Abstract
Annexin A2 (AnxA2) was originally identified as a substrate of the pp60v-src oncoprotein in transformed chicken embryonic fibroblasts. It is an abundant protein that associates with biological membranes as well as the actin cytoskeleton, and has been implicated in intracellular vesicle fusion, the organization of membrane domains, lipid rafts and membrane-cytoskeleton contacts. In addition to an intracellular role, AnxA2 has been reported to participate in processes localized to the cell surface including extracellular protease regulation and cell-cell interactions. There are many reports showing that AnxA2 is differentially expressed between normal and malignant tissue and potentially involved in tumour progression. An important aspect of AnxA2 function relates to its interaction with small Ca2+-dependent adaptor proteins called S100 proteins, which is the topic of this review. The interaction between AnxA2 and S100A10 has been very well characterized historically; more recently, other S100 proteins have been shown to interact with AnxA2 as well. The biochemical evidence for the occurrence of these protein interactions will be discussed, as well as their function. Recent studies aiming to generate inhibitors of S100 protein interactions will be described and the potential of these inhibitors to further our understanding of AnxA2 S100 protein interactions will be discussed.
Tables of Links
| LIGANDS | |
|---|---|
| ATP | Histamine |
| Bepridil | Ketoconazole |
| cAMP | Trifluoperazine |
| Forskolin | Von Willebrand factor |
| Heparin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
AnxA2 structure
Like all annexins, AnxA2 is a slightly curve-shaped protein with a convex and a concave side. It consists of a highly conserved core domain of four homologous repeats of 70–80 amino acids called the annexin repeats and a unique 30-amino-acid long N-terminal ‘head domain’ (which is also referred to as tail domain or N-terminal interaction domain in some literature) (Waisman, 1995; Gerke et al., 2005). The core domain region, encompassing residues 31–338, has binding sites for calcium, phospholipids, heparin and F-actin. Although the core domain is conserved among the annexins, subtle differences between them have been noticed. For example, the AnxA2 core domain is subtly different in its Ca2+ sensitivity in Ca2+-dependent membrane interactions (Drucker et al., 2013). Thus, different annexins may have different functions in the cell.
The head domain of AnxA2 contains a number of features relatively unique to this particular annexin. The first 12 residues of the head domain constitute the binding site for S100A10, a member of the S100 protein family (Johnsson et al., 1986, 1988). This region also encompasses a binding site for tissue plasminogen activator (tPA) mapped to residues 7–12 (Johnsson et al., 1988; Hajjar et al., 1998) and a nuclear export signal (NES) mapped to residues 3–13 (Eberhard et al., 2001). Residues Tyr23, Ser11 and Ser25 of AnxA2 can be phosphorylated by Src family tyrosine kinases and serine kinases respectively (Gould et al., 1986; Khanna et al., 1986; Powell and Glenney, 1987; Jost and Gerke, 1996).
The interaction of AnxA2 with S100 proteins
Perhaps one of the most clearly defined features that characterize AnxA2 is its capacity to interact with members of the S100 protein family to yield so-called heterotetrameric complexes, consisting of an S100 protein dimer and two AnxA2 proteins ((S100AXX-AnxA2)2). S100 proteins are a group of small Ca2+-binding proteins with molecular weight of 10–12 kDa (Donato, 1999, 2003). With the exception of S100A10, they contain Ca2+-binding EF-hand motifs, and are regarded as the largest family grouping within the EF-hand protein superfamily. Rather uniquely, compared with other EF-hand proteins, S100 monomers contain two different EF hands with distinct affinities for calcium: a canonical C-terminal EF hand (KD ≈ 10–50 μM) and a pseudo-canonical N-terminal EF hand (KD ≈200–500 μM). S100 proteins always function as dimers, mostly homodimers, but sometimes heterodimers: S100A1/B, S100A8/A9, S100A1/A4 and S100A1/P (Odink et al., 1987; Duda et al., 1996; Tarabykina et al., 2000; Wang et al., 2004). Ca2+ binding induces a conformational change in the S100 proteins due to repositioning of helix III from a near antiparallel position to helix IV to a nearly perpendicular position. Thus, a compact and closed conformation opens up and exposes a large hydrophobic area which is capable of recognizing and binding potential targets (Malashkevich et al., 2008). S100A10 is unique among S100 proteins in that it is locked in a permanently open conformation, comparable to the Ca2+-bound configuration of the other S100 proteins. Many S100 proteins play a role in cancer prognosis or progression (Schlagenhauff et al., 2000; Davies et al., 2002; Cross et al., 1996; Vimalachandran et al., 2005; De Petris et al., 2009) and some of them are suggested as biomarkers to certain types of cancer (Hamberg et al., 2003; Nedjadi et al., 2009; Tsuna et al., 2009).
The classic AnxA2-binding S100 protein is S100A10, which was identified as a binding partner almost 30 years ago (Erikson et al., 1984; Gerke and Weber, 1985a, b). Binding to S100A10 occurs at the helical AnxA2 N-terminus (Glenney et al., 1986; Johnsson et al., 1988; Kube et al., 1992; Rety et al., 1999). The AnxA2 N-terminus is accommodated in the free hydrophobic space between helix III and helix IV of the S100A10 dimer (Rety et al., 1999) (Figure 1). Interaction with the AnxA2 N-terminus appears sufficient for binding since proteolytic removal of this N-terminus from purified AnxA2 (cleaved at Gly14) (Johnsson et al., 1988) or deletion of residues 1–14 from recombinant AnxA2 (e.g. Semov et al., 2005) results in a complete loss of the interaction with S100A10. Removal of the first methionine of the primary AnxA2 translation product as well as acetylation of the serine at position 2 is necessary for AnxA2 binding to S100A10 (Johnsson et al., 1988; Becker et al., 1990; Konig et al., 1998; Nazmi et al., 2012). In addition to the acetyl group, specific hydrophobic residues are crucial for binding with S100A10 (Becker et al., 1990; Rety et al., 1999).
Figure 1.
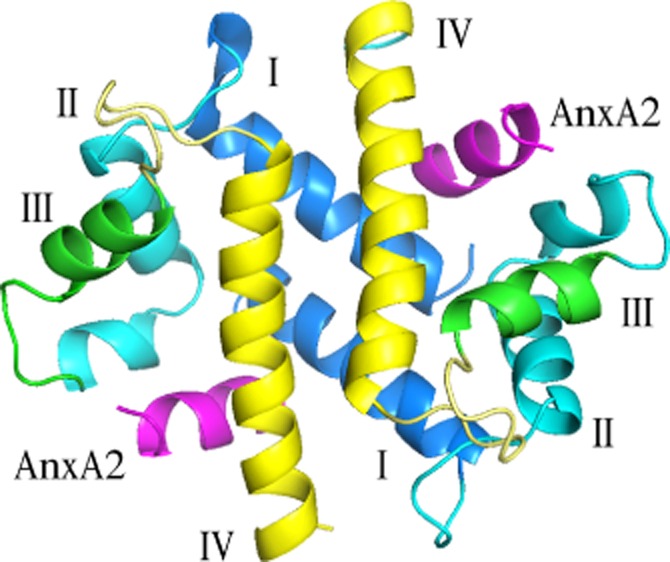
Structure of S100A10 binding with annexin A2 peptide displayed as ribbon diagram. S100 proteins are coloured blue green yellow while the AnxA2 N-terminus is coloured magenta (PDB: 1BT6) (Rety et al., 1999).
Recent studies showed that in addition to S100A10, three other S100 proteins, S100A6, S100A4 and S100A11, are also able to bind to AnxA2 (Table 1). Various techniques have been employed to investigate the binding between these S100 proteins and AnxA2. Isothermal titration calorimetry of 16 different S100 proteins with the AnxA2 N-terminus identified S100A10 and S100A11 as binding partners (Streicher et al., 2009). The interaction between S100A11 and AnxA2 was also demonstrated by nuclear magnetic resonance (NMR), isothermal titration calorimetry and immunoprecipitation (Rintala-Dempsey et al., 2006). Although not observed using isothermal titration calorimetry, S100A4 can accommodate the AnxA2 N-terminus based upon nuclear NMR and immunoprecipitation evidence (Semov et al., 2005). The NMR studies indicated that Glu6, Asp10, Leu42, Phe45, Ile82, Phe89 and Pro94 in S100A4 could be involved in the binding with AnxA2 and these amino acids are in similar positions to key amino acids in S100A10 involved in the binding to AnxA2 (Glu5, Glu9, Phe38, Phe41, Leu78, Tyr85 and Met90 in S100A10) (Semov et al., 2005). Finally, an AnxA2 complex with S100A6 was identified using affinity chromatography and immunoprecipitation methods (Zeng et al., 1993) and further confirmed by biochemistry and spectrometry methods (Filipek et al., 1995; Nedjadi et al., 2009). Given the similarity of the calcium-bound conformations of S100A4, S100A6 and S100A11 to the S100A10 conformation, it may be argued that AnxA2 is accommodated in a similar fashion in each of these S100 proteins.
