Abstract
Background and Purpose
The urinary bladder urothelium expresses various receptors and in response to chemical and mechanical stimuli releases mediators, thereby modulating bladder sensory pathways. Transient receptor potential vanilloid 1 (TRPV1) ion channels and nerve growth factor (NGF) in those cells are implicated in this modulatory effect and play a role in sensitizing pain-related afferent pathways during inflammation. In this study, we investigated the interaction between NGF and TRPV1 channels in urothelial cells.
Experimental Approach
Urothelial cells from female Sprague-Dawley rat bladders were cultured to quantify membrane expression of TRPV1 channels and capsaicin-induced ATP release in the presence of NGF alone or with TrKA or PI3K inhibitors. Pain scores from rats with cyclophosphamide (CYP)-induced bladder inflammation were assessed after treatment with a TrkA antagonist. Bladders (from control and CYP rats) were collected and analysed for NGF content and TRPV1 channel expression.
Key Results
Cultured cells responded to NGF with increased TRPV1 channel expression in the cell membrane and increased release of ATP. Both responses were blocked by either a TrkA antagonist or a PI3K inhibitor. Treatment in vivo with the TrkA antagonist alleviated pain symptoms and reduced CYP-induced NGF overexpression in the mucosa. Furthermore, in urothelial cells from animals with bladder inflammation, expression of TRPV1 channels in the membrane was significantly increased.
Conclusions and Implications
During bladder inflammation, increased production of NGF in urothelial cells induced increased expression and activity of TRPV1 channels in the cell membrane. This effect was primarily mediated by the PI3K pathway.
Tables of Links
| LIGANDS |
|---|
| Capsaicin |
| Chelerythrine chloride |
| GW441756 |
| NGF |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b,).
Introduction
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a debilitating condition in which the intense suprapubic and pelvic pain reported by patients significantly impairs their quality of life (Parsons, 2004). The aetiology is still unclear and the syndrome is difficult to manage since no effective long-term treatment has been found (Bjorling et al., 2011).
Several animal models that mimic symptoms of BPS/IC have been developed. The most commonly used include chemically induced cystitis, in which animals show marked signs of nociception and vesical inflammation that alters the normal bladder function. Those modifications occur following alteration of the properties of bladder nerve fibres including changes in ion channel expression (Nazif et al., 2007). Sensory neurons that innervate the inflamed tissue become more excitable resulting in the phenomena of hyperalgesia and allodynia (Yoshimura and de Groat, 1999; Nazif et al., 2007). More recently, considerable interest has been directed to the role of the bladder epithelium (urothelium) in the modulation of afferent signals during inflammatory conditions. Mounting evidence supports a sensory function for this specialized epithelium as urothelial cells are now recognized to express a variety of sensory receptors, allowing them to respond in an autocrine fashion to the neurotransmitters and growth factors they produce and those released by other cells present in the lamina propria (Birder, 2010). In addition, the growth factors and neurotransmitters produced and released by urothelial cells also act on underlying nerve fibres coursing the bladder wall, influencing bladder reflex activity (Birder, 2010).
An example of an important trophic factor produced by urothelial cells is the nerve growth factor (NGF). This neurotrophin is also produced by bladder detrusor smooth muscle cells and its expression levels were already shown to be elevated during BPS/IC (Lowe et al., 1997; Pinto et al., 2010). NGF acts by binding in part to its high-affinity receptor tyrosine kinase A (TrkA), a cell surface transmembrane protein expressed in urothelial cells and primary afferents. Activation of TrkA by NGF leads to dimerization of the receptor and phosphorylation of different residues that promote the activation of several intracellular signalling cascades (Allen and Dawbarn, 2006; Steers and Tuttle, 2006), including the ERK and the PI3K/Akt pathways (Obata and Noguchi, 2004). Recent studies performed in adult sensory neurons showed that the activation of these pathways regulates neuronal responses with important roles in pain-signalling systems (Pezet and McMahon, 2006).
The transient receptor potential vanilloid 1 (TRPV1) ion channel is a pivotal component of sensory pathways. This protein constitutes a non-selective cationic channel that is activated by several nociceptive chemical and physical stimuli such as noxious heat and vanilloid compounds including capsaicin (Caterina et al., 1997). In the urinary bladder, TRPV1 channels are expressed in primary afferents and urothelial cells (Birder et al., 2001; Avelino et al., 2002). Its role on bladder pain perception is already recognized since the activation of the receptor induces the development of hyperreflexia and pain associated with cystitis (Charrua et al., 2007). A relationship between NGF and TRPV1 channels has recently been established. In vivo, bladder inflammation, together with bladder hyperactivity, is accompanied by increased expression of NGF and TRPV1 channels (Liu et al., 2009; 2014,; Dornelles et al., 2014), suggesting a link between both systems. Accordingly, recent studies using TRPV1 knockout (KO) mice revealed that these animals were unable to develop bladder overactivity induced by chronic administration of NGF (Frias et al., 2012). Using in vitro approaches, other studies have established the molecular mechanisms suggesting the interaction of NGF and TRPV1 channels. In sensory neurons, binding of NGF to TrkA activates a PI3K pathway, promoting TRPV1 channel trafficking to the membrane (Stein et al., 2006). The channel is inserted into the membrane via regulated exocytosis and an increased sensitivity to capsaicin was observed in the presence of NGF (Zhang et al., 2005). PKC activation also promotes a rapid vesicular transport of TRPV1 channels to the membrane (Morenilla-Palao et al., 2004). However, the modulation of TRPV1 channels by NGF on non-neuronal cells such as the urothelium has not been demonstrated.
