Abstract
The efficacy of boron neutron capture therapy relies on the selective delivery of boron carriers to malignant cells. p-Boronophenylalanine (BPA), a boron delivery agent, has been proposed to be localized to cells through transporter-mediated mechanisms. In this study, we screened aromatic amino acid transporters to identify BPA transporters. Human aromatic amino acid transporters were functionally expressed in Xenopus oocytes and examined for BPA uptake and kinetic parameters. The roles of the transporters in BPA uptake were characterized in cancer cell lines. For the quantitative assessment of BPA uptake, HPLC was used throughout the study. Among aromatic amino acid transporters, ATB0,+, LAT1 and LAT2 were found to transport BPA with Km values of 137.4 ± 11.7, 20.3 ± 0.8 and 88.3 ± 5.6 μM, respectively. Uptake experiments in cancer cell lines revealed that the LAT1 protein amount was the major determinant of BPA uptake at 100 μM, whereas the contribution of ATB0,+ became significant at 1000 μM, accounting for 20–25% of the total BPA uptake in MCF-7 breast cancer cells. ATB0,+, LAT1 and LAT2 transport BPA at affinities comparable with their endogenous substrates, suggesting that they could mediate effective BPA uptake in vivo. The high and low affinities of LAT1 and ATB0,+, respectively, differentiate their roles in BPA uptake. ATB0,+, as well as LAT1, could contribute significantly to the tumor accumulation of BPA at clinical dose.
Keywords: Amino acid transporter, BNCT, BPA, cancer cell lines, drug delivery
Boron neutron capture therapy (BNCT) is a therapeutic modality for malignant tumors using the nuclear capture and fission reactions that occur when boron-10 (10B) is irradiated with neutron beams. This reaction, in theory, only kills 10B-containing cells because the destructive effect of the alpha particles and lithium nuclei, which are produced by the reaction, is limited to the immediate vicinity of the reaction (5–9 μm, approximately one cell diameter).1 Thus, the efficacy of BNCT relies on the selective delivery of 10B to malignant cells. Although various boron delivery agents have been proposed, only two compounds are currently under consideration in clinical trials, p-boronophenylalanine (BPA) and sodium borocaptate.1,2 The latter is an anionic polyhedral borane icosahedron containing 12 boron atoms. This compound is thought to enter tumor cells by passive diffusion through the plasma membrane.3,4 In contrast, BPA is localized to tumor cells by selective uptake mediated by transporters.5
Alterations of transporter expression in tumor cells, an adaption to altered tumor metabolism, offer opportunities for selective drug delivery.6 In this context, aromatic amino acid transporters upregulated in tumor cells are expected to play an important role in the delivery of BPA, a phenylalanine analogue. Previous studies have suggested that system L transporters, particularly LAT1, are involved in the transport of BPA.5,7,8 The expression of LAT1 is highly upregulated in various cancers, where it is thought to contribute to tumor growth by increasing amino acids supply.9–11 Since we found LAT1 as a first system L transporter, we have examined its functional properties and revealed that a PET tracer, 3-fluoro-L-α-methyl-tyrosine, is taken up by LAT1 into tumors.12–15
This study provides a BPA transport profile of human aromatic amino acid transporters. In previous studies, the mechanisms of BPA uptake were studied by using non-specific transporter inhibitors and/or exploiting Na+-dependence of the uptake.5,7,8 However, such approaches cannot separate transporter isoforms. In the present study, we have expressed each transporter in Xenopus oocytes to examine whether it transports BPA. Here, we have identified transporters responsible for BPA uptake among aromatic amino acid transporters. We have, furthermore, characterized their transport kinetics and roles in BPA transport in cancer cells.
Materials and Methods
Materials
Chemicals and cell media were purchased from Wako Pure Chemicals (Osaka, Japan) unless otherwise specified. L-isomer of BPA was from Sigma-Aldrich (St Louis, MO, USA). 4-Fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F), a fluorescence-derivatization agent, was from Dojindo (Kumamoto, Japan). Pancreatic cancer MIA PaCa−2, cervical cancer HeLa S3, and breast cancer MCF-7 cell lines were from the American Type Culture Collection (Manassas, VA, USA). Pancreatic cancer cell line T3M4 was from Riken Cell Bank (Tsukuba, Japan). Hepatocarcinoma FLC-4 was a gift from Dr. Seishi Nagamori.16 ASF104 serum-free medium was from Ajinomoto (Tokyo, Japan). Fetal bovine serum was from Gibco (Gibco Life Technologies, Grand Island, NY, USA).
Transport assay in Xenopus oocytes
The human cDNAs for transporters are listed in Table1; the cDNAs of the transporters, collectrin and 4F2hc were cloned into pcDNA3.1(+) (Invitrogen Life Technologies, Carlsbad, CA, USA) by standard recombinant methods. Capped complementary RNAs (cRNA) were transcribed from linearized cDNA plasmid templates by T7 mMESSAGE mMACHINE kit (Ambiom Life Technologies, Austin, TX, USA).
Table 1.
p-Boronophenylalanine (BPA) uptake by human aromatic amino acid transporters
| Transport system | Gene | Protein | Na+ dependence | BPA transport |
|---|---|---|---|---|
| B0 | SLC6A19 | B0AT1 | Dependent | − |
| B0,+ | SLC6A14 | ATB0,+ | Dependent | + |
| L1 | SLC7A5 | LAT1 | Independent | + |
| SLC7A8 | LAT2 | Independent | + | |
| L2 | SLC43A1 | LAT3 | Independent | − |
| SLC43A2 | LAT4 | Independent | − | |
| T | SLC16A10 | TAT1 | Independent | − |
Xenopus oocytes expressing each transporter were incubated for 30 min in uptake buffer containing 100 μM BPA with or without Na+ on the basis of Na+ dependence. Oocyte lysate was separated by HPLC for BPA detection. +, positive for BPA uptake; −, negative for BPA uptake.
