Abstract
Background and purpose
Itch is associated with increased sensitization to nociceptive stimuli. We investigated whether 3-iodothyroacetic acid (TA1), by releasing histamine, induces itch and increases sensitization to noxious and painful heat stimuli.
Experimental Approach
Itch was evaluated after s.c. administration of TA1 (0.4, 1.32 and 4 μg·kg−1). Mice threshold to noxious (NHT) and to painful heat stimuli were evaluated by the increasing-temperature hot plate (from 45.5 to 49.5°C) or by the hot plate (51.5°C) test, respectively, 15 min after i.p. injection of TA1 (0.4, 1.32 and 4 μg·kg−1). Itch, NHT and pain threshold evaluation were repeated in mice pretreated with pyrilamine. Itch and NHT were also measured in HDC+/+ and HDC−/− following injection of saline or TA1 (1.32, 4 and 11 μg·kg−1; s.c. and i.p.). pERK1/2 levels were determined by Western blot in dorsal root ganglia (DRG) isolated from CD1 mice 15 min after they received (i.p.): saline, saline and noxious heat stimulus (46.5°C), TA1 (0.1, 0.4, 1.32, 4 μg·kg−1) or TA1 1.32 μg·kg−1 and noxious heat stimulus.
Key Results
TA1 0.4 and 1.32 μg·kg−1 induced itch and reduced NHT; pyrilamine pretreatment prevented both of these effects. TA1 4 μg·kg−1 (i.p.) reduced pain threshold without inducing itch or modifying NHT. In HDC−/− mice, TA1 failed to induce itch and to reduce NHT. In DRG, pERK1/2 levels were significantly increased by noxious heat stimuli and by TA1 0.1, 0.4 and 1.32 μg·kg−1; i.p.
Conclusions and Implications
Increased TA1 levels induce itch and an enhanced sensitivity to noxious heat stimuli suggesting that TA1 might represent a potential cause of itch in thyroid diseases.
Tables of Links
| LIGANDS | |
|---|---|
| 3-iodothyronamine | Histamine |
| Capsaicin | Pyrilamine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,b,c).
Introduction
3-iodothyroacetic acid (TA1) is an endogenous compound produced by oxidative deamination of 3-iodothyronamine (T1AM), probably representing a terminal metabolite of thyroid hormone.
T1AM and TA1, pharmacologically administered to rodents, modify animal behaviour and metabolism since they favour learning, reduce pain threshold and increase plasma glycaemia (Manni et al., 2013; Musilli et al., 2014). Recently, we reported that these effects are dependent on the release of histamine, providing evidence for a novel signalling pathway connecting thyronamine derivatives with the histaminergic system (Musilli et al., 2014).
Histamine interacts with four receptor subtypes and plays a major role in several processes including memory (Köhler et al., 2011), inflammation, hyperalgesia and itch (Sikand et al., 2011). Itch is a cutaneous sensation which provokes the desire to scratch, and it is usually associated with a generalized pain sensation unrelated to the intensity of the nociceptive input (Gronroos et al., 1997; Schelmz, 2014). The reason for this enhanced sensitivity of nociceptive neurons of dorsal root ganglia (DRG) has been the subject of extensive investigations aimed at determining if the association between itch and increased nociceptive sensitivity is the result of specific peripheral afferent itch-sensitive fibres (Sikand et al., 2011; Nakagawa and Hiura, 2013) that communicate with efferent nociceptive pathways. In fact, pain and itch share mediators at the cellular and molecular levels, and there is evidence that itch-inducing chemicals may activate some nociceptive primary afferent fibres, suggesting the existence of a neuron subpopulation activated by pruritogenic and also by painful stimuli (Ross et al., 2014).
Histamine type 1 receptors (H1 receptors) and transient activated receptor channels type 1 (TRPV1), both expressed in sensory C fibres of the DRG, might be involved in the increased sensitivity of sensorial C fibres mediating itch and the response to noxious heat stimuli (Nakagawa and Hiura, 2013). In particular, TRPV1 senses small changes in environmental temperature but also stimuli that are painful and hurt. H1 receptor activation is also involved in itch and in histamine-induced hyper-response to noxious heat. Interestingly, a facilitatory role for the H1 receptor on capsaicin-sensitive TRPV1 of DRG has been demonstrated (Kajihara et al., 2010).
The present investigation was aimed at testing the hypothesis that TA1, by promoting histamine release, might induce pruritus and, at the same time, modify the threshold to noxious and painful heat stimuli.
Methods
Animals
All animal care and experimental procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications no. 80-23, revised 1996) and were approved by the Animal Care Committee of the Department of Pharmacology, University of Florence, in compliance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS no. 123) and the European Community Council Directive of 24 November 1986 (86/609/EEC). All efforts were made to minimize the number of animals used and their suffering.
A total of 100 male mice (CD1 strain; 20–30 g) from Harlan-Nossan (Udine, Italy) were used. Animals were kept at 22 ± 1°C with a 12 h light–dark cycle (light on at 07:00 h) and were fed a standard laboratory diet with water ad libitum. Five mice were housed per cage.
