Abstract
Background and Purpose
Growing evidence shows cannabidiol (CBD) modulates some of the effects of Δ9-tetrahydrocannabinol (THC). CBD is a constituent of some strains of recreational cannabis but its content is highly variable. High CBD strains may have less memory-impairing effects than low-CBD strains and CBD can reverse behavioural effects of THC in monkeys. CBD/THC interactions in rodents are more complicated as CBD can attenuate or exacerbate the effects of THC. This study was undertaken to determine if CBD could reverse hypothermia or hypolocomotor effects caused by THC in rats.
Experimental Approaches
Male Sprague-Dawley rats were prepared with radiotelemetry devices and then given doses of THC (10–30 mg·kg−1, i.p.) with or without CBD. Experiments determined the effect of simultaneous or 30 min pretreatment with CBD in a 1:1 ratio with THC, as well as the effect of CBD in a 3:1 ratio. Additional experiments determined the effects of pretreatment with the cannabinoid CB1 receptor antagonist SR141716 (rimonabant).
Key Results
CBD did not attentuate THC-induced hypothermia or hypolocomotion but instead exaggerated these effects in some conditions. The antagonist SR141716 blocked hypolocomotor effects of THC for the first hour after injection and the hypothermia for 6 h; thus validating the pharmacological model.
Conclusions and Implications
There is no evidence from this study that elevated CBD content in cannabis could provide protection from the physiological effects of THC, in rats.
Tables of Links
| TARGETS |
|---|
| GPCR |
| CB1 receptor |
| LIGANDS |
|---|
| CBD, cannabidiol |
| SR141716, rimonabant |
| THC, Δ9tetrahydrocannabinol |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Cannabidiol (CBD) is a constituent of some strains of recreational cannabis plant material; the content of CBD-enriched strains is, however, highly variable in the market (Morgan et al., 2010; Burgdorf et al., 2011). CBD has been viewed as an inactive constituent of cannabis, for example, it produces minimal disruption of behavioural tasks in humans, monkeys or rodents (Belgrave et al., 1979; Lichtman et al., 1995; Winsauer et al., 1999). There is growing evidence, particularly in human and non-human primates, that CBD may modulate the effects of the primary psychoactive cannabis constituent, Δ9-tetrahydrocannabinol (THC). CBD may function as an allosteric modulator (antagonist) at cannabinoid CB1 and CB2 receptor subtypes (Turkanis and Karler, 1986; Petitet et al., 1998; Mechoulam et al., 2007), which provides a potential pharmacological mechanism for any in vivo effects observed. A recent review argues, however, that CBD influence on THC-related effects may be mediated through non-CB1 receptor mechanisms (McPartland et al., 2015).
The evidence that CBD can attenuate or modify the effects of THC in humans is several fold. A combined cannabinoid oral/mucosal spray Sativex (it delivers a 1:1 ratio of CBD : THC) has been approved in Canada for spasticity associated with multiple sclerosis following many clinical trials (see Oreja-Guevara, 2012). The mechanism of the therapeutic effect of CBD is not well established, but the addition of CBD to the THC may permit higher doses of THC to be administered without significant ‘adverse effects’, including subjective ‘high’ (Robson, 2011; Schoedel et al., 2011) and cognitive/behavioural impairment (Wade et al., 2004). Furthermore, a mixed CBD/THC cannabis extract improved EEG mis-match negativity activation whereas THC did not (Juckel et al., 2007) but CBD/THC- and THC-only treatment both reduced P300 amplitude (Roser et al., 2008). In a different vein, Morgan and colleagues showed that smoking CBD-enriched marijuana did not cause deficits of immediate and delayed prose recall caused by CBD-poor cannabis (Morgan et al., 2010) and users habitually exposed to CBD-containing cannabis may have relatively preserved recognition memory versus CBD-poor cannabis users (Morgan et al., 2012). The limits of human field studies (varying CBD/THC dose, no control of individuals who select CBD-rich vs. CBD-poor cannabis) and human laboratory studies (limited dosing ranges of CBD vs THC) have motivated animal studies to investigate how CBD modulates the effects of THC. Finally, a recent study from this laboratory found that CBD could ameliorate or reverse some motor coordination and object-spatial memory effects of THC in macaque monkeys when CBD and THC were administered in equal amount (Wright et al., 2013b). This is important given that CBD : THC ratios in street cannabis do not typically exceed 1:1 (Morgan et al., 2010; Burgdorf et al., 2011).
The available evidence on interactive effects of CBD and THC in rodent models presents a more complicated picture. While CBD can reverse conditioned place aversion produced by 10 mg·kg−1 THC in rats (Vann et al., 2008), CBD may potentiate the anxiogenic and locomotor suppressant effects of THC in rats treated chronically (Klein et al., 2011). In addition CBD/THC interactions may depend on the pretreatment offset (see Zuardi et al., 2012). When CBD is administered 30 min (or up to 24 h) prior to THC in rats or mice, a potentiation can be observed whereas co-administration results in blockade or amelioration of THC effects. The picture may be complicated even further by a suggestion that CBD/THC ratios on the order of 8 are necessary for antagonistic properties and only 1.8 for potentiation of THC-related effects in rodents (Zuardi et al., 1984).