The present study addressed this issue by examining the effect of NGF on primary cultures of rat urinary bladder urothelial cells, with particular attention to the location and sensitivity of TRPV1 channels as well as the signalling pathways involved. In addition, an animal model of bladder hypersensitivity induced by cyclophosphamide (CYP) was used to analyse the in vivo role of the NGF receptor in terms of nociceptive behaviour. Overall, the present study shows the effect of NGF on the surface expression of TRPV1 channels of urothelial cells and stresses the importance of the TrkA receptor and downstream pathways that may play a role in mediating painful symptoms of BPS/IC.
Methods
Animals
All animal care and experimental procedures complied with the American Physiological Society's Guide for the care and use of laboratory animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. A total of 66 animals from a commercial vendor were used in the experiments described here. Experiments were performed with female Sprague-Dawley rats (250–300 g), maintained under 12 h light/dark cycles with free access to food and water.
Cell culture
Animals were killed (100% CO2) and urinary bladders were excised and placed in minimal essential medium (MEM) (Invitrogen, Grand Island, NY, USA) containing penicillin/streptomycin/fungizone (1%; Invitrogen) and HEPES (Invitrogen). The bladders were cut open, gently stretched with the epithelial side up, pinned in a Sylgard-coated dish and incubated overnight at 4°C in MEM with dispase 2 mg·mL−1 (Invitrogen). On the following day, the epithelium was gently scraped from the underlying tissue, placed in a culture flask and treated with 0.25% trypsin to dissociate urothelial cells. Following dissociation, the cell suspension was placed in MEM containing FBS and centrifuged at 416× g for 15 min. The cells were resuspended in CNT-16 (CellnTEC, Bern, Switzerland), plated and incubated at 37°C with 5% CO2 and used within 24–48 h.
Cell surface biotinylation
Cells from normal (n = 10) or CYP-inflamed (n = 18, see below) rats were cultured as described above and plated on 24-well dish plates at a concentration of 2 × 105 cells/mL. Cell surface proteins (TRPV1 channels) were biotinylated with Sulfo-NHS-SS-Biotin using Pierce Cell Surface Protein Isolation Kit (Thermo Scientific, Rockford, IL, USA). Different experimental conditions were tested by incubating cells with NGF (100 ng·mL−1) for 15 min or NGF in the presence of a TrkA antagonist (GW441756, 500 nM, n = 4) (Zhang et al., 2014; Jung and Kim, 2008), a PI3K inhibitor (PP121, 5 μM, n = 4) (Apsel et al., 2008) or a PKC inhibitor (chelerythrine chloride, CC, 1 μM, n = 4) (Herbert et al., 1990; Ayar et al., 2014) during 30 min at 37°C before further experimentation. Whole cell lysates and membrane proteins (eluted with SDS-PAGE buffer) were isolated and subjected to Western blotting using a standard protocol. Levels of TRPV1 channels were measured by densitometry and normalized with α-tubulin.
ATP release
Urothelial cells were cultured as described above and plated on 35 mm collagen-coated culture dishes in a concentration of 2 × 105 cells mL−1. Cells were superfused with HBSS buffer (5 mM KCl, 0.3 mM KH2PO4, 138 mM NaCl, 4 mM NaHCO3, 0.3 mM NaH2PO4, 5.6 mM glucose, 10 mM HEPES, 2 mM CaCl2, 1 mM MgCl2; pH 7.4) using a peristaltic pump (flow rate 0.4 mL·min−1) and 100 μL of perfusate collected every 60 s. Samples were taken for 15 min before perfusion with the TRPV1 agonist capsaicin (0.5 μM, n = 11), for 20 min during capsaicin perfusion and for 15 min during washout with HBSS. To analyse the influence of NGF in the capsaicin-induced ATP release, responses of control cells were compared with cells pretreated with NGF (100 ng·mL−1, n = 11) for 15 min at 37°C prior to further experimentation. In addition, responses were also measured after pretreatment of cells with the TrkA antagonist (GW441756, 500 nM, n = 9), the PI3K inhibitor (PP121, 5 μM, n = 9) or the TRPV1 antagonist capsazepine (50 μM, n = 8). ATP levels were quantified immediately after sample collection using a luciferin-luciferase reagent and bioluminescence measured using a luminometer. The blank readings (HBSS only) were subtracted from the luminescence readings of each sample and the standard curve was plotted using GraphPad Prism software (La Jolla, CA, USA). ATP released from each culture dish was calculated with reference to the standard curve and expressed in AUC units.
CYP-induced cystitis/TrkA antagonist administration
Acute bladder inflammation was induced by an i.p. injection of 150 mg·kg−1 of CYP. Control animals received an i.p. injection of vehicle (saline). CYP is metabolized in the liver to acrolein, an irritant compound that is excreted in the urine, eliciting an extreme bladder irritation. Animals were killed 24 or 48 h after CYP injection. The TrkA antagonist GW441756 (IC50 = 2 nM) or its vehicle was injected at a dose of 0.5 mg per rat, i.p., 24 h prior to CYP injection and every day for 48 h. GW441756 is a potent and selective inhibitor of the TrkA receptor and its selectivity, cell viability studies and safety for in vivo use have been described earlier (Zhang et al., 2014).