The transport assay was carried out as previously described.12 Briefly, oocytes were micro-injected with cRNA: 25 ng for equimolar mixture of collectrin and B0AT1, 4F2hc and LAT1 or LAT2; 12.5 ng for LAT4; and 25 ng for the others. B0AT1 cRNA was co-injected with that of collectrin for efficient membranous expression. Similarly, LAT1 and LAT2 were coexpressed with 4F2hc. The injected oocytes were incubated at ∽18°C for 2 days for LAT1 + 4F2hc and LAT2 + 4F2hc, and for 3 days for the others. The oocytes were then subjected to uptake assay for designated incubation time in the uptake buffer containing BPA at designated concentrations. For Na+-dependent uptake, Na+ uptake buffer (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2·2H2O, 1 mM MgCl2·6H2O, and 5 mM HEPES, pH 7.5) or Na+-free uptake buffer was used. In Na+ free uptake buffer, NaCl was replaced with 96 mM choline chloride. Uptake was terminated by washing three times with an ice-cold uptake buffer.
High performance liquid chromatography analysis
Amino acid derivatization were reported elsewhere17. Samples were prepared and separated by HPLC as described below. Each oocyte was homogenized in 100 μL water by sonication and spun at 15,000g at 4°C for 10 min. After carefully removing the yolk fraction by aspiration, 20 μL supernatant was vigorously mixed with 180 μL methanol for deproteinization, and spun at 15,000g at 4°C for 10 min. Cell samples were similarly prepared. Protein concentrations were determined by bicinchoninic acid assay (Thermo Scientific, Rockford, IL, USA) before deproteinizing. One hundred microliters of the supernatant was dried in a vacuum chamber. The sediment was resolved in 20 μL of 200 mM sodium borate (pH 8.0). For fluorescence derivatization, 5 μL of 40 mM NBD-F in acetonitrile (MeCN) was added and heated at 60°C for 2 min. Derivatizing reaction was terminated by adding 75 μL of 0.5% trifluoroacetic acid (TFA) aqueous solution. Twenty microliters of the sample was separated by a NANOSPACE SI-2 HPLC system (Shiseido, Tokyo, Japan) with a fluorescence detector. The analytical column was Capcell Pak C18 MGII S5 (250 × 2.0 mm i.d.). The mobile phase was MeCN–TFA–water (27.5:0.05:72.5, vol/vol/vol) with a flow rate of 200 μL/min. Isocratic elution was carried out for 15 min. The BPA in samples was quantified by comparing the peak height with that of a BPA standard of known amount.
Cell culture
MIA Paca-2 cells were grown in Dulbecco's modified Eagle's medium (DMEM). HeLa S3 cells were in Eagle's minimum essential medium. MCF-7 cells were in DMEM with insulin (0.01 mg/mL), sodium pyruvate (1 mM), and NEAA. FLC-4 cells were in ASF104 serum-free medium. T3M4 cells were in RPMI-1640 medium. Cell lines were cultured at 37°C with 5% CO2. Media were supplemented with FBS (10%) and penicillin/streptomycin (100 U/mL).
Membrane fractionation and Western blot analysis
Cultured cells were collected and pelleted at 5000g for 3 min at 4°C. The cell pellet was sonicated in 500 μL ice-cold PBS (pH 7.4) containing Complete EDTA-free protease inhibitor cocktail (Roche, Mannheim, Germany). Homogenate was cleared at 1000g for 10 min. The supernatant was centrifuged at 10,000g for 10 min. The resultant supernatant was ultracentrifuged at 391,000g for 30 min. The membrane pellet was washed twice with ice-cold PBS and re-suspended in 50 μL PBS with 1% NP-40. The protein concentrations were determined by bicinchoninic acid assay. Protein samples were separated on a 10% polyacrylamide gel. The gel was blotted onto a PVDF membrane at 100 V for 30 min. The membrane was probed in TBST buffer (10 mM Tris, 150 mM, pH 7.6 adjusted with HCl) containing 5% skim milk with anti-SLC6A14/ATB0,+ (1:1000; MBL, Nagoya, Japan) followed by goat anti-rabbit IgG HRP-conjugated (1:5000; Jackson, West Grove, PA, USA), or anti-LAT1 antibody (1:5000; Trans Genic Inc., Kumamoto, Japan) followed by goat anti-rabbit IgG HRP-conjugated (1:5000; Jackson). Na+-K+ ATPase was detected as loading control by anti-Na+-K+ ATPase antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by goat anti-mouse IgG HRP-conjugated (1:5000; Jackson). The antibody-treated membrane was developed by ECL Prime Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK) and imaged by LAS-4000 mini version 2.0 (Fujifilm, Tokyo, Japan).
Transport assay in cultured cells
The FLC-4, MCF-7, MIA PaCa-2, HeLa S3 and T3M4 cells were seeded on a 24-well plate at 1.2 × 105, 2 × 105, 0.8 × 105, 1.2 × 105 and 1 × 105 cells/well. Cells were incubated for 2 days. The uptake experiment was carried out as described18 in Hanks' balanced salt solution or Na+-free Hanks' balanced salt solution in which NaCl was replaced with equivalent concentration of choline chloride.
For RNA interference, MCF-7 cells were seeded at 1.2 × 105 cells/well on a 24-well plate 24 h prior to transfection with RNAiMax (Invitrogen Life Technologies). The cells were subjected to uptake experiments after 48 h of transfection. The siRNAs used in this study were: non-targeting controls siRNA #1 (D-001810-01-05) and #2 (D-001810-01-20) from Thermo Scientific; and LAT1 siRNA #3 (s15653), #4 (s15654), and #5 (s15655) from Ambion Life Technologies.
