 | 22.4 |
| No | 45 | 77.6 |
| Survival, months, mean ± SD | ||
| Delay between colectomy and hepatectomy | 21.54 ± 21.85 | |
| Overall survival after hepatectomy | 58.20 ± 3.10 | |
| Disease-free survival after hepatectomy | 26.20 ± 2.08 | |
| Duration of follow-up after hepatectomy | 60.95 ± 20.76 | |
| Tumoral status at end date | ||
| Remission | 28 | 48.3 |
| Metastasis | 30 | 51.7 |
| Anatomo-pathological features, %, mean ± SD | ||
| Necrosis in liver sample (n = 47) | 30.8 ± 25.9 | |
| Tumoral tissue in liver sample (n = 47) | 88.4 ± 16.7 | |
Quantitative RT-PCR
Total RNAs were extracted using the NucleoSpin RNA II kit (Macherey Nagel, Düren, Germany) following manufacturer's protocol. cDNAs were prepared as previously described.23,24 Amplification of cDNA was carried out using the Power SYBR Green kit PCR Master Mix and the real-time detection of amplification product was carried out using a LightCycler 480 instrument (Roche Basel, Switzerland). Each marker was assayed in triplicate in three independent experiments. Expression levels of genes were normalized to the mRNA level of GAPDH and HPRT1 housekeeping genes. Results were expressed in reference to the individuals who never received chemotherapy as follows: the results of control groups were averaged and set to 1 for comparison with gene expression of the individuals who received chemotherapy. Primer information is given in Table S1.
Immunohistochemistry
Immunohistochemical staining was carried out on 4-μm-thick sections from the formalin-fixed embedded tissues using an automated immunostainer Benchmark XT (Ventana, Strasbourg, France). Tissue sections were incubated with rabbit anti-CXCR2 antibody (ab14935) (1:100; Abcam Cambridge, UK). The slides were counterstained with Harris hematoxylin and coverslipped with glycerol. Negative controls consisted of similar sections processed without the primary antibody. Positive external controls consisted of normal colonic mucosa sections. Histologic assessment was carried out by two pathologists. Cells were considered positive if cytoplasmic or membranous staining was present. The periphery, the center of tumors, and the surrounding normal tissues were assessed.
Statistical analysis
Results were expressed as mean ± SD, median, maximum, and minimum for continuous variables and as frequencies and percentages for categorical variables.
The disease-free survival after hepatectomy was defined as the time between the date of hepatectomy of the date of last follow-up or relapse. No patient had experienced death before relapse. The overall survival was calculated as the time between the date of hepatectomy and the date of last follow-up or death.
Comparisons of frequencies were done using a χ2-test or Fisher's exact test.
For continuous parameters, the assumption of normality was tested by Shapiro–Wilk test. For continuous parameters with normal distribution, the comparisons of groups were carried out using Student's t-test or anova. Otherwise, we used the Mann–Whitney U-test or the Kruskal–Wallis rank test.
The disease-free and overall survival curves were estimated using the Kaplan–Meier method and compared according the groups using the log–rank test. For continuous parameters, we used Cox's proportional hazard model and the hazard ratios with 95% confidence intervals were calculated.
The following parameters were tested for their association with survival (bivariate analyses): expression in quantitative RT-PCR (qRT-PCR) of genes of interest, chemotherapy treatments, and confounding factors (T, N, M, location of the primary colorectal tumor, synchronous liver resection or not with colectomy, age at colorectal surgery, synchronous or metachronous liver metastases, chemotherapy administered in a time <6 months). When a significant relationship between survival and parameters of expression in qRT-PCR existed, we identified an optimal cut-off by using the maximization of the hazard ratio. The quantitative variables were dichotomized by identifying the cut-off that maximizes the log–rank test.
Multivariable Cox regression was used to analyze the relationship between survival and the treatments after adjustment for other parameters related to survival. The parameters included in this multivariable analysis were the parameters having a P-value < 0.05 in the bivariate analyses.
A P-value < 0.05 was considered statistically significant.
All statistical analyses were carried out by means of SAS software (SAS Institute Inc., Cary, NC, USA).
