Abstract
Neglected tropical diseases are a group of some 17 diseases that afflict poor and predominantly rural people in developing nations. One significant disease that contributes to substantial morbidity in endemic areas is schistosomiasis, caused by infection with one of five species of blood fluke belonging to the trematode genus Schistosoma. Although there is one drug available for treatment of affected individuals in clinics, or for mass administration in endemic regions, there is a need for new therapies. A prominent target organ of schistosomes, either for drug or vaccine development, is the peculiar epithelial syncytium that forms the body wall (tegument) of this parasite. This dynamic layer is maintained and organized by concerted activity of a range of proteins, among which are the abundant tegumentary annexins. In this review, we will outline advances in structure–function analyses of these annexins, as a means to understanding tegument cell biology in host–parasite interaction and their potential exploitation as targets for anti-schistosomiasis therapies.
Table of Links
This Table lists key protein targets in this document, which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al.2013a, b, c).
Neglected tropical diseases (NTDs)
NTDs include some 17 lesser known chronic infections that affect poor and disenfranchised people, primarily, but not exclusively, in developing nations (Hotez et al., 2007; Hotez and Fenwick, 2009). Chronic infections caused by NTDs lead to many adverse outcomes in affected populations and contribute substantially to human morbidity. In addition to microbial and protozoan diseases, NTDs include a number of helminth infections, such as diseases caused by flatworm parasites, notably schistosomiasis, echinococcosis and liver fluke diseases, as well as roundworm parasites, such as the major soil transmitted helminth infections (ascariasis, trichuriasis and hookworm diseases). Although no individual NTD rivals the major infectious threats of HIV, malaria or tuberculosis in terms of global impact of disease, collectively, the NTDs contribute substantially to morbidity throughout the world (Engels and Savioli, 2006).
A number of factors present major challenges for the development of new treatments for NTDs. Firstly, NTDs are chronic diseases, which may reside in affected people as lifelong infections. Secondly, NTDs are not always associated with human mortality and the burden of these diseases can be subtle, hidden among such other measures of disease burden as hindered development, poor cognitive function and chronic ailments. Thirdly, as stated, NTDs often affect the poorest of the poor, people often unable to pay for medical treatments, especially for chronic illnesses. Hence, these diseases receive less attention than other, immediately life-threatening infectious diseases. Lastly, poor development of infrastructure systems in impoverished countries also irreversibly impacts on efficient drug distribution for the treatment of these NTDs (Chimbari et al., 2004).
Among the NTDs of major interest is the suite of diseases known as human schistosomiasis. These diseases are caused by infection with any of a number of species of the genus Schistosoma, a taxon of platyhelminth trematodes, commonly known as blood flukes (and historically, known as the agents of bilharzia) (Ross et al., 2002). Five species are the main contributors to human schistosomiasis, Schistosoma mansoni (Sm), S. japonicum, S. mekongi, S. intercalatum and S. haematobium. Transmission of the parasites to humans takes place in freshwater, typically in regions of poor sanitation where human excreta contaminate water bodies. The egg hatches in freshwater to liberate a larva, which searches for and infects a species of snail. Schistosomes, like other trematodes, display high host specificity for their snail host. The distribution of a schistosome species is largely dependent on the geographic distribution of its snail host.
Schistosomes infect over 200 million people in approximately 74 nations, the majority of which are in Sub-Saharan Africa and the Middle East (Steinmann et al., 2006). Distinct foci of schistosomiasis also occur in Asia (China, the Philippines, along the Mekong River and Indonesia), as well as South America (notably Brazil and some Caribbean Islands). Disability-adjusted life years lost to human schistosomiasis in 2010 were measured at 48/100 000, an increase of 20% on estimates made in 1990 (Murray et al., 2013).
There is a distinct dichotomy in schistosomiasis in relation to the host responsiveness to various life stages. On the one hand, the invasive larvae and adult parasites are largely able to avoid immunosurveillance of the hosts. To that effect, these parasites employ a series of strategies including rapid development, stealth-like host interfaces and immunosuppression (Wilson, 2009). On the other hand, the active secretion of immunogenic molecules by eggs provokes an intense immune response (Burke et al., 2009). This phenomenon is characterized by a strong granulocytic response around the egg in affected tissues that may lead to fibrosis, particularly in the liver. The intense response enables the escape of the eggs from the host. The cellular infiltrate forces a schistosome egg across the vascular endothelium and into tissues of luminal organs, such as the intestinal lining, the bladder wall or genital organs, driving the egg ultimately into the lumen, from which the egg is voided into the environment. The bulk of chronic disease in schistosomiasis is related to host responses against parasite eggs deposited in the blood vessels surrounding the gut (Sm and S. japonicum) or bladder and genital organs (S. haematobium) (Ross et al., 2002). However, it has proven more effective to direct control towards killing adult worms or the invasive larvae that establish infection so that the deposition of eggs is stopped.
Schistosomes belong to the Clade Lophotrochozoa of the Kingdom Animalia. The multicellular animals are monophyletic (Walker et al., 2011) and there are substantial similarities among the many cellular, biochemical and molecular adaptations in different animal clades. The search for effective treatments against schistosomiasis thus needs to exploit key molecular and conformational differences between target molecules of these parasites and their hosts. This review explores work focused on the search for novel molecular targets of therapeutics and prophylactics, and examines new insights from studies of a primary site of host interaction, the schistosome tegument. Some annexins of schistosomes are abundant molecules in the proteome of the schistosomes. These proteins are found in close association with the apical membrane of the tegument of the parasites. In view of their abundance, distribution and distinctive structure, these proteins are of interest, both as targets and as vehicles to understand the dynamic nature of the apical membrane complex of these parasites, a complex that is crucial for survival of the parasites in their hosts.
Treatments for schistosomiasis – drugs
There currently exist few drugs for treatment of schistosomiasis: praziquantel, metrifonate, oxamniquine and artemether (Cioli et al., 1995; Ross et al., 2002; Bartley et al., 2008). All of these drugs have proven useful for therapeutic treatment of individuals in the clinic or of communities in mass drug administration. Of the four drugs, oxamniquine is only effective against schistosomiasis mansoni; resistance to this drug by the parasite is known and the mechanism of resistance elucidated (Valentim et al., 2013). Metrifonate is only effective against urinary schistosomiasis, caused by S. haematobium, and its use is hampered by a complex administration schedule with multiple doses required over a 2 week period. Frequently, this therapy is met with a low rate of compliance among patients. Furthermore, the drug is labile in warm climates and is thus less useful in field settings. Combination therapy using praziquantel and metrifonate has been effective for urinary schistosomiasis (Danso-Appiah et al., 2009).
Artemether is a β-methyl ether derivative of artemisinin, a compound derived from the sweet wormwood Artemesia annua. Artemisinin and its derivatives are highly effective against haematophagous parasites, notably malaria, but they have also proven effective against schistosome infection (Liu et al., 2012). One recent report suggests that artemisinin is acted upon by elemental iron in the iron-rich environment of haematophagous parasites and the complex, in turn, inhibits calcium transport (Shandilya et al., 2013). Concerns about resistance to artemisinin and its derivatives by the more insidious human disease of falciparum malaria has precluded the use of artemether against schistosomiasis where the two diseases are co-endemic (Bergquist et al., 2005; Utzinger et al., 2007).