Statistics
Student's t-test was used for statistical analyses. P < 0.05 was taken as significant. Each experiment was carried out in triplicate. Representative results are shown in figures.
Results
Determination of transporter-mediated BPA uptake
Standard BPA and amino acid mixture were separated by HPLC after pre-column derivatization with NBD-F. NBD-BPA was eluted between NBD-alanine and NBD-proline (Fig.1a,b). NBD-BPA (0.1 pmol/injection) was detected with our HPLC specifications. BPA standards were linearly quantified from 0.1 to 12 pmol with a correlation coefficient r = 0.9995. To establish HPLC determination of BPA in biological sample, BPA transported into Xenopus oocytes by LAT1 was separated by HPLC (Fig.1c,d). The BPA peak of LAT1-expressing oocyte was higher than that of the control, indicating that BPA uptake by LAT1 was detected by this analytical procedure. Quantitative estimation of BPA in biological sample was carried out when the BPA peak height was more than two times higher than the background. To assess the recovery of BPA, samples prepared from oocytes were exogenously supplemented with 1 pmol of BPA before homogenization. The recovery was estimated to be 97 ± 1.25% (mean ± SD, n = 3).
Figure 1.
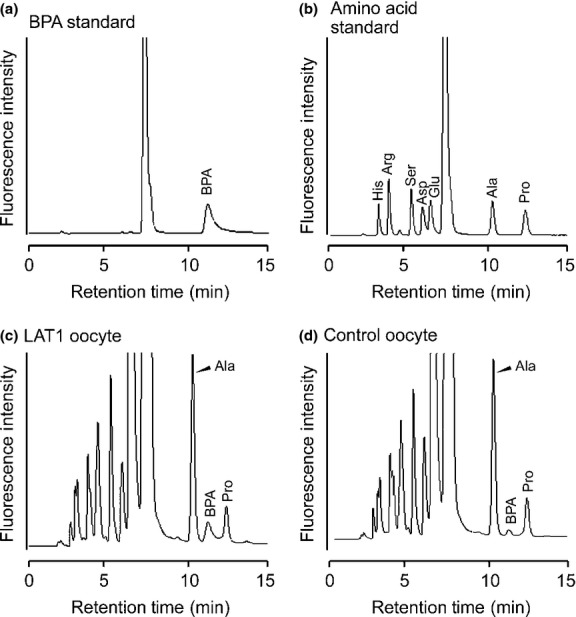
Separation of p-boronophenylalanine (BPA) by HPLC. Chromatograms showing the separation of a BPA standard (0.2 pmol) (a), amino acid standards (0.2 pmol each) (b), a sample from the oocyte expressing LAT1 (c), and the control oocyte not expressing LAT1 (d). (a, b) The BPA peak was identified by retention time and spike study (not shown). Similarly, by comparison with amino acid standards and a spike study (not shown), the peaks neighboring BPA in oocyte samples were identified as alanine (Ala) and proline (Pro). (c, d) The LAT1-expressing oocyte and non-expressing control oocyte were incubated in the uptake buffer containing BPA. Samples from the oocytes were separated by HPLC. The increased BPA peak height in (c) showed that the uptake of BPA was mediated by LAT1. Arg, arginine; Asp, aspartic acid; Glu, glutamic acid; His, histidine; Ser, serine.
Identification of human amino acid transporters mediating BPA uptake
The HPLC-based uptake assay was applied to screen over human aromatic amino acid transporters to identify BPA transporters. The transporters listed in Table1 were selected based on the substrate selectivity in which phenylalanine or tyrosine is preferred.19 The selected amino acid transporters were functionally expressed in Xenopus oocytes (Fig. S1). The experimental and control oocytes were subjected to uptake experiments followed by HPLC detection. As shown in Figure2 and Table1, ATB0,+, LAT1 and LAT2 were found to transport BPA.
Figure 2.
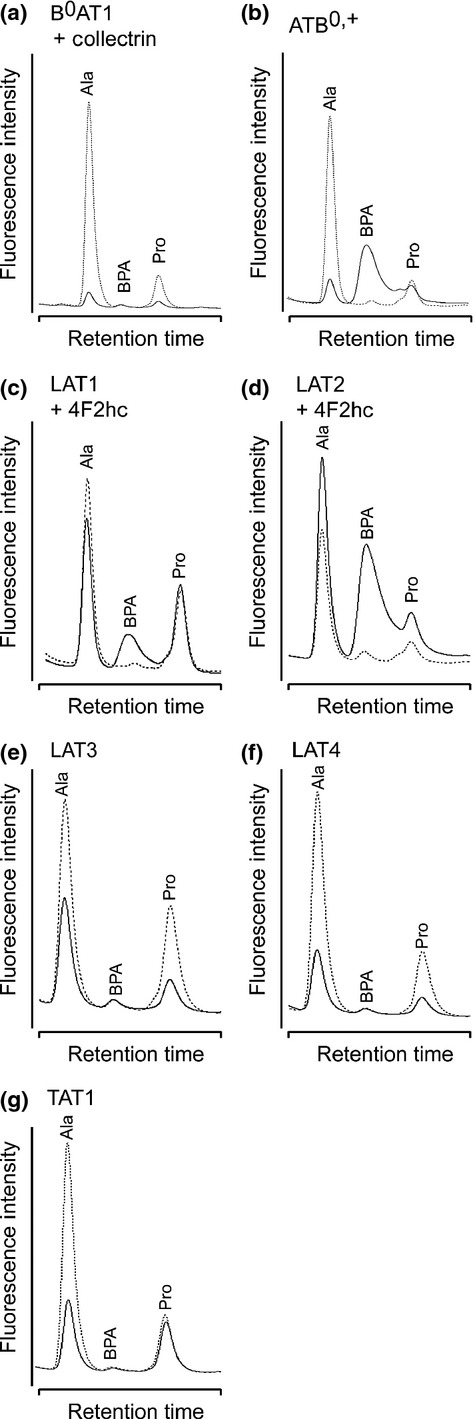
Chromatograms of p-boronophenylalanine (BPA) taken up by aromatic amino acid transporters. Chromatograms for oocytes expressing each transporter (solid line) and non-expressing control oocytes (dotted line) are overlaid on the same scaling. In ATB0,+ (b), LAT1 (c) and LAT2 (d), the BPA peaks were significantly higher than those in the controls, but not in the others. The endogenous alanine (Ala) and proline (Pro) peaks were frequently found to be lower in the oocytes expressing transporters, probably because of the translational consumption of endogenous amino acids.