This study was therefore designed to determine if CBD attenuates, potentiates or extends the duration of hypothermia and hypomotility produced by acute THC in rats. A radiotelemetry system, shown effective at distinguishing dose-dependent effects of even closely related stimulant drugs (Crean et al., 2006, 2007; Taffe et al., 2006), was selected to minimize any potential anxiogenic or anxiolytic effects of THC which might interact with rectal sampling, as well as providing a full time course of effects. The outcomes of simultaneous CBD/THC injection were compared with those of giving CBD 30 min before THC to determine if this altered the qualitative effects of CBD/THC combinations. Further investigation examined whether a 3:1 CBD : THC ratio altered the effects of CBD, compared with a 1:1 ratio. A final study determined the effect of the cannabinoid CB1 receptor antagonist SR141716 (rimonabant) on the thermoregulatory response to THC to serve as a positive control condition to demonstrate mechanistic specificity.
Methods
Animals
All animal care and experimental procedures were conducted under protocols approved by the Institutional Care and Use Committee of The Scripps Research Institute (Protocol #08–0053-2,3). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 13 animals were used in the experiments described here. Male Sprague-Dawley (Harlan; Livermore, CA, USA) rats were housed in humidity- and temperature-controlled (23 ± 2°C) conditions, on 12:12 hour light : dark cycles. Animals entered the laboratory at approximately 10 weeks of age. Group 1 rats (n = 5) were 14 weeks old and weighed 362–405 g at the start of this study. Group 2 rats (n = 8) were 18 weeks old and weighed 400–459 g at the start of this study. Animals had ad libitum access to food and water in their home cages.
Radiotelemetry
Rats were anaesthetized with an isoflurane/oxygen vapour mixture (isoflurane 5% induction, 1–3% maintenance) and sterile radiotelemetry transmitters (Data Sciences International, St. Paul, MN, USA; TA-F40) were implanted in the abdominal cavity through an incision along the abdominal midline posterior to the xyphoid space. Absorbable sutures were used to close the abdominal muscle incision and the skin incision was closed with tissue adhesive. A minimum of 7 days was allowed for surgical recovery prior to starting an experiment. For the first 3 days of the recovery period, an antibiotic (cephazolin; 0.4 g·mL−1; 2.0 mL·kg−1, s.c.) and an analgesic (flunixin; 2.5 mg·mL−1; 2.0 mL·kg−1, s.c.) were administered daily.
Animals were evaluated in clean standard plastic homecages (thin layer of bedding) in a dark testing room, separate from the vivarium, during the (vivarium) dark cycle. Radiotelemetry transmissions were collected via telemetry receiver plates (Data Sciences International; RPC-1) placed under the cages as described in prior investigations (Wright et al., 2012; Aarde et al., 2013; Miller et al., 2013). Test sessions started with a 5–10 minute interval to ensure data collection, followed by i.p. injection of drug. The ambient temperature for the studies was 23.5 ± 1°C.
Studies
The order of group 1 studies was (i) simultaneous 30 mg·kg−1 CBD and 30 mg·kg−1 THC; (ii) 30-min pretreatment of 30 mg·kg−1 CBD followed by 30 mg·kg−1 THC; (iii) 30 min pretreatment of 60 mg·kg−1 CBD followed by 20 mg·kg−1 THC. A single 10 mg·kg−1 THC challenge was inserted between studies 2 and 3. Treatment conditions (including vehicle and solo-drug challenges) were randomized within the studies, with active THC doses administered no more frequently than a 7 day interval. All challenge studies were completed during a 16 week interval. Group 2 had a prior history of five acute exposures to THC (one including 4 mg·kg−1 SR141716, i.p. pretreatment) via inhalation in sessions lasting 10–30 min, administered no more frequently than a 7 day interval.
Data analysis
The body temperature and activity rate (counts per minute) were collected on a 5 min schedule but are expressed as hourly averages for statistical analysis of the group 1 study and 30 min averages for the group 2 study. In the case of the pretreatment studies, the data are expressed relative to the second injection time. Any missing values were interpolated from the values before and after the lost time point. Data were analysed with two way anova including repeated measures factors for the treatment condition and the time post-injection. Any significant main effects were followed with post hoc analysis using Tukey's correction for all comparisons. All analysis used Prism 6 for Windows (v. 6.02; GraphPad Software, Inc, San Diego, CA, USA).
Materials
THC was administered i.p. in doses of 10, 20 and 30 mg·kg−1, CBD was administered i.p. in doses of 30 and 60 mg·kg−1 and SR141716 (rimonabant) was administered i.p. in a dose of 4 mg·kg−1. The dose selection for THC was based on assessment of published data and pilot studies showing this to be the effective range for consistent effects. For injection, CBD, SR141716 or THC was suspended in a vehicle of 95% ethanol, Cremophor EL and saline in a 1:1:8 ratio. The THC was provided by the US National Institute on Drug Abuse and the CBD and SR141716 were purchased from Cayman Chemical (Ann Arbor, MI, USA).