Table 1.
S100 proteins known to bind AnxA2
| AnxA2 targets | IP | X-ray | ITC | Biochemistry method | Spectrometry or chromatography method |
|---|---|---|---|---|---|
| S100A10 | Erikson et al., 1984 | Rety et al., 1999 | Streicher et al., 2009 | Johnsson et al., 1988 | – |
| Gerke et al., 2002 | Li et al., 2010 | ||||
| Gerke et al., 1985b | Streicher et al., 2009 | ||||
| S100A4 | Semov et al., 2005 | – | – | – | Semov et al., 2005 |
| S100A6 | Zeng et al., 1993 | – | – | Filipek et al., 1995 | Zeng et al., 1993 |
| Filipek et al., 1995 | Filipek et al., 1995 | ||||
| Nedjadi et al., 2009 | Nedjadi et al., 2009 | ||||
| S100A11 | Rintala-Dempsey et al., 2006 | – | Streicher et al., 2009 | – | Rintala-Dempsey et al., 2006 |
| Rintala-Dempsey et al., 2006 |
IP, immunoprecipitation; ITC, isothermal titration calorimetry; X-ray, crystallography.
Several studies have investigated the affinity of the AnxA2 N-terminus for S100 proteins. Using nuclear magnetic resonance techniques, a dissociation constant of 3.3 ± 0.6 μM was derived for the binding of AnxA2 to S100A11 by titrating an AnxA2 N-terminus peptide into calcium-bound S100A11 (Rintala-Dempsey et al., 2006). Isothermal titration calorimetry experiments have also been used to determine the dissociation constant of this interaction (Streicher et al., 2009). It was found that the binding isotherm could not be fitted to the simplest binding model, but fitted into a sequential binding model suggesting that the interaction involves non-symmetric binding to the two AnxA2 peptides: one binding site on the dimer needs to be occupied before the second binding event can take place. Thus, two dissociation constants were determined for the binding of the AnxA2 N-terminus to S100A11: 1.7 ± 1.2 μM and 9.2 ± 1.9 μM. A very similar scenario applied to the binding of the AnxA2 N-terminus to S100A10 albeit that the two sequential binding events appeared to have identical dissociation constants of 0.5 ± 0.4 μM (Streicher et al., 2009). A comparable dissociation constant of 1.3 ± 0.3 μM was measured independently for this interaction using equilibrium dialysis and fluorescence resonance transfer techniques (Li et al., 2010) and for the binding of full-length AnxA2 to S100A10 (Nazmi et al., 2012).
The structure of the full AnxA2-S100 complex
In appreciating the well-established binding mode of the AnxA2 N-terminus to S100A10, the structure of the full complex is more speculative. Modelling the complex between the S100A10 dimer and the AnxA2 N-terminus with the solved AnxA2 core domain suggests two plausible configurations. In one model, the S100A10 dimer bridges two AnxA2 molecules arranged in opposite orientation whereas the second model predicts the S100A10 dimer to sit on top of two AnxA2 molecules arranged side by side (Sopkova-de Oliveira Santos et al., 2000). It is extremely difficult to ascertain which of these models would prevail in vivo; however, individually they can explain various functions of the AnxA2 protein and therefore both conformations may exist. In terms of how these various conformations are regulated, it is of interest that the interaction of AnxA2 with the S100A10 dimer takes place at the very end of the protein, leaving a loop region between this S100A10 recognition region and the AnxA2 core domain. Flexibility in this loop may allow the one or the other conformation. Electron microscopy data of membrane bridges at pH 7.4 in the presence of Ca2+ suggest that the dimer of S100A10 be located in the centre of the protein density, with one molecule of AnxA2 facing the bilayer on each side (Lambert et al., 1997). However, AnxA2 can also move around S100A10 as a hinge to acquire a more open conformation where the S100A10 subunit is held away from the phospholipid bilayer (Lambert et al., 2004; Menke et al., 2004; Illien et al., 2010), compatible with the suggested models (Sopkova-de Oliveira Santos et al., 2000). A third more stretched conformation has been observed experimentally at mild acidic pH in the absence of Ca2+ (Illien et al., 2010). Cellular studies indicated that acidic pH can support the membrane binding of the (S100A10-AnxA2)2 heterotetramer complex and it is perhaps this stretched conformation that is responsible for this (Monastyrskaya et al., 2008). Interestingly, the putative hinge region of the annexin head domain is subject to phosphorylation and phosphorylation events may also regulate the specific loop architecture and conformation of the tetramer (Grindheim et al., 2014).
The cellular complex of AnxA2 and S100 proteins
The ratio of monomeric AnxA2 to (S100A10-AnxA2)2 can vary widely, and the differences in ratio are due to coordinated expression of AnxA2 and S100A10 (Munz et al., 1997) as well as post-translational control (Puisieux et al., 1996). Interfering with the expression of one of the partners in the (S100A10-AnxA2)2 complex affects the expression of the other partner, indicating their intimate relationship in vivo. In most reports, knockdown of AnxA2 affects the levels of S100A10; however, the reverse has also been observed in some cases and thus the ‘direction’ of the regulatory effect seems to differ between cell types. For example, in endothelial cells, knockdown of AnxA2 not only results in the disappearance of AnxA2 but also in that of S100A10, while knockdown of S100A10 does not affect expression of AnxA2 (Brandherm et al., 2013). In complex with S100A10, AnxA2 may protect S100A10 from being rapidly polyubiquitinated and degraded (He et al., 2008). Interestingly, the residues 86–95 subject to ubiquitination of S100A10 are the residues responsible for binding with the AnxA2 N-terminal, suggesting that once ubiquitinated, S100A10 may not bind AnxA2 anymore. The amount of S100A10 in the cell would thus dictate the amount of the (S100A10-AnxA2)2 complex in the cell (Figure 2). This may be important to determine the intracellular fate of AnxA2. Although AnxA2 itself can associate with cellular membranes (Zobiack et al., 2001), S100A10 binding increases the Ca2+ sensitivity of AnxA2 and its capacity to bind membranes and (submembranous) F-actin (Ikebuchi and Waisman, 1990; Harder and Gerke, 1994; Filipenko and Waisman, 2001; Monastyrskaya et al., 2007). Depletion of S100A10 by RNA silencing or phosphorylation on Ser11 on AnxA2 (which inhibits interaction with S100A10) disrupts the membrane association of AnxA2 (Regnouf et al., 1995; Deora et al., 2004). Similar observations have been made for S100A6 which has also been proposed to interact with AnxA2. Depletion of S100A6 from pancreatic cancer cells was accompanied by diminished levels of membrane AnxA2 associated with a pronounced reduction in the motility of pancreatic cancer cells (Nedjadi et al., 2009). Under certain conditions of stress, AnxA2 can become expressed on the cell surface, in a mechanism that requires the interaction with S100A10 as well as phosphorylation of AnxA2 on tyrosine at position 23 (Deora et al., 2004).
Figure 2.
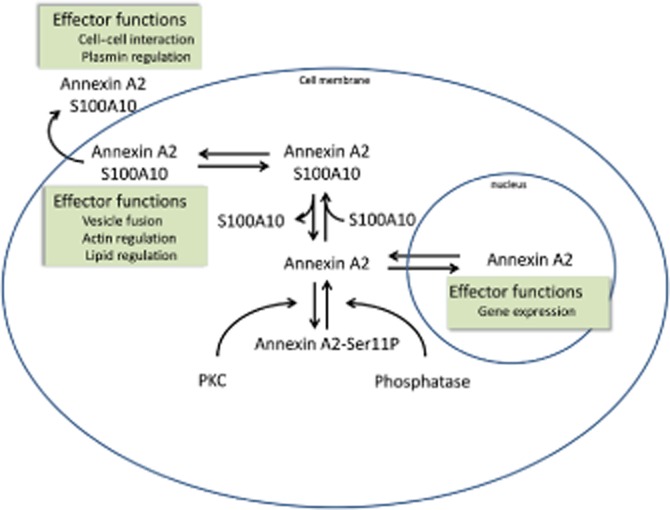
Simplified diagram to illustrate some aspects of the cellular regulation of AnxA2 by S100 protein interactions.
Sequestration of AnxA2 by S100A10 in the cytosol also prevents its nuclear localization (Eberhard et al., 2001). In prostate cancer cells, monomeric AnxA2 can localize to the nucleus where it acts as negative regulator of cell proliferation (Liu et al., 2003). Phosphorylation of AnxA2 may be important for its nuclear localization (Chiang et al., 1996; Eberhard et al., 2001). AnxA2 contains a functional NES sequence at the N-terminal which allows export via the Ran/exportin-mediated export pathway (Eberhard et al., 2001). This sequence overlaps with the binding site of AnxA2 with S100A10.