Time- and concentration-dependent uptake of BPA by ATB0,+, LAT1 and LAT2
The dependence of BPA transport on uptake time and BPA concentration was examined. The uptake of BPA by each transporter was linear over 30 min (Fig.3). Thus, uptakes were measured for 30 min to determine kinetic parameters. The BPA transports by ATB0,+, LAT1 and LAT2 were saturable and followed the Michaelis–Menten kinetics (Fig.4). The Michaelis–Menten constants (Km) and Vmax values were determined by the Lineweaver–Burk plot (Fig.4) and summarized in Table2.
Figure 3.
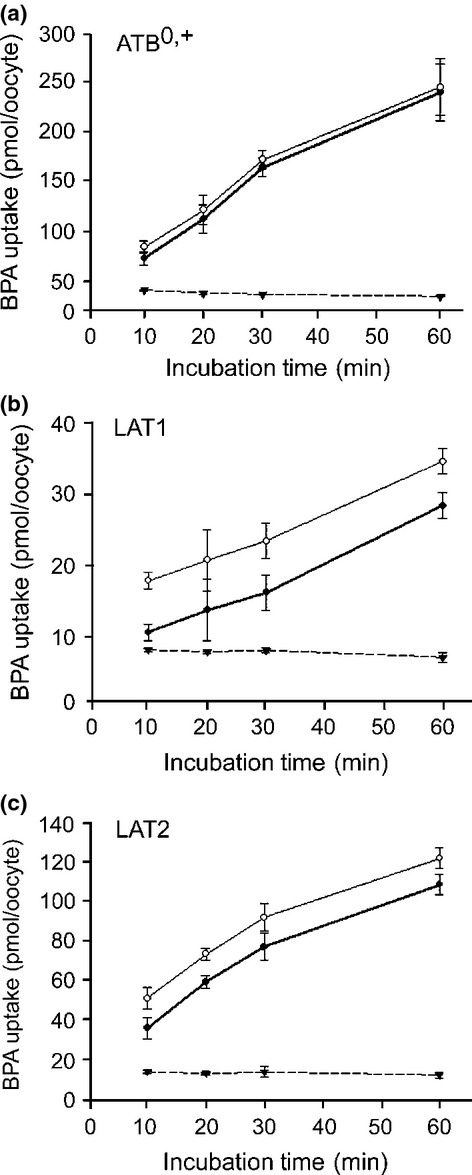
Time-dependent uptake of p-boronophenylalanine (BPA). Uptakes of BPA by oocytes expressing the indicated transporter (○) and by non-expressing controls (▾) were measured over 60 min in Na+ uptake buffer containing 50 μM BPA for ATB0,+ (a), and in Na+-free uptake buffer for LAT1 (b) and LAT2 (c). The time-course of the uptake was determined at 50 μM BPA because the measurement became less accurate for the 10- and 20-min time points at lower concentrations. The transporter-mediated uptakes (•) were determined by subtracting the uptake in non-expressing control oocytes (▾) from the uptake in oocytes expressing each transporter (○). Uptake by ATB0,+ and LAT2 linearly increased up to 30 min (a, c). Uptake by LAT1 was linear over 60 min (b). Each data point represents the mean ± SEM of three to five oocytes.
Figure 4.
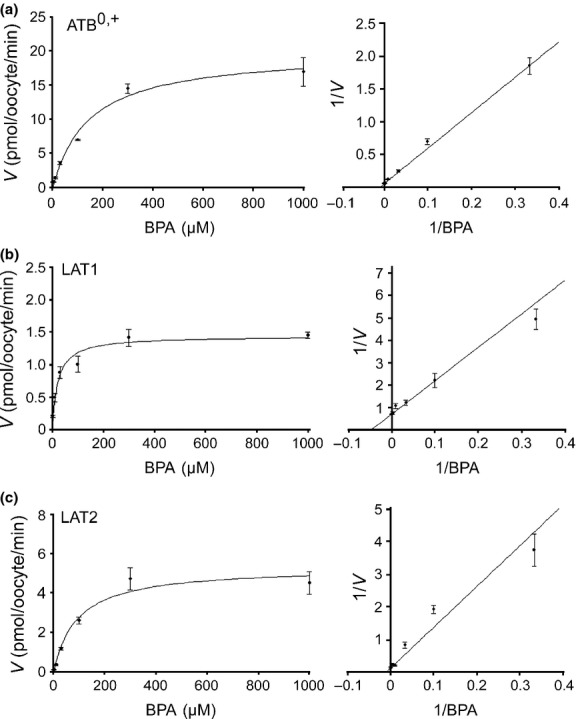
Concentration-dependent uptake of p-boronophenylalanine (BPA). Uptake of BPA was measured for 30 min at 3–1000 μM for ATB0,+ (a), LAT1 (b) and LAT2 (c). The velocity (V) of transporter-mediated uptake was determined by subtracting the uptake in non-expressing control oocytes from the uptake in oocytes expressing each transporter. Representative Michaelis–Menten fitting (left panels) and Lineweaver–Burk plot (right panels) are shown for each transporter. Each data point represents the mean ± SEM of five to seven oocytes.