All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
HDC−/− mice
Histidine decarboxylase knockout (HDC−/−) and their wild-type littermate mice (HDC+/+; 129/Sv background; 18–22 g of body weight) were a kind gift from Dr Ohtsu, Japan (Ohtsu et al., 2001). The animals were housed by four to six in standard transparent laboratory cages in a temperature-controlled room (22 ± 1°C) and kept in a 12 h light–dark cycle (lights on at 07:00 h) with access to food and water ad libitum.
Detection of the HDC gene
The genotypes were confirmed at birth by amplifying the DNA prepared from tale biopsies by PCR, as described by Anaclet et al. (2009).
Itch
Itch was evaluated by measuring the time of onset, intensity and duration of scratching behaviour.
CD1 or HDC mice were put into an observation cylinder (d = 24.5 cm) for about 10 min to acclimatize, as described by Kuraishi et al. (1995) with minor modifications. Mice were then injected s.c., to allow a rapid activation of dermal mastocytes, with 200 μL of TA1 (0.4, 1.32 and 4 mg·kg−1 and 1.32, 4 and 11 mg·kg−1 for CD1 and HDC respectively; n = 10 for each dose) or vehicle (saline; Veh) into the caudal part of their back. Immediately after the injection, mice were put back into the observation cylinder (one animal per cylinder). In another set of experiments, pyrilamine (10 mg·kg−1), an antagonist of H1 receptors, was administered i.p. 30 min before the injection of TA1 or vehicle.
The time of onset and intensity of scratching were evaluated by three independent operators which observed animals over a 20 min period. Results are expressed as the mean ± SEM.
The increasing-temperature hot plate test to measure noxious heat threshold (NHT)
NHT was determined by a manually controlled increasing-temperature hot plate, as described by Almási et al. (2003) with minor modifications. CD1 or HDC−/− or HDC+/+ mice received i.p. (to obtain systemic bioavailability of TA1 a condition potentially including peripheral and central mechanisms underlying the hyperalgesic effects) TA1 (0.4, 1.32 and 4 or 11 μg·kg−1) or vehicle (n = 10 for each group) and, after 15 min, they were placed on the hot plate device set at 45.5 or 46.5°C in the case of CD1 or HDC mice respectively. Mice were then taken off the plate and put back in the cage for 1 min allowing the temperature of the plate to increase of one degree. Mice were then again placed on the plate and their behaviour was observed for 60 s. This procedure was repeated three or four times. In fact, the last observation was carried out at the temperature of 47.5 or 49.5°C in the case of CD1 or HDC mice respectively. The latency of a licking, flinching or jumping response was measured, with a cut-off time of 60 s.
Determination of mice pain threshold
CD1 mice received i.p. TA1 (0.4, 1.32 and 4 μg·kg−1) or vehicle (n = 10 for each group) and, after 15 min, they were placed on the hot plate device set at 51.5°C. The latency of licking, flinching or jumping response was measured. The cut-off time was set at 45 s to minimize skin damage.
In another set of experiments, mice received an i.p. injection of pyrilamine (10 mg·kg−1), or zolantidine, an antagonist at H2 receptors (5 mg·kg−1), or vehicle (saline) 15 min before receiving TA1 (0.4, 1.32 and 4 μg·kg−1) or saline (i.p.). Fifteen minutes later, mice were placed on the hot plate and the test was repeated.
Isolation of rat DRG neurons for electrophysiology
Wistar rats (7–10 days old) were killed by cervical dislocation followed by decapitation. DRG neurons were prepared as previously described (Vellani et al., 2004). Briefly, 20–30 ganglia were isolated from the full length of the spinal column following removal of the spinal cord.
After incubation in collagenase (2.5 mg·mL−1) for 1 h at 37°C, ganglia were mechanically triturated with a 45 μm sterile needle. The cell suspension was filtered in 40 μm Nylon filter (BD Falcon, Franklin Lakes, NJ, USA), centrifuged and re-suspended in DMEM (Gibco, Monza, Italy) supplemented with 50 U·mL−1 penicillin, 0.05 mg·mL−1 streptomycin (Invitrogen), 1% L-glutamine (Invitrogen), 10% FBS (Gibco), 50 ng·mL−1 nerve growth factor (Promega, Milano, Italy) and 1.25 μg·mL−1 cytosine β-D-arabinofuranoside (Ara-C, Sigma, St. Louis LO, USA). DRG neurons were plated onto 13 mm borosilicate cover glass previously coated with poly L-lysine (100 μg·mL−1; Sigma Aldrich) and laminin (10 μg·mL−1, Sigma Aldrich). The medium was changed after 24 h. All electrophysiology recordings were made within 72 h from dissociation.