The current drug of choice for treatment of schistosomiasis is praziquantel. This drug has been used in mass treatment campaigns in many countries and remains a primary tool in the war against the disease (Knopp et al., 2013). The mode of action of praziquantel remains unknown, although recent developments strongly suggest a role for the drug in calcium homeostasis in the parasites and notably in calcium transport complexes (Greenberg, 2005; You et al., 2013). The drug remains highly effective for a wide range of flatworm diseases of humans and domestic animals. Praziquantel has been deployed for mass drug administration in endemic regions and has been successful in pushing the disease from high to low endemicity (Geary, 2012). This major achievement has been facilitated in part by reductions in costs associated with manufacture of the drug, and the development of public–private partnerships that have led to the distribution of the drug to many impoverished communities where schistosomiasis is endemic.
Despite its high efficacy, praziquantel has limitations (Geary, 2012). The drug is only effective against adult or pre-adult forms (Greenberg, 2005). Furthermore, praziquantel confers no protection against subsequent infection and people may become reinfected within days of treatment (Ross et al., 2002). Treatment failures for S. mansoni and S. haematobium infections have been observed, and the presence of resistant strains has been demonstrated experimentally (Greenberg, 2013). Although widespread resistance to praziquantel has not been observed clinically, the application of the drug in mass treatment campaigns may result in new resistant forms emerging and new replacement drugs and formulations are needed (Geary, 2012).
Prevention of schistosomiasis – vaccines
Many experts within the schistosomiasis community argue that continued application of a single drug, praziquantel, for single treatments and as a mass control strategy is problematic and not likely to lead to effective control of the disease. The alternative, a subunit vaccine, has thus been promoted as an important alternative strategy for the control and elimination of schistosomiasis (Bergquist et al., 2008; McManus and Loukas, 2008; Loukas et al., 2011; Kupferschmidt, 2013).
Optimism for a vaccine rests on observations from the 1970s on host responses to radiation-attenuated (RA) cercariae in experimental infections (Bickle et al., 1979a, b). A cercaria is the larval stage that penetrates human skin to initiate infection. This stage transforms rapidly in human skin to become a host-adapted larva, the schistosomulum. This larva then follows a set pattern of migration and development over the following days and weeks, passing along vasculature through the lung and liver. In the liver, a male parasite will mate with a female and carry her to mesenteric or pelvic circulation, the final destination being parasite species specific (Wilson, 2009). It was shown that infection of humans with live, RA parasites led to strong protection against subsequent challenge infections with normal cercariae (Correa-Oliveira et al., 2000; Ribeiro de Jesus et al., 2000). Vaccination of animal models with RA cercariae has thus led to an adult worm burden reduction in experimental schistosomiasis of 60–70% (Bickle et al., 1979a, b; Caulada-Benedetti et al., 1991; Coulson et al., 1998; McManus, 1999; Dillon et al., 2008). The molecular mechanism of protection with RA is unclear; however, the immune response appears to result from transcriptional suppression in the attenuated parasites during the early stage of development (Dillon et al., 2008). Transcriptional suppression in RA was observed for a variety of genes including those encoding tegument proteins, members of signalling pathways associated with GPCRs, neurotransmitters and cytoskeletal components. The major lessons learned from these studies are that parasite killing is largely dependent on host–parasite interaction during the host establishment phase of the parasites, that is, within the first week after infection. During this time, the cercaria undergoes an extensive remodelling of its surface body wall, the tegument and becomes transcriptionally active for a series of molecules associated with surface dynamics and nutrient absorption (Gobert et al., 2009b), compared with the cercaria. Indeed, some of the promising vaccine candidates come from this tissue, and it seems that vaccine targeting of this layer is crucial for parasite killing.
Despite the high level of protection available with radiation-attenuated vaccines, the unstable lifespan, delivery problems and safety problems of these modified cercariae makes them unsuitable for further development as a vaccine (Bergquist et al., 2008). Therefore, efforts have been directed to discover and identify suitable protective antigens from schistosomes, leading to the development of recombinant vaccines, DNA vaccines, peptide–epitope-based vaccines, multivalent vaccines and chimeric vaccines (McManus and Loukas, 2008).
Of the vaccines trialled, a number have been promoted for human trials, including the Bilvax vaccine based on a 28 kDa S. haematobium glutathione-S-transferase, which has entered phase 3 clinical trials, and a S. mansoni tetraspanin (Sm-TSP-2) (Tran et al., 2006), which has entered phase 1 trials (Loukas et al., 2011; Kupferschmidt, 2013). Other vaccines presented at a recent vaccine discovery workshop sponsored by the Bill and Melinda Gates Foundation in the United States (Kupferschmidt, 2013) identified additional vaccines still in experimental development, including Sm14, a fatty acid binding protein, a calpain (Smp80) from S. mansoni, and Sj23, a TSP, a triose-phosphate isomerase, an insulin receptor, and paramyosin from S. japonicum (Zhu et al., 2004,2006; Siddiqui et al., 2005; Tendler and Simpson, 2008; You et al., 2012). An advantage of vaccination strategies against the zoonotic S. japonicum is that the parasite is found in a variety of domesticated animals, including water buffalo and goats in China. Researchers involved in controlling this species in China and the Philippines have developed vaccines for use in animals as transmission-blocking vaccines, based on modelling of transmission dynamics in endemic regions (McManus et al., 2009). Antigen discovery studies are still progressing using a variety of immunomics and proteomic approaches. It is now widely appreciated that targeted approaches are required for antigen discovery, and there is continuing interest in considering fundamental cell biological and developmental understanding with molecular advances.
The tegument of schistosomes
The tegument, or body wall, of schistosomes is a dynamic host-adapted interface between the parasite and its vascular environment. The tegument is a highly polarized syncytium and possesses functional analogy with transporting epithelia, including the gut lining or the syncytiotrophoblasts of the human placenta. The tegument plays significant roles in nutrient uptake, immune evasion and modulation, excretion, osmoregulation, sensory reception, and signal transduction (Jones et al., 2004; Kusel et al., 2007; Castro-Borges et al., 2011). Given the importance of the schistosome tegument in nutrition and immune evasion, proteins of this surface layer are recognized as prime candidates to target for vaccine and therapeutic drug development (Loukas et al., 2007).
Ultrastructure of schistosome tegument
The tegument is formed as a single syncytium that covers the entire body and is continuous with other epithelia (Figures 2), notably the foregut lining (Silk et al., 1969). This surface cytoplasmic layer is a highly ordered structure with distinct transporting regions, secretory components and absorptive adaptations. A peculiarity of the layer is the presence of a dual membrane complex that forms the apical extremity of the tegument cytoplasm (Hockley, 1973; Hockley and McLaren, 1973; Castro-Borges et al., 2011).
Figure 2.