Results
Simultaneous administration, 1:1 CBD-THC ratio
Temperature
Body temperature was reduced by THC (30 mg·kg−1, i.p.) and the simultaneous injection of CBD (30 mg·kg−1, i.p.) enhanced the magnitude of hypothermia (Figure 1). The anova confirmed significant main effects of time after injection [F (3, 36) = 44.60; P < 0.0001], drug treatment condition [F(2, 12) = 21.63; P < 0.0001] and the interaction of factors [F (6, 36) = 8.15; P < 0.0001] on temperature. The post hoc test confirmed that temperature was significantly lower than the respective vehicle treatment following CBD-THC (all 4 h) and Veh-THC (2–4 h after injection). There was also a significant difference between CBD-THC and Veh-THC conditions in the third hour after injection.
Figure 1.
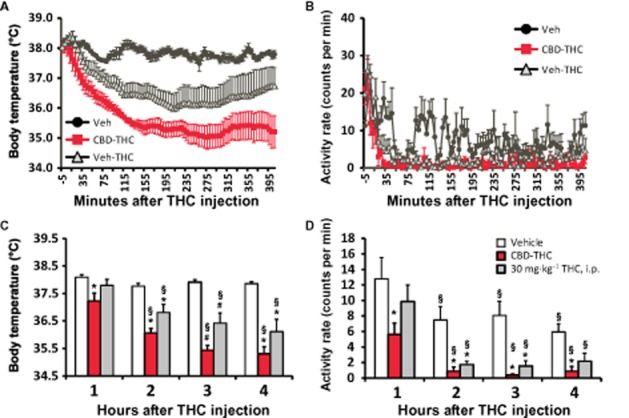
Telemetered body temperature (A and C) and activity rate (B and D) after treatment with 30 mg·kg−1 THC with 30 mg·kg−1 CBD or the vehicle, i.p., administered simultaneously. A vehicle-only control condition (Veh) is also shown. A and B display the data as collected (5 min intervals) and (C and D) depict the hourly averages used for analysis. Values shown are means ± SEM (n = 5). *P < 0.05, significantly different from Veh (only); #P < 0.05, significantly different from both other conditions; §P < 0.05, significantly different from the first hour (within treatment condition).
Activity
THC also reduced activity, but there was no additional effect of CBD on hypolocomotion. The anova confirmed significant main effects of hours after injection [F (3, 36) = 23.98; P < 0.0001], drug treatment condition [F (2, 12) = 10.78; P < 0.005], but not of the interaction of factors [F (6, 36) = 0.92; P = 0.50], on activity. The post hoc test confirmed that activity was significantly lower than the corresponding vehicle condition after CBD-THC (all at 4 h) and after Veh-THC (hours 2–3).
Thirty minute pretreatment, 1:1 CBD-THC ratio
Temperature
The administration of CBD (30 mg·kg−1, i.p.) 30 min before THC (30 mg·kg−1, i.p.) also enhanced the magnitude of hypothermia (Figure 2). The anova confirmed main effects of time after injection [F (3, 12) = 21.29; P < 0.0001], drug treatment condition [F (3, 12) = 7.15; P < 0.01] and the interaction of factors [F (9, 36) = 8.39; P < 0.0001] on temperature. The post hoc test confirmed that temperature was significantly lower than both Veh-Veh and CBD-Veh treatment following CBD-THC (2–4 h) and Veh-THC (3–4 h after injection); the Veh-THC condition also differed significantly from the Veh-Veh condition in the second hour after injection. There was also a significant difference between CBD-THC and Veh-THC conditions in the fourth hour after injection. Post hoc analysis within treatment condition confirmed that body temperature was significantly lower than the first hour following treatment with CBD-THC (2–4 h after injection) and with Veh-THC (hours 2–4). Significant differences between hours 3–4 and hour 2 were confirmed for the CBD-THC condition as well.
Figure 2.
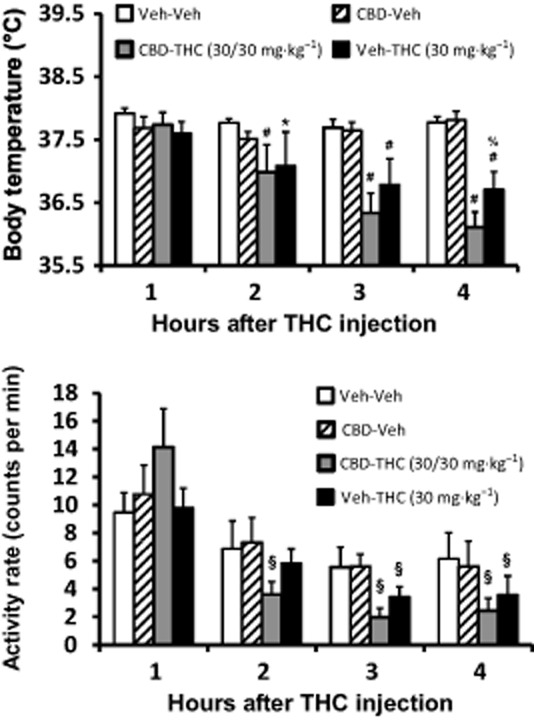
Telemetered body temperature (top) and activity rate (bottom) after treatment with 30 mg·kg−1 THC with and without 30 mg·kg−1 CBD administered 30 min prior. Veh control conditions are also shown. Values shown are means ± SEM (n = 5). *P < 0.05, significantly different from Veh-Veh (only); #P < 0.05, significantly different from Veh-Veh and CBD-Veh; %P < 0.05, significantly different between CBD-THC and Veh-THC; §P < 0.05, significantly different in activity compared with the first hour (within treatment condition). See text for effects of time on temperature.