Results
Study population
The mean age at the time of primary tumor resection was 61 ± 10.6 years. The primary tumor location was right or transversal colon in 10 cases, left colon in 28 cases, and rectum in 17 cases. Most patients (38 of 55; 69.1%) received adjuvant chemotherapy following the resection of the primary tumor. Among the 58 samples of liver metastases, 34 (58.6%) originated from patients preoperatively treated with the FOLFOX regimen or capecitabine alone (n = 19; 55.9%), with the FOLFIRI regimen (n = 2; 5.9%), or with the FOLFIRI regimen associated with bevacizumab (n = 13; 38.2%) (Table1). The mean follow-up after hepatectomy was 60.9 ± 20.8 months. The mean overall survival after curative hepatectomy was 58.2 ± 3.1 months, and the mean disease-free survival was 26.2 ± 2.1 months (Table2). One patient who died from postoperative peritonitis following the hepatic resection was excluded from the survival analysis.
Table 1.
Clinicopathologic details of patients with liver metastases of colorectal cancer who underwent colectomy (n = 55)
| No. of patients | % | |
|---|---|---|
| Age at time of colectomy, years, mean ± SD | 61 ± 10.6 | |
| Gender (n = 55) | ||
| Male | 38 | 69.1 |
| Female | 17 | 30.9 |
| Primary tumor depth of invasion, pT (n = 50) | ||
| T1 + T2 | 8 | 16.0 |
| T3 | 34 | 68.0 |
| T4 | 8 | 16.0 |
| Primary tumor lymph nodes status, pN (n = 50) | ||
| N0 | 25 | 50.0 |
| N1 + N2 | 25 | 50.0 |
| Primary tumor distant metastasis, pM (n = 55) | ||
| M0 | 34 | 61.8 |
| M1 | 21 | 38.2 |
| Hepatectomy synchronous to colectomy (n = 55) | ||
| Yes | 8 | 14.5 |
| No | 47 | 85.5 |
| Adjuvant chemotherapy to colectomy (n = 55) | ||
| Yes | 38 | 69.1 |
| No | 17 | 30.9 |
Overexpression of CXCR2 and CXCL7 is correlated to overall and disease-free survival
We first compared survival time after hepatectomy according to the expression level of CXCR2 in metastatic liver tissue in the total population of patients (Fig.1). An overexpression of CXCR2, with a cut-off value at 2, was associated with significant reduction in patient's overall and disease-free survival. The mean overall survival was 65.4 ± 3.3 months in patients showing a CXCR2 expression level below 2 (n = 32) compared to 29.8 ± 1.7 months for the high CXCR2 expression group (n = 19; P = 0.012) (Fig.1a). Similarly, with a cut-off at 2, the mean disease-free survival was significantly poorer in the high CXCR2 expression group than in the low CXCR2 expression group (15.4 ± 2.0 months vs 31.8 ± 2.6 months; P = 0.002) (Fig.1a).
Figure 1.