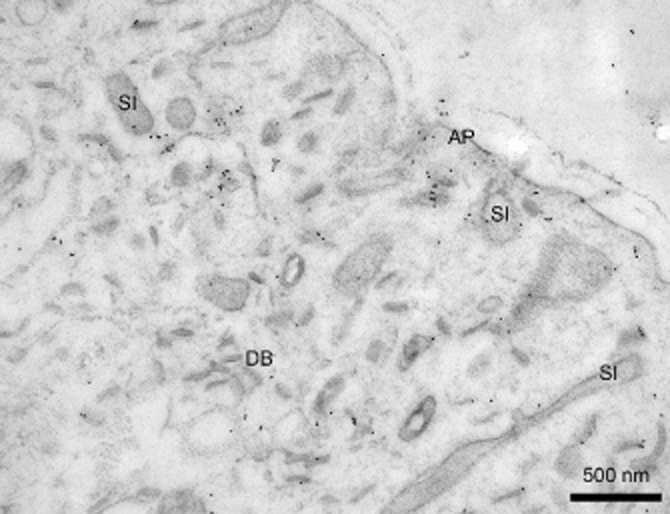
Immuno-electron microscopy of Sm-Anx-B22, transmission electron microscopy, using indirect immunocytochemistry incorporating 10 nm protein-A gold particles. Sm-Anx-B22 was localized to surface invaginations (SI) and other membrane compartments associated with the apical plasma membrane complex (AP). After Leow et al. (2014).
The developmental activity of cercarial transformation referred to above appears first and foremost to involve alteration of the apical membrane of these parasites soon after invasion (Hockley and McLaren, 1973; Skelly and Shoemaker, 1996, 2001; Keating et al., 2006). The single-unit membrane of the cercaria, with its highly immunogenic glycocalyx, becomes replaced by a host-adapted dual membrane system, consisting of the membrane proper, overlain by an additional unit membrane, the membranocalyx. Although the membranocalyx is depauperate of parasite-derived proteins, the underlying membrane is decorated with abundant membrane proteins (Braschi and Wilson, 2006). Membrane repair and maintenance is an ongoing process, as evidenced by abundant cytoplasmic inclusions and molecule associated with the apical membranes.
The advantage to schistosomes in possessing a syncytial tegument is poorly understood, but appears to be an important strategy that ensures survival of parasites in the vascular environment. Invaginations of the surface membrane complex, as well as in the basal membrane of the cytoplasm (Hockley, 1973; Gobert et al., 2003; Skelly and Wilson, 2006), are structural evidence of high turnover of these membranes (Brouwers et al., 1999), a process that is related to nutrient uptake and a way of avoiding the host immune response by internalizing antibodies and removing possible antigenic molecules from the surface (Skelly and Wilson, 2006). Membrane internalization and translocation events are driven by a complex interplay of multiple membrane proteins including the TSP-enriched microdomains (TEMs) (Tran et al., 2010; Jia et al., 2014). The TEMs are protein complexes formed about a membrane-resident TSPs, which act as scaffold proteins for the multiple fusion and scission activities of plasma membrane (Hemler, 2008). For S.mansoni, TEM residents include a variety of proteins strongly linked to the apical plasma membrane, including schistosome annexins B30, Sm29, a dysferlin, calpain, fructose-biphosphate aldolase, heat shock protein 70 and actin (Jia et al., 2014).
The tegument is supported by cell bodies that lie embedded in the parasite parenchyma (Hockley, 1973; Gobert et al., 2003). The apical cytoplasm of the tegument and the cell bodies are linked by cytoplasmic bridges, which traverse the muscle bundles lying beneath the parasite tegument (Figure 1B) (Hockley, 1973; Gobert et al., 2003). Tegumentary cell bodies contain the synthetic machinery of the syncytium, including endoplasmic reticulum and Golgi apparatus, and produce abundant vesicular products that are trafficked to the tegument along cytoplasmic bridges (Figure 3).
Figure 1.
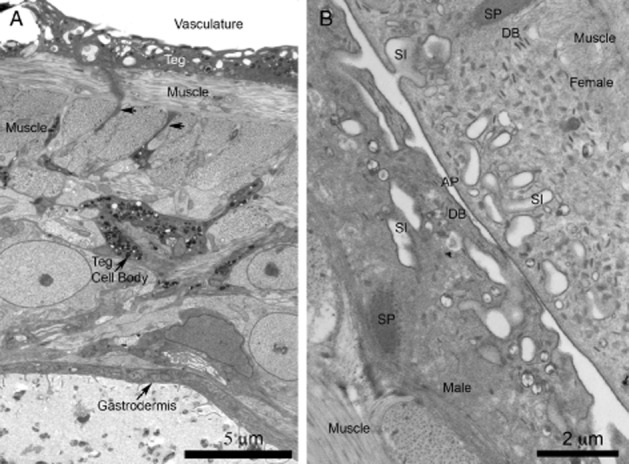
Tegument of Schistosoma mansoni by transmission electron microscopy. (A) Low magnification image from a cross-section of an adult. The image shows the apical cytoplasm of the tegument (Teg), which is the interface with the host vasculature. The syncytial cytoplasm rests on bands of musculature and is supported and maintained by tegumentary cell bodies. That depicted is rich in vesicles that are transported to the apical cytoplasm along cytoplasmic bridges (arrows). The parasite digestive system, lined by a syncytial epidermis called a gastrodermis, lies deep within the body. (B) High magnification view of the teguments of paired male and female adults. The apical membrane complex (AP) consists of a plasma membrane overlain by a subsidiary membrane-like structure, the membranocalyx, evident only after special fixation/staining of TEM tissues using uranyl acetate. The apical cytoplasm infolds frequently as surface invaginations, sometimes with secondary caveola-like outpocketings appearing. Other bodies decorate the tegument, including discoid bodies (DB). Tegumentary spines (SP), used for adhesion, are observed. Original figures.
Figure 3.
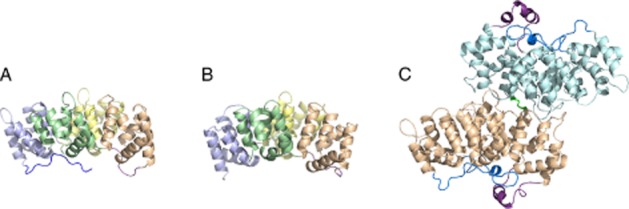
Cartoon representations of (A) human annexin A5, (B) α1-giardin from Giardia intestinalis and (C) the dimerized Schistosoma mansoni annexin Sm-Anx-B22. For annexin A5 and α1-giardin, the annexin repeats are shown in different colours. For all annexins shown the N-terminus is coloured blue and the II/III linker in magenta. Note the distinctly longer linker in Sm-Anx-B22, which packs against the N-terminal domain. The disulphide bond between Cys173 (molecule 1) and Cys173 (molecule 2) is rendered in green. Protein structures were rendered with PyMOL (DeLano, 2002).
The molecular interactions driving membrane formation during transformation and in repair and renewal are far from understood, but the abundance of adaptor and chaperone proteins associated with the apical membrane complex (Table 1) and abundance of membrane vesicles (Figures 2) suggest a continuous cycle of renewal and repair throughout adult life of the parasite.
Table 1.