Activity
The hypolocomotor effect of THC was not affected by the administration of CBD 30 min before (Figure 2). The statistical analysis confirmed that there were significant main effects of time after injection [F (3, 12) = 32.26; P < 0.0001] and drug treatment condition [F (3, 12) = 4.61; P < 0.05], but not of the interaction of factors [F (9, 36) = 1.83; P = 0.10], on activity. The post hoc test confirmed that activity was significantly lower than the first hour after treatment with CBD-THC (2–4 h after injection) and after Veh-THC (hours 3–4).
Thirty minute pretreatment, 3:1 CBD-THC ratio
Temperature
A lower dose of THC (20 mg·kg−1, i.p.) still reduced body temperature but a threefold higher dose of CBD (60 mg·kg−1, i.p.) had no effect on this response when administered 30 min before THC (Figure 3). The anova confirmed main effects of time after injection [F (3, 12) = 22.38; P < 0.0001] and the interaction of factors [F (9, 36) = 10.31; P < 0.0001], but not drug treatment condition [F (3, 12) = 2.14; P = 0.15], on temperature. The post hoc test confirmed that temperature was significantly lower than the respective vehicle condition temperature following CBD-THC (2–4 h after injection) and Veh-THC (1, 3–4 h after injection). Temperature was lower than both Veh-Veh and CBD-Veh conditions 3–4 h after CBD-THC or Veh-THC. There were no significant differences between CBD-THC and Veh-THC conditions at any time after injection. The post hoc test also confirmed that temperature was lower compared with the first hour after Veh (2–4 h), CBD-Veh (second hour), Veh-THC (3–4 h, which also differed from 2 h). Temperature following CBD-THC was significantly different between all hourly observations, except for the 1 versus 2 h comparison.
Figure 3.
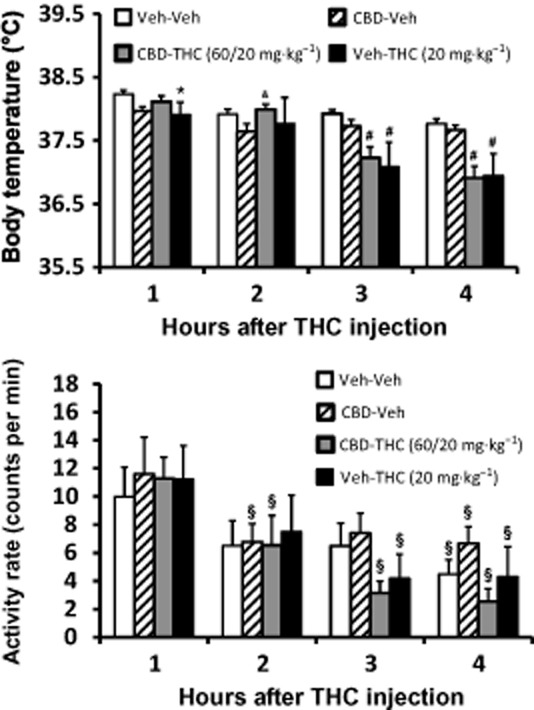
Telemetered body temperature (top) and activity rate (bottom) after treatment with 20 mg·kg−1 (lower panels) THC with and without 60 mg·kg−1 CBD administered 30 min prior. Veh control conditions are also shown. Values shown are means ± SEM (n = 5). *P < 0.05, significantly different from Veh-Veh (only); #P < 0.05, significantly different from Veh-Veh and CBD-Veh; &P < 0.05, significantly different from CBD-Veh (only); §P < 0.05, significantly different in activity compared with the first hour (within treatment condition). See text for effects of time on temperature.
Activity
The threefold higher CBD dose likewise did not modulate the reduction of activity caused by 20 mg·kg−1 THC (Figure 3). There was a significant main effect of time after injection [F (3, 12) = 21.91; P < 0.0001], but not of drug treatment condition [F (3, 12) = 1.58; P = 0.25] or of the interaction of factors [F (9, 36) = 0.89; P = 0.54], on activity. Post hoc analysis confirmed that activity was lower than the first hour for the fourth hour after Veh, the second and fourth hours after CBD-Veh, the third and fourth hour after Veh-THC and second, third and fourth hours after treatment with CBD-THC.