Electrophysiology
Extracellular solution contained (in mM): 140 NaCl, 2 CaCl2, 1 MgCl2, 3 KCl, 10 HEPES, 10 D(+) glucose, adjusted to pH = 7.3 with NaOH, osmolarity 300–310 mosmol·L−1. The pipette was filled with an intracellular solution containing (in mM): 120 K-gluconate, 15 KCl, 10 HEPES, 5 EGTA, 2 MgCl2, 5 Na2phosphocreatine, 0.3 NaGTP, 2 MgATP and adjusted to pH = 7.3 with KOH, osmolarity 310–315 mosmol·L−1. Cells were continuously perfused with extracellular solution using a gravity-fed perfusion system. The stock solution of capsaicin (Sigma) was 10 mM in DMSO. Voltage-clamp experiments were performed using a Warner PC-505B patch clamp amplifier (Warner, Handen, CT, USA) and digitalized with a Digidata 1440 A and Clampex 10 (Axon, Sunnyvale, CA, USA). Pipettes, resistance 3–5 MΩ, were pulled from borosilicate capillaries (Harvard Apparatus, London, UK) using a Narishige PP830 vertical puller (Narishige International Ltd, London, UK). Pipette capacitance transients were cancelled while no whole-cell compensation was used. Cells were held at −60 mV. Signals were sampled at 10 kHz, low-pass filtered at 1 kHz. All recordings were made at 21–23°C.
Identification of signalling activated in mice DRG by TA1 and by temperature
DRG were freshly isolated, using the same procedure as described for rats, from mice receiving the following treatments:
i.p. injection of saline and killed after 15 min;
saline i.p. and, after 15 min, mice were put on the hot plate set at 46.5°C until a response such as licking, jumping or flicking was observed, after which they were killed.
1.32 μg·kg−1 TA1 i.p. and, after 15 min, put on the hot plate set at 46.5°C, their NHT, waiting for licking, jumping or flicking, after which they were killed.
0.1, 0.4. 1.32 and 4 μg·kg−1 TA1 was injected i.p. and the mice killed after 15 min.
This experiment was repeated twice.
DRG were stored in 0.5% SDS at −80°C until they were used for Western blot analysis.
Western blot analysis
Proteins (20 μg) isolated from DRG were separated on 10% SDS-PAGE and transferred into PVDF membranes (120 min at 100 V) using standard procedures. Blots were incubated overnight at 4°C with specific antibody against ERK1/2 phosphorylated on Thr202/Tyr204 (pERK1/2, Cell Signalling Technology, Denver, MA, USA) and total ERK (Cell Signalling Technology) as reference protein. Primary antibodies were diluted in PBS containing 1% albumin and 0.05% Tween. The antigen–antibody complexes were visualized using appropriate secondary antibodies (1:10 000, diluted in PBS containing 1% albumin and 0.05% tween) and left for 1 h at room temperature. Blots were then extensively washed and developed using an enhanced chemiluminescence detection system (Pierce, Rodano, Italy). Exposition and developing time were standardized for all blots. Densitometric analysis of scanned images was performed on a Macintosh iMac computer using the public domain NIH Image program. Results represent mean ± SEM of three different gels and they are expressed as arbitrary units (AU), consisting of the ratio between the expression levels of the protein of interest and of GAPDH.
Statistical analysis
Data are expressed as mean ± SEM of independent experiments. Statistical analysis was performed by one-way anova, followed by Student-Newman-Keuls multiple comparison post hoc test. When the experimental setting included only two groups, unpaired t-test was used. The threshold of statistical significance was set at P < 0.05. Data analysis was performed by GraphPad Prism 5.0 statistical program (GraphPad software, San Diego, CA, USA).
Results
TA1 injected s.c. induced itch: effect of pyrilamine pretreatment
Histamine is among the main endogenous mediators of itch (Rosa and Fantozzi, 2013). As shown in Figure 1, itch occurred 10 min after an injection of 0.4 μg·kg−1 TA1 and persisted for 5 min. After 1.32 μg·kg−1 TA1 administration, scratching occurred within 5 min and persisted for an additional 15 min. Interestingly, following 4 μg·kg−1 TA1, the onset, intensity and duration of scratching were similar to those observed after saline treatment (Figure 1A).
Figure 1.