Apical membrane complex-associated proteins of schistosomes
| Membranocalyx associated | Proteins | Putative role |
|---|---|---|
| Tetraspanin-2 | Mediators of tetraspanin-enriched microdomain in surface membrane complex; membrane remodelling | |
| 8 kDa low-molecular weight protein? | Secreted protein | |
| Sm13 | Uncertain, membrane protein | |
| Sm29 | Uncertain role, but potential ligand of tetraspanin | |
| Sm200 | Uncertain role | |
| Bound host proteins-CD48, 90, immunoglobulins, complement factors | Host molecules | |
| Inter-membrane space | 8 kDa low-molecular weight protein? | Secreted protein |
| Annexin B30 | Membrane maintenance | |
| Alkaline phosphatase | Hydrolase | |
| Carbonic anhydrase | Metalloenzyme | |
| 8 kDa low-molecular weight protein? | Secreted protein | |
| CD59 | Complement inhibition | |
| Plasma membrane | PDE | PDE |
| Apyrase | Apyrase | |
| Tetraspanins | Membrane recycling, tetraspanin-enriched microdomains | |
| Glucose transporter (SGTP-4) | Glucose uptake | |
| Aquaporins | Water and small solute transport | |
| Na/K ATPase | Na/K ATPase | |
| Anion channel | ||
| Plasmolipins | Tetraspanin myelin proteins, membrane rafts | |
| Cytosolic | Dysferlin | Calcium-dependent membrane fusion events; component of tetraspanin-enriched microdomains in tegument |
| Calpain? | Protease | |
| Tegument allergen-like proteins (Sm 22.6, Sm21.7) | Dynein-like motor function; calcium binding | |
| GAPDH | GAPDH | |
| Heat shock protein (HSP70) | Chaperone | |
| Actin | Cytoskeleton | |
| Dynein light chains | Dynein-like motor function |
Evidence or proposed location in the tegument is derived from the review of proteomic analyses of the tegument of Schistosoma mansoni by Wilson (2012). In many cases, the link between the protein and unit membrane is inferred and further experimental evidence is required. The model is based on a static two-dimensional structure only and ignores the dynamic nature of the schistosome tegument. The membrane complex is a dual membrane system, consisting of a unit membrane overlaid by an additional membrane structure, the so-called membranocalyx. Only one annexin is listed here, although it is known that multiple annexins are present in the tegument of schistosomes.
Tegument proteins as vaccine targets
In mice immunized with tegument extract of newly transformed S. mansoni schistosomula, an induced Th1-type protection has been observed, which damages the adult worm tegument layer and reduces egg number and parasite burden in challenge infections (Smithers et al., 1990). Therefore, it is currently believed that tegument proteins of schistosomes are a priority in antigen discovery. Proteins potentially exposed at its surface during intra-mammalian stages are possibly the most susceptible targets for vaccine development (Loukas et al., 2007). A challenge in studies of schistosome biology is the elucidation of when and how during the infection targets are exposed to the host immune recognition. According to models of the S. mansoni tegument, the primary vaccine target Sm-TSP-2 (tetraspanin-2) occurs in the plasma membrane, that is, it lies hidden from the host under the membranocalyx (Wilson, 2012). Recent immunolocalization data suggest the molecule is even more hidden from the host, in adult parasites at least, occurring predominantly in association with surface invaginations of the hosts and in subsidiary membranes (Schulte et al., 2013).
Analysis of the schistosome proteome has vastly increased the speed of identification of tegument proteins (Braschi and Wilson, 2006; Mulvenna et al., 2010; Castro-Borges et al., 2011; Jia et al., 2014) (Table 1). A series of experiments have allowed different proteins to be assigned to the distinct membrane fractions of the apical membrane complex (Wilson, 2012), although these assignments are likely to be crude and require further analysis by refined localization tools. A range of molecules has been identified including glucose transporters, proteases and other enzymes, receptors, chaperones and structural proteins (Mulvenna et al., 2010; Castro-Borges et al., 2011; Wilson, 2012). Further confirmation of the co-location of many of these molecules has come from interaction studies of Sm-TSP-2 (Jia et al., 2014), which show strong interactions, as stated, with a range of surface-linked molecules including annexin B30, alkaline phosphatase, actin, an aldolase, calpain, HSP70, dysferlin and Sm29, a schistosome-specific molecule. These interacting partners widen the pool of available molecules for vaccination studies.
Among the dominant surface-related proteins is S. mansoni annexin B30 (hereafter Sm-Anx-B30) (Castro-Borges et al., 2011; Cantacessi et al., 2013; Jia et al., 2014). This molecule is strongly associated with the tegument (Tararam et al., 2010) and the Sm-TSP-2 TEMs, although how it binds to other proteins is undetermined. Sm-Anx-B30 lies in direct association with the apical plasma membrane (C. Leow, unpubl. obs.). Three other S. mansoni annexins, namely, Sm-Anx B7a, B22 and B5a, have been shown in various studies of different schistosomes to be located to the tegument. The abundance, as well as the peculiar features of annexins of some parasite groups, makes them potential targets for therapies.
Schistosome annexins
Annexins are a family of proteins that are able to bind to acidic phospholipid membranes. Their membrane-binding mode includes formation of a ternary complex involving the protein, the calcium ions and the membrane. The survey of group B annexins from different invertebrate taxa revealed that the proteins occur in the vast majority of species studied so far (Cantacessi et al., 2013). The abundant annexin proteins are conspicuously evident in many parasite groups, including a series of arthropod vectors of disease, as well as basal metazoans, but are apparently absent from others, notably the Mollusca. Using structure-based amino acid sequence alignments and phylogenetic analyses, the recent analysis provided a robust classification for this protein group, enabling information on structure–functional relationships of these proteins, as well as to assign names to sequences with ambiguous annotations in public databases (Cantacessi et al., 2013). It was immediately apparent in phylogenetic analyses that gene duplication in divergent clades was the major evolutionary event in annexins' genesis, particularly in schistosomes. The highest representation of annexin was found in S. mansoni with 13 annexins, many distributed on two chromosomes, suggesting linkage (Cantacessi et al., 2013).
Evidence gained from tissue-specific transcriptional and proteomic profiling of adult parasites suggests that the different schistosome annexins are expressed differentially throughout the body of the parasites (Gobert et al., 2009a). As stated, Sm-Anx-B7a, B22 and B30 are distinctly associated with the syncytial tegument (Braschi and Wilson, 2006; Mulvenna et al., 2010). Our tissue-specific transcriptomic survey of female S. japonicum indicated that different annexins were expressed preferentially by different cell types, with the gut lining expressing annexin B7 and B22, while the vitelline gland expressed annexin B5 (Gobert et al., 2009a). Although S. japonicum is a distinctive parasite, as it diverged early from other species of Schistosoma, similar patterns of annexin expression might reasonably be expected to be conserved within the genus. The abundance of annexin B7 and B22 in gut and tegument allows the postulate that these molecules may be epithelial annexin in these parasites, and thus associated with syncytial epithelia. Importantly, both the gastrodermis and the tegument are predicted to have high membrane turnover and reshaping (Nawaratna et al., 2011).