Tissue preparation for elisa
Rat (control, CYP and CYP + GW441756 treated) bladders (n = 27) were harvested and collected in HBSS medium (5 mM KCl, 0.3 mM KH2PO4, 138 mM NaCl, 4 mM NaHCO3, 0.3 mM NaH2PO4, 5.6 mM glucose, 10 mM HEPES; pH 7.4). Bladders were longitudinally opened and mucosa was stripped and collected into 300 μL of membrane lysis buffer (0.3 M NaCl, 50 mM Tris-HCl and 0.5% Triton X-100) containing complete protease inhibitor cocktail (1 tablet/10 mL; Roche, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (Sigma Aldrich, St. Louis, MO, USA; 1:100). In this procedure, the term ‘mucosa’ is used instead of urothelium because the presence of some scarce suburothelial nerve terminals in the collected tissue cannot be excluded. Tissue was homogenized at 6 ms for 30 s in a Fast-Prep homogenizer (MP Biomedical, Santa Ana, CA, USA) and centrifuged at 14 000× g for 15 min at 4°C. The supernatant was collected and the pellet was resuspended in 150 μL of membrane lysis buffer. The suspensions were incubated on ice, homogenized and centrifuged again as previously. Supernatants from each centrifugation were collected and combined. Protein concentration was determined using the Pierce BCA protein assay (Thermo Scientific). NGF levels were determined in the mucosa from control versus CYP-treated animals (24 and 48 h, n = 6 per group) and compared with NGF levels in mucosa from animals pretreated with TrkA antagonist both alone and also prior to CYP (GW441756 + CYP 24 h and GW441756 + CYP 48 h, n = 3 per group).
NGF elisa
Mucosa lysates were diluted with 4 volumes of Dulbecco's PBS (2.68 mM KCl, 137 mM NaCl, 1.47 mM KH2PO4, 8.1 mM Na2HPO4, 0.9 mM CaCl2 × 2H2O, 0.5 mM MgCl2 × 6H2O; pH 7.35). Samples were treated with 1N HCl to pH 2.0–3.0 for 15 min at room temperature and neutralized with 1N NaOH to pH 7.5–8.0. Samples were then assayed in duplicate by elisa according to the manufacturer's instructions. Plates were read at 450 nm using a SpectraFluor Plus (Tecan, Maennedorf, Switzerland) and the absorbance of the blank value (the mean of the assay wells without sample) was subtracted from each sample's absorbance. NGF tissue values were normalized against the protein concentrations of each sample and expressed as picograms per microgram of protein.
Behaviour tests
Nociceptive behaviour
Decreased breathing rate, eye closure and immobile posture with rounded back were considered as signs of pain based on the study of Saitoh et al. (2010). The behaviour (n = 3 each group) was observed for 10 min intervals during a 60 min period in control (saline injected), CYP and CYP + GW441756 treated animals 24 and 48 h after CYP injection. The absence or presence of ‘nociceptive behaviour’ was examined and scored as one positive event of ‘nociceptive behaviour’ when the immobility associated with reduced breathing frequency occurred during each interval. The number of positive events of nociceptive behaviour was then counted in each rat for the 60 min total observation period. Observations were made without knowledge of the treatments.
Mechanical hyperalgesia
All animals were handled daily prior to behaviour testing to avoid stress and fear-motivated behaviours. To assess mechanical sensitivity, rats were placed in individual observation chambers atop a metal mesh floor and allowed to acclimatize for 15 min. Cutaneous sensitivity was assessed in the lower abdomen and in the hindpaw using the von Frey monofilaments (rated at 2, 4, 6, 8, 15, 26, 60 and 100 g). Filaments were applied for 5 s perpendicularly to the plantar surface and tested five times with an interval of 30 s between filaments. A response was considered positive when the animal reacted to the filament (paw withdraw or licking) in at least three of five filament applications. The test was performed at day 0, before any procedure, and 24 and 48 h after CYP administration or its vehicle. Observations were made without knowledge of the treatments.
Data analysis
Data were analysed using one-way anova followed by Newman–Keuls/Dunnett's multiple comparison test or two-way anova followed by Tukey's multiple comparison test. Statistical significance was considered with P < 0.05.
Materials
CYP (Endoxan) was purchased from Baxter Oncology, Halle, Germany. The TrkA antagonist (GW441756), the PI3K inhibitor (PP121) and the PKC inhibitor CC were purchased from Tocris Bioscience (Bristol, UK). elisa kit for NGF was obtained from Promega (Madison, WI, USA). Capsaicin, capsazepine and the ATP luminescence kit were obtained from Sigma Aldrich.
Results
NGF increases expression of TRPV1 channels in the cell membrane of urothelial cells
Exogenous NGF was applied to cultures of primary urothelial cells for 15 min. After the incubation period, the expression levels of the TRPV1 channel on the cell surface were examined using biotinylation of receptor membrane. Incubation with NGF induced a 242% increase of the TRPV1 expression within the cell membrane fraction as compared with control (Figure 1). In order to examine whether this effect may be due to NGF receptor signalling, we used an antagonist of the TrkA receptor (GW441756). Urothelial cells previously incubated with the TrkA antagonist, in the presence of NGF, showed levels of TRPV1 channels similar to those in non-treated cells (25% change), indicating that the membrane expression of TRPV1 channels is dependent on NGF binding to TrkA. Therefore, we investigated the TrkA-mediated downstream signalling pathways that may be regulating TRPV1 channel expression. Inhibition of the PI3K and PKC pathway (using PP121 and CC, respectively) was also tested in these cells in the presence of NGF. Inhibition of either PI3K or PKC induced a 79 and 93% increase of TRPV1 channel expression that was not significantly different from that observed in non-treated cells. Hence, either inhibitor prevented the effects of NGF on the translocation of TRPV1 channels to the urothelial cell membrane (Figure 1).
Figure 1.