As mentioned above, loss of S100A10 has also been observed to affect the levels of AnxA2, in particular in studies in which S100A10 was removed by genetic deletion. In S100A10 knockout mice, the AnxA2 level decreased in spleen, kidneys, lungs and liver, but was not affected in intestine (Madureira et al., 2012), suggesting that S100A10 could stabilize and regulate the level of AnxA2. However, studies in nociceptor neurons indicated that genetic deletion of S100A10 did not affect AnxA2 levels (Foulkes et al., 2006).
Functions associated with the AnxA2-S100 complex
Biochemical reconstitution experiments, mouse genetic deletion models and RNA interference studies have yielded much information on the effector functions of individual annexins and S100 proteins, including AnxA2 and S100A10. It is beyond the scope of this review to discuss all the evidence and the reader is referred to recent excellent reviews in this area (Gerke and Moss, 2002; Rescher and Gerke, 2004; Gerke et al., 2005; Kwon et al., 2005; Flood and Hajjar, 2011; Madureira et al., 2011; Bharadwaj et al., 2013; Luo and Hajjar, 2013). However, a few recent relevant examples are cited here to provide an indication of the effector function of (S100A10-AnxA2)2 and related complexes in the cell. It is perhaps useful to consider these in relation to the two conformational models cited above. While it is very difficult to ascertain precisely which model applies to a particular cellular context, in a broad simplification one could say that in one conformation, the ‘opposite conformation’, the tetramer can bring together different cell membranes, while in the other conformation, the ‘lateral conformation’, the tetramer can act as a platform for association with other proteins. This classification should, however, not be taken as absolute, since the former conformation could accommodate additional proteins and the latter can serve to juxtapose membranes.
The former conformation points to a role in membrane trafficking, secretory or endocytic processes. A recently presented example shows that the (S100A10-AnxA2)2 complex is involved in the secretion of von Willebrand factor (vWF), which is stored in the Weibel–Palade bodies (secretory granules) of endothelial cells (Knop et al., 2004; Brandherm et al., 2013). It is normally released by agonists that raise intracellular Ca2+ or cAMP levels and a functional (S100A10-AnxA2)2 complex is required for the forskolin-induced, cAMP-dependent release of vWF (Knop et al., 2004; Brandherm et al., 2013). Forskolin triggers dephosphorylation of AnxA2 (Borthwick et al., 2007), mediated by a calcineurin-like phosphatase. This stabilizes the (S100A10-AnxA2)2 complex and promotes vWF release (Brandherm et al., 2013). When the (S100A10-AnxA2)2 complex cannot form, cAMP-dependent vWF secretion is compromised (Brandherm et al., 2013). At present, it is not clear whether additional protein interactions contribute to the secretion of vWF such as observed in secretory processes in stimulated chromaffin cells. In these cells, AnxA2 directly interacts with S100A10 to form a tetramer at the plasma membrane (Chasserot-Golaz et al., 2005; Umbrecht-Jenck et al., 2010). S100A10 can interact with vesicle-associated membrane protein 2 (VAMP2) which may act as docking factor for S100A10. Prevention of S100A10 binding to VAMP2 inhibits the translocation of annexin-A2 to the plasma membrane. In bronchial epithelial cells, AnxA2 associates with collagen VI and the SNARE proteins SNAP-23 and VAMP2 at secretory vesicle membranes, and as such has been implicated in the collagen VI secretion pathway (Dassah et al., 2014). It is not clear whether this also involves the S100A10 protein interaction.
Localized at the cell surface, AnxA2 has been implicated in cell-cell interactions and cell adhesion. AnxA2 provides a signal for interaction with and phagocytosis of apoptotic cells, most likely via interactions with phosphatidyl serine on the juxtaposed apoptotic cell surface (Fan et al., 2004; Law et al., 2009; Fang et al., 2012). AnxA2 expressed on apoptotic cells themselves binds complement factors as signal for cell-cell interaction and phagocytosis (Leffler et al., 2010; Martin et al., 2012). Furthermore, the (S100A10-AnxA2)2 complex has been implicated in tight junction maintenance in epithelial MDCK cell monolayers in a model in which AnxA2 is associated with the lipid membrane with the S100A10 dimer bridging two AnxA2 molecules (Lee et al., 2004, 2008). The binding of surface AnxA2 to surface S100A10 also contributes to heterotypic cell-cell interactions between breast tumour cells and microvascular endothelial cells. An AnxA2 molecule present on an opposing cell, such as a breast cancer cell, can bridge to the endothelial cell by interacting with surface-localized S100A10 located on the latter (Myrvang et al., 2013).
A wide range of platform functions of the (S100A10-AnxA2)2 complex have been suggested. Early research revealed that as well as bridging phospholipid vesicles and binding biological membranes, the (S100A10-AnxA2)2 complex displays binding and bundling of F-actin (Gerke and Weber, 2002). This occurs at physiological Ca2+ concentrations in the μM range (Ikebuchi and Waisman, 1990; Regnouf et al., 1991). This activity can be specifically inhibited by pre-incubation of F-actin with a nonapeptide to the actin-binding site of AnxA2 at residues 286–294 (Jones et al., 1992). The (S100A10-AnxA2)2 complex is important for the organization of F-actin at lipid rafts and for the dynamic regulation and remodelling of the actin cytoskeleton (Hayes et al., 2004, 2006). As such, AnxA2 has been implicated in various cellular processes that involve the actin cytoskeleton.
One of the other AnxA2 partners, S100A11, is required for efficient plasma membrane repair which may support the survival of invasive cancer cells (Jaiswal et al., 2014). During cell migration and invasion, cells are exposed to physical stress. Injury to the cell membrane occurring during this process results in entry of calcium into the cell which in turn can trigger recruitment of S100A11 and AnxA2 to the site of injury (Jaiswal et al., 2014). The complex of S100A11 with AnxA2 directs polymerization of cortical F-actin and excision of the damaged part of the plasma membrane thereby resealing the plasma membrane (Jaiswal et al., 2014).
On endothelial cells, the (S100A10-AnxA2)2 complex has been proposed as endothelial surface platform for tPA and plasminogen (Plgn), aiding the conversion to plasmin. Several somewhat conflicting models exist to explain the exact contributions of these proteins individually (or as a complex) to the plasmin activation process with either AnxA2 (Cesarman et al., 1994; Hajjar et al., 1994, 1998; Flood and Hajjar, 2011; Luo and Hajjar, 2013) or S100A10 (Kassam et al., 1998; MacLeod et al., 2003; Madureira et al., 2011; Bharadwaj et al., 2013) proposed as the main receptor of tPA and Plgn. Both models implicate the binding of S100A10 and AnxA2 in the regulation of the surface proteases and the fact that genetic deletion of either protein shows roles for both proteins in maintenance of vascular patency, fibrin resolution, cell migration and neoangiogenesis also suggests a very close relationship between them, most likely because they act as a complex in these processes (Ling et al., 2004; Huang et al., 2011; Madureira et al., 2011; Phipps et al., 2011; Surette et al., 2011).
Like the complex of AnxA2 and S100A10, the complex of AnxA2 and S100A4 may also regulate the tPA/Plgn cascade on the cell surface. Addition of S100A4 to umbilical vein endothelial cells stimulated tPA/Plgn on these cells. This stimulation was reversible upon addition of a synthetic peptide based upon the AnxA2 N-terminus, suggesting that a complex between S100A4 and AnxA2 is involved in tPA/Plgn regulation (Semov et al., 2005). This scenario may be relevant when tumour cells produce S100A4 which, once bound to endothelial cells and activating pericellular proteases, can aid the growth of blood vessels into the tumour.
While the F-actin-binding and protease-regulating platform functions of the (S100A10-AnxA2)2 complex are not clearly defined in structural terms, interactions with the protein AHNAK and SMARCA3 have been solved by protein crystallography. AHNAK, a Hebrew word for ‘giant’, is a 629 kDa protein involved in membrane repair (Shtivelman et al., 1992; Zhang et al., 2004). A multi-protein complex of (S100A10-AnxA2)2 and AHNAK is a target of dysferlin, a core protein in wound repairing process of ‘injured’ epithelial cells (Huang et al., 2007). The minimal binding site of AHNAK protein, AHNAK5654–5673, with the (S100A10-AnxA2)2 complex has been mapped (De Seranno et al., 2006; Rezvanpour et al., 2011) and was used to derive a crystal structure (Ozorowski et al., 2013). The 20-amino-acid length AHNAK peptide binds asymmetrically across the (S100A10-AnxA2)2 complex. Hydrogen bonding between backbone atoms of ANNAK peptides and (S100A10-AnxA2)2 and the hydrophobic interaction of ANNAK side chains with S100A10 are the main binding forces responsible for the ternary complex (Ozorowski et al., 2013).