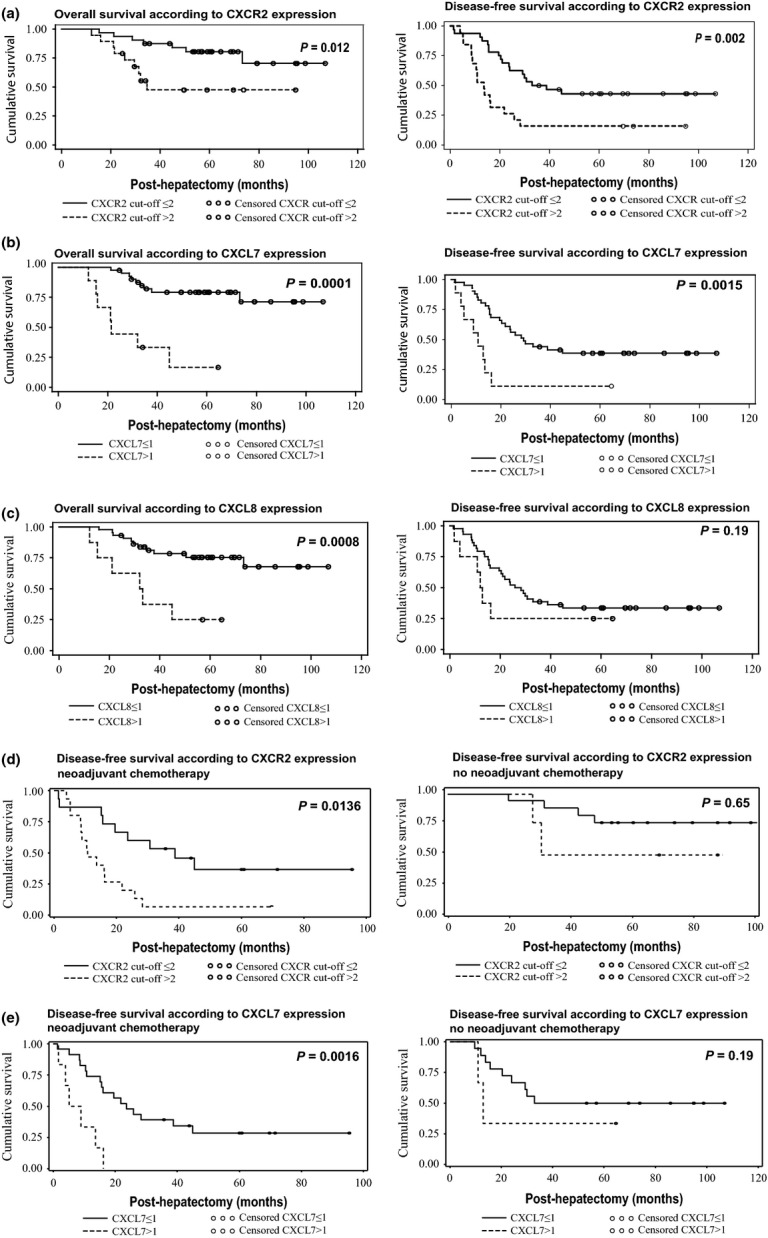
Overall and disease-free survival according to CXCR2, CXCL7, and CXCL8 expression in metastatic colon cancer. (a) Overall and disease-free survival according to CXCR2 expression. Cut-off level, 2. (b) Overall and disease-free survival according to CXCL7 expression. Cut-off level, 1. (c) Overall and disease-free survival according to CXCL8 expression. Cut-off level, 1. (d) Disease-free survival according to CXCR2 expression analyzed in two groups of patients, those who received or did not receive neoadjuvant chemotherapy prior to hepatic surgery. Cut-off level, 2. (e) Disease-free survival according to CXCL7 expression analyzed in the same two groups of patients. Cut-off level, 1.
Likewise, we compared patients’ overall and disease-free survival according to the expression of the two main ligands of CXCR2, CXCL7 and CXCL8.
Overexpression of CXCL7 was significantly correlated with poorer overall and disease-free survival (Fig.1b). The CXCL7 cut-off of expression was identified to be 1. The mean overall survival was 64.4 ± 3.0 months in patients showing a CXCL7 expression below 1 (n = 41) and 28.0 ± 4.67 months in patients with a CXCL7 expression above 1 (n = 9; P < 0.0001). The mean disease-free survival was also significantly poorer in the high CXCL7 expression group than in the low CXCL7 expression group with a CXCL7 cut-off at 1 (9.9 ± 1.8 months vs 29.2 ± 2.4 months, respectively; P = 0.0015).
Overexpression of CXCL8 was significantly correlated with poorer overall survival but not with disease-free survival (Fig.1c). The discriminating CXCL8 cut-off of expression was identified to be 1. According to this cut-off, the mean overall survival was 62.8 ± 30.1 months in the low CXCL8 expression group (n = 44) compared to 31 ± 4.9 months in the high expression group (n = 8; P = 0.0008). The mean disease-free survival was also poorer in the high CXCL8 expression group than in the low CXCL8 expression group (11.2 ± 2.0 months vs 27.7 ± 2.3 months, respectively), but with no statistical significance (P = 0.19).