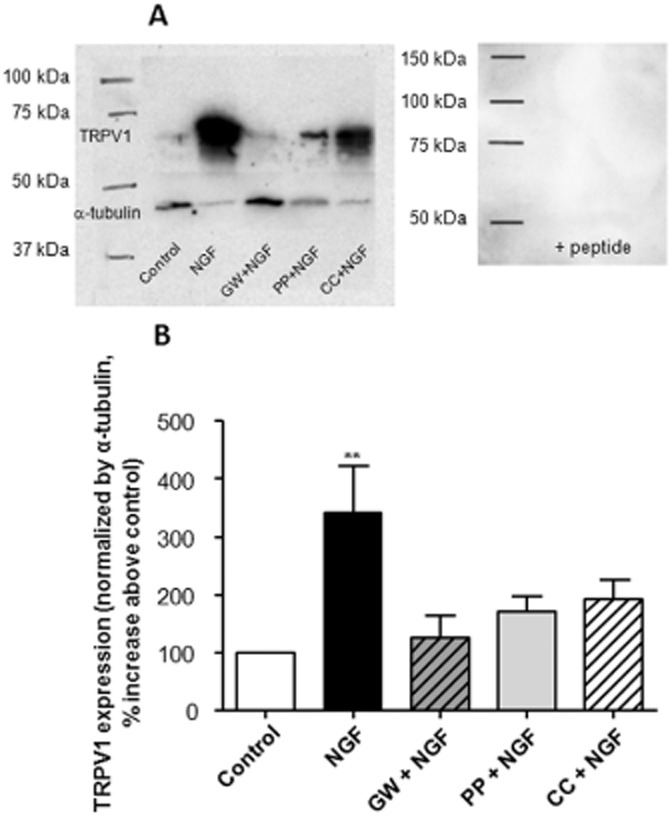
Expression of urothelial membrane TRPV1 channels. The expression of TRPV1channels was quantified by cell surface biotinylation after isolation of the membrane fraction of urothelial cells. (A) Western blot analysis of TRPV1 channels in membrane extracts under different conditions (left) and after pre-incubation of the TRPV1 antibody with the blocking peptide (right). (B) Graph represents the quantification of the expression of TRPV1 channels after normalization with α-tubulin. The presence of NGF in the cell culture increased expression of TRPV1 channels (n = 10). The presence of GW441756 (GW; TrkA antagonist) or inhibitors of PI3K (PP) or PKC (CC) (n = 4) counteracted the NGF effect showing levels of TRPV1 channels similar to the control. **P < 0.05, significantly different from control; one-way anova followed by Newman–Keuls multiple comparison test.
NGF increases the capsaicin-induced release of ATP from urothelial cells
The incubation of urothelial cells with capsaicin, the pungent compound that activates TRPV1 channels, induced the release of ATP (8.7 ± 1.3 AUC). This capsaicin-induced ATP release was increased to 16.6 ± 2.9 AUC when urothelial cells were previously incubated with NGF (Figure 2). As in the previous experiments, to demonstrate whether NGF acts via TrkA receptor-activated pathways, cells were pre-incubated with GW441756 or with PP121. These agents prevented the NGF-induced increases in ATP release by capsaicin, with 73 and 81% reductions (4.9 ± 0.9 and 3.5 ± 0.7 AUC, respectively) when compared with responses to capsaicin in cells treated with NGF alone. As a control, the ATP release was also measured in the presence of capsazepine, a non-specific TRPV1 channel antagonist with a higher specificity for this receptor. Under these conditions, there was no ATP release, indicating that it occurs via TRPV1 channels.
Figure 2.
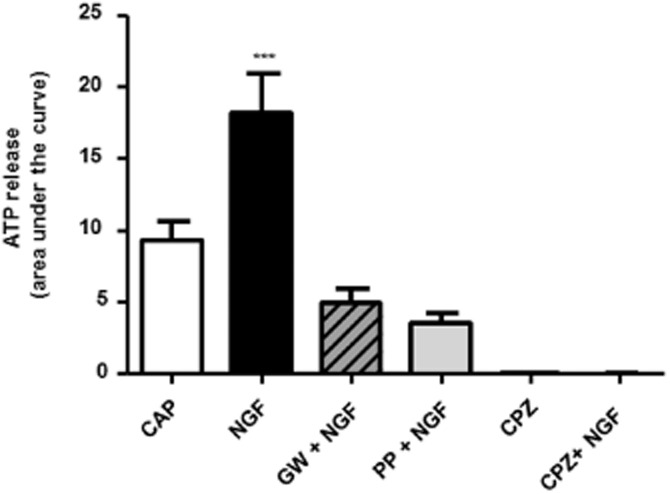
ATP release induced by capsaicin. Urothelial cells release ATP in the presence of capsaicin (CAP; 0.5 mM, n = 11). Treatment with NGF elicited a larger release of ATP with capsaicin (n = 9). The incubation of urothelial cells with the TrkA antagonist (GW; n = 9) or a PI3K inhibitor (PP, n = 9) in the presence of NGF blocked its effect and the ATP remained at control or baseline levels. The TRPV1 channel antagonist capsazepine (CPZ) completely blocked the release of ATP from these cells. ***P < 0.0001, significantly different from capsaicin alone; one-way anova followed by Newman–Keuls multiple comparison test.
Systemic administration of the TrkA antagonist improves pain behaviour induced by bladder inflammation
Bladder inflammation was induced by administration of CYP. These animals showed a freezing behaviour characterized by rounded back, immobile posture and closed eyes that have been already described by other groups (Boucher et al., 2000; Auge et al., 2013). This altered behaviour was observed as early as 4 h after CYP administration and was significantly higher 24 and 48 h later when compared with saline-injected animals (Figure 3). In addition, inflamed bladders analysed at the end of the experiment showed clear signs of inflammation such as oedema and petechial haemorrhage on the bladder surface (Boucher et al., 2000).