It was recently found that SMARCA3, a protein involved in chromatin remodelling in different nuclear processes in an ATP-dependent manner (Debauve et al., 2008), is also a target of the (S100A10-AnxA2)2 complex (Oh et al., 2013). The co-crystal structure of SMARCA3 with this complex shows that the binding site is very similar to that of AHNAK peptide: two SMARCA3 peptides symmetrically bind to the (S100A10-AnxA2)2 complex at the ‘back’ of S100A10 reaching into a small hydrophobic cavity created by the ‘C-terminal’ of AnxA2 peptide and helix IV(IV′) (Oh et al., 2013). It was observed that the (S100A10-AnxA2)2 complex can increase the DNA binding affinity of SMARCA3 and help SMARCA3 localize to the nuclear matrix; this would require S100A10 to be present in the nucleus.
A peptide toolbox to study AnxA2-S100 protein interactions
Given the detailed knowledge of the binding interactions between the N-terminus of AnxA2 and S100A10, various groups have reported the use of peptides based upon this N-terminus to perform competition experiments with the aim of disrupting the endogenous complex of the two proteins and understanding its functions. An isolated acetylated synthetic peptide comprising residues 1–14 of AnxA2 can disrupt a preformed complex between S100A10 and a labelled annexin 1–14 peptide (O'Connell et al., 2010). Furthermore, the same peptide also disrupts a preformed full-length (S100A10-AnxA2)2 complex (Konig et al., 1998). Thus, it is feasible to use synthetic peptides to disrupt endogenous complexes between AnxA2 and S100A10 in vivo (Table 2).
Table 2.
Peptide inhibitors used to elucidate the function of annexin A2 protein interactions
| Peptide | Test system | Observation | Reference |
|---|---|---|---|
| AA2 (1–14) | Chloride channel activation measured by patch clamp | Reduced by acetylated but not by non-acetylated peptide | Nilius et al., 1996 |
| AA2 (1–14) | Purified AnxA2/S100A10 complex binding to liposomes | Loss of binding to liposomes in the presence of peptide | Konig et al., 1998 |
| AA2 (1–14) | vWF release after micro injection | Reduced by acetylated but not by non-acetylated peptide | Knop et al., 2004 |
| AA2 (1–14) | Formation of epithelial cell tight junctions in vitro | Reduced by peptide | Lee et al., 2004 |
| AA2 (1–14) | FGF- and VEGF-driven angiogenesis into Matrigel plug in vivo | 80% decrease in vascularization by the peptide | Ling et al., 2004 |
| AA2 (7–12) | Pancreatic cancer cell migration | Inhibited by peptide at high concentrations | Diaz et al., 2004 |
| AA2 (1–14) | S100A4-induced, tPA-mediated plasminogen activation on endothelial cells | Inhibited by the peptide | Semov et al., 2005 |
| AA2 (1–12) | Adhesion of embryonic stem cells to annexin A2 in vitro | ∼80% inhibition | Jung et al., 2007 |
| AA2 (1–14) | AnxA2/S100A10 complex formation with CFTR in co-immunoprecipitates | Binding of AnxA2 reduced by acetylated but not by non-acetylated peptide | Borthwick et al., 2007 |
| AA2 (1–12) | Adhesion of prostate cancer cells to endothelial cell monolayer | Reduced by peptide | Shiozawa et al., 2008 |
| AA2 (1–12) | Homing of prostate cancer cells to bone marrow in vivo (metastasis) | Reduced by peptide | Shiozawa et al., 2008 |
| AA2 (1–14) | Adhesion of breast cancer cells to endothelial cell monolayers | Reduced by acetylated but not by scrambled peptide | Myrvang et al., 2013 |
CFTR, cystic fibrosis transmembrane conductance regulator; FGF, fibroblast growth factor.
Because of their nature, peptide interference studies have largely been confined to scenarios where AnxA2 and S100A10 are localized at the outer face of the plasma membrane, or where the peptide could somehow be introduced into the cells, for example, by microinjection or in patch clamp experiments (Table 2). Very elegant studies have been performed in which the action of an acetylated peptide was compared with a non-acetylated peptide. It is known that the non-acetylated version of AnxA2 (or its N-terminus) binds weakly to S100A10 dimers, therefore it is expected that such a peptide cannot disrupt the endogenous complex (Becker et al., 1990). By studying both peptides in parallel, a convincing argument can be made for or against the involvement of the (S100A10-AnxA2)2 complex in a cellular process under study. In this way, it was shown that an acetylated version of the annexin N-terminus peptide reduced the volume activation of a chloride current in pulmonary artery endothelial cells, whereas a non-acetylated version of the same peptide did not affect the current, implicating the S100A10 protein interaction with AnxA2 in activation of these ion currents (Nilius et al., 1996). The same strategy has revealed the involvement of the (S100A10-AnxA2)2 complex in histamine induced secretion of vWF from endothelial cells (Knop et al., 2004).
The AnxA2 N-terminus peptide is able to compete with cell-cell interactions between breast cancer cells and endothelial cells while a scrambled peptide is not (Myrvang et al., 2013). It was observed that AnxA2 is present on the surface of breast cancer cells, and S100A10 on the surface of endothelial cells. Thus, these proteins may function as bridge between these cell types in cell-cell interactions. Similar studies using competing N-terminus peptides indicate that (S100A10-AnxA2)2 complexes are involved in tight junction assembly between kidney epithelial cells, suggesting a role in cell-cell interactions (Lee et al., 2004).
The adhesion of prostate cancer cells to bone marrow endothelial cell is also inhibited by AnxA2 N-terminus peptides. A putative AnxA2 receptor has been identified on prostate cancer cells, which may aid the cell-cell interaction (Shiozawa et al., 2008).
A peptide based upon the AnxA2 N-terminus, but not a scrambled peptide, has been shown to inhibit neoangiogenesis into Matrigel plugs (Ling et al., 2004), suggesting that protein interactions at the AnxA2 N-terminus participate in this process. The peptide may compete with an endogenous (S100A10-AnxA2)2 complex at the endothelial cell surface, or alternatively it may inhibit directly the interaction between AnxA2 and tPA.
This last scenario illustrates the power of the use of synthetic peptides in elucidating the involvement of the (S100A10-AnxA2)2 complex in physiological processes but also the problem, since additional interactions are possible (at least in principle) to explain the observations.
Chemical manipulation of the AnxA2-S100 protein interaction
Protein interactions are generally considered not amenable to blockade with small molecules. This is largely because they involve shallow and extensive interfaces with no features that could support effective small molecule binding. However, there are cases of successful targeting of protein interactions. For example, the interaction between Mdm2 and p53, and the interaction between Bcl2 and Bak have both been explored pharmacologically using small molecule inhibitors, which have subsequently shown promise as therapeutic agents (Shangary and Wang, 2008; 2009; Gandhi et al., 2011). It is of interest that both p53 and Bak contain a short helical sequence that docks into a well-defined groove-like feature on the surface of the respective binding partners, which in both cases constitutes a small globular protein. The protein interaction between the AnxA2 N-terminus and S100A10 proteins similarly involves a well-defined and comparatively deep concave binding pocket accommodating a small helical peptide. The AnxA2 N-terminus conceals approximately 660 Å2 of solvent-accessible surface area in the lipophilic pocket of S100A10 (Rety et al., 1999). Most of the binding energy derives from hydrophobic interactions in the innermost portion of the pocket and from charge-enhanced H-bonds with the carboxyls of E5 and E9 of S100A10 (Becker et al., 1990; Rety et al., 1999). Residues V3, I6, L7 and L10 alone displace approximately 430 Å2 of solvent-accessible surface area. This is a size of binding pocket that is within reach of what are commonly considered drug-like molecules (Lipinski, 2004).
A receptor-guided as well as a ligand-guided virtual screening approach was recently used to identify a novel class of small molecules that inhibit the interaction between AnxA2 and S100A10 (Reddy et al., 2011; 2012; 2014) (Figure 3). This virtual screening approach allowed the identification of candidate blockers that were able to dock into the AnxA2-binding site on S100A10 or that mimicked the binding pose of the AnxA2 N-terminus as defined in the complex crystal structure (Rety et al., 1999). Candidate molecules were then screened in a biochemical FRET assay that measured the binding between the AnxA2 N-terminus and the S100A10 protein. This identified two classes of compounds: 3-hydroxy-1H-pyrrol-2(5H)-one analogues and substituted 1,2,4-triazoles as effective blockers of the binding of S100A10 and AnxA2 (Reddy et al., 2011; 2012). The docking suggested that both kinds of inhibitors could bind to three pockets on S100A10 that are normally occupied by an acetyl, valine and leucine moiety on the AnxA2 N-terminus (Reddy et al., 2011; 2012). Selected blockers were also able to inhibit the interaction of the native complex of AnxA2 and S100A10 and some were shown to inhibit the complex inside the cell. These compounds may be used to further elucidate the function of the (S100A10-AnxA2)2 complex.