THC dose comparison
Temperature
A final analysis (Figure 4) compared the effects of administered THC dose by contrasting the vehicle and 30 mg·kg−1 THC dose conditions from the same-dose 30 min pretreatment study (Figure 2) with the 20 mg·kg−1 dose in the asymmetrical dose study (Figure 3) and a single 10 mg·kg−1 THC challenge conducted in between those studies. Analysis of the temperature data confirmed main effects of time after injection [F (3, 12) = 9.75; P < 0.005] and the interaction [F (9, 36) = 3.50; P < 0.005], but not of treatment condition, [F (3, 12) = 1.40; P = 0.29]. The post hoc analysis confirmed that temperature was significantly lower than the vehicle condition in the first hour after injection of 10 mg·kg−1, in the second hour after injection of 10 or 30 mg·kg−1 and in the third and fourth hours after injection of 10, 20 or 30 mg·kg−1 THC. Correspondingly, temperature was lower compared with the first hour after both 20 mg·kg−1 (3–4 h) and 30 mg·kg−1 (2–4 h) THC and compared with the second hour after 20 mg·kg−1 (3–4 h) injection. Significant differences in body temperature were also confirmed between the 10 and 20 mg·kg−1 treatment conditions 1 and 2 h after injection of THC.
Figure 4.
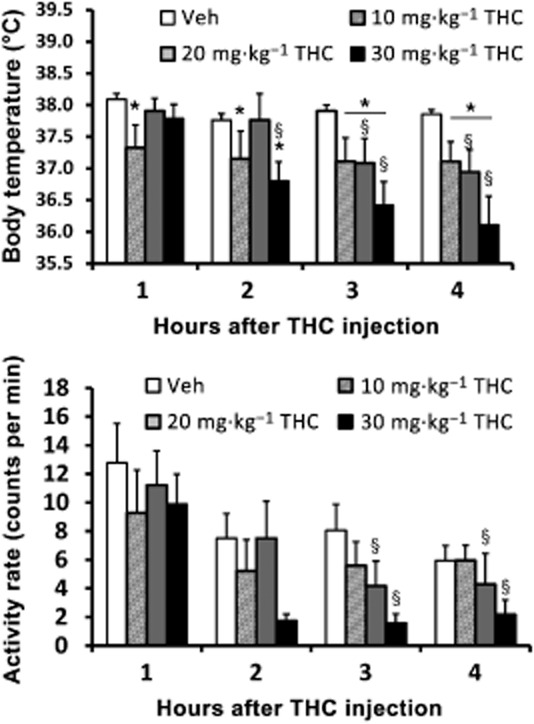
Telemetered body temperature (upper) and activity rate (lower) after treatment with 10 mg·kg−1 THC is compared with data after administration of 20 or 30 mg·kg−1 THC as reported in Figure 2 in the Veh-THC conditions. Values shown are means ± SEM (n = 5). *P < 0.05, significantly different from Veh (only); §P < 0.05, significant differences from the first hour (within treatment condition).
Activity
Analysis of the activity data (Figure 4) confirmed a main effect of time after injection [F (3, 12) = 19.15, P < 0.0001], but not of treatment condition [F (3, 12) = 0.36, P = 0.78] or of dose [F (9, 36) = 0.49, P = 0.87]. Post hoc analysis confirmed that activity was lower than the first hour for the third and fourth hours after treatment with 20 or 30 mg·kg−1 THC but no other comparisons confirmed differential activity.
CB1 receptor antagonism
Temperature
Treatment of rats with 4 mg·kg−1 SR141716, i.p., 15 min before THC (20 mg·kg−1, i.p.) significantly attenuated the hypothermic response. The anova confirmed main effects of time after injection [F (12, 84) = 33.24; P < 0.0001], SR/Veh pretreatment condition [F (1, 7) = 44.19; P < 0.0005] and the interaction of factors [F (12, 84) = 37.99; P < 0.0001] on temperature. The post hoc test confirmed that temperature was significantly different between Veh and SR141716 pretreatment conditions from 90 to 390 min after THC administration. Within the SR141716 pretreatment condition, body temperature was lower than at 30 min (60–270, 330–360 min after THC) and higher than at 90 (270–390 min after THC), 120 (270–290 min after THC), 150 (300–390 min after THC), 180 (300 min after THC) and 210 (300 min after THC) minute time points. In contrast, body temperature following vehicle pretreatment and 20 mg·kg−1 THC, i.p. was significantly lower than the 30 or 60 min time points at all following points, as well as lower than the temperature at 90 (150–390 min),120 (210–390 min) and 150 (270–330 min) minutes after THC.
Activity
The anova confirmed main effects of time post-injection [F (12, 84) = 25.46; P < 0.0001], SR141716 /Veh pretreatment condition [F (1, 7) = 7.57; P = 0.028] but not the interaction of factors [F (12, 84) = 1.46; P = 0.15] on activity. Post hoc analysis confirmed that activity was only significantly higher in the SR141716 pretreatment condition relative to vehicle pretreatment 60 min after THC injection.
Discussion
This study used a minimally invasive radiotelemetry procedure to determine the effect of CBD on hypothermia and hypolocomotion induced by i.p. THC. The major finding was that CBD failed to attenuate the effects of THC on activity and thermoregulation, and in fact potentiated the effects of THC in some cases. CBD was ineffectual at blocking THC-mediated hypothermia and locomotor suppression when administered either simultaneously or as a pretreatment 30 min before THC injection. The study also found that there was no differential effect when CBD was administered 30 min prior to THC at three times the dose. Finally, CBD had no effect on body temperature or activity when administered in the absence of THC, consistent with many earlier findings of the lack of effect of treatment with CBD alone. The methodological approach was effective as body temperature was reduced significantly for up to 6 h after 20–30 mg·kg−1 THC, i.p. as predicted for rats (Tulunay et al., 1981; Wiley et al., 2007), and the effect was reversed by pretreatment with the CB1 receptor antagonist SR141716 (rimonabant) as had been shown in both rats (Nava et al., 2000) and monkeys (Taffe, 2012b). Activity was also suppressed by THC administration, although this effect was only reversed in the first hour by pretreatment with the antagonist. The effects of THC were roughly dose-dependent and reproducible across several challenges in the same animals, which further validate this model.