Structure–function observations of schistosome annexins
Observations made by us and others in the recent past point towards a potential use of parasite annexins as therapeutic targets. These findings include (i) immunoreactivity of some parasite annexins (Hongli et al., 2002; Palm et al., 2003; Weiland et al., 2003; Gao et al., 2007; Weeratunga et al., 2012; Leow et al., 2014); (ii) localization of certain parasite annexins to areas of potential exposure and/or structural integrity (Braschi and Wilson, 2006; Jia et al., 2014); and (iii) the existence of a unique structural feature, including the extended helical linker between repeats II and III (Figure 3), in parasite annexins that differentiates them from host annexins (Hofmann et al., 2010; Weeratunga et al., 2012; Leow et al., 2014).
The extended linker region is a primary source of variation between some group B and group A annexins. Many group B annexins, including those from the cestode Taenia solium; annexin B36 (nex-4) from the model nematode Caenorhabditis elegans; and some Group E annexins, including α-12- and α-19 giardin, possess an unusually long linker segment between repeats II and III on the concave side of the protein (Hofmann et al., 2010) (Figure 3). Whereas the typical length of this linker in annexin ranges from 10 to 15 amino acids, the linker peptide of these groups B and E range from 25 to 38 amino acids (Hofmann et al., 2010). Secondary structure predictions consistently indicate that this elongated linker region adopts an α-helical structure, and the recent crystal structure of Sm-Anx-B22 provided the anticipated experimental proof (Leow et al., 2014). We hypothesize that this additional α-helical element on the concave side of the molecule may provide a target for immunological therapeutics (Hofmann et al., 2010). It is tempting to speculate that other parasite annexins with an extended II/III linker peptide may adopt a very similar conformation. A comparison of the extent of the N-terminal domains for annexins with the unique linker shows that such a fold may be possible for most of them.
The crystal structure of Sm-Anx-B22 confirms the presence of the predicted α-helical segment in the II/III linker and also reveals a covalently linked head-to-head dimer (Leow et al., 2014). Sm-Anx-B22 and its homologues from S. japonicum (Cantacessi et al., 2013) and S. bovis (de la Torre-Escudero et al., 2012) are the only B annexins known to date that possess an exposed cysteine residue in the IIDE loop (Cys173), a position where most other annexins possess a serine residue. In Sm-Anx-B22, the involvement of Cys173 in an inter-molecular disulphide bond as well as several intimate electrostatic side chain interactions add to the stabilization of the unique head-to-head dimer topology where the dimer interface is exclusively located in module II/III. Structurally, this is significantly different to other annexin head-to-head dimers (Hofmann et al., 2010), where the dimer interface comprises the entire convex surface of both molecules.
In addition, from the calcium-bound crystal structure of Sm-Anx-B22, canonical as well as novel calcium binding sites can been identified, which seems to be a recurring motif in parasite annexins. Intriguingly, the dimer arrangement observed in the annexin B22 crystal structure revealed the presence of two non-anticipated prominent features: a potential non-canonical membrane-binding site and a potential binding groove opposite of the former (Figure 4).
Figure 4.
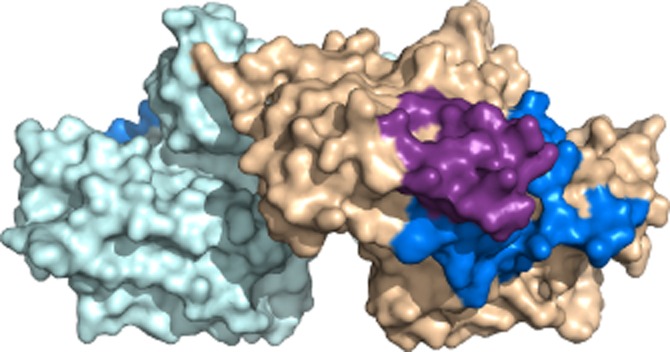
Surface-rendered model of Schistosoma mansoni annexin Sm-Anx-B22. A distinctive groove, lying at the junction of the two partners of the dimer (coloured light blue and tan) may give the protein an adaptor function. The N-terminus (dark blue) and II/II linker region (magenta) of one partner are shown. Figure prepared with PyMOL (DeLano, 2002).
Annexins in schistosomes
A variety of roles have been proposed for annexins. In vertebrates, annexins are known to display a broad range of biological activities including response to inflammation, membrane traffic and adhesion, anticoagulation, signal transduction, developmental processes and membrane repair (Bouter et al., 2011; Draeger et al., 2011). In parasites, annexins are suggested to be involved in maintenance of membrane structure (Peattie et al., 1989; Tararam et al., 2010), anti-inflammatory activity (Zhang et al., 2007) and fibrinolytic activity (de la Torre-Escudero et al., 2012). Annexins may thus have distinct roles in enabling survival of parasites when they are within the hosts. Some annexins are speculated to be involved in redox reactions and the regulation of reactive oxygen molecules in plants (Hofmann et al., 2003) (Konopka-Postupolska et al., 2011) and in mammals (Tanaka et al., 2004; Madureira et al., 2011; Madureira and Waisman, 2013).
Localization of Sm-Anx-B22 by fluorescence and electron microscopy in different species of schistosomes (Tararam et al., 2010; de la Torre-Escudero et al., 2012; Leow et al., 2014) demonstrates that the molecule is strongly associated with the tegument and the plasma membrane structures of the apical regions of the tegument of adult parasites (Figure 2). The molecule is expressed in human-parasitic phases of the parasite life cycle, suggesting a major role in surface membrane dynamics during life in the human host. Although Sm-Anx-B22 shares many structural similarities with other annexins, its dimeric nature as well as the unique extended linker region suggests that this molecule is co-adapted to function in the peculiar syncytial environment of the tegument of these parasites (Leow et al., 2014). Among the peculiarities, there is a prominent groove that occurs within the dimeric species (Figure 4). This groove is postulated to enable the Sm-Anx-B22 dimer to assume an adaptor function, linking the apical membrane complex with proteins.
Sm-Anx-B22 possesses another unique feature, namely, the external arrangement of the II/III linker that is reflexed over the N-terminal region of the molecule. There is now substantial evidence that annexins, and indeed other molecules of the schistosome tegument, adopt unique conformations that might be exploited for therapeutics or prophylaxis as they distinguish the parasite proteins from homologous proteins in the host.
The question remains as to how these unique regions might be targeted if we are to develop anti-schistosomiasis therapies directed against these molecules. Undoubtedly, as with all areas of investigation concerning annexins from a wide variety of organisms, more structure–function analyses of the proteins in cells is required. For schistosomes the peculiarities of the annexins, including the extended II/III linker regions, non-canonical calcium binding sites and other molecular anomalies are of interest, not only for enhancing fundamental understanding of membrane dynamics, but also for designing anti-parasite targets.
Being highly adapted to life within hosts, helminth parasites present considerable difficulties in functional genomics analyses. They are not easily cultivated outside of the host and require molecular signalling from their host to develop fully. Furthermore, transgenesis studies for schistosomes remain in their infancy, although these parasites are amenable to RNA-interfering technologies. Thus, studies of annexins of schistosomes present some challenges. Two important outcomes of the recent comparative analysis of invertebrate annexins (Cantacessi et al., 2013) is the occurrence of common structural motifs in some group B (invertebrate) and group E (Giardia) annexins and the growing diversity of annexins among the single celled protists. The encouraging result suggests that there is substantial information to be gained from comparative studies among parasites that are less tractable in laboratory models and readily culturable invertebrate model species and parasites. These comparative structure–function investigations as models for understanding annexins' function at the host–parasite interface, as heterologous expression systems of parasite annexins and as targets of inhibitor and drugs assays, will prove invaluable as we move towards developing targeted therapies for parasites of socio-economic importance.