Figure 3.
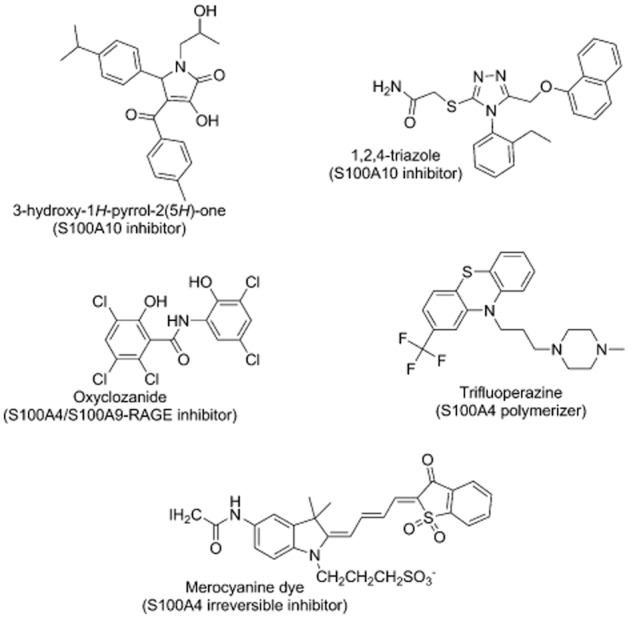
Chemical structures of S100 protein interaction inhibitors.
The compound withaferin A has been shown to bind to the N-terminus of AnxA2 via covalent bonding to the cysteine residue at position 9 (Ozorowski et al., 2012). This residue is solvent exposed in the (S100A10-AnxA2)2 complex and withaferin A did not inhibit the protein interaction between the proteins. However, it may inhibit functions of the monomeric form of AnxA2.
In addition to the S100A10 AnxA2 blockers described above, a number of additional S100A4 protein blockers have been described that could conceivably be useful in understanding interactions between S100 proteins and annexins. Merocyanine can covalently bind to Cys81 of S100A4 and act as an irreversible inhibitor of the binding of S100A4 to myosin IIA (Garrett et al., 2008). Cys81 is a part of the hydrophobic area on S100A4 that could possibly be involved in the binding with AnxA2 (Semov et al., 2005), indicating that interactions with AnxA2 may also be inhibited by this compound. A set of Food and Drug Administration-approved drugs was tested for their ability to inhibit the Ca2+-induced conformational change of S100A4 as determined by a fluorescence increase of the linked biosensor. This identified a number of phenothiazine compounds. Phenothiazines were found to defunctionalize S100A4 by polymerizing the protein (Malashkevich et al., 2010). Other compounds affecting the S100A4 conformation included ketoconazole, bepridil and nicergoline (Garrett et al., 2008). Bepridil can inhibit the myosin IIA filament depolarizing effect of S100A4. It is not known whether compounds like these interfere with the AnxA2-binding properties of S100A4.
Oxyclozanide has been shown to inhibit the interaction between S100A4 and receptors for advanced glycation end products (RAGEs) or toll-like receptor 4 (TLR4) (Bjork et al., 2013). It appears to bind to the homodimeric form or to an S100A4/A9 heterodimer to interfere with the binding with RAGE and TLR4 (Bjork et al., 2013). These interactions are implicated in inflammation and tumour growth (Foell and Roth, 2004; Apetoh et al., 2007; Gebhardt et al., 2008; Bjork et al., 2009) as well as cell matrix invasion (Yammani et al., 2006). Again, it remains to be established whether this compound interferes with the AnxA2-binding properties of S100A4.
Conclusion
The interaction between S100 proteins and AnxA2 plays a role in various processes in the cell. The recent identification of small molecule inhibitors of this interaction, combined with known peptidic inhibitors, will allow further functional elucidation of these complexes.
Acknowledgments
We acknowledge the support of Cancer Research UK and the Biotechnology and Biological Sciences Research Council UK.
Glossary
- AnxA2
annexin A2
- Plgn
plasminogen
- tPA
tissue plasminogen activator
Conflict of interest
There is no conflict of interest to disclose.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic receptors. Br J Pharmacol. 2013a;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Becker T, Weber K, Johnsson N. Protein-protein recognition via short amphiphilic helixes; a mutational analysis of the binding site of annexin II for p11. EMBO J. 1990;9:4207–4213. doi: 10.1002/j.1460-2075.1990.tb07868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj A, Bydoun M, Holloway R, Waisman D. Annexin A2 heterotetramer: structure and function. Int J Mol Sci. 2013;14:6259–6305. doi: 10.3390/ijms14036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork P, Bjork A, Vogl T, Stenstrom M, Liberg D, Olsson A, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7:800–812. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork P, Kallberg E, Wellmar U, Riva M, Olsson A, He Z, et al. Common interactions between S100A4 and S100A9 defined by a novel chemical probe. PLoS ONE. 2013;8:e63012. doi: 10.1371/journal.pone.0063012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick LA, McGaw J, Conner G, Taylor CJ, Gerke V, Mehta A, et al. The formation of the cAMP/protein kinase A-dependent annexin 2-S100A10 complex with cystic fibrosis conductance regulator protein (CFTR) regulates CFTR channel function. Mol Biol Cell. 2007;18:3388–3397. doi: 10.1091/mbc.E07-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandherm I, Disse J, Zeuschner D, Gerke V. cAMP-induced secretion of endothelial von Willebrand factor is regulated by a phosphorylation/dephosphorylation switch in annexin A2. Blood. 2013;122:1042–1051. doi: 10.1182/blood-2012-12-475251. [DOI] [PubMed] [Google Scholar]
- Cesarman GM, Guevara CA, Hajjar KA. An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J Biol Chem. 1994;269:21198–21203. [PubMed] [Google Scholar]
- Chasserot-Golaz S, Vitale N, Umbrecht-Jenck E, Knight D, Gerke V, Bader MF. Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol Biol Cell. 2005;16:1108–1119. doi: 10.1091/mbc.E04-07-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YP, Davis RG, Vishwanatha JK. Altered expression of annexin II in human B-cell lymphoma cell lines. Biochim Biophys Acta. 1996;1313:295–301. doi: 10.1016/0167-4889(96)00103-6. [DOI] [PubMed] [Google Scholar]
- Cross SS, Hamdy FC, Deloulme JC, Rehman I. Expression of S100 proteins in normal human tissues and common cancers using tissue microarrays: S100A6, S100A8, S100A9 and S100A11 are all overexpressed in common cancers. Histopathology. 2005;46:256–269. doi: 10.1111/j.1365-2559.2005.02097.x. [DOI] [PubMed] [Google Scholar]
- Dassah M, Almeida D, Hahn R, Bonaldo P, Worgall S, Hajjar KA. Annexin A2 mediates secretion of collagen VI, pulmonary elasticity and apoptosis of bronchial epithelial cells. J Cell Sci. 2014;127(Pt 4):828–844. doi: 10.1242/jcs.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BR, O'Donnell M, Durkan GC, Rudland PS, Barraclough R, Neal DE, et al. Expression of S100A4 protein is associated with metastasis and reduced survival in human bladder cancer. J Pathol. 2002;196:292–299. doi: 10.1002/path.1051. [DOI] [PubMed] [Google Scholar]
- De Petris L, Orre LM, Kanter L, Pernemalm M, Koyi H, Lewensohn R, et al. Tumor expression of S100A6 correlates with survival of patients with stage I non-small-cell lung cancer. Lung Cancer. 2009;63:410–417. doi: 10.1016/j.lungcan.2008.06.003. [DOI] [PubMed] [Google Scholar]
- De Seranno S, Benaud C, Assard N, Khediri S, Gerke V, Baudier J, et al. Identification of an AHNAK binding motif specific for the Annexin2/S100A10 tetramer. J Biol Chem. 2006;281:35030–35038. doi: 10.1074/jbc.M606545200. [DOI] [PubMed] [Google Scholar]
- Debauve G, Capouillez A, Belayew A, Saussez S. The helicase-like transcription factor and its implication in cancer progression. Cell Mol Life Sci. 2008;65:591–604. doi: 10.1007/s00018-007-7392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem. 2004;279:43411–43418. doi: 10.1074/jbc.M408078200. [DOI] [PubMed] [Google Scholar]
- Diaz VM, Hurtado M, Thomson TM, Reventos J, Paciucci R. Specific interaction of tissue-type plasminogen activator (t-PA) with annexin II on the membrane of pancreatic cancer cells activates plasminogen and promotes invasion in vitro. Gut. 2004;53:993–1000. doi: 10.1136/gut.2003.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- Drucker P, Pejic M, Galla HJ, Gerke V. Lipid segregation and membrane budding induced by the peripheral membrane binding protein annexin A2. J Biol Chem. 2013;288:24764–24776. doi: 10.1074/jbc.M113.474023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda T, Goraczniak RM, Sharma RK. Molecular characterization of S100A1-S100B protein in retina and its activation mechanism of bovine photoreceptor guanylate cyclase. Biochemistry. 1996;35:6263–6266. doi: 10.1021/bi960007m. [DOI] [PubMed] [Google Scholar]