The CBD-mediated enhancement of hypothermia is similar to an earlier result in rats in which equal doses were administered simultaneously (Fernandes et al., 1974). In that study, CBD acted mostly to prolong the duration of hypothermia, whereas here it also changed the magnitude of temperature reduction, at least when administered at a 1:1 dose ratio either simultaneously or at a 15 min offset (Figures 1 and 2). Similarly, when CBD ws given 20 min before an equal dose of THC, it also slightly potentiated behavioural impairment in rats (Klein et al., 2011). There was some evidence for a dose ratio dependency of the enhancement of hypothermia as it was not observed in the experiments using the 3:1 CBD : THC treatment (Figure 3). This may be similar to the clinical observation that cannabis strains of 6% THC/7.5% CBD produced equivalent therapeutic relief in chronic pain in MS patients as did 19% THC/>1% CBD strain without any difference in the amount of cannabis used per day (Brunt et al., 2014). That finding is consistent with a CBD-mediated enhancement of THC effects, rather than a diminution.
The present study also showed that thermoregulatory and activity responses to THC in the rat may not model the expected outcome in humans quite as well as studies in non-human primates. Human studies have shown that CBD : THC ratios in cannabis strains that approach 1:1 may prevent cognitive disruption associated with low CBD strains of approximately equal THC content (Morgan et al., 2010, 2012). We have previously shown that CBD reversed the effect of behaviourally impairing doses of THC when administered at a 1:1 ratio in the macaque monkey and these effects were similar when CBD was injected either simultaneously with THC or with a 20 min pretreatment interval (Wright et al., 2013b). Furthermore, the dose ranges for behavioural impairment in monkeys (Taffe, 2012a; Wright et al., 2013a) overlap with those that produce significant hypothermia (Taffe, 2012b).
The magnitude and duration of hypothermia produced by 20 mg·kg−1 THC i.p. in the two treatment groups was very similar (Figures 1 and 5), demonstrating the consistency of the effect across minor differences in animal age and body weight. The delayed effect of SR141716 on hypothermia induced by 20 mg·kg−1 THC, i.p., in the validation study was unexpected since most thermoregulatory investigations, particularly in mice, appear to demonstrate immediate blockade of THC-induced hypothermia. Nevertheless, SR141716 pretreatment affected a prolactin response to THC at 90, but not 20, min after injection in rats (Fernandez-Ruiz et al., 1997). Similarly, SR141716 attenuated hypothermia at 30, but not 10, min after injection of the CB1 receptor full agonist WIN 55,212-2 in mice (Son et al., 2010). The rather modest effect of SR141716 on hypolocomotion may be a reflection of the fact that the activity measure was not highly sensitive or perhaps because tetrad effects of THC may be maximal at different doses, for example see Marshell et al. (2014). In other studies in mice, the first time point for temperature measurement was 30 min or 60 min after a CB1 receptor agonist was administered (Fox et al., 2001) so it is difficult to definitively establish the time course of effects. Interestingly, when SR141716 was administered 20 min after THC, it appeared to ameliorate hypothermia within 10 min (Nava et al., 2000). Overall, the present results are mostly consistent with the published data available and further experiments with varied SR141716 /THC administration offsets would be required to fully characterize the response within the first 30 min of THC injection. It is possible, for example, that prior exposure to THC in these animals had a conditioning effect or induced some unknown neuroplastic effect that was only effective in the first hour after injection.
Figure 5.
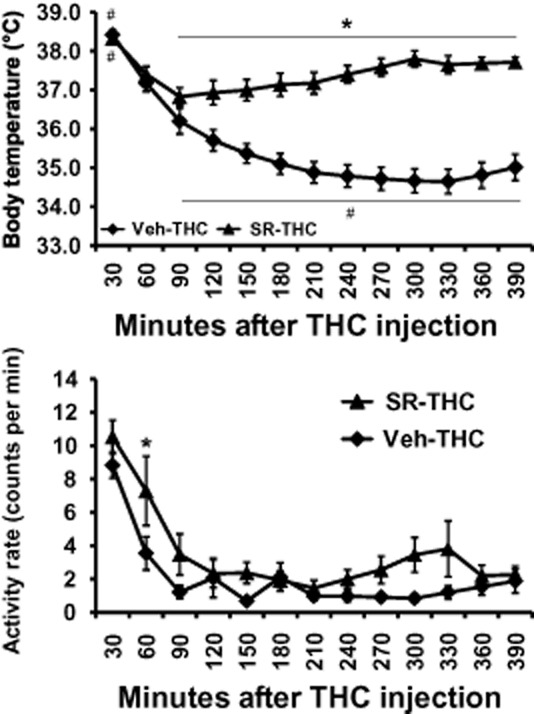
Telemetered body temperature (upper) and activity rate (lower) after treatment with 20 mg·kg−1 THC with 4 mg·kg−1 SR141716 or the vehicle administered, i.p., 15 min prior to THC. Values shown are means ± SEM (n = 8). *P < 0.05, significant differences between treatment conditions; #P < 0.05, significantly different from the 60 min time point (within treatment) for temperature.