Table 2.
Kinetic parameters of p-boronophenylalanine transport
| Transporter | Km, μM | Vmax, pmol/oocyte/min |
|---|---|---|
| ATB0,+ | 137.4 ± 11.7 | 19.7 ± 0.9 |
| LAT1 | 20.3 ± 0.8 | 1.32 ± 0.2 |
| LAT2 | 88.3 ± 5.6 | 5.8 ± 0.9 |
Values represent the mean ± SEM of three separate experiments. Km, Michaelis–Menten constant.
Boronophenylalanine uptake in cancer cell lines and expression of LAT1and ATB0,+
LAT2 expression is associated with normal tissues, whereas LAT1 and ATB0,+ are known for their relevance to malignant tumors.6,9,20 To assess the contribution of LAT1 and ATB0,+ in BPA uptake, uptake rates were measured in cancer cell lines with various expression levels of LAT1 and ATB0,+. The LAT1 protein amounts were compared in Western blot analysis: low in FLC-4, medium in MCF-7 and MIA PaCa-2, and high in HeLa S3 and T3M4 cells (Fig.5a). In contrast, ATB0,+ protein was strongly detected in MCF-7 and also weakly in T3M4 cells, but not in the other cell lines (Fig.5b). The BPA uptake rates were proportional to the LAT1 protein levels (Fig.5a,c). We examined the Na+-dependence of BPA uptake, because ATB0,+ is Na+-dependent whereas LAT1 is not. There were no significant differences in the uptakes at 100 μM BPA between Na+-free and normal Na+ conditions (Fig.5c).
Figure 5.
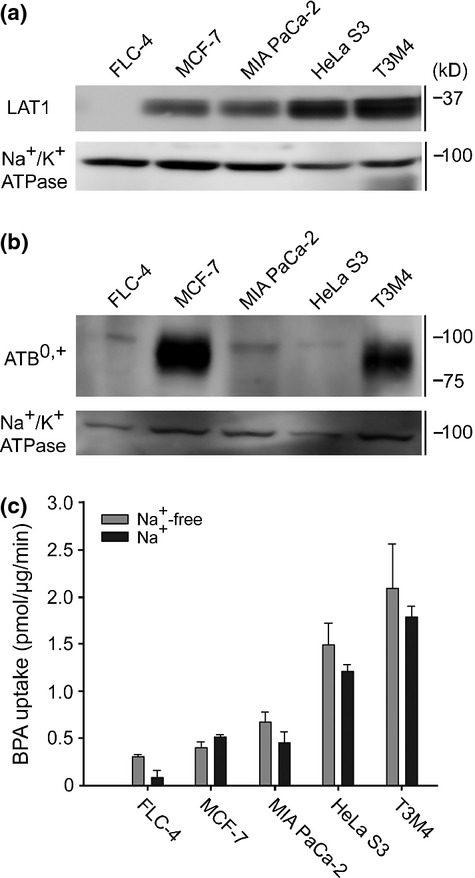
Transporter expression and p-boronophenylalanine (BPA) uptake in cancer cell lines. (a) Expression level of LAT1 was analyzed by western blotting using crude membrane fraction. The cell lines were ordered in increasing LAT1 amounts. Na+/K+ ATPase was used as loading control. (b) Similarly, the expression of ATB0,+ was analyzed. ATB0,+ was expressed in MCF-7 cells, to a lesser degree in T3M4 cells, and not detected in the other cell lines. (c) Uptake of BPA in cell lines was measured for 5 min in the presence or absence of Na+. The presence of Na+ did not significantly affect the BPA uptakes in the cell lines. A representative result was shown with the mean ± SEM (n = 4).
Increased contribution of ATB0,+at higher BPA concentration
ATB0,+ has a lower affinity to BPA than LAT1 (Table2). Thus, ATB0,+ is proposed to work at higher BPA concentrations. Consistent with this, a significant Na+-dependent uptake of BPA was observed at 1000 μM in MCF-7 cells (Fig.6a). To reduce the contribution of LAT1 to BPA uptake in MCF-7 cells, the LAT1 expression was silenced by RNA interference. Treatment of MCF-7 cells with siRNAs depleted the LAT1 protein amount to ∽20% (Fig.6b). Among the siRNAs tested, control siRNAs and LAT1 siRNAs had a similar impact on the LAT1 protein amount (Fig.6b) and BPA uptake (Fig. S2). After LAT1 was knocked down in 1000 μM BPA, the residual component was further decreased by removing Na+ and also inhibited by lysine, a high-affinity substrate of ATB0,+ (Km, ∽100 μM)21 but not interactive with LAT112 (Fig.6c). This Na+-dependent and lysine-inhibitable component is proposed to be mediated by ATB0,+. Such component was only found at 1000 μM, accounting for at least 20–25% of the total BPA uptake (Fig.6c). In 100 μM of BPA, the residual component after LAT1 knockdown was not dependent on Na+ (Fig.6c).
Figure 6.