Figure 3.
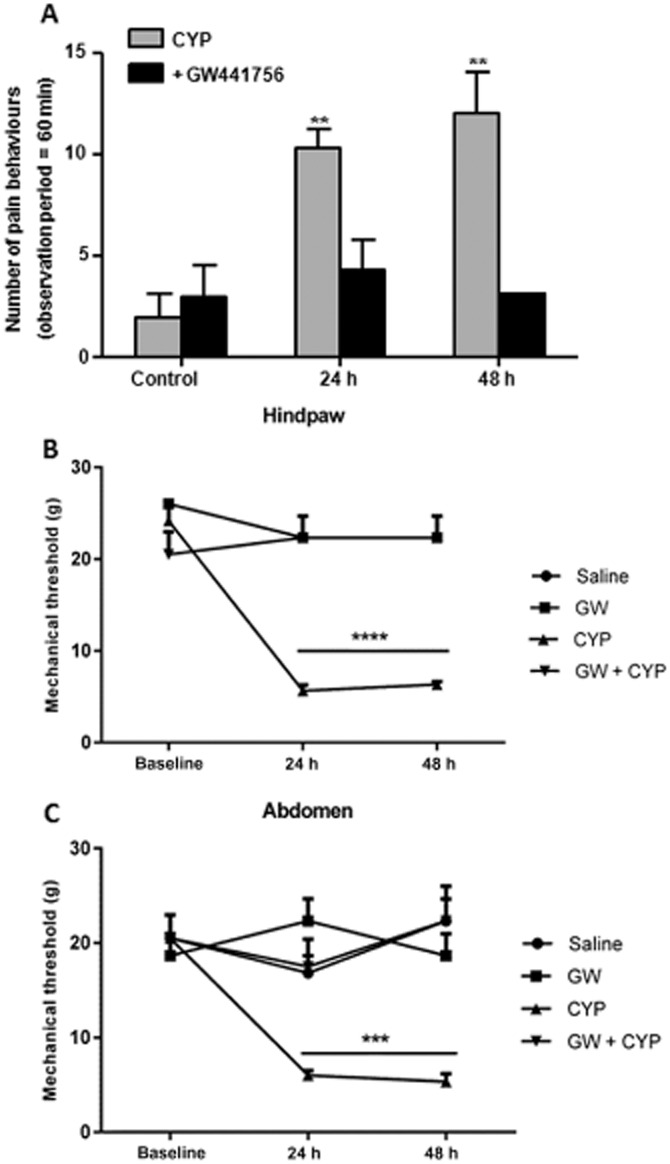
Behaviour of CYP-treated rats after treatment with a TrkA antagonist. (A) Pain behaviour observed measured at 10 min intervals over a period of 60 min. A nociceptive event was considered positive if animals showed decreased breathing rate associated with immobile posture. (B–C) von Frey test was used to evaluate the mechanical sensitivity to the von Frey monofilaments in the abdomen (B) and in the hindpaw (C). Animals with bladder inflammation have a significant increase of the pain behaviour as well as cutaneous sensitivity on the abdomen and hindpaws 24 and 48 h after CYP injection when compared with saline-injected animals (A, B and C respectively). Those with bladder inflammation that were previously treated with the TrkA antagonist GW441756 showed pain behaviours similar to non-inflamed animals (A). Accordingly, these inflamed animals also experienced an improvement of the mechanical hypersensitivity in the hindpaws (B) and abdomen (C) when previously treated with GW441756. **P < 0.05; significantly different from control; one-way anova followed by Newman–Keuls multiple comparison test. ***P < 0.05, significantly different from control, one-way anova.
The systemic administration of GW441756 by itself did not induce any different behaviour on control animals. However, in CYP-inflamed animals that were treated with GW441756, the CYP-induced pain behaviour was prevented. These animals did not actually show any signs of pain, behaving similarly to the other controls even if previously injected with CYP at both 24 and 48 h after injection (Figure 3).
Inflammation induced by CYP is also associated with an increased cutaneous sensitivity as well as referred visceral pain that can be assessed in the hindpaws and in the lower abdominal area (Frias et al., 2013). In the present work, the von Frey monofilaments were used to evaluate cutaneous sensitivity in all experimental groups. Animals injected with saline or GW441756 showed normal values of cutaneous sensitivity in both the hindpaws and abdomen. Animals with CYP inflammation had increased sensitivity 24–48 h after CYP administration. However, animals with CYP inflammation that were also treated with GW441756, did not develop cutaneous hypersensitivity in the hindpaws and abdomen (Figure 3). This may indicate that the TrkA receptor antagonist prevents NGF binding and consequent activation of pain-signalling pathways.
Bladder inflammation elicits an increase of NGF in the bladder mucosa
Urinary bladders were collected from all experimental groups and mucosa was stripped from the underlying tissue. An elisa for NGF was performed and protein levels were quantified by established methods. Bladders from control animals or from animals injected with GW441756 alone showed similar levels of NGF in the mucosa. In contrast, inflammation increased the mucosal NGF content above control at 24 h (201% increase) and 48 h (497% increase) after CYP administration. The treatment of CYP animals with GW441756 showed decreased NGF mucosal content particularly when compared with CYP-inflamed animals for 48 h (Figure 4). Although this decrease of 52% (48 h CYP vs. GW441756 + 48 h CYP) was not statistically significant, it still suggested that the antagonistic actions of GW441756 may have a preventive role in the development of bladder inflammation.
Figure 4.