- Eberhard DA, Karns LR, VandenBerg SR, Creutz CE. Control of the nuclear-cytoplasmic partitioning of annexin II by a nuclear export signal and by p11 binding. J Cell Sci. 2001;114(Pt 17):3155–3166. doi: 10.1242/jcs.114.17.3155. [DOI] [PubMed] [Google Scholar]
- Erikson E, Tomasiewicz HG, Erikson RL. Biochemical characterization of a 34-kilodalton normal cellular substrate of pp60v-src and an associated 6-kilodalton protein. Mol Cell Biol. 1984;4:77–85. doi: 10.1128/mcb.4.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Krahling S, Smith D, Williamson P, Schlegel RA. Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol Biol Cell. 2004;15:2863–2872. doi: 10.1091/mbc.E03-09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YT, Lin CF, Wang CY, Anderson R, Lin YS. Interferon-gamma stimulates p11-dependent surface expression of annexin A2 in lung epithelial cells to enhance phagocytosis. J Cell Physiol. 2012;227:2775–2787. doi: 10.1002/jcp.23026. [DOI] [PubMed] [Google Scholar]
- Filipek A, Wojda U, Lesniak W. Interaction of calcyclin and its cyanogen-bromide fragments with Annexin-II and Glyceraldehyde-3-Phosphate Dehydrogenase. Int J Biochem Cell Biol. 1995;27:1123–1131. doi: 10.1016/1357-2725(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Filipenko NR, Waisman DM. The C terminus of annexin II mediates binding to F-actin. J Biol Chem. 2001;276:5310–5315. doi: 10.1074/jbc.M009710200. [DOI] [PubMed] [Google Scholar]
- Flood EC, Hajjar KA. The annexin A2 system and vascular homeostasis. Vascul Pharmacol. 2011;54:59–67. doi: 10.1016/j.vph.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004;50:3762–3771. doi: 10.1002/art.20631. [DOI] [PubMed] [Google Scholar]
- Foulkes T, Nassar MA, Lane T, Matthews EA, Baker MD, Gerke V, et al. Deletion of annexin 2 light chain p11 in nociceptors causes deficits in somatosensory coding and pain behavior. J Neurosci. 2006;26:10499–10507. doi: 10.1523/JNEUROSCI.1997-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett SC, Hodgson L, Rybin A, Toutchkine A, Hahn KM, Lawrence DS, et al. A biosensor of S100A4 metastasis factor activation: inhibitor screening and cellular activation dynamics. Biochemistry. 2008;47:986–996. doi: 10.1021/bi7021624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Fuerstenberger G, Mueller-Decker K, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Gerke V, Weber K. Calcium-dependent conformational-changes in the 36-kda subunit of intestinal protein-I related to the cellular 36-kDa target of Rous sarcoma virus tyrosine kinase. J Biol Chem. 1985a;260:1688–1695. [PubMed] [Google Scholar]
- Gerke V, Weber K. The regulatory chain in the p36-kD substrate complex of viral tyrosine-specific protein-kinases is related in sequence to the S-100 protein of glial-cells. EMBO J. 1985b;4:2917–2920. doi: 10.1002/j.1460-2075.1985.tb04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Glenney JR, Jr, Boudreau M, Galyean R, Hunter T, Tack B. Association of the S-100-related calpactin I light chain with the NH2-terminal tail of the 36-kDa heavy chain. J Biol Chem. 1986;261:10485–10488. [PubMed] [Google Scholar]
- Gould KL, Woodgett JR, Isacke CM, Hunter T. The protein-tyrosine kinase substrate p36 is also a substrate for protein kinase C in vitro and in vivo. Mol Cell Biol. 1986;6:2738–2744. doi: 10.1128/mcb.6.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindheim AK, Hollas H, Ramirez J, Saraste J, Trave G, Vedeler A. Effect of serine phosphorylation and ser25 phospho-mimicking mutations on nuclear localisation and ligand interactions of annexin A2. J Mol Biol. 2014;426:2486–2499. doi: 10.1016/j.jmb.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J Biol Chem. 1994;269:21191–21197. [PubMed] [Google Scholar]
- Hajjar KA, Mauri L, Jacovina AT, Zhong F, Mirza UA, Padovan JC, et al. Tissue plasminogen activator binding to the annexin II tail domain. Direct modulation by homocysteine. J Biol Chem. 1998;273:9987–9993. doi: 10.1074/jbc.273.16.9987. [DOI] [PubMed] [Google Scholar]
- Hamberg AP, Korse CM, Bonfrer JMG, de Gast GC. Serum S100B is suitable for prediction and monitoring of response to chemoimmunotherapy in metastatic malignant melanoma. Melanoma Res. 2003;13:45–49. doi: 10.1097/00008390-200302000-00008. [DOI] [PubMed] [Google Scholar]
- Harder T, Gerke V. The annexin II2p11(2) complex is the major protein component of the triton X-100-insoluble low-density fraction prepared from MDCK cells in the presence of Ca2. Biochim Biophys Acta. 1994;1223:375–382. doi: 10.1016/0167-4889(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Rescher U, Gerke V, Moss SE. Annexin-actin interactions. Traffic. 2004;5:571–576. doi: 10.1111/j.1600-0854.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by annexin 2. EMBO J. 2006;25:1816–1826. doi: 10.1038/sj.emboj.7601078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K-L, Deora AB, Xiong H, Ling Q, Weksler BB, Niesvizky R, et al. Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p11. J Biol Chem. 2008;283:19192–19200. doi: 10.1074/jbc.M800100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Deora AB, He KL, Chen K, Sui G, Jacovina AT, et al. Hypoxia-inducible factor-1 drives annexin A2 system-mediated perivascular fibrin clearance in oxygen-induced retinopathy in mice. Blood. 2011;118:2918–2929. doi: 10.1182/blood-2011-03-341214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Laval SH, van Remoortere A, Baudier J, Benaud C, Anderson LVB, et al. AHNAR, a novel component of the dysferlin protein complex, redistributes to the cytoplasm with dysferlin during skeletal muscle regeneration. FASEB J. 2007;21:732–742. doi: 10.1096/fj.06-6628com. [DOI] [PubMed] [Google Scholar]
- Ikebuchi NW, Waisman DM. Calcium-dependent regulation of actin filament bundling by lipocortin-85. J Biol Chem. 1990;265:3392–3400. [PubMed] [Google Scholar]
- Illien F, Finet S, Lambert O, Ayala-Sanmartin J. Different molecular arrangements of the tetrameric annexin 2 modulate the size and dynamics of membrane aggregation. Biochim Biophys Acta. 2010;1798:1790–1796. doi: 10.1016/j.bbamem.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Lauritzen SP, Scheffer L, Sakaguchi M, Bunkenborg J, Simon SM, et al. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nat Commun. 2014;5:3795. doi: 10.1038/ncomms4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N, Vandekerckhove J, Van Damme J, Weber K. Binding sites for calcium, lipid and p11 on p36, the substrate of retroviral tyrosine-specific protein kinases. FEBS Lett. 1986;198:361–364. doi: 10.1016/0014-5793(86)80437-9. [DOI] [PubMed] [Google Scholar]
- Johnsson N, Marriott G, Weber K. p36, the major cytoplasmic substrate of src tyrosine protein-kinase, binds to its p11 regulatory subunit via a short amino-terminal amphiphatic helix. EMBO J. 1988;7:2435–2442. doi: 10.1002/j.1460-2075.1988.tb03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PG, Moore GJ, Waisman DM. A nonapeptide to the putative F-actin binding site of annexin-II tetramer inhibits its calcium-dependent activation of actin filament bundling. J Biol Chem. 1992;267:13993–13997. [PubMed] [Google Scholar]
- Jost M, Gerke V. Mapping of a regulatory important site for protein kinase C phosphorylation in the N-terminal domain of annexin II. Biochim Biophys Acta. 1996;1313:283–289. doi: 10.1016/0167-4889(96)00101-2. [DOI] [PubMed] [Google Scholar]
- Jung Y, Wang J, Song J, Shiozawa Y, Wang J, Havens A, et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110:82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassam G, Le BH, Choi KS, Kang HM, Fitzpatrick SL, Louie P, et al. The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry. 1998;37:16958–16966. doi: 10.1021/bi981713l. [DOI] [PubMed] [Google Scholar]
- Khanna NC, Tokuda M, Chong SM, Waisman DM. Phosphorylation of p36 in vitro by protein kinase C. Biochem Biophys Res Commun. 1986;137:397–403. doi: 10.1016/0006-291x(86)91223-4. [DOI] [PubMed] [Google Scholar]