Acknowledgments
We gratefully acknowledge funding of our laboratories by the National Health and Medical Research Council (A. H., M. K. J.). C. Y. L. was supported by a scholarship from the Malaysian Government and Universiti Sains Malaysia ASTS scholarship. C. W. was supported by a NHMRC Australian Biomedical Research Fellowship.
Glossary
- Anx
annexin
- NTDs
neglected tropical diseases
- RA
radiation attenuated
- Sm
Schistosoma mansoni
- TEMs
tetraspanin-enriched microdomains
- TSP
tetraspanin
Conflict of interest
None.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013a;170:1449–1458. [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14:Transporters. Br J Pharmacol. 2013b;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley PB, Glanfield A, Li Y, Stanisic DI, Duke M, Jones MK, et al. Artemether treatment of prepatent Schistosoma japonicum induces resistance to reinfection in association with reduced pathology. Am J Trop Med Hyg. 2008;78:929–935. [PMC free article] [PubMed] [Google Scholar]
- Bergquist R, Utzinger J, Chollet J, Shu-Hua X, Weiss NA, Tanner M. Triggering of high-level resistance against Schistosoma mansoni reinfection by artemether in the mouse model. Am J Trop Med Hyg. 2005;71:774–777. [PubMed] [Google Scholar]
- Bergquist R, Utzinger J, McManus DP. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis. 2008;2:e244. doi: 10.1371/journal.pntd.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle QD, Dobinson T, James ER. The effects of gamma-irradiation on migration and survival of Schistosoma mansoni schistosomula in mice. Parasitology. 1979a;79:223–230. doi: 10.1017/s0031182000053300. [DOI] [PubMed] [Google Scholar]
- Bickle QD, Taylor MG, Doenhoff MJ, Nelson GS. Immunization of mice with gamma-irradiated intramuscularly injected schistosomula of Schistosoma mansoni. Parasitology. 1979b;79:209–222. doi: 10.1017/s0031182000053294. [DOI] [PubMed] [Google Scholar]
- Bouter A, Gounou C, Berat R, Tan S, Gallois B, Granier T, et al. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nat Commun. 2011;2:270. doi: 10.1038/ncomms1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Prot. 2006;5:347–356. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- Brouwers JF, Skelly PJ, Van Golde LM, Tielens AG. Studies on phospholipid turnover argue against sloughing of tegumental membranes in adult Schistosoma mansoni. Parasitology. 1999;119:287–294. doi: 10.1017/s0031182099004679. [DOI] [PubMed] [Google Scholar]
- Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- Cantacessi C, Seddon JM, Miller TL, Leow CY, Thomas L, Mason L, et al. A genome-wide analysis of annexins from parasitic organisms and their vectors. Sci Rep. 2013;3:2893. doi: 10.1038/srep02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Borges W, Dowle A, Curwen RS, Thomas-Oates J, Wilson RA. Enzymatic shaving of the tegument surface of live schistosomes for proteomic analysis: a rational approach to select vaccine candidates. PLoS Negl Trop Dis. 2011;5:e993. doi: 10.1371/journal.pntd.0000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulada-Benedetti Z, Al-Zamel F, Sher A, James S. Comparison of Th1- and Th2-associated immune reactivities stimulated by single versus multiple vaccination of mice with irradiated Schistosoma mansoni cercariae. J Immunol. 1991;146:1655–1660. [PubMed] [Google Scholar]
- Chimbari MJ, Chirebvu E, Ndlela B. Malaria and schistosomiasis risks associated with surface and sprinkler irrigation systems in Zimbabwe. Acta Trop. 2004;89:205–213. doi: 10.1016/j.actatropica.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Cioli D, Pica-Mattoccia L, Archer S. Antischistosomal drugs: past, present … and future? Pharmacol Ther. 1995;68:35–85. doi: 10.1016/0163-7258(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Correa-Oliveira R, Caldas IR, Gazzinelli G. Natural versus drug-induced resistance in Schistosoma mansoni infection. Parasitol Today. 2000;16:397–399. doi: 10.1016/s0169-4758(00)01740-3. [DOI] [PubMed] [Google Scholar]
- Coulson PS, Smythies LE, Betts C, Mabbott NA, Sternberg JM, Wei XG, et al. Nitric oxide produced in the lungs of mice immunized with the radiation-attenuated schistosome vaccine is not the major agent causing challenge parasite elimination. Immunology. 1998;93:55–63. doi: 10.1046/j.1365-2567.1998.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso-Appiah A, Garner P, Olliaro PL, Utzinger J. Treatment of urinary schistosomiasis: methodological issues and research needs identified through a Cochrane systematic review. Parasitology. 2009;136:1837–1849. doi: 10.1017/S0031182009005939. [DOI] [PubMed] [Google Scholar]
- DeLano W. 2002. The PyMOL molecular graphics system. Available at: http://www.pymol.org (accessed 12/1/2011)
- Dillon GP, Feltwell T, Skelton J, Coulson PS, Wilson RA, Ivens AC. Altered patterns of gene expression underlying the enhanced immunogenicity of radiation-attenuated schistosomes. PLoS Negl Trop Dis. 2008;2:e240. doi: 10.1371/journal.pntd.0000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger A, Monastyrskaya K, Babiychuk EB. Plasma membrane repair and cellular damage control: the annexin survival kit. Biochem Pharmacol. 2011;81:703–712. doi: 10.1016/j.bcp.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Engels D, Savioli L. Reconsidering the underestimated burden caused by neglected tropical diseases. Trends Parasitol. 2006;22:363–366. doi: 10.1016/j.pt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Yan HL, Ding FX, Lu YM, Sun SH. Annexin B1 at the host–parasite interface of the Taenia solium cysticercus: secreted and associated with inflammatory reaction. Acta Trop. 2007;101:192–199. doi: 10.1016/j.actatropica.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Geary TG. Are new anthelminthics needed to eliminate human helminthiasis? Curr Opin Infect Dis. 2012;25:709–717. doi: 10.1097/QCO.0b013e328359f04a. [DOI] [PubMed] [Google Scholar]
- Gobert GN, Stenzel DJ, McManus DP, Jones MK. The ultrastructural architecture of the adult Schistosoma japonicum tegument. Int J Parasitol. 2003;33:1561–1575. doi: 10.1016/s0020-7519(03)00255-8. [DOI] [PubMed] [Google Scholar]