We then studied the correlations in the two subgroups of patients, having received or not a neoadjuvant chemotherapy prior to hepatic surgery (Fig.1d,e). We found that both CXCR2 and CXCL7 overexpression correlated with high significance with shorter overall and disease-free survival in the subgroup of patients treated by neoadjuvant chemotherapy and hepatic surgery but not in the subgroup of patients treated by hepatic surgery only. These data show that a poorer clinical outcome segregates with the increased CXCR2 and CXCL7 transcript levels and concerns patients who received neoadjuvant chemotherapy, suggesting that neoadjuvant chemotherapy would preselect for resistant tumors showing a deregulation of the CXCR2/CXCL7 axis.
CXCR2 and CXCL7 are independent factors of overall and disease-free survival
Multivariate analyses were carried out considering the level of expression of one of these markers of interest and the known prognostic factors, including TNM classification of primary colorectal tumor (M status and T3 or T4 stage), treatment or no treatment with neoadjuvant chemotherapy before hepatectomy, and treatment or no treatment with neoadjuvant bevacizumab before hepatectomy (Table S2).
By multivariate analysis, CXCR2 was an independent factor of poorer overall and disease-free survival (P = 0.008 and P = 0.004, respectively) after adjustment with the administration of neoadjuvant chemotherapy and synchronous versus metachronous pattern of the metastatic disease. With a cut-off level of CXCR2 expression at 2, CXCR2 was independently associated with decreasing of overall and disease-free survival (P = 0.029 and P = 0.01, respectively) after adjustment for treatment with neoadjuvant chemotherapy and neoadjuvant bevacizumab. The actuarial overall survival after hepatectomy was significantly linked to the levels of expression of CXCR2 and of its ligand CXCL7, when data were adjusted to the administration of neoadjuvant bevacizumab (P = 0.01 and P = 0.007, respectively). The relative risk (95% confidence interval) of recurrence was 1.036 (1.006–1.066) every time CXCR2 increased by 1 unit. In multivariate analysis, CXCR4 and CXCL8 were not associated with overall or disease-free survival. Multivariate analysis carried out on the subgroup of patients having received a neoadjuvant chemotherapy regimen also identified CXCR2 and CXCL7 as independent prognostic factors of poorer overall and disease-free survival in this subgroup of patients.
Therefore, the CXCL7/CXCR2 axis appears to be a crucial pathway promoting further metastatic recurrence in patients treated with neoadjuvant chemotherapy and hepatic surgery for metastatic colon cancer.
Neoadjuvant chemotherapy before hepatectomy increases the expression of CXCR2 in colorectal liver metastasis
We compared the median CXCR2 expression levels in the two subgroups of patients having received, or not received, a neoadjuvant chemotherapy regimen. The median CXCR2 expression was more than threefold higher in the treated group (n = 30) than in the control group (n = 21) (1.89 [0.02–50.92] vs 0.55 [0.07–3.22], respectively; P = 0.016) (Fig.2a). Median CXCL7 expression was nearly threefold higher in the treated group than in the control group (0.40 [0.00–7.85] vs 0.15 [0.01–7.88], respectively) but without reaching statistical significance (P = 0.127) (Fig.2b). Neoadjuvant chemotherapy did not impact the median CXCL8 expression level (Fig.2c). In addition, no significant difference was found between median CXCL2, CXCL6, and MIF expression levels between the two subgroups of patients. Also contrasting with our previous findings obtained with a series of 23 patients,7 we could not confirm an increase in CXCR4 expression in treated versus untreated patients with this larger series of 55 patients (Fig.2c).
Figure 2.