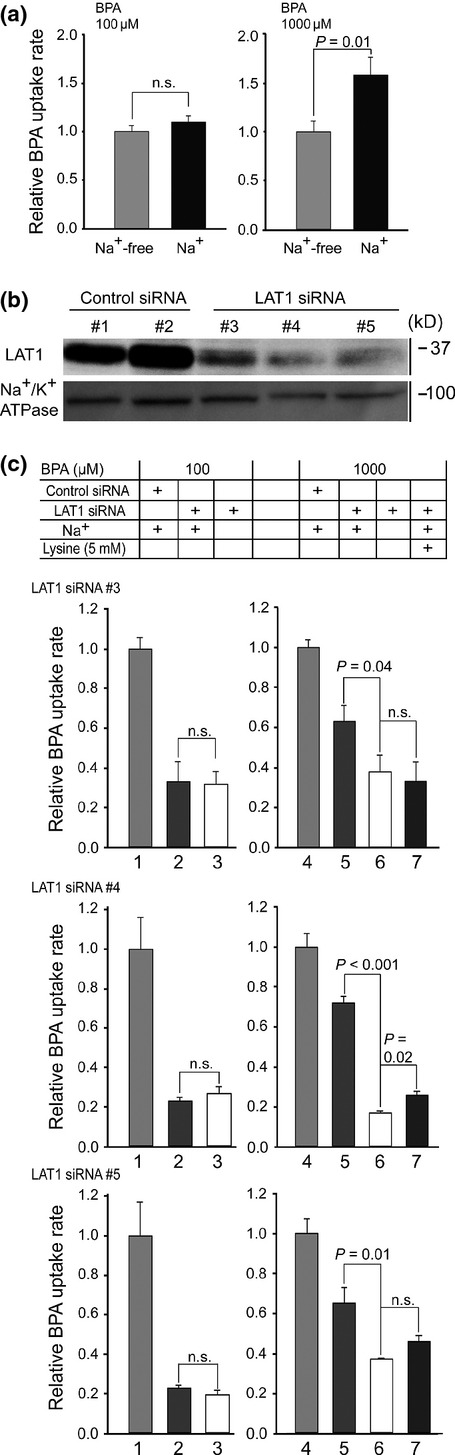
Differential roles of ATB0,+ and LAT1 in p-boronophenylalanine (BPA) uptake. (a) Uptake of BPA in MCF-7 cells was measured for 10 min at 100 μM (left) and 1000 μM (right) BPA. At 1000 μM, the uptake rate in the presence of Na+ increased by ∽1.4-fold from the Na+-free uptake. (b) MCF-7 cells were transfected with non-targeting control siRNA #1 and #2, and LAT1-targeting siRNA #3, #4 and #5. The siRNAs #3–#5 achieved a ∽80% reduction of LAT1 protein amount. (c) Uptake by ATB0,+ was separated by LAT1 knockdown and lysine inhibition. The uptakes in the presence of Na+ and with mock knockdown were set at 1.0 at each BPA concentration (bars 1 and 4). At 100 μM BPA, the uptake levels with LAT1 knockdown did not differ regardless of Na+ (bars 2 and 3). At 1000 μM, LAT1 knockdown left a significant Na+-dependent component (bars 5 and 6). Overall, although with various statistical significances, the BPA uptake in bar 5 was inhibited by 5 mM lysine to a level similar to bar 6 (bar 7). This Na+-dependent and lysine-inhibitable component accounted for at least 20–25% of the total uptake. siRNA#1 was used for control siRNA. n.s., not significant.
Discussion
Previously published reports suggested that system L transporters mediate the entry of BPA into tumor cells.5,7,8 The present study clarified this by separately expressing transporters in Xenopus oocytes; LAT1 and LAT2 transport BPA whereas LAT3 and LAT4 do not. In addition, ATB0,+ was proved to be a BPA transporter. ATB0,+ has been shown to be upregulated in malignant tumors as discussed later and proposed to be a potential therapeutic target.6,20,22,23 ATB0,+ transports all amino acids except glutamate and aspartate by using a Na+ and Cl− gradient.21 This broad selectivity of ATB0,+ holds potential to be used for targeted drug delivery.6
The range of Km values for large neutral amino acids is 10–30 μM in LAT1 and 40–120 μM in LAT2.13,24 ATB0,+ transports 18 amino acids at various affinities with Km values ranging from 10 to 600 μM.21 The BPA Km values for these transporters fall within the endogenous substrate ranges, suggesting that these transporters could mediate effective BPA uptake in vivo (Table2). LAT1 and ATB0,+ differentially uptake BPA, depending on its concentration. At 100 μM BPA, LAT1 appears to be a key BPA transporter because the BPA uptake rate was Na+-independent and positively correlated with the amount of LAT1 protein (Fig.5). At 1000 μM, ATB0,+ measurably transports BPA, accounting for 20–25% of total uptake in MCF-7 cells (Fig.6c). This ratio is comparable to the ATB0,+ contribution in the uptake of iodine-123-α-methyl tyrosine, a single photon emission computed tomography tracer, in glioma cells.25 Clinically, blood BPA concentrations reach ∽2000 μM when administering 250 mg BPA/kg body weight,26 suggesting that the contribution of ATB0,+ could be significant at the clinical dose of BPA if the tumors express ATB0,+.