A minor caveat to the study is that it is possible that interactive effects would be observed at a lower dose of THC. The dose range for this study was selected based on published data and pilot studies showing consistent effects on the temperature and activity end points that were selected. Thus, it seems most likely that lower doses would simply make the THC-related effects too variable for interpretation; but the possibility CBD/THC interactions would be detectable at lower doses cannot be definitively excluded. Likewise, although the treatment intervals resulted in approximately the same magnitude of effects of identical THC doses across the repeated measures design, it is possible that minor degrees of tolerance were produced. Given the primary interpretive comparisons of interest for this study, however, it is unclear how minor degrees of tolerance would affect the major conclusions.
In conclusion, this investigation found no evidence that CBD could ameliorate the thermoregulatory or hypolocomotor effects of THC when administered either simultaneously or prior to THC. Increasing the ratio of CBD : THC from 1:1 to 3:1 had no differential effect. As both street marijuana products and the medication Sativex do not exceed a 1:1 CBD : THC ratio, and drugs are administered simultaneously, the present studies have the best construct validity for translational inferences. It cannot be excluded that a longer pretreatment interval or much larger CBD : THC ratio might produce a different result, but it is difficult to establish relevance of such approaches to the actual use of available strains of cannabis (Morgan et al., 2010; Burgdorf et al., 2011). Overall, there was no evidence from this study that elevated CBD content in cannabis would provide protection from the physiological effects of THC.
Acknowledgments
The authors are grateful to the other principal investigators of the Scripps Center for Cannabis Addiction Neurobiology, Drs. Barbara J. Mason, Loren H. Parsons and Susan F. Tapert for many helpful discussions. This work was supported by USPHS grants DA024194, DA024105 and DA035482. The National Institutes of Health/NIDA had no direct input on the design, conduct, analysis or publication of the findings. This is manuscript #28040 from The Scripps Research Institute.
Glossary
- CBD
cannabidiol
- SR141716
5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- THC
Δ9tetrahydrocannabinol
Author contributions
M. A. T. designed the study with significant input from K. M. C. and S. A. V. K. M. C. and S. A. V. performed the research and conducted initial data analysis. M. A. T. conducted statistical analysis of data, created figures and wrote the paper. M. A. T., K. M. C. and S. A. V. revised the final draft and approved of the submitted version of the manuscript.
Conflict of interest
The authors have no competing interests.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgrave BE, Bird KD, Chesher GB, Jackson DM, Lubbe KE, Starmer GA, et al. The effect of cannabidiol, alone and in combination with ethanol, on human performance. Psychopharmacology (Berl) 1979;64:243–246. doi: 10.1007/BF00496070. [DOI] [PubMed] [Google Scholar]
- Brunt TM, van Genugten M, Honer-Snoeken K, van de Velde MJ, Niesink RJ. Therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade cannabis. J Clin Psychopharmacol. 2014;34:344–349. doi: 10.1097/JCP.0000000000000129. [DOI] [PubMed] [Google Scholar]
- Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug Alcohol Depend. 2011;117:59–61. doi: 10.1016/j.drugalcdep.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA. Effects of (+/−)3,4-methylenedioxymethamphetamine, (+/−)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience. 2006;142:515–525. doi: 10.1016/j.neuroscience.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Taffe MA. Oral administration of (+/−)3,4-methylenedioxymethamphetamine and (+)methamphetamine alters temperature and activity in rhesus macaques. Pharmacol Biochem Behav. 2007;87:11–19. doi: 10.1016/j.pbb.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M, Schabarek A, Coper H, Hill R. Modification of delta9-THC-actions by cannabinol and cannabidiol in the rat. Psychopharmacologia. 1974;38:329–338. doi: 10.1007/BF00429130. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz JJ, Munoz RM, Romero J, Villanua MA, Makriyannis A, Ramos JA. Time course of the effects of different cannabimimetics on prolactin and gonadotrophin secretion: evidence for the presence of CB1 receptors in hypothalamic structures and their involvement in the effects of cannabimimetics. Biochem Pharmacol. 1997;53:1919–1927. doi: 10.1016/s0006-2952(97)00168-8. [DOI] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, et al. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Juckel G, Roser P, Nadulski T, Stadelmann AM, Gallinat J. Acute effects of Δ9-tetrahydrocannabinol and standardized cannabis extract on the auditory evoked mismatch negativity. Schizophr Res. 2007;97:109–117. doi: 10.1016/j.schres.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, et al. Cannabidiol potentiates Delta(9)-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) 2011;218:443–457. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL, et al. In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Delta-THC in mice: inhalation versus intraperitoneal injection. Pharmacol Biochem Behav. 