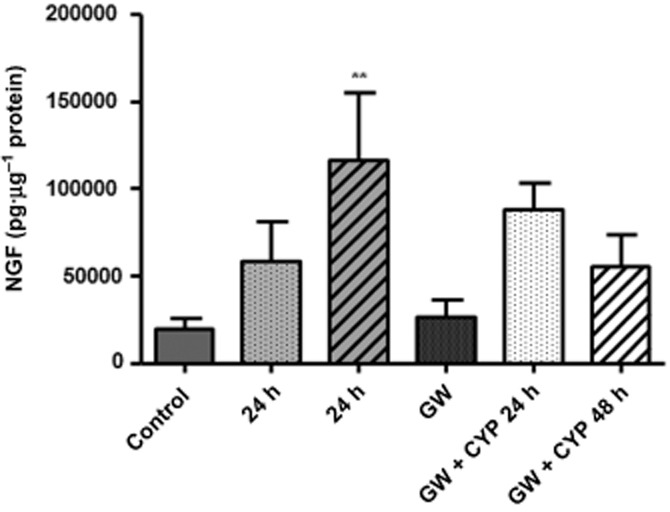
Quantification of the NGF content in the bladder mucosa. Mucosa was stripped from bladders of all experimental groups (control, CYP 24 and 48 h, GW441756 and CYP + GW441756, 24 and 48 h) and the content of NGF was measured by elisa. Animals treated with saline or GW441756 alone showed similar levels of mucosal NGF. CYP-treated rats showed an increase in mucosal neurotrophin content that was significantly higher 48 h after CYP (n = 6) injection, and this was reduced by pretreatment with GW441756 (n = 3). **P < 0.05, significantly different from control; one-way anova followed by Dunnett's multiple comparison tests.
Bladder inflammation elicits increased expression of TRPV1 channels in the membrane of urothelial cells
Primary cell cultures of urothelial cells collected from animals with bladder inflammation were analysed for the membrane expression of TRPV1 channels. The analysis revealed that the expression of TRPV1 channels exhibited a 471% increase 48 h after CYP administration when compared with saline-injected controls (Figure 5). These results indicate that during bladder inflammation, the expression of TRPV1 channels significantly increases in the urothelial membrane.
Figure 5.
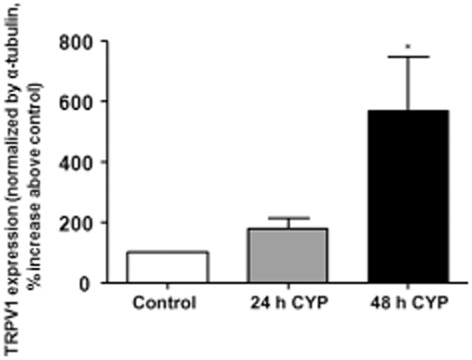
TRPV1 channel expression in urothelial cells. Use of Western immunoblotting revealed that urothelial membrane expression (expressed as % above control) of TRPV1 channels was significantly increased by CYP administration (n = 6 per group). *P < 0.05, significantly different from control; one-way anova followed by Newman–Keuls multiple comparison test.
Discussion and conclusions
Studies have lent support to the idea that the bladder urothelium exhibits a variety of receptors and transmitters typically found in neurons. The coexistence of TRPV1 channels and TrkA receptors has already been described and shown to be essential in bladder control and sensitivity (Charrua et al., 2007; Frias et al., 2012). The present study demonstrated that NGF regulates the expression and function of urothelial TRPV1 channels. Further, as described in other studies (Charrua et al., 2009; Kullmann et al., 2009), our findings demonstrate the presence of TRPV1 channels within the urothelial plasma membrane that can be regulated by trophic factors. A relationship between NGF and TRPV1 channels has been previously demonstrated in sensory neurons. Studies using dorsal root ganglion (DRG) neurons showed that NGF can modulate the translocation of TRPV1 channels to the cell membrane (Morenilla-Palao et al., 2004). Similar to DRG neurons, NGF also facilitated expression of TRPV1 channels in urothelial membranes, which was prevented by the NGF receptor (TrkA) antagonist. Importantly, this was not likely to be dependent on alteration in protein expression as the NGF incubation was of short duration and changes in membrane expression of TRPV1 channels were detected soon after NGF treatment.
The downstream signalling pathways activated by NGF that could lead to altered expression levels of TRPV1 channels were also investigated. Previous studies showed that, in neuronal cells, NGF binds to its high-affinity receptor TrkA, leading to the activation of the PI3K pathway (Bonnington and McNaughton, 2003; Zhuang et al., 2004), which is known to facilitate trafficking of TRPV1 channels (Zhang et al., 2005). Another study suggested that this mechanism also includes the activation of PKC which promotes a rapid vesicular transport of TRPV1 channels to the membrane by a mechanism involving the soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor (SNARE) (Morenilla-Palao et al., 2004). Our experiments using inhibitors of these pathways revealed that TRPV1 channel traffic in urothelial cells operates as it does in sensory neurons.
The functional state of TRPV1 channels was evaluated by quantification of ATP release by capsaicin stimulation, a well-established method to assess the functional activity of this ion channel (Beckel and Birder, 2012; Sadananda et al., 2012). We observed that NGF treatment potentiated ATP release in response to this vanilloid, suggesting an increased availability and/or sensitivity of TRPV1 channels on the cell surface. This process was dependent on TrKA activation via a PI3K and PKC signalling pathway.