- Gobert GN, McManus DP, Nawaratna S, Moertel L, Mulvenna J, Jones MK. Tissue specific profiling of females of Schistosoma japonicum by integrated laser microdissection microscopy and microarray analysis. PLoS Negl Trop Dis. 2009a;3:e469. doi: 10.1371/journal.pntd.0000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert GN, Moertel L, Brindley PJ, McManus DP. Developmental gene expression profiles of the human pathogen Schistosoma japonicum. BMC Genomics. 2009b;10:128. doi: 10.1186/1471-2164-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RM. Are Ca2+ channels targets of praziquantel action? Int J Parasitol. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Greenberg RM. New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology. 2013;140:1534–1546. doi: 10.1017/S0031182013000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME. Targeting of tetraspanin proteins – potential benefits and strategies. Nat Rev Drug Discov. 2008;7:747–758. doi: 10.1038/nrd2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockley DJ. Ultrastructure of the tegument of Schistosoma. Adv Parasitol. 1973;11:233–305. doi: 10.1016/s0065-308x(08)60188-8. [DOI] [PubMed] [Google Scholar]
- Hockley DJ, McLaren DJ. Schistosoma mansoni: changes in the outer membrane of the tegument during development from cercaria to adult worm. Int J Parasitol. 1973;3:13–25. doi: 10.1016/0020-7519(73)90004-0. [DOI] [PubMed] [Google Scholar]
- Hofmann A, Delmer DP, Wlodawer A. The crystal structure of annexin Gh1 from Gossypium hirsutum reveals an unusual S3 cluster. Eur J Biochem. 2003;270:2557–2564. doi: 10.1046/j.1432-1033.2003.03612.x. [DOI] [PubMed] [Google Scholar]
- Hofmann A, Osman A, Leow CY, Driguez P, McManus DP, Jones MK. Parasite annexins – new molecules with potential for drug and vaccine development. Bioessays. 2010;32:967–976. doi: 10.1002/bies.200900195. [DOI] [PubMed] [Google Scholar]
- Hongli Y, Shuhan S, Ruiwen C, Yingjun G. Cloning and functional identification of a novel annexin subfamily in Cysticercus cellulosae. Mol Biochem Parasitol. 2002;119:1–5. doi: 10.1016/s0166-6851(01)00383-8. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- Jia X, Schulte L, Loukas A, Pickering D, Pearson M, Mobli M, et al. Solution structure, membrane interactions and protein binding partners of the tetraspanin Sm-TSP-2, a vaccine antigen from the human blood fluke Schistosoma mansoni. J Biol Chem. 2014;289:7151–7163. doi: 10.1074/jbc.M113.531558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MK, Gobert GN, Zhang L, Sunderland P, McManus DP. The cytoskeleton and motor proteins of human schistosomes and their roles in surface maintenance and host–parasite interactions. Bioessays. 2004;26:752–765. doi: 10.1002/bies.20058. [DOI] [PubMed] [Google Scholar]
- Keating JH, Wilson RA, Skelly PJ. No overt cellular inflammation around intravascular schistosomes in vivo. J Parasitol. 2006;92:1365–1369. doi: 10.1645/GE-864R.1. [DOI] [PubMed] [Google Scholar]
- Knopp S, Person B, Ame SM, Mohammed KA, Ali SM, Khamis IS, et al. Elimination of schistosomiasis transmission in Zanzibar: baseline findings before the onset of a randomized intervention trial. PLoS Negl Trop Dis. 2013;7:e2474. doi: 10.1371/journal.pntd.0002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Hofmann A. Structure, function and membrane interactions of plant annexins: an update. Plant Sci. 2011;181:230–241. doi: 10.1016/j.plantsci.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt K. A worm vaccine, coming at a snail's pace. Science. 2013;339:502–503. doi: 10.1126/science.339.6119.502. [DOI] [PubMed] [Google Scholar]
- Kusel JR, Al-Adhami BH, Doenhoff MJ. The schistosome in the mammalian host: understanding the mechanisms of adaptation. Parasitology. 2007;134:1477–1526. doi: 10.1017/S0031182007002971. [DOI] [PubMed] [Google Scholar]
- Leow CY, Willis C, Osman A, Mason L, Simon A, Smith BJ, et al. Crystal structure and immunological properties of the first annexin from Schistosoma mansoni – insights into the structural integrity of the schistosomal tegument. FEBS J. 2014;281:1209–1225. doi: 10.1111/febs.12700. [DOI] [PubMed] [Google Scholar]
- Liu R, Dong HF, Jiang MS. Artemisinin: the gifts from traditional Chinese medicine not only for malaria control but also for schistosomiasis control. Parasitol Res. 2012;110:2071–2074. doi: 10.1007/s00436-011-2707-7. [DOI] [PubMed] [Google Scholar]
- Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2007;37:257–263. doi: 10.1016/j.ijpara.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Loukas A, Gaze S, Mulvenna JP, Gasser RB, Brindley PJ, Doolan DL, et al. Vaccinomics for the major blood feeding helminths of humans. OMICS. 2011;15:567–577. doi: 10.1089/omi.2010.0150. [DOI] [PubMed] [Google Scholar]
- Madureira PA, Waisman DM. Annexin A2: the importance of being redox sensitive. Int J Mol Sci. 2013;14:3568–3594. doi: 10.3390/ijms14023568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madureira PA, Hill R, Miller VA, Giacomantonio C, Lee PW, Waisman DM. Annexin A2 is a novel cellular redox regulatory protein involved in tumorigenesis. Oncotarget. 2011;2:1075–1093. doi: 10.18632/oncotarget.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus DP. The search for a vaccine against schistosomiasis – a difficult path but an achievable goal. Immunol Rev. 1999;171:149–161. doi: 10.1111/j.1600-065x.1999.tb01346.x. [DOI] [PubMed] [Google Scholar]
- McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus DP, Li Y, Gray DJ, Ross AG. Conquering ‘snail fever’: schistosomiasis and its control in China. Expert Rev Anti Infect Ther. 2009;7:473–485. doi: 10.1586/eri.09.17. [DOI] [PubMed] [Google Scholar]
- Mulvenna J, Moertel L, Jones MK, Nawaratna S, Lovas EM, Gobert GN, et al. Exposed proteins of the Schistosoma japonicum tegument. Int J Parasitol. 2010;40:543–554. doi: 10.1016/j.ijpara.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2013;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Nawaratna S, McManus DP, Moertel L, Gobert GN, Jones MK. Gene atlasing of digestive and reproductive tissues in Schistosoma mansoni. PLoS Negl Trop Dis. 2011;5:e1043. doi: 10.1371/journal.pntd.0001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm JE, Weiland ME, Griffiths WJ, Ljungstrom I, Svard SG. Identification of immunoreactive proteins during acute human giardiasis. J Infect Dis. 2003;187:1849–1859. doi: 10.1086/375356. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie DA, Alonso RA, Hein A, Caulfield JP. Ultrastructural localization of giardins to the edges of disk microribbons of Giarida lamblia and the nucleotide and deduced protein sequence of alpha giardin. J Cell Biol. 1989;109:2323–2335. doi: 10.1083/jcb.109.5.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro de Jesus A, Araujo I, Bacellar O, Magalhaes A, Pearce E, Harn D, et al. Human immune responses to Schistosoma mansoni vaccine candidate antigens. Infect Immun. 