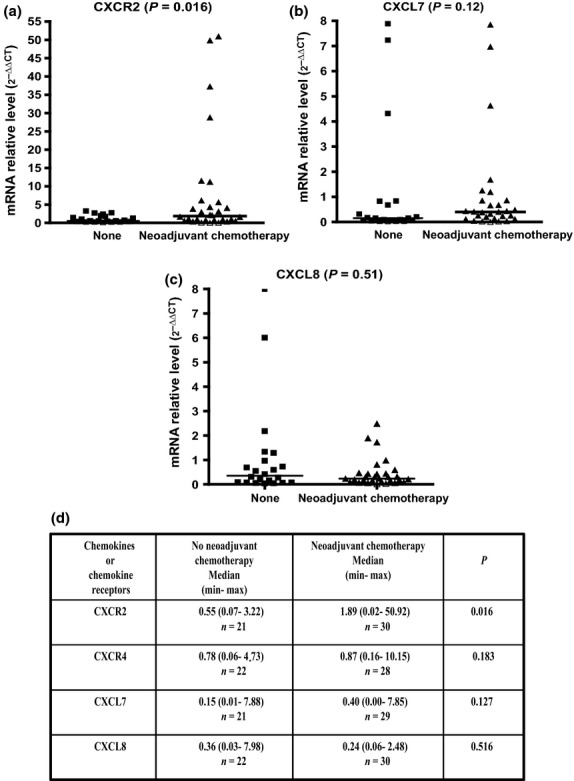
Comparison of chemokine and chemokine receptor transcript levels in liver metastases from patients treated or not treated with neoadjuvant chemotherapy prior to hepatic surgery. (a) CXCR2 expression. (b) CXCL7 expression. (c) CXCL8 expression. (d) Synthesis of results for CXCR2, CXCR4, CXCL7, and CXCL8 expression.
Bevacizumab was given in combination with conventional chemotherapies to 13 of the 34 (38.2%) patients who received neoadjuvant chemotherapy. In order to analyze the effect of the addition of an anti-VEGF therapy, and bevacizumab in particular, on the expression of CXCR2, CXCL7, and CXCL8, we compared the expression of these genes in the liver metastases from patients treated with neoadjuvant chemotherapy with bevacizumab versus patients treated by neoadjuvant chemotherapy without bevacizumab. No significant difference was found either for CXCR2 or CXCL7 according to the addition or not of bevacizumab (Fig.3a,b).
Figure 3.
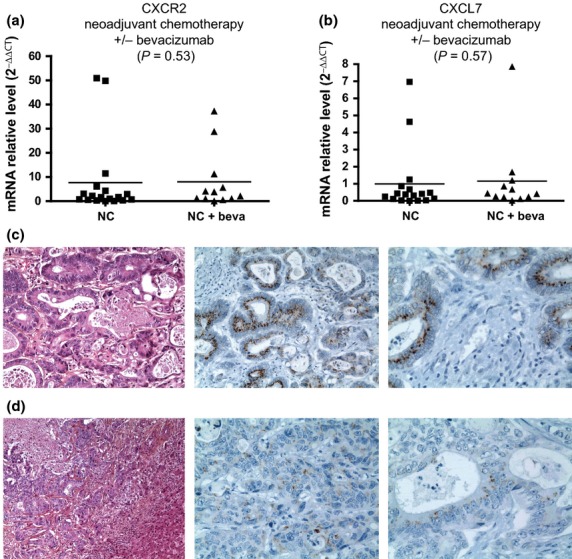
CXCR2 and CXCL7 transcript levels and CXCR2 immunostaining in liver metastases from patients who received neoadjuvant chemotherapy (NC) associated with, or not associated with, bevacizumab (beva). (a, b) Comparison of CXCR2 and CXCL7 transcript levels from patients treated by neoadjuvant chemotherapy with or without bevacizumab prior to hepatic surgery. (c, d) Histologic examination with hematoxylin, eosin, saffron and Astra blue and CXCR2 immunostaining of liver metastases from two patients treated by neoadjuvant chemotherapy with (c) or without (d) bevacizumab (c,d magnification, ×100 and ×400, respectively).
Immunohistochemical analyses of liver colorectal metastasis showed a marked CXCR2 expression in tumor epithelial cells from treated patients (Fig.3c,d). A very weak CXCR2 staining was also present in the tumoral capillaries or endothelial cells. CXCR2 immunostaining was weak or not detected in the healthy liver parenchyma near the metastatic area. CXCR2 expression was more pronounced in the group of patients treated with neoadjuvant chemotherapy combined with bevacizumab than in the control group, both in terms of proportion of CXCR2-positive tumor cells and intensity of immunostaining.