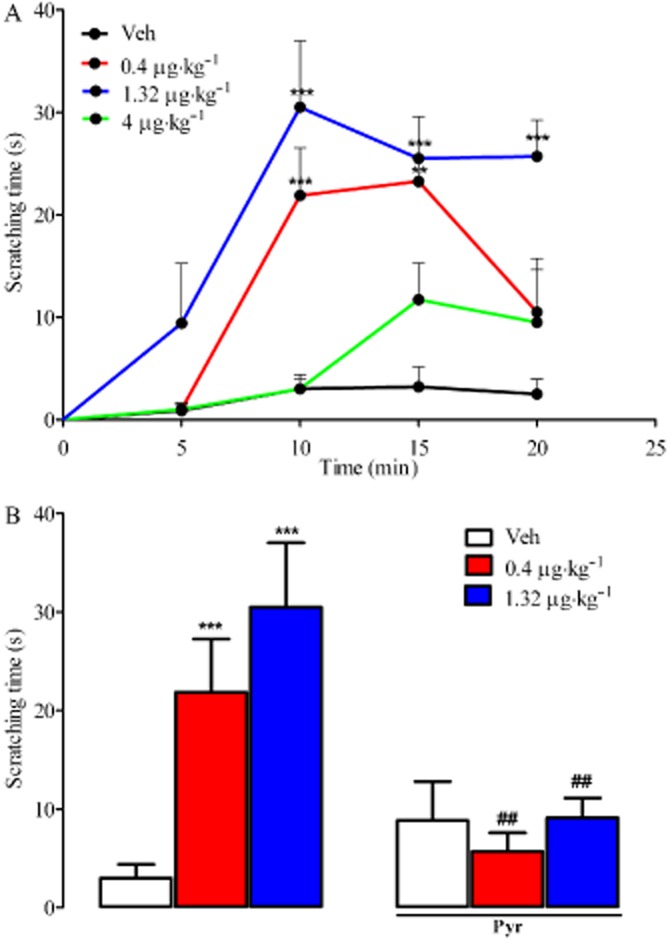
TA1 induces itch: the effect of pyrilamine pretreatment. Mice were treated s.c. with saline (Veh) or with TA1 (0.4, 1.32 and 4 μg·kg−1) and observed for the time of onset and intensity of a scratching behaviour as described in Methods (A). Results are mean ± SEM. n = 10 in each group. **P < 0.01 versus Veh; ***P < 0.001 versus Veh. (B) Itch was also measured in mice pretreated with 10 mg·kg−1 pyrilamine (i.p.) or saline (i.p.) 30 min before 0.4, 1.32 and 4 μg·kg−1 TA1 or saline s.c. The intensity of itch after 10 min from TA1 (0.4, 1.32 μg·kg−1) was reported. Results are mean ± SEM. n = 10 in each group. ***P < 0.001 versus Veh; ##P < 0.01 versus TA1 in the absence of pyrilamine.
In mice pretreated with pyrilamine (10 mg·kg−1 i.p.), the intensity of pruritus induced by TA1 (0.4 and 1.32 μg·kg−1) was significantly lower than that observed in mice not receiving pyrilamine (Figure 1B).
Determination of the NHT of CD1 mice
CD1 mice injected i.p. with saline did not show licking, flinching or jumping, in the 45.5–46.5°C temperature range. Instead, mice responded at the temperature of 47.5°C, which was therefore considered as their NHT (Figure 2A).
Figure 2.

The NHT of CD1 mice is reduced by TA1: the effect of pyrilamine pretreatment. Mice were treated i.p. with saline (Veh) or TA1 (0.4, 1.32 and 4 μg·kg−1) (A). After 15 min from Veh or TA1 injection their threshold to noxius heat (from 45.5 to 47.5°C) was measured as described in Methods. In another set of experiments, mice were pretreated with 10 mg·kg−1 pyrilamine (i.p.) or Veh (i.p) 15 min before 1.32 μg·kg−1 TA1 i.p. (B). The NHT of the mice was measured as described in ‘Methods’. Results are mean ± SEM of 10 mice per group. *P < 0.05 versus Veh; ***P < 0.001 versus Veh.
TA1 decreases the NHT of CD1 mice: the effect of pyrilamine pretreatment
In animals treated with 0.4 μg·kg−1 TA1, NHT decreased to 46.5°C. When the plate reached the temperature of 47.5°C, the latency to respond was similar to that observed in saline-treated mice.
The NHT of CD1 mice decreased to 45.5°C following 1.32 μg·kg−1 TA1. The latency to respond was also significantly lower than that observed in saline-injected mice at the temperature of 46.5°C, but not at 47.5°C (Figure 2A).
At the dose of 4 μg·kg−1, TA1 did not modify the NHT, which remained set at 47.5°C (Figure 2A).
In order to verify the involvement of histamine in the TA1-induced reduction of NHT, experiments were repeated in mice pretreated with pyrilamine (10 mg·kg−1; i.p.) or saline. Pyrilamine per se did not modify the NHT, which remained set at 47.5°C. However, pyrilamine pretreatment completely abolished the reduction in the NHT to 45.5 and 46.5°C induced by 0.4 (data not shown) and 1.32 μg·kg−1 TA1 (Figure 2B).
TA1, at the dose of 4 μg·kg−1, injected i.p.-induced hyperalgesia
To ascertain whether the same doses of TA1 active in reducing NHT of CD1 mice were also able to reduce their threshold to painful stimuli, mice were injected i.p. with TA1 (0.4, 1.32 and 4 μg·kg−1) and, after 15 min, they were placed on the hot plate set at 51.5°C. As shown in Figure 3, mice treated with 0.4 and 1.32 μg·kg−1 TA1 or with vehicle showed a similar latency to respond. In contrast, with 4 μg·kg−1 TA1, a significant reduction in the latency to respond was observed (Figure 3). The hyperalgesic effect of 4 μg·kg−1 TA1 was completely abolished in mice pretreated with pyrilamine (10 mg·kg−1; i.p.), but not with zolantidine (5 mg·kg−1; i.p.), 15 min before the TA1 injection (Figure 3).