- Knop M, Aareskjold E, Bode G, Gerke V. Rab3D and annexin A2 play a role in regulated secretion of vWF, but not tPA, from endothelial cells. EMBO J. 2004;23:2982–2992. doi: 10.1038/sj.emboj.7600319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig J, Prenen J, Nilius B, Gerke V. The annexin II-p11 complex is involved in regulated exocytosis in bovine pulmonary artery endothelial cells. J Biol Chem. 1998;273:19679–19684. doi: 10.1074/jbc.273.31.19679. [DOI] [PubMed] [Google Scholar]
- Kube E, Becker T, Weber K, Gerke V. Protein-protein interaction studied by site-directed mutagenesis. Characterization of the annexin II-binding site on p11, a member of the S100 protein family. J Biol Chem. 1992;267:14175–14182. [PubMed] [Google Scholar]
- Kwon M, MacLeod TJ, Zhang Y, Waisman DM. S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors. Front Biosci. 2005;10:300–325. doi: 10.2741/1529. [DOI] [PubMed] [Google Scholar]
- Lambert O, Gerke V, Bader MF, Porte F, Brisson A. Structural analysis of junctions formed between lipid membranes and several annexins by cryo-electron microscopy. J Mol Biol. 1997;272:42–55. doi: 10.1006/jmbi.1997.1183. [DOI] [PubMed] [Google Scholar]
- Lambert O, Cavusoglu N, Gallay J, Vincent M, Rigaud JL, Henry JP, et al. Novel organization and properties of annexin 2-membrane complexes. J Biol Chem. 2004;279:10872–10882. doi: 10.1074/jbc.M313657200. [DOI] [PubMed] [Google Scholar]
- Law AL, Ling Q, Hajjar KA, Futter CE, Greenwood J, Adamson P, et al. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol Biol Cell. 2009;20:3896–3904. doi: 10.1091/mbc.E08-12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DB, Jamgotchian N, Allen SG, Kan FW, Hale IL. Annexin A2 heterotetramer: role in tight junction assembly. Am J Physiol Renal Physiol. 2004;287:F481–F491. doi: 10.1152/ajprenal.00175.2003. [DOI] [PubMed] [Google Scholar]
- Lee DB, Jamgotchian N, Allen SG, Abeles MB, Ward HJ. A lipid-protein hybrid model for tight junction. Am J Physiol Renal Physiol. 2008;295:F1601–F1612. doi: 10.1152/ajprenal.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler J, Herbert AP, Norstrom E, Schmidt CQ, Barlow PN, Blom AM, et al. Annexin-II, DNA, and histones serve as factor H ligands on the surface of apoptotic cells. J Biol Chem. 2010;285:3766–3776. doi: 10.1074/jbc.M109.045427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Reddy TRK, Fischer PM, Dekker LV. A Cy5-labelled S100A10 tracer used to identify inhibitors of the protein interaction with annexin A2. Assay Drug Dev Technol. 2010;8:85–95. doi: 10.1089/adt.2009.0218. [DOI] [PubMed] [Google Scholar]
- Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, et al. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J Clin Invest. 2004;113:38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Liu J, Rothermund Christy A, Ayala-Sanmartin J, Vishwanatha Jamboor K. Nuclear annexin II negatively regulates growth of LNCaP cells and substitution of ser 11 and 25 to glu prevents nucleo-cytoplasmic shuttling of annexin II. BMC Biochem. 2003;4:10. doi: 10.1186/1471-2091-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Hajjar KA. Annexin A2 system in human biology: cell surface and beyond. Semin Thromb Hemost. 2013;39:338–346. doi: 10.1055/s-0033-1334143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod TJ, Kwon M, Filipenko NR, Waisman DM. Phospholipid-associated annexin A2-S100A10 heterotetramer and its subunits: characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin. J Biol Chem. 2003;278:25577–25584. doi: 10.1074/jbc.M301017200. [DOI] [PubMed] [Google Scholar]
- Madureira PA, Surette AP, Phipps KD, Taboski MA, Miller VA, Waisman DM. The role of the annexin A2 heterotetramer in vascular fibrinolysis. Blood. 2011;118:4789–4797. doi: 10.1182/blood-2011-06-334672. [DOI] [PubMed] [Google Scholar]
- Madureira PA, O'Connell PA, Surette AP, Miller VA, Waisman DM. The biochemistry and regulation of S100A10: a multifunctional plasminogen receptor involved in oncogenesis. J Biomed Biotechnol. 2012;2012:353687. doi: 10.1155/2012/353687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malashkevich VN, Varney KM, Garrett SC, Wilder PT, Knight D, Charpentier TH, et al. Structure of Ca2+-bound S100A4 and its interaction with peptides derived from nonmuscle myosin-IIA. Biochemistry. 2008;47:5111–5126. doi: 10.1021/bi702537s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malashkevich VN, Dulyaninova NG, Ramagopal UA, Liriano MA, Varney KM, Knight D, et al. Phenothiazines inhibit S100A4 function by inducing protein oligomerization. Proc Natl Acad Sci U S A. 2010;107:8605–8610. doi: 10.1073/pnas.0913660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Leffler J, Blom AM. Annexin A2 and A5 serve as new ligands for C1q on apoptotic cells. J Biol Chem. 2012;287:33733–33744. doi: 10.1074/jbc.M112.341339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke M, Ross M, Gerke V, Steinem C. The molecular arrangement of membrane-bound annexin A2-S100A10 tetramer as revealed by scanning force microscopy. Chembiochem. 2004;5:1003–1006. doi: 10.1002/cbic.200400004. [DOI] [PubMed] [Google Scholar]
- Monastyrskaya K, Babiychuk EB, Hostettler A, Rescher U, Draeger A. Annexins as intracellular calcium sensors. Cell Calcium. 2007;41:207–219. doi: 10.1016/j.ceca.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Monastyrskaya K, Tschumi F, Babiychuk EB, Stroka D, Draeger A. Annexins sense changes in intracellular pH during hypoxia. Biochem J. 2008;409:65–75. doi: 10.1042/BJ20071116. [DOI] [PubMed] [Google Scholar]
- Munz B, Gerke V, Gillitzer R, Werner S. Differential expression of the calpactin I subunits annexin II and p11 in cultured keratinocytes and during wound repair. J Invest Dermatol. 1997;108:307–312. doi: 10.1111/1523-1747.ep12286470. [DOI] [PubMed] [Google Scholar]
- Myrvang HK, Guo X, Li C, Dekker LV. Protein interactions between surface annexin A2 and S100A10 mediate adhesion of breast cancer cells to microvascular endothelial cells. FEBS Lett. 2013;587:3210–3215. doi: 10.1016/j.febslet.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Nazmi AR, Ozorowski G, Pejic M, Whitelegge JP, Gerke V, Luecke H. N-terminal acetylation of annexin A2 is required for S100A10 binding. Biol Chem. 2012;393:1141–1150. doi: 10.1515/hsz-2012-0179. [DOI] [PubMed] [Google Scholar]
- Nedjadi T, Kitteringham N, Campbell F, Jenkins RE, Park BK, Navarro P, et al. S100A6 binds to annexin 2 in pancreatic cancer cells and promotes pancreatic cancer cell motility. Br J Cancer. 2009;101:1145–1154. doi: 10.1038/sj.bjc.6605289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Gerke V, Prenen J, Szucs G, Heinke S, Weber K, et al. Annexin II modulates volume-activated chloride currents in vascular endothelial cells. J Biol Chem. 1996;271:30631–30636. doi: 10.1074/jbc.271.48.30631. [DOI] [PubMed] [Google Scholar]
- O'Connell PA, Surette AP, Liwski RS, Svenningsson P, Waisman DM. S100A10 regulates plasminogen-dependent macrophage invasion. Blood. 2010;116:1136–1146. doi: 10.1182/blood-2010-01-264754. [DOI] [PubMed] [Google Scholar]
- Odink K, Cerletti N, Bruggen J, Clerc RG, Tarcsay L, Zwadlo G, et al. 2 calcium-binding proteins in infiltrate macrophages of rheumatoid-arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- Oh Y-S, Gao P, Lee K-W, Ceglia I, Seo J-S, Zhang X, et al. SMARCA3, a chromatin-remodeling factor, is required for p11-dependent antidepressant action. Cell. 2013;152:831–843. doi: 10.1016/j.cell.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozorowski G, Ryan CM, Whitelegge JP, Luecke H. Withaferin A binds covalently to the N-terminal domain of annexin A2. Biol Chem. 2012;393:1151–1163. doi: 10.1515/hsz-2012-0184. [DOI] [PubMed] [Google Scholar]