We also studied TRPV1 channel modulation using an animal model of the BPS/IC syndrome. In this case, bladder inflammation was induced by a single CYP administration which has been used to mimic aspects of BPS/IC (Dinis et al., 2004; Pinto et al., 2010; Frias et al., 2013; Dornelles et al., 2014). This model is accompanied by an up-regulation of endogenous NGF (Vizzard, 2000; Zhang and Qiao, 2012) as well as by an increased sensitivity of the TRPV1 channel (Dang et al., 2013). Studies performed in BPS/IC patients also showed that both the protein and the mRNA encoding NGF are overexpressed in the mucosa of human bladders (Liu and Kuo, 2007; Liu et al., 2014). Another study revealed an increase of NGF in the urine of those patients (Pinto et al., 2010). Regarding TRPV1 channels, two recent studies showed increased gene and protein expression of the TRPV1 channel in bladder mucosal biopsies from BPS/IC patients that also correlated with the intensification of the symptoms (Homma et al., 2013; Liu et al., 2014). A recent study performed in rats with CYP-induced cystitis also observed an up-regulation of the mRNA encoding TRPV1 channels in the inflamed animals compared with controls which correlated with an increased immunoreactivity for this receptor in the urothelial layer (Dornelles et al., 2014). In our study, we also observed an increase of NGF expression in the mucosa of CYP-inflamed animals. This increased expression of mucosal NGF correlated with the increased expression of membrane TRPV1 channels and increased response to a nociceptive stimulus.
The relation between TRPV1 channels and NGF-induced pain is now well established in somatic and visceral pain as well as bladder hyperactivity with the use of TRPV1-KO mice (Chuang et al., 2001; Frias et al., 2012). Our study indicates that NGF may play a role in inducing or modulating nociceptive symptoms via neuronal and non-neuronal mechanisms. Behavioural observations showed that rats exposed to TrkA inhibition prior and during induction of bladder inflammation with CYP neither exhibit nociceptive behaviour nor exhibit increases in membrane expression of NGF or TRPV1 channels.
Modulation of NGF/TrkA binding by systemic administration of TrkA antagonists has been performed by other investigators. For example, in animal models of visceral inflammatory pain, both the thermal and mechanical hyperalgesias were prevented by use of NGF neutralizing antibodies or TrkA-IgG fusion molecules (Mantyh et al., 2011). In animal models of BPS/IC, urinary frequency and peripheral pain have also been shown to be reduced by intrathecal administration or intravesical instillation of a TrkA antagonist (Guerios et al., 2008; Cruz, 2014). However, preclinical evidence did not result in successful trials in BPS/IC patients. BPS/IC patients who received a humanized monoclonal antibody against NGF, although they experienced improved pain and urgency, also complained of adverse secondary effects such as paraesthesia and arthralgia (Cruz, 2014). Ideally, research into agents with increased specificity may lessen the number of adverse events and could be beneficial for patient treatment.
Overall, our findings suggest that NGF plays an important role in pain behaviour and in the facilitation of TRPV1 channels trafficked to the urothelial cell membrane. This receptor movement correlated with the increased sensitivity of the urothelial cell to chemical stimuli and involves activation of NGF receptor TrkA and associated intracellular pathways.
The significant reduction of the pain behaviour induced by bladder inflammation by the systemic administration of a TrkA antagonist further supports a role for NGF in visceral pain/hypersensitivity. Studies exploring the involvement of neurotrophins and modulation of receptor/ion channel activity in both neuronal and non-neuronal cells will undoubtedly increase our understanding of mechanisms underlying visceral pain states and open new possibilities for much needed treatments.
Acknowledgments
This research was supported by NIH grants R37 DK54824 and R01 DK57284 (to L. A. B.) and a Fulbright Research Scholar Award (A. C.).
Glossary
- BPS/IC
bladder pain syndrome /interstitial cystitis
- CYP
cyclophosphamide
- DRG
dorsal root ganglion
- NGF
nerve growth factor
- NSF
N-ethylmaleimide sensitive factor
- SNARE
soluble N-ethylmaleimide sensitive fusion attachment receptor
- TrkA
tyrosine kinase receptor A
- TRPV1
transient receptor potential vanilloid 1
Author contributions
A. C. and A. S. W-J.: Research design and data acquisition, data interpretation, writing of the manuscript. S. S.: Research data acquisition. C. D. C.: Research design, drafting and critical revision of the manuscript. F. C.: Data interpretation and critical revision of the manuscript. A. A.: Data interpretation, drafting and critical revision of the manuscript. L. B.: Directed and financed the research, data interpretation and critical revision of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013a;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci. 2006;110:175–191. doi: 10.1042/CS20050161. [DOI] [PubMed] [Google Scholar]
- Apsel B, Blair JA, Gonzalez B, Nazif TM, Feldman ME, Aizenstein B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008;4:691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auge C, Chene G, Dubourdeau M, Desoubzdanne D, Corman B, Palea S, et al. Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder. Eur J Pharmacol. 2013;707:32–40. doi: 10.1016/j.ejphar.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. doi: 10.1016/s0306-4522(01)00496-1. [DOI] [PubMed] [Google Scholar]