From the Michaelis–Menten curves in Figure4, both LAT1 and ATB0,+ are extrapolated to be almost saturated to transport BPA at the clinical concentrations. Despite the saturated kinetics, an animal study using a regimen of increasing dose from 250 to 1000 mg BPA/kg body weight resulted in an increase in tumor boron concentration.27 Also, the extension of infusion time increased boron accumulation in tumor.27,28 Wyss et al.29 observed that intratumor blood flow is heterogeneous in low-grade glioma and so is the uptake of an amino acid PET tracer. Such heterogeneous vasculature possibly generates various local BPA concentrations within the tumor mass. Higher-dose and longer-infusion protocol might help raise the local concentrations in poorly perfused regions, resulting in homogeneous BPA distribution. Clinically, a high-dose and longer-infusion regimen (900 mg BPA/kg, 6-h infusion) is reported to extend patients' survival.1,30
In addition to the transporter kinetics, expression levels of LAT1 and ATB0,+ in tumor tissues are thought to impact on BPA accumulation. Tumors where BNCT is of clinical interest are head and neck cancers, melanoma, glioma and hepatic metastases of colorectal cancer.31 The expressions of LAT1 and ATB0,+ in head and neck cancers, melanoma and glioma were compared using data from the Human Protein Atlas project (http://www.proteinatlas.org/) (Fig. S3).32 Head and neck cancers and melanomas appeared to have higher expression profiles of these transporters over other tumors (Fig. S3). Recent successful applications of BNCT to head and neck cancers may rely on the abundance of LAT1 and ATB0,+ in situ. LAT1 or ATB0,+ was moderately or weakly detected in the glioma specimens in Figure S3. However, in two other studies, LAT1 expression was detected in all glioma specimens with increasing immunostaining intensity over grade (Table S1);33,34 this finding is important because BNCT is indicated for high-grade gliomas.1,31 Expression studies in liver metastases of colorectal cancer are scarce with one study reporting the upregulation of ATB0,+.35
In contrast, LAT2 is expressed in normal tissues.11 An expressed sequence tag analysis showed a negative association of LAT2 with tumor tissues.9 Based on these findings, the main role of LAT2 in the BPA pharmacokinetics is proposed to lower the tumor-to-normal (T/N) ratio of BPA accumulation. This hypothesis could be appropriately tested by using primary specimens containing both tumor and adjacent normal tissues, not by cultured cells.
On a methodological note, HPLC was extensively used throughout this study to detect and quantify BPA, instead of direct-current plasma atomic emission spectrometry (DCP-AES). Our HPLC method derivatizes BPA with NBD-F and detects NBD-BPA with a detection limit of 0.1 pmol per injection, whereas DCP-AES requires a minimum of 16 nmol BPA/sample for detection.36 Di Pierro et al.36 reported an HPLC application with o-phthalaldehyde derivatization and a similar detection limit. Such derivatization methods are applicable to compounds with amino groups with which the derivatizing reagents react.
p-Boronophenylalanine is well accumulated in tumor cells with a low T/N ratio, whereas sodium borocaptate has a high T/N ratio with weak accumulation.4 The T/N ratio of BPA might be improved by modifying BPA to be more LAT1-specific. Recently, we reported that the methylation of the α-carbon in tyrosine confers LAT1 selectivity.15 The PET tracer 3-fluoro-L-α-methyl-tyrosine is a successful example of modifying an existing substrate for LAT1 selectivity.15
In conclusion, the screening and kinetic analysis of aromatic amino acid transporters revealed that LAT1, LAT2 and ATB0,+ transport BPA at high, medium and low affinity, respectively. Their affinities for BPA are comparable with those of the endogenous substrate, suggesting that they could mediate effective BPA uptake in vivo. Uptake experiments in cancer cell lines revealed differential roles of LAT1 and ATB0,+ in BPA uptake depending on the concentrations. It is proposed that ATB0,+, as well as LAT1, could contribute significantly to the tumor accumulation of BPA at clinical dose.
Acknowledgments
The authors thank Shiseido Co., Ltd., (Tokyo, Japan) for their technical support in using the HPLC system. P. Wongthai was supported by Kasetsart Veterinary Development Funds.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Fig. S1. Functional expression of aromatic amino acid transporters.
Fig. S2. Equivalent effectiveness of LAT1 siRNAs.
Fig. S3. Expression profile of ATB0,+ and LAT1 in cancers.
Table S1. LAT1 expression in gliomas
References
- Barth RF, Vicente MGH, Harling OK, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I, Ono K, Sakurai Y, et al. Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot. 2004;61:1069–73. doi: 10.1016/j.apradiso.2004.05.059. [DOI] [PubMed] [Google Scholar]
- Yoshida F, Matsumura A, Yamamoto T, Kumada H, Nakai K. Enhancement of sodium borocaptate (BSH) uptake by tumor cells induced by glutathione depletion and its radiobiological effect. Cancer Lett. 2004;215:61–7. doi: 10.1016/j.canlet.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Miyatake S, Kajimoto Y, et al. Pharmacokinetic study of BSH and BPA in simultaneous use for BNCT. J Neurooncol. 2006;78:227–32. doi: 10.1007/s11060-005-9099-4. [DOI] [PubMed] [Google Scholar]
- Wittig A, Sauerwein WA, Coderre JA. Mechanisms of transporters of p-borono-phenylalanine through the cell membrane in vitro. Radiat Res. 2000;153:173–80. doi: 10.1667/0033-7587(2000)153[0173:motopb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Detta A, Cruickshank GS. L-amino acid transporter-1 and boronophenylalanine-based boron neutron capture therapy of human brain tumors. Cancer Res. 2009;69:2126–32. doi: 10.1158/0008-5472.CAN-08-2345. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Kurihara H, Honda N, et al. Predominant contribution of L-type amino acid transporter to 4-borono-2-18F-fluoro-phenylalanin uptake in human glioblastome cells. Nucl Med Biol. 2013;40:625–9. doi: 10.1016/j.nucmedbio.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime. Semin Cancer Biol. 2005;15:254–66. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Endou H. Heterodimeric amino acid transporters: molecular biology and pathological and pharmacological relevance. Curr Drug Metab. 2001;2:229–354. doi: 10.2174/1389200013338324. [DOI] [PubMed] [Google Scholar]