2014;124C:40–47. doi: 10.1016/j.pbb.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JM, Duncan M, Di Marzo V, Pertwee R. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–753. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol – recent advances. Chem Biodivers. 2007;4:1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- Miller ML, Creehan KM, Angrish D, Barlow DJ, Houseknecht KL, Dickerson TJ, et al. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone) Drug Alcohol Depend. 2013;127:248–253. doi: 10.1016/j.drugalcdep.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected] Br J Psychiatry. 2010;197:285–290. doi: 10.1192/bjp.bp.110.077503. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Gardener C, Schafer G, Swan S, Demarchi C, Freeman TP, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med. 2012;42:391–400. doi: 10.1017/S0033291711001322. [DOI] [PubMed] [Google Scholar]
- Nava F, Carta G, Gessa GL. Permissive role of dopamine D(2) receptors in the hypothermia induced by delta(9)-tetrahydrocannabinol in rats. Pharmacol Biochem Behav. 2000;66:183–187. doi: 10.1016/s0091-3057(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Oreja-Guevara C. Clinical efficacy and effectiveness of Sativex, a combined cannabinoid medicine, in multiple sclerosis-related spasticity. Expert Rev Neurother. 2012;12:3–8. doi: 10.1586/ern.12.11. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitet F, Jeantaud B, Reibaud M, Imperato A, Dubroeucq MC. Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of delta9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63:PL1–PL6. doi: 10.1016/s0024-3205(98)00238-0. [DOI] [PubMed] [Google Scholar]
- Robson P. Abuse potential and psychoactive effects of delta-9-tetrahydrocannabinol and cannabidiol oromucosal spray (Sativex), a new cannabinoid medicine. Expert Opin Drug Saf. 2011;10:675–685. doi: 10.1517/14740338.2011.575778. [DOI] [PubMed] [Google Scholar]
- Roser P, Juckel G, Rentzsch J, Nadulski T, Gallinat J, Stadelmann AM. Effects of acute oral Delta9-tetrahydrocannabinol and standardized cannabis extract on the auditory P300 event-related potential in healthy volunteers. Eur Neuropsychopharmacol. 2008;18:569–577. doi: 10.1016/j.euroneuro.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Chen N, Hilliard A, White L, Stott C, Russo E, et al. A randomized, double-blind, placebo-controlled, crossover study to evaluate the subjective abuse potential and cognitive effects of nabiximols oromucosal spray in subjects with a history of recreational cannabis use. Hum Psychopharmacol. 2011;26:224–236. doi: 10.1002/hup.1196. [DOI] [PubMed] [Google Scholar]
- Son MH, Kim HD, Chae YN, Kim MK, Shin CY, Ahn GJ, et al. Peripherally acting CB1-receptor antagonist: the relative importance of central and peripheral CB1 receptors in adiposity control. Int J Obes. 2010;34:547–556. doi: 10.1038/ijo.2009.253. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Delta(9)tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J Psychopharmacol. 2012a;26:1299–1306. doi: 10.1177/0269881112443743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA. Delta9-tetrahydrocannabinol attenuates MDMA-induced hyperthermia in rhesus monkeys. Neuroscience. 2012b;201:125–133. doi: 10.1016/j.neuroscience.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN. Hyperthermia induced by 3,4-methylenedioxymethamphetamine in unrestrained rhesus monkeys. Drug Alcohol Depend. 2006;82:276–281. doi: 10.1016/j.drugalcdep.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulunay FC, Ayhan IH, Portoghese PS, Takemori AE. Antagonism by chlornaltrexamine of some effects of delta 9-tetrahydrocannabinol in rats. Eur J Pharmacol. 1981;70:219–224. doi: 10.1016/0014-2999(81)90217-x. [DOI] [PubMed] [Google Scholar]
- Turkanis SA, Karler R. Cannabidiol-caused depression of spinal motoneuron responses in cats. Pharmacol Biochem Behav. 1986;25:89–94. doi: 10.1016/0091-3057(86)90235-2. [DOI] [PubMed] [Google Scholar]
- Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, et al. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2008;94:191–198. doi: 10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10:434–441. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- Wiley JL, O'Connell MM, Tokarz ME, Wright MJ., Jr Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, et al. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS ONE. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vandewater SA, Parsons LH, Taffe MA. Δ9Tetrahydrocannabinol impairs reversal learning but not extra-dimensional shifts in rhesus macaques. Neuroscience. 2013a;235:51–58. doi: 10.1016/j.neuroscience.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vandewater SA, Taffe MA. Cannabidiol attenuates deficits of visuospatial associative memory induced by Delta(9) tetrahydrocannabinol. Br J Pharmacol. 2013b;170:1365–1373. doi: 10.1111/bph.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Teixeira NA, Karniol IC. Pharmacological interaction of the effects of delta 9-trans-tetrahydrocannabinol and cannabidiol on serum corticosterone levels in rats. Arch Int Pharmacodyn Ther. 1984;269:12–19. [PubMed] [Google Scholar]
- Zuardi AW, Hallak JE, Crippa JA. Interaction between cannabidiol (CBD) and (9)-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology (Berl) 2012;219:247–249. doi: 10.1007/s00213-011-2495-x. [DOI] [PubMed] [Google Scholar]