- Ayar A, Ozcan M, Alcin E, Serhatlioglu I, Ozcan S, Kutlu S, et al. Oxytocin activates calcium signaling in rat sensory neurons through a protein kinase C-dependent mechanism. J Physiol Biochem. 2014;70:43–48. doi: 10.1007/s13105-013-0278-z. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Birder LA. Differential expression and function of nicotinic acetylcholine receptors in the urinary bladder epithelium of the rat. J Physiol. 2012;590(Pt 6):1465–1480. doi: 10.1113/jphysiol.2011.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA. Urothelial signaling. Auton Neurosci. 2010;153:33–40. doi: 10.1016/j.autneu.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol Urodyn. 2011;30:673–682. doi: 10.1002/nau.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551(Pt 2):433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher M, Meen M, Codron JP, Coudore F, Kemeny JL, Eschalier A. Cyclophosphamide-induced cystitis in freely-moving conscious rats: behavioral approach to a new model of visceral pain. J Urol. 2000;164:203–208. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007;177:1537–1541. doi: 10.1016/j.juro.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Charrua A, Reguenga C, Cordeiro JM, Correiade-Sa P, Paule C, Nagy I, et al. Functional transient receptor potential vanilloid 1 is expressed in human urothelial cells. J Urol. 2009;182:2944–2950. doi: 10.1016/j.juro.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001;165:975–979. [PubMed] [Google Scholar]
- Cruz CD. Neurotrophins in bladder function: what do we know and where do we go from here? Neurourol Urodyn. 2014;33:39–45. doi: 10.1002/nau.22438. [DOI] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced cystitis reduces ASIC channel but enhances TRPV1 receptor function in rat bladder sensory neurons. J Neurophysiol. 2013;110:408–417. doi: 10.1152/jn.00945.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis P, Charrua A, Avelino A, Cruz F. Intravesical resiniferatoxin decreases spinal c-fos expression and increases bladder volume to reflex micturition in rats with chronic inflamed urinary bladders. BJU Int. 2004;94:153–157. doi: 10.1111/j.1464-4096.2004.04855.x. [DOI] [PubMed] [Google Scholar]
- Dornelles FN, Andrade EL, Campos MM, Calixto JB. Role of CXCR2 and TRPV1 in functional, inflammatory and behavioural changes in the rat model of cyclophosphamide-induced haemorrhagic cystitis. Br J Pharmacol. 2014;171:452–467. doi: 10.1111/bph.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias B, Charrua A, Avelino A, Michel MC, Cruz F, Cruz CD. Transient receptor potential vanilloid 1 mediates nerve growth factor-induced bladder hyperactivity and noxious input. BJU Int. 2012;110(8 Pt B):E422–E428. doi: 10.1111/j.1464-410X.2012.11187.x. [DOI] [PubMed] [Google Scholar]
- Frias B, Allen S, Dawbarn D, Charrua A, Cruz F, Cruz CD. Brain-derived neurotrophic factor, acting at the spinal cord level, participates in bladder hyperactivity and referred pain during chronic bladder inflammation. Neuroscience. 2013;234:88–102. doi: 10.1016/j.neuroscience.2012.12.044. [DOI] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R111–R122. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Homma Y, Nomiya A, Tagaya M, Oyama T, Takagaki K, Nishimatsu H, et al. Increased mRNA expression of genes involved in pronociceptive inflammatory reactions in bladder tissue of interstitial cystitis. J Urol. 2013;190:1925–1931. doi: 10.1016/j.juro.2013.05.049. [DOI] [PubMed] [Google Scholar]
- Jung EJ, Kim DR. Apoptotic cell death in TrkA-overexpressing cells: kinetic regulation of ERK phosphorylation and caspase-7 activation. Mol Cells. 2008;26:12–17. [PubMed] [Google Scholar]
- Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol. 2009;296:F892–F901. doi: 10.1152/ajprenal.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BL, Yang F, Zhan HL, Feng ZY, Zhang ZG, Li WB, et al. Increased severity of inflammation correlates with elevated expression of TRPV1 nerve fibers and nerve growth factor on interstitial cystitis/bladder pain syndrome. Urol Int. 2014;92:202–208. doi: 10.1159/000355175. [DOI] [PubMed] [Google Scholar]
- Liu HT, Kuo HC. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology. 2007;70:463–468. doi: 10.1016/j.urology.2007.04.038. [DOI] [PubMed] [Google Scholar]
- Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009;104:1476–1481. doi: 10.1111/j.1464-410X.2009.08675.x. [DOI] [PubMed] [Google Scholar]
- Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–577. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. 2011;115:189–204. doi: 10.1097/ALN.0b013e31821b1ac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology. 2007;69(4 Suppl):24–33. doi: 10.1016/j.urology.2006.08.1108. [DOI] [PubMed] [Google Scholar]
- Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Parsons CL. Diagnosing chronic pelvic pain of bladder origin. J Reprod Med. 2004;49(3 Suppl):235–242. [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Pinto R, Lopes T, Frias B, Silva A, Silva JA, Silva CM, et al. Trigonal injection of botulinum toxin A in patients with refractory bladder pain syndrome/interstitial cystitis. Eur Urol. 2010;58:360–365. doi: 10.1016/j.eururo.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Sadananda P, Kao FC, Liu L, Mansfield KJ, Burcher E. Acid and stretch, but not capsaicin, are effective stimuli for ATP release in the porcine bladder mucosa: are ASIC and TRPV1 receptors involved? Eur J Pharmacol. 2012;683:252–259. doi: 10.1016/j.ejphar.2012.02.041. [DOI] [PubMed] [Google Scholar]
- Saitoh C, Yokoyama H, Chancellor MB, de Groat WC, Yoshimura N. Comparison of voiding function and nociceptive behavior in two rat models of cystitis induced by cyclophosphamide or acetone. Neurourol Urodyn. 2010;29:501–505. doi: 10.1002/nau.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers WD, Tuttle JB. Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol. 2006;3:101–110. doi: 10.1038/ncpuro0408. [DOI] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Descamps O, Hart MJ, Poksay KS, Spilman P, Kane DJ, et al. Paradoxical effect of TrkA inhibition in Alzheimer's disease models. J Alzheimers Dis. 2014;40:605–617. doi: 10.3233/JAD-130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QL, Qiao LY. Regulation of IGF-1 but not TGF-beta1 by NGF in the smooth muscle of the inflamed urinary bladder. Regul Pept. 2012;177:73–78. doi: 10.1016/j.regpep.2012.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