- Nakada N, Mikami T, Hana K, et al. Unique and selective expression of L-amino acid transporter 1 in human tissue as well as being an aspect of oncofetal protein. Histol Histopathol. 2014;29:217–27. doi: 10.14670/HH-29.217. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H. Miyamoto Ki, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–32. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Uchino H, Kanai Y, Kim DK, et al. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol. 2002;61:729–37. doi: 10.1124/mol.61.4.729. [DOI] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Otani Y, et al. Fluorine-18-alpha-methyltyrosine positron emission tomography for diagnosis and staging of lung cancer: a clinicopathologic study. Clin Cancer Res. 2007;13:6369–78. doi: 10.1158/1078-0432.CCR-07-1294. [DOI] [PubMed] [Google Scholar]
- Wiriyasermkul P, Nagamori S, Tominaga H, et al. Transport of 3-fluoro-L-α-methyl-tyrosine by tumor-upregulated L-type amino acid transporter 1: a cause of the tumor uptake in PET. J Nucl Med. 2012;53:1253–61. doi: 10.2967/jnumed.112.103069. [DOI] [PubMed] [Google Scholar]
- Laurent T, Murase D, Tsukioka S, Matsuura T, Nagamori S, Oda H. A novel human hepatoma cell line, FLC-4, exhibits highly enhanced liver differentiation functions through the three-dimensional cell shape. J Cell Physiol. 2012;227:2898–906. doi: 10.1002/jcp.23033. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Oyama T, Itoh Y, Hamase K. Enantioselective two-dimensional high-performance liquid chromatographic determination of amino acids; analysis and physiological significance of D-amino acids in mammals. Chromatography. 2014;35:49–57. [Google Scholar]
- Khunweeraphong N, Nagamori S, Wiriyasermkul P, et al. Establishment of stable cell lines with high expression of heterodimers of human 4f2hc and human amino acid transporter LAT1 or LAT2 and delineation of their differential interaction with α-alkyl moieties. J Pharmacol Sci. 2012;119:368–80. doi: 10.1254/jphs.12124FP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo S, Aurich MK, Jonsson JJ, Thiele I. Membrane transporters in a human genome-scale metabolic knowledgebase and their implications for disease. Front Physiol. 2014;5:91. doi: 10.3389/fphys.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Chiotellis A, Keller C, et al. Imaging tumor ATB0,+ transport activity by PET with the cationic amino acid O-2((2-[18F]fluoroethyl)methyl-amino)ethyltyrosine. Mol Imaging Biol. 2014;16:412–20. doi: 10.1007/s11307-013-0711-2. [DOI] [PubMed] [Google Scholar]
- Sloan JL, Mager S. Cloning and functional expression of a human Na+ and Cl−–dependent neutral and cationic amino acid transporter B0+ J Biol Chem. 1999;274:23740–5. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- Karunakaran S, Ramachandran S, Coothankandaswamy V, et al. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286:31830–8. doi: 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran S, Umapathy NS, Thangaraju M, et al. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem J. 2008;414:343–55. doi: 10.1042/BJ20080622. [DOI] [PubMed] [Google Scholar]
- Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional expression of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–51. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- Riemann B, Kopka K, Stögbauer F, et al. Kinetic parameters of 3-[123I]iodo-L-α-methyl tyrosine ([123I]IMT) transport in human GOS3 glioma cells. Nucl Med Biol. 2001;28:293–7. doi: 10.1016/s0969-8051(01)00191-3. [DOI] [PubMed] [Google Scholar]
- Coderre JA, Elowitz EH, Chadha M, et al. Boron neutron capture therapy for glioblastoma multiforme using p-boronophenylalanine and epithermal neutrons: trial design and early clinical results. J Neurooncol. 1997;33:141–52. doi: 10.1023/a:1005741919442. [DOI] [PubMed] [Google Scholar]
- Joel DD, Coderre JA, Micca PL, Nawrocky MM. Effect of dose and infusion time on the delivery of p-boronophenylalanine for neutron capture therapy. J Neurooncol. 1999;41:213–21. doi: 10.1023/a:1006176901713. [DOI] [PubMed] [Google Scholar]
- Smith DR, Chandra S, Barth RF, Yang W, Joel DD, Coderre JA. Quantitative imaging and microlocalization of boron-10 in brain tumors and infiltrating tumor cells by SIMS ion microscopy: relevance to neutron capture therapy. Cancer Res. 2001;61:8179–87. [PubMed] [Google Scholar]
- Wyss MT, Hofer S, Hefti M, et al. Spatial Heterogeneity of low-grade gliomas at the capillary level: a PET study on tumor blood flow and amino acid uptake. J Nucl Med. 2007;48:1047–52. doi: 10.2967/jnumed.106.038489. [DOI] [PubMed] [Google Scholar]
- Henriksson R, Capala J, Michanek A, et al. Boron neutron capture therapy (BNCT) for glioblastoma multiforme: a phase II study evaluating a prolonged high-dose of boronophenylalanine (BPA) Radiother Oncol. 2008;88:183–91. doi: 10.1016/j.radonc.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Barth RF, Coderre JA, Vicente MG, Blue ET. Boron Neutron Capture Therapy of Cancer: current Status and Future Prospects. Clin Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–50. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- Haining Z, Kawai N, Miyake K, et al. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin Pathol. 2012;12:4. doi: 10.1186/1472-6890-12-4. . doi: 10.1186/1472-6890-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawashiro H, Otani N, Shinomiya N, et al. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int J Cancer. 2006;119:484–92. doi: 10.1002/ijc.21866. [DOI] [PubMed] [Google Scholar]
- Gupta N, Miyauchi S, Martindale RG, et al. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim Biophys Acta. 2005;1741:215–23. doi: 10.1016/j.bbadis.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Di Pierro D, Lazzarino G, Pastore FS, et al. Determination of boronophenylalanine in biological samples using precolumn o-phthalaldehyde derivatization and reversed-phase high-performance liquid chromatography. Anal Biochem. 2000;284:301–6. doi: 10.1006/abio.2000.4715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Functional expression of aromatic amino acid transporters.
Fig. S2. Equivalent effectiveness of LAT1 siRNAs.
Fig. S3. Expression profile of ATB0,+ and LAT1 in cancers.
Table S1. LAT1 expression in gliomas