Discussion
Currently, treatment of synchronous or metachronous colorectal liver metastases requires neoadjuvant chemotherapy before hepatic surgery. Actually, neoadjuvant chemotherapy is administered when liver metastases are initially unresectable or marginally resectable (more than five bilateral nodules). Conventionally, chemotherapy regimens consist of 5FU and folinic acid combined with irinotecan or oxaliplatin scheduled over six preoperative treatments.25 Hepatic metastases are resected between 2 and 5 weeks after the last chemotherapy treatment,26 or after 5–6 weeks when bevacizumab is used.27 However, results have been disappointing, with a significant failure rate.25,28
In this report, we have shown that CXCR2 transcript levels were correlated with reduced disease-free and overall survival in patients with metastatic colon cancer. These data support the potential implication of CXCR2-dependent signalling pathways in tumor relapse. Furthermore, 5FU-based neoadjuvant chemotherapy for metastatic colon cancer patients increased the levels of CXCR2 transcripts in liver metastases and CXCR2 overexpression was associated with poorer outcome, suggesting that chemotherapy may preselect for more aggressive colon cancer cells with a deregulated CXCR2 axis. The identification of CXCR2 overexpression as a predictive factor for cancer relapse and poor survival was confirmed in multivariate analysis excluding confounding factors such as treatment with neoadjuvant chemotherapy or anti-VEGF neoadjuvant biotherapy. In tumors, the CXCR2 receptor has been primarily characterized on tumor cells, endothelial cells, infiltrating neutrophils, and tumor-associated macrophages.29 CXCR2 immunostaining in colorectal liver metastases showed that chemotherapy-induced overexpression of CXCR2 is associated with the malignant status of epithelial cells. It was shown that activation of CXCR2 present on cancer cells promotes cell proliferation, cell migration, and invasion. Blockade of CXCR2 signalling with a CXCR2 antagonist inhibits proliferation and invasion of colon carcinoma cells in in vitro assays and growth of tumor xenografts in immunodeficient mice.30 CXCR2 activation in cancer cells can occur in an autocrine-dependent manner. Transfection of the CXCL8 gene in colon cancer cells increases their proliferative and invasive capacity.30,31 An autocrine loop involving growth-related oncogenes CXCL1 and CXCL2 promotes the proliferation of oesophageal cancer cells.32
However, data from published reports brought clear evidence that CXCR2 receptor expressed in cells of the microenvironment also represents a critical component of tumors. Deficiency or inhibition of CXCR2 reduce inflammation-driven tumorigenesis and spontaneous intestinal adenocarcinoma in mouse models of intestinal tumorigenesis.33 CXCR2 blockade reduces neutrophil infiltration in tumors and inhibits tumor growth.34 In the tumor microenvironment, CXCR2 expression on endothelial cells is also relevant to tumor angiogenesis.35 CXCR2 and CXCL8 promote angiogenesis and growth of mouse tumor grafts.18 In pancreatic ductal adenocarcinoma, CXCR2 expressed on cancer-associated fibroblasts mediates tumor–stroma interactions and promotes tumor progression.36 Interleukin-8 signalling pathways also mediate the mobilization of immature myeloid cells in CXCL8 transgenic mice.37 Although CXCR2 expressed on microenvironmental cells can play a role in the progression of several tumors including colon tumors, our data show that CXCR2 expression in liver metastases is primarily found in the malignant cells and correlates to relapse of metastatic disease.