Figure 3.
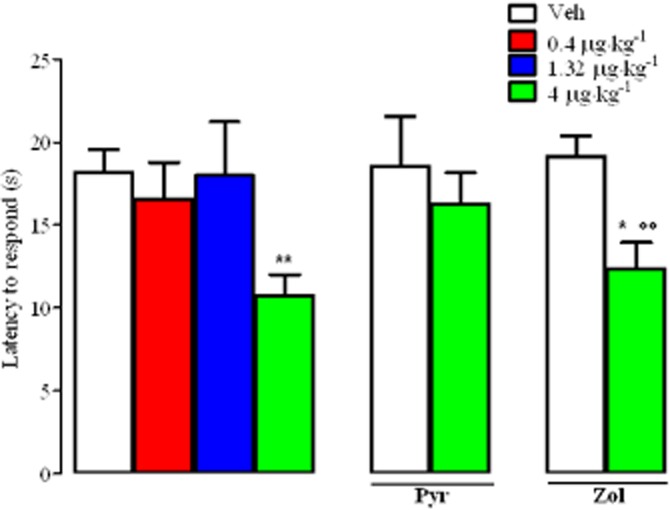
TA1 reduces pain threshold of CD1 mice: the effect of pyrilamine or zolantidine pretreatment. Mice were treated i.p. with saline (Veh) or TA1 (0.4, 1.32 and 4 μg·kg−1). After 15 min, their pain threshold was measured as described in Methods by the hot plate test (51.5°C). Experiments were repeated in mice pretreated (i.p.) with 10 mg·kg−1 pyrilamine or zolatidine (5 mg·kg−1) or saline (Veh) 15 min before 4 μg·kg−1 TA1. Results are mean ± SEM obtained from 10 mice for each group. *P < 0.05 versus Veh; **P < 0.01 versus Veh; °°P < 0.01 versus zolantidine alone.
Heat exposure and TA1 activated ERK1/2 in mouse DRG
Spinal extracellular signal-regulated kinases (ERKs) have been identified as mediators of nociceptive plasticity and of noxious stimulation (Watkins et al., 1997; Wiertelak et al., 1997). Therefore, we investigated the effects of noxious heat, TA1 or a combination of both stimuli on ERK 1/2 activation in our experimental model.
The baseline level of pERK1/2 in DRG was very low, while strong ERK1/2 activation was observed in DRG prepared from mice treated with saline i.p. and then placed on the hot plate set at 46.5°C (Figure 4A and B).
Figure 4.
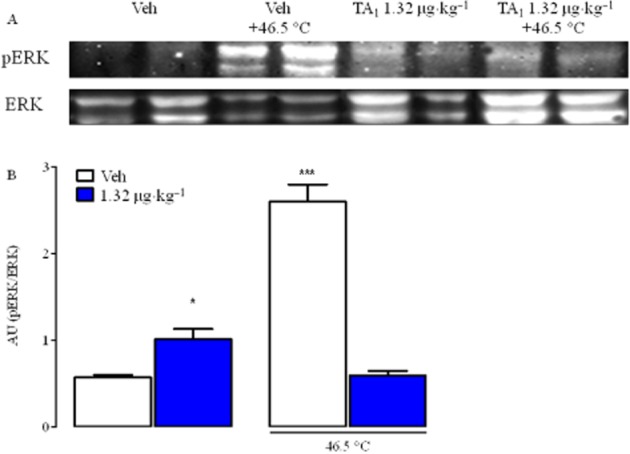
Noxious heat temperature (NHT) and TA1 induces pERK1/2 in DRG from CD1 mice. DRG were isolated from mice randomly treated with saline, TA1 1.32 μg·kg−1 with and without noxious heat stimulus and analysed for pERK1/2 levels by Western blot as described in Methods. (A) pERK1/2 in DRG protein lysates. A representative experiment is shown. (B) Densitometric analysis of pERK1/2 levels in DRG from CD1 mice. Results represent mean ± SEM of two different gels. *P < 0.05 versus Veh; ***P < 0.001 versus Veh.
TA1 1.32 μg·kg−1 activated ERK 1/2 to a lesser extent than noxious heat but higher than saline treatment.
In mice receiving both TA1 (1.32 μg·kg−1) and the noxious heat stimulus (46.5°C), ERK 1/2 activation was similar to that observed in mice treated with saline.
pERK1/2 levels did not increase in parallel with TA1 doses
Increased pERK1/2 compared to the baseline, where measured in DRG from mice receiving 0.1, 0.4 and 1.32 μg·kg−1 TA1, reached a maximum with 0.4 μg kg−1 TA1. Interestingly, in mice treated with 4 μg·kg−1 TA1, pERK1/2 levels were not different from basal levels (Figure 5A and B).
Figure 5.