- Ozorowski G, Milton S, Luecke H. Structure of a C-terminal AHNAK peptide in a 1:2:2 complex with S100A10 and an acetylated N-terminal peptide of annexin A2. Acta Crystallogr D Biol Crystallogr. 2013;69:92–104. doi: 10.1107/S0907444912043429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps KD, Surette AP, O'Connell PA, Waisman DM. Plasminogen receptor S100A10 is essential for the migration of tumor-promoting macrophages into tumor sites. Cancer Res. 2011;71:6676–6683. doi: 10.1158/0008-5472.CAN-11-1748. [DOI] [PubMed] [Google Scholar]
- Powell MA, Glenney JR. Regulation of calpactin I phospholipid binding by calpactin I light-chain binding and phosphorylation by p60v-src. Biochem J. 1987;247:321–328. doi: 10.1042/bj2470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisieux A, Ji J, Ozturk M. Annexin II up-regulates cellular levels of p11 protein by a post-translational mechanism. Biochem J. 1996;313:51–55. doi: 10.1042/bj3130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy TR, Li C, Guo X, Fischer PM, Dekker LV. Design, synthesis and SAR exploration of tri-substituted 1,2,4-triazoles as inhibitors of the annexin A2-S100A10 protein interaction. Bioorg Med Chem. 2014;22:5378–5391. doi: 10.1016/j.bmc.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy TRK, Li C, Guo X, Myrvang HK, Fischer PM, Dekker LV. Design, synthesis, and structure-activity relationship exploration of 1-substituted 4-aroyl-3-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one analogues as inhibitors of the annexin A2-S100A10 protein interaction. J Med Chem. 2011;54:2080–2094. doi: 10.1021/jm101212e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy TRK, Li C, Fischer PM, Dekker LV. Three-dimensional pharmacophore design and biochemical screening identifies substituted 1,2,4-triazoles as inhibitors of the annexin A2-S100A10 protein interaction. ChemMedChem. 2012;7:1435–1446. doi: 10.1002/cmdc.201200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnouf F, Rendon A, Pradel LA. Biochemical characterization of annexins I and II isolated from pig nervous tissue. J Neurochem. 1991;56:1985–1996. doi: 10.1111/j.1471-4159.1991.tb03457.x. [DOI] [PubMed] [Google Scholar]
- Regnouf F, Sagot I, Delouche B, Devilliers G, Cartaud J, Henry JP, et al. In vitro' phosphorylation of annexin 2 heterotetramer by protein kinase C. Comparative properties of the unphosphorylated and phosphorylated annexin 2 on the aggregation and fusion of chromaffin granule membranes. J Biol Chem. 1995;270:27143–27150. doi: 10.1074/jbc.270.45.27143. [DOI] [PubMed] [Google Scholar]
- Rescher U, Gerke V. Annexins – unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- Rety S, Sopkova J, Renouard M, Osterloh D, Gerke V, Tabaries S, et al. The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat Struct Biol. 1999;6:89–95. doi: 10.1038/4965. [DOI] [PubMed] [Google Scholar]
- Rezvanpour A, Santamaria-Kisiel L, Shaw GS. The S100A10-annexin A2 complex provides a novel asymmetric platform for membrane repair. J Biol Chem. 2011;286:40174–40183. doi: 10.1074/jbc.M111.244038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintala-Dempsey AC, Santamaria-Kisiel L, Liao Y, Lajoie G, Shaw GS. Insights into S100 target specificity examined by a new interaction between S100A11 and annexin A2. Biochemistry. 2006;45:14695–14705. doi: 10.1021/bi061754e. [DOI] [PubMed] [Google Scholar]
- Schlagenhauff B, Schittek B, Ellwanger U, Stroebel W, Blum A, Schwarz M, et al. Significance of serum protein S100 levels in screening for melanoma metastasis: does protein S100 enable early detection of melanoma recurrence? Melanoma Res. 2000;10:451–459. doi: 10.1097/00008390-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Semov A, Moreno MJ, Onichtchenko A, Abulrob A, Ball M, Ekiel I, et al. Metastasis-associated protein S100A4 induces angiogenesis through interaction with annexin II and accelerated plasmin formation. J Biol Chem. 2005;280:20833–20841. doi: 10.1074/jbc.M412653200. [DOI] [PubMed] [Google Scholar]
- Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14:5318–5324. doi: 10.1158/1078-0432.CCR-07-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, et al. Annexin II/Annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105:370–380. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E, Cohen FE, Bishop JM. A human gene (AHNAK) encoding an unusually large protein with a 1.2-mu-m polyionic rod structure. Proc Natl Acad Sci U S A. 1992;89:5472–5476. doi: 10.1073/pnas.89.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopkova-de Oliveira Santos J, Oling FK, Rety S, Brisson A, Smith JC, Lewit-Bentley A. S100 protein-annexin interactions: a model of the (Anx2-p11) (2) heterotetramer complex. Biochim Biophys Acta. 2000;1498:181–191. doi: 10.1016/s0167-4889(00)00095-1. [DOI] [PubMed] [Google Scholar]
- Streicher WW, Lopez MM, Makhatadze GI. Annexin I and annexin II N-terminal peptides binding to S100 protein family members: specificity and thermodynamic characterization. Biochemistry. 2009;48:2788–2798. doi: 10.1021/bi8019959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette AP, Madureira PA, Phipps KD, Miller VA, Svenningsson P, Waisman DM. Regulation of fibrinolysis by S100A10 in vivo. Blood. 2011;118:3172–3181. doi: 10.1182/blood-2011-05-353482. [DOI] [PubMed] [Google Scholar]
- Tarabykina S, Kriajevska M, Scott DJ, Hill TJ, Lafitte D, Derrick PJ, et al. Heterocomplex formation between metastasis-related protein S100A4 (Mts1) and S100A1 as revealed by the yeast two-hybrid system. FEBS Lett. 2000;475:187–191. doi: 10.1016/s0014-5793(00)01652-5. [DOI] [PubMed] [Google Scholar]
- Thiel C, Osborn M, Gerke V. The tight association of the tyrosine kinase substrate annexin II with the submembranous cytoskeleton depends on intact p11- and Ca(2+)-binding sites. J Cell Sci. 1992;103(Pt 3):733–742. doi: 10.1242/jcs.103.3.733. [DOI] [PubMed] [Google Scholar]
- Tsuna M, Kageyama S-I, Fukuoka J, Kitano H, Doki Y, Tezuka H, et al. Significance of S100A4 as a prognostic marker of lung squamous cell carcinoma. Anticancer Res. 2009;29:2547–2554. [PubMed] [Google Scholar]
- Umbrecht-Jenck E, Demais V, Calco V, Bailly Y, Bader MF, Chasserot-Golaz S. S100A10-mediated translocation of annexin-A2 to SNARE proteins in adrenergic chromaffin cells undergoing exocytosis. Traffic. 2010;11:958–971. doi: 10.1111/j.1600-0854.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- Vimalachandran D, Greenhalf W, Thompson C, Luttges J, Prime W, Campbell F, et al. High nuclear S100A6 (Calcyclin) is significantly associated with poor survival in pancreatic cancer patients. Cancer Res. 2005;65:3218–3225. doi: 10.1158/0008-5472.CAN-04-4311. [DOI] [PubMed] [Google Scholar]
- Waisman DM. Annexin II tetramer: structure and function. Mol Cell Biochem. 1995;149–150:301–322. doi: 10.1007/BF01076592. [DOI] [PubMed] [Google Scholar]
- Wang GZ, Zhang S, Fernig DG, Spiller D, Martin-Fernandez M, Zhang HM, et al. Heterodimeric interaction and interfaces of S100A1 and S100P. Biochem J. 2004;382:375–383. doi: 10.1042/BJ20040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4 – role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–2911. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- Zeng FY, Gerke V, Gabius HJ. Identification of Annexin-II, Annexin-VI and Glyceraldehyde-3-Phosphate Dehydrogenase as Calcyclin-binding proteins in bovine heart. Int J Biochem. 1993;25:1019–1027. doi: 10.1016/0020-711x(93)90116-v. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Wietgrefe SW, Li QS, Shore MD, Duan LJ, Reilly C, et al. Roles of substrate availability and infection of restina and activated CD4(+) T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 2004;101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobiack N, Gerke V, Rescher U. Complex formation and submembranous localization of annexin 2 and S100A10 in live HepG2 cells. FEBS Lett. 2001;500:137–140. doi: 10.1016/s0014-5793(01)02604-7. [DOI] [PubMed] [Google Scholar]