CXCR2 ligands include CXCL1, 2, and 3, epithelial cell-derived neutrophil-activating peptide-78)/CXCL5, granulocyte chemotactic protein-2/CXCL6, CXCL7, and CXCL8. These chemokines can promote tumor growth through the recruitment of protumorigenic neutrophils and stimulation of angiogenesis.19,38,39 The important role of CXCL8 was particularly brought to evidence by the expression of human CXCL8 in mice that do not have the CXCL8 gene.18,37 Among the CXCR2 ligands analyzed in this study, we identified CXCL7 as a predictive factor of shorter disease-free and overall survival of patients. Few studies have been carried out on the potential role of this CXCR2 ligand in tumorigenesis. In breast cancer cells, transfection of CXCL7 stimulates Matrigel invasion and secretion of the lymphangiogenic factors VEGF-C and VEGF-D.40,41 Interestingly, the production of CXCL7 by mesenchymal stem cells was recently shown to be induced by interaction with breast cancer stem cells. A positive feedback cytokine loop between the two cell types was identified in which the cancer cells secrete IL6, which mediates the homing of the mesenchymal stem cells and the induction of CXCL7 production.42 A growing body of evidence suggests that drug-resistant cancer cell subpopulations may be enriched with cancer stem cells.43 In our study, it is possible that the elevated CXCL7 levels are related to the presence of cancer stem cells.
Our work shows for the first time the role of the CXCL7/CXCR2 signaling pathway in liver metastases as a predictive factor of metastatic recurrence and poor outcome in metastatic CRC. Furthermore, 5FU-based chemotherapy regimens increase the expression levels of CXCL7 and CXCR2, which are both strongly correlated with poor outcome, thus providing one explanation for the higher aggressiveness and metastatic potential of relapsed drug-resistant tumors. Tumor dormancy has been recently proposed as a mechanism allowing drug-resistant cancer stem cells to persist in a latent state in the body before tumor relapse.44
In conclusion, our data provide new potential therapeutic opportunities with the selective blockage of the CXCR2/CXCL7 signaling pathway.
Acknowledgments
The authors thank the technical team of the Department of Anatomo-Pathology of Saint-Vincent de Paul Hospital (Lille, France) and Laurence Wicquart (Tumorothèque du Centre Régional de Référence en Cancérologie, CHRU-Lille, France). This work was supported by Ligue contre le Cancer (Comité du Nord), INSERM, SIRIC ONCO-Lille Grant INCa-DGOS-Inserm 6041, “Contrat de Plan Etat Région” CPER Cancer 2007–2013 (IVS).
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Table S1. Primer sequences used for quantitative RT-PCR on human liver metastasis samples of colorectal adenocarcinoma.
Table S2. Multivariate analysis of overall and disease-free survival in patients with liver metastases of colorectal adenocarcinoma.
References
- Majek O, Gondos A, Jansen L, et al. Survival from colorectal cancer in Germany in the early 21st century. Br J Cancer. 2012;106:1875–80. doi: 10.1038/bjc.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JR, Blazeby JM, Fayers P, et al. Patient-reported outcomes after hepatic resection of colorectal cancer metastases. J Clin Oncol. 2012;30:1364–70. doi: 10.1200/JCO.2011.38.6177. [DOI] [PubMed] [Google Scholar]
- Chan KM, Chiang JM, Lee CF, et al. Outcomes of resection for colorectal cancer hepatic metastases stratified by evolving eras of treatment. World J Surg Oncol. 2011;9:174. doi: 10.1186/1477-7819-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou A, Aloia TA, Brouquet A, Vauthey JN. Recent advances in the curative treatment of colorectal liver metastases. Gastrointest Cancer Res. 2011;4:S2–8. [PMC free article] [PubMed] [Google Scholar]
- Sorbye H, Mauer M, Gruenberger T, et al. Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983) Ann Surg. 2012;255:534–9. doi: 10.1097/SLA.0b013e3182456aa2. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Dessein AF, Stechly L, Jonckheere N, et al. Autocrine induction of invasive and metastatic phenotypes by the MIF-CXCR4 axis in drug-resistant human colon cancer cells. Cancer Res. 2010;70:4644–54. doi: 10.1158/0008-5472.CAN-09-3828. [DOI] [PubMed] [Google Scholar]
- Oladipo O, Conlon S, O'Grady A, et al. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br J Cancer. 2011;104:480–7. doi: 10.1038/sj.bjc.6606055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke H, Struyf S, Laureys G, Van Damme J. The expression and role of CXC chemokines in colorectal cancer. Cytokine Growth Factor Rev. 2011;22:345–58. doi: 10.1016/j.cytogfr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–31. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–44. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- Rombouts EJ, Pavic B, Lowenberg B, Ploemacher RE. Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004;104:550–7. doi: 10.1182/blood-2004-02-0566. [DOI] [PubMed] [Google Scholar]
- Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–53. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- Terada H, Urano T, Konno H. Association of interleukin-8 and plasminogen activator system in the progression of colorectal cancer. Eur Surg Res. 2005;37:166–72. doi: 10.1159/000085964. [DOI] [PubMed] [Google Scholar]
- Rubie C, Frick VO, Pfeil S, et al. Correlation of IL-8 with induction, progression and metastatic potential of colorectal cancer. World J Gastroenterol. 2007;13:4996–5002. doi: 10.3748/wjg.v13.i37.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Choi I, Ning Y, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106:1833–41. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- Heidemann J, Ogawa H, Dwinell MB, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–15. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- Cheng WL, Wang CS, Huang YH, Tsai MM, Liang Y, Lin KH. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Ann Oncol. 2011;22:2267–76. doi: 10.1093/annonc/mdq739. [DOI] [PubMed] [Google Scholar]
- Matsubara J, Honda K, Ono M, et al. Reduced plasma level of CXC chemokine ligand 7 in patients with pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:160–71. doi: 10.1158/1055-9965.EPI-10-0397. [DOI] [PubMed] [Google Scholar]
- Van Seuningen I, Perrais M, Pigny P, Porchet N, Aubert JP. Sequence of the 5’-flanking region and promoter activity of the human mucin gene MUC5B in different phenotypes of colon cancer cells. Biochem J. 2000;348(Pt 3):675–86. [PMC free article] [PubMed] [Google Scholar]
- Skrypek N, Duchene B, Hebbar M, Leteurtre E, van Seuningen I, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene. 2013;32:1714–23. doi: 10.1038/onc.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger M, Eipeldauer S, Hacker S, et al. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur J Surg Oncol. 2009;35:515–20. doi: 10.1016/j.ejso.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Millet G, Truant S, Leteurtre E, et al. Volumetric analysis of remnant liver regeneration after major hepatectomy in bevacizumab-treated patients: a case-matched study in 82 patients. Ann Surg. 2012;256:755–61. doi: 10.1097/SLA.0b013e31827381ca. ; discussion 61–2. [DOI] [PubMed] [Google Scholar]
- Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906–11. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- Ning Y, Labonte MJ, Zhang W, et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther. 2012;11:1353–64. doi: 10.1158/1535-7163.MCT-11-0915. [DOI] [PubMed] [Google Scholar]
- Ning Y, Manegold PC, Hong YK, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–49. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071–7. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- Jamieson T, Clarke M, Steele CW, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–44. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzyman S, Barry ST, Ashton S, et al. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J Cancer. 2011;129:847–58. doi: 10.1002/ijc.25987. [DOI] [PubMed] [Google Scholar]
- Addison CL, Daniel TO, Burdick MD, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–77. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- Ijichi H, Chytil A, Gorska AE, et al. Inhibiting Cxcr2 disrupts tumor–stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–17. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaha S, Dubeykovskiy AN, Tomita H, et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144:155–66. doi: 10.1053/j.gastro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:768–78. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Tang Z, Yu M, Miller F, Berk RS, Tromp G, Kosir MA. Increased invasion through basement membrane by CXCL7-transfected breast cells. Am J Surg. 2008;196:690–6. doi: 10.1016/j.amjsurg.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Yu M, Berk R, Kosir MA. CXCL7-mediated stimulation of lymphangiogenic factors VEGF-C, VEGF-D in human breast cancer cells. J Oncol. 2010;2010:939407. doi: 10.1155/2010/939407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–24. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA. ERK1/2 and p38alpha/beta signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res. 2011;17:5850–7. doi: 10.1158/1078-0432.CCR-10-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer sequences used for quantitative RT-PCR on human liver metastasis samples of colorectal adenocarcinoma.
Table S2. Multivariate analysis of overall and disease-free survival in patients with liver metastases of colorectal adenocarcinoma.