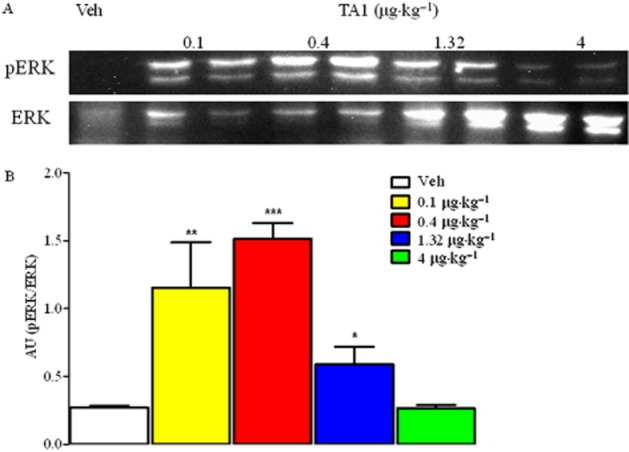
pERK1/2 levels in DRG from mice receiving increasing TA1 doses. DRG were isolated from mice randomly treated with saline or TA1 (0.1, 0.4, 1.32 and 4 μg·kg−1) and analysed for pERK1/2 levels by Western blot as described in Methods. (A) pERK1/2 in DRG protein lysates. A representative experiment is shown. (B) Densitometric analysis of pERK1/2 levels in DRG from CD1 mice. Results represent mean ± SEM of two different gels. *P < 0.05 versus Veh; **P < 0.01 versusVeh; ***P < 0.001 versus Veh.
TA1 did not activate TRPV1 in rat DRG neurons
TRPV1 is the channel involved in sensing temperature over the range 43.5–48.5°C and plays an important role in itch (Biro et al., 2007; Brito et al., 2014).
In order to verify whether TA1 had any direct effect on the TRPV1 channel, electrophysiological studies were performed on rat cultured primary sensory neurons Recordings were obtained from morphologically identified nociceptors (diameter less than 25 μm). Capsaicin was used to confirm the expression of functional TRPV1 channels and to study the effect of TA1 on TRPV1-mediated current. In control conditions, 150 nM capsaicin applied for 2 min induced a slow and transient inward current in 5 out of 5 neurons patched (−189.6 ± 10.5 pA; n = 5, Figure 6B). TA1 (1, 10, 100 nM) had no effect on whole-cell current when applied alone (n = 5, Figure 5A) nor did it modulate the capsaicin-dependent inward current when pre-incubated for 10 min before the addition of capsaicin (−198 ± 13.5 pA; P = 0.632 vs. capsaicin alone; n = 5; Figure 6A and B).
Figure 6.

Effect of TA1 on TRPV1 channels. (A) Typical whole-cell current traces recorded from putative nociceptors in culture. TA1 (1, 10 or 100 nM) shows no effect per se on whole-cell current in capsaicin-sensitive neurons (n = 5). (B) Left, representative traces obtained from cultured DRG neurons showing the capsaicin-induced, TRPV1-mediated currents in control and in presence of TA1. Right, scatter graph comparing the effect of capsaicin (150 nM) in control conditions and in neurons pre-incubated with TA1 (10 min, 10 nM). Pre-incubation with TA1 did not modulate capsaicin induced-inward currents (P = 0.632; n = 5).
TA1 failed to induce itch in HDC mice
Itch and scratching were completely absent following s.c. injection of TA1 (1.32, 4 and 11 μg·kg−1) in HDC+/+ but also in HDC−/− mice (data not shown).
NHT in HDC mice
As shown in Figure 7A, HDC+/+ mice injected i.p. with saline responded only when the plate reached the temperature of 49.5°C. This temperature was then considered as their NHT.
Figure 7.

NHT of HDC+/+ and HDC−/− mice: effect of TA1 administration. HDC+/+ were treated i.p. with saline (Veh) or TA1 (1.32, 4 and 11 μg·kg−1). After 15 min, their threshold to noxious heat (from 46.5°C to 49.5°C) was measured as described in Methods (A). In another set of experiments mice were pretreated with 10 mg·kg−1 pyrilamine (i.p.) or Veh (i.p.) 15 min before, 1.32 and 4 μg·kg−1 TA1 i.p. (B). HDC−/− mice were treated i.p. with saline (Veh) or with TA1 (1.32 μg·kg−1) to measure their NHT as described in Methods (C). Results are the means ± SEM of observations from 10 mice. *P < 0.05 versus Veh; **P < 0.01 versus Veh; #P < 0.05 and ##P < 0.01 versus TA1 without pyrilamine respectively. Results are the means ± SEM of observation from 10 mice.
TA1 reduced the NHT of HDC+/+ mice: effect of pyrilamine pretreatment
The NHT was not modified by 0.4 μg·kg−1 TA1 i.p. (data not shown) while it was reduced to 48.5°C following 1.32, 4 and 11 μg·kg−1 TA1 (i.p.). The reduction was not dose-related (Figure 7A). HDC+/+ treated i.p. with TA1 (1.32, 4 and 11 μg·kg−1) or with Veh (i.p.) showed a similar latency to respond at 49.5°C (Figure 7A).