2000;68:2797–2803. doi: 10.1128/iai.68.5.2797-2803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, et al. Schistosomiasis. N Engl J Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- Schulte L, Lovas E, Green K, Mulvenna J, Gobert GN, Morgan G, et al. Tetraspanin-2 localisation in high pressure frozen and freeze-substituted Schistosoma mansoni adult males reveals its distribution in membranes of tegumentary vesicles. Int J Parasitol. 2013;43:785–793. doi: 10.1016/j.ijpara.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Shandilya A, Chacko S, Jayaram B, Ghosh I. A plausible mechanism for the antimalarial activity of artemisinin: a computational approach. Sci Rep. 2013;3:2513. doi: 10.1038/srep02513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AA, Pinkston JR, Quinlin ML, Kavikondala V, Rewers-Felkins KA, Phillips T, et al. Characterization of protective immunity induced against Schistosoma mansoni via DNA priming with the large subunit of calpain (Sm-p80) in the presence of genetic adjuvants. Parasite. 2005;12:3–8. doi: 10.1051/parasite/2005121003. [DOI] [PubMed] [Google Scholar]
- Silk MH, Spence IM, Gear JH. Ultrastructural studies of the blood fluke – Schistosoma mansoni. I. The integument. S Afr J Med Sci. 1969;34:1–10. [PubMed] [Google Scholar]
- Skelly PJ, Shoemaker CB. Rapid appearance and asymmetric distribution of glucose transporter SGTP4 at the apical surface of intramammalian-stage Schistosoma mansoni. Proc Natl Acad Sci U S A. 1996;93:3642–3646. doi: 10.1073/pnas.93.8.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly PJ, Shoemaker CB. The Schistosoma mansoni host-interactive tegument forms from vesicle eruptions of a cyton network. Parasitology. 2001;122(Pt 1):67–73. doi: 10.1017/s0031182000007071. [DOI] [PubMed] [Google Scholar]
- Skelly PJ, Wilson RA. Making sense of the schistosome surface. Adv Parasitol. 2006;63:185–284. doi: 10.1016/S0065-308X(06)63003-0. [DOI] [PubMed] [Google Scholar]
- Smithers SR, Hackett F, Braga V, Simpson AJ. Immunoblotting identifies additional antigens recognised by mice protectively vaccinated with adult Schistosoma mansoni tegumental membranes. Parasitol Res. 1990;76:454–456. doi: 10.1007/BF00933557. [DOI] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Akatsuka S, Ozeki M, Shirase T, Hiai H, Toyokuni S. Redox regulation of annexin 2 and its implications for oxidative stress-induced renal carcinogenesis and metastasis. Oncogene. 2004;23:3980–3989. doi: 10.1038/sj.onc.1207555. [DOI] [PubMed] [Google Scholar]
- Tararam CA, Farias LP, Wilson RA, Leite LC. Schistosoma mansoni Annexin 2: molecular characterization and immunolocalization. Exp Parasitol. 2010;126:146–155. doi: 10.1016/j.exppara.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Tendler M, Simpson AJ. The biotechnology-value chain: development of Sm14 as a schistosomiasis vaccine. Acta Trop. 2008;108:263–266. doi: 10.1016/j.actatropica.2008.09.002. [DOI] [PubMed] [Google Scholar]
- de la Torre-Escudero E, Manzano-Roman R, Siles-Lucas M, Perez-Sanchez R, Moyano JC, Barrera I, et al. Molecular and functional characterization of a Schistosoma bovis annexin: fibrinolytic and anticoagulant activity. Vet Parasitol. 2012;184:25–36. doi: 10.1016/j.vetpar.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–840. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- Tran MH, Freitas TC, Cooper L, Gaze S, Gatton M, Jones MK, et al. Suppression of mRNAs encoding tegument tetraspanins from Schistosoma mansoni results in impaired tegument turnover. PLoS Pathog. 2010;6:e1000840. doi: 10.1371/journal.ppat.1000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzinger J, Xiao SH, Tanner M, Keiser J. Artemisinins for schistosomiasis and beyond. Curr Opin Investig Drugs. 2007;8:105–116. [PubMed] [Google Scholar]
- Valentim CL, Cioli D, Chevalier FD, Cao X, Taylor AB, Holloway SP, et al. Genetic and molecular basis of drug resistance and species-specific drug action in schistosome parasites. Science. 2013;42:1385–1389. doi: 10.1126/science.1243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G, Dorrell RG, Schlacht A, Dacks JB. Eukaryotic systematics: a user's guide for cell biologists and parasitologists. Parasitology. 2011;138:1638–1663. doi: 10.1017/S0031182010001708. [DOI] [PubMed] [Google Scholar]
- Weeratunga SK, Osman A, Hu NJ, Wang CK, Mason L, Svard S, et al. Alpha-1 giardin is an annexin with highly unusual calcium-regulated mechanisms. J Mol Biol. 2012;423:169–181. doi: 10.1016/j.jmb.2012.06.041. [DOI] [PubMed] [Google Scholar]
- Weiland ME, Palm JE, Griffiths WJ, McCaffery JM, Svard SG. Characterisation of alpha-1 giardin: an immunodominant Giardia lamblia annexin with glycosaminoglycan-binding activity. Int J Parasitol. 2003;33:1341–1351. doi: 10.1016/s0020-7519(03)00201-7. [DOI] [PubMed] [Google Scholar]
- Wilson RA. The saga of schistosome migration and attrition. Parasitology. 2009;136:1581–1592. doi: 10.1017/S0031182009005708. [DOI] [PubMed] [Google Scholar]
- Wilson RA. Proteomics at the schistosome–mammalian host interface: any prospects for diagnostics or vaccines? Parasitology. 2012;139:1178–1194. doi: 10.1017/S0031182012000339. [DOI] [PubMed] [Google Scholar]
- You H, Gobert GN, Duke MG, Zhang W, Li Y, Jones MK, et al. The insulin receptor is a transmission blocking veterinary vaccine target for zoonotic Schistosoma japonicum. Int J Parasitol. 2012;42:801–807. doi: 10.1016/j.ijpara.2012.06.002. [DOI] [PubMed] [Google Scholar]
- You H, McManus DP, Hu W, Smout MJ, Brindley PJ, Gobert GN. Transcriptional responses of in vivo praziquantel exposure in schistosomes identifies a functional role for calcium signalling pathway member CamKI. PLoS Pathog. 2013;9:e1003245. doi: 10.1371/journal.ppat.1003254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang KH, Guo YJ, Lu YM, Yan HL, Song YL, et al. Annexin B1 from Taenia solium metacestodes is a newly characterized member of the annexin family. Biol Chem. 2007;388:601–610. doi: 10.1515/BC.2007.071. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Si J, Harn DA, Yu C, Liang Y, Ren J, et al. The protective immunity of a DNA vaccine encoding Schistosoma japonicum Chinese strain triose-phosphate isomerase in infected BALB/C mice. Southeast Asian J Trop Med Pub Health. 2004;35:518–522. [PubMed] [Google Scholar]
- Zhu Y, Si J, Harn DA, Xu M, Ren J, Yu C, et al. Schistosoma japonicum triose-phosphate isomerase plasmid DNA vaccine protects pigs against challenge infection. Parasitology. 2006;132:67–71. doi: 10.1017/S0031182005008644. [DOI] [PubMed] [Google Scholar]