To verify whether the reduction of the NHT of HDC+/+ mice was the consequence of H1 receptor activation, mice were pretreated with pyrilamine (10 mg·kg−1) or with saline (i.p.) 15 min before saline or TA1 treatment (i.p.).
As shown in Figure 7B, pyrilamine pretreatment (i.p.) completely prevented the effect of 1.32 and 4 and 11 μg·kg−1 TA1.
TA1 fails to affect the NHT of HDC−/− mice
HDC−/− mice injected i.p. with saline started licking only when the plate reached the temperature of 49.5°C, showing the same NHT of HDC+/+. In HDC−/− mice, 1.32 μg·kg−1 TA1 (i.p.) failed to produce any reduction in the NHT, which remained set at 49.5°C (Figure 7C).
Discussion
In this study, we showed for the first time that TA1, one of the end metabolites of thyroid hormone, induces itch and reduces the NHT of mice, by promoting histamine release. Our results suggest that increased endogenous TA1 levels may be considered as a potential cause of itch, which is associated with an augmented sensitivity to thermal stimuli. In addition, our results support the clinical usefulness of H1 receptor antagonists in controlling itch associated with thyroid diseases.
In particular, we found that TA1 at the doses of 0.4 and 1.32 μg·kg−1 (but not of 4 μg·kg−1) induced pruritus, which was modulated by pyrilamine, thus indicating it was mediated by H1 receptor activation. Surprisingly, TA1 failed to induce itch in HDC mice, irrespective of their histamine content, thus suggesting that this strain is less sensitive to TA1 effects than CD1 mice. The reason for this difference is, as yet, unknown. However, since endogenous TA1 levels have been reported to be lower (i.e. undetectable by the current assay technique) in HDC mice (Musilli et al., 2014), an alternative hypothesis is that the concentration achieved after pharmacological administration was not sufficient to induce behavioural effects.
At the same doses that induced itch, TA1 reduced the NHT of CD1 mice. Interestingly, the physiological NHT of mice was not modified by pyrilamine, whereas pyrilamine prevented the TA1-induced reduction of NHT. These results suggest that (i) TA1 reduces the threshold of sensory neuron activation by temperature; (ii) histamine is not involved in the physiological setting of this threshold, at least in our experimental conditions; (iii) TA1-induced reduction of NHT depends on H1 receptor activation.
The hypothesis that TA1-induced reduction of the NHT is mediated by histamine release is further supported by results obtained in HDC mice. In fact, TA1 (1.32, 4 and 11 μg·kg−1) reduced the NHT only in HDC+/+ and not in HDC−/− animals.
Activation of ERK1/2 is a typical event induced by noxious heat stimuli (Firner et al., 2006) but also by histamine receptor activation (Dandekar and Khan, 2011). Interestingly, the pathway(s) activated by TA1 also lead to ERK1/2 activation. Accordingly, increased pERK1/2 levels were found in DRG following exposure to either noxious temperatures or TA1, confirming TA1 as a potential mediator in neuronal or glial cells (Donnerer and Liebmann, 2010). However, the effects of TA1 and noxious temperature were not additive. To interpret these results, we suggest that the transduction pathways activated by noxious heat and TA1 share ERK1/2 activation, but the presence of both stimuli may lead to desensitization of the kinase. It is well known that desensitization may occur from over-activation of one target or of a different ones transmitting to a common intracellular cascade. Both possibilities might occur in the case of TA1. Since histamine does not appear to be involved in the physiological setting of the NHT, one could speculate that temperature and TA1 activate different downstream targets which have in common ERK1/2. In line with the desensitization hypothesis, we found that pERK1/2 levels in DRG did not increase in parallel with TA1 doses but, instead, pERK1/2 reached a maximum of activation at 0.4 μg·kg−1 and then it decreased resulting no differences in the levels at 4 μg·kg−1 TA1 from basal levels. Interestingly, following 4 μg·kg−1 TA1, mice did not experience itch or a reduction in NHT.
The latter result suggests that, depending on the levels of TA1, and then on the amount of histamine released, a specific sequence of temperature sensors with their intracellular cascades is recruited. Electrophysiological recordings exclude a role for TRPV1 as a sensor in our conditions. In particular, TA1 did not behave as a direct channel activator, a possibility suggested by its acidic nature, and it did not modify capsaicin-induced current. However, the lack of TA1 (i.e. histamine) facilitatory role on TRPV opening (Kajihara et al., 2010), could be due to the limitation of our models including the low number of neurons where TRPV1 and H1 receptors are co-localized.
In conclusion, our results show that TA1-induced itch, as well as a reduction in NHT, and these effects were mediated by H1 receptor activation following histamine release.
Lastly and remarkably, we found that CD1 and HDC mice showed different NHT, which was set at 47.5 and 49.5°C in CD1 and HDC (both wild-type and knockout mice) respectively. These results suggest that as observed for itch, NHT is strain-dependent but its value does not depend on endogenous histamine release. It is not known whether CD1 and HDC show a different expression of H1 receptors or TRPV1 or of other thermal sensors, in DRG. However, as we previously demonstrated, HDC mice have undetectable endogenous levels of TA1 (Musilli et al., 2014). We now add the information that HDC mice show a lower sensitivity to the effects of TA1, namely itch and a reduction in NHT. In the light of our present findings, the apparent absence of TA1 in HDC mice might indeed play a role in the different behavioural response of the two strains of mice.
It is well established that 1–4% of patients with thyroid diseases experience generalized pruritus, without flare (Ward and Bernhard, 2005), the pathophysiology of which is largely unknown, but it can be relieved by antihistamines (Hiramanek, 2004). Interestingly, hypothyroid patients also show a reduced pain threshold (Guieu et al., 1993). In line with this, the results of the present study show that TA1 is an inducer of itch, an effect accompanied by thermal hyperalgesia, and that both effects can be controlled by pyrilamine.
The present results suggest that TA1 accumulation might be involved in the onset of itch in thyroid diseases. This hypothesis should be tested by assaying TA1 levels in patients with this condition.
Furthermore, our findings confirm the existence of a novel signalling pathway integrating histamine and this putative thyroid hormone metabolite.
Acknowledgments
We thank Dr. Scanlan from the University of Oregon (USA) for supplying us with T1AM, and Mrs. Nadia Trevisan for helping in animal housing. This work was supported by a grant from the University of Florence.
Glossary
- DRG
dorsal root ganglia
- H1 receptor
histamine type 1 receptor
- HDC−/−
histidine decarboxylase knockout mice
- HDC+/+
histidine decarboxylase wild-type mice
- NHT
noxious heat threshold
- T1AM
3-iodothyronamine
- TA1
3-iodothyroacetic acid
- TRPV1
transient activated receptor channel type 1
Author contributions
A. L. designed and performed the behavioural and biochemical experiments; G. D S. performed the behavioural experiments; F. R. performed the electrophysiological experiments; A. M. analysed the electrophysiological data; C. M. performed the biochemical experiments; R. Z. and L. R. analysed the data and wrote the paper.
Conflict of interest
The authors state the absence of any conflict of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almási R, Pethő G, Bölcskei K, Szolcsányi J. Effect of resiniferatoxin on the noxious heat threshold temperature in the rat: a novel heat allodynia model sensitive to analgesics. Br J Pharmacol. 2003;139:49–58. doi: 10.1038/sj.bjp.0705234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP, et al. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-outmouse models. J Neurosci. 2009;29:14423–14438. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro T, Tóth BI, Marincsák R, Dobrosi N, Géczy T, Ralf P. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim Biophys Acta. 2007;1772:1004–1021. doi: 10.1016/j.bbadis.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Brito R, Sheth S, Mukherjea D, Rybak LP, Ramkumar V. TRPV1: a potential drug target for treating various diseases. Cells. 2014;3:517–545. doi: 10.3390/cells3020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar RD, Khan MM. Regulation of ERK2 phosphorylation by histamine in splenocytes. Immunopharmacol Immunotoxicol. 2011;33:250–258. doi: 10.3109/08923973.2010.499913. [DOI] [PubMed] [Google Scholar]
- Donnerer J, Liebmann I. Dorsal root ganglion neurons respond with prolonged extracellular signal-regulated protein kinase phosphorylation following noxious heat and cold stimulation. Neurosci Lett. 2010;472:109–113. doi: 10.1016/j.neulet.2010.01.064. [DOI] [PubMed] [Google Scholar]
- Firner M, Greffrath W, Treede DR. Phosphorylation of extracellular signal-related protein kinase is required for rapid facilitation of heat-induced currents in rat dorsal root ganglion neurons. Neuroscience. 2006;143:253–263. doi: 10.1016/j.neuroscience.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Gronroos M, Reunala T, Kartamaa M, Pertovaara A. Altered skin sensitivity in chronic itch: role of peripheral and central mechanisms. Neurosci Lett. 1997;228:199–202. doi: 10.1016/s0304-3940(97)00405-9. [DOI] [PubMed] [Google Scholar]
- Guieu R, Harley JR, Blin O, Pouget J, Serratrice G. Nociceptive threshold in hypothyroid patients. Acta Neurol. 1993;15:183–188. [PubMed] [Google Scholar]
- Hiramanek N. Itch: a symptom of occult disease. Aust Fam Physician. 2004;33:7. [PubMed] [Google Scholar]
- Kajihara Y, Murakami AM, Imagawa AT, Otsuguro BK, Itoa AS, Ohtaa TC. Histamine potentiates acid-induced responses mediating transient receptor potential v1 in mouse primary sensory neurons. Neuroscience. 2010;166:292–304. doi: 10.1016/j.neuroscience.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler CA, da Silva WC, Benetti F, Bonini JS. Histaminergic mechanisms for modulation of memory systems. Neural Plast. 2011;2011:328602. doi: 10.1155/2011/328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Manni ME, De Siena G, Saba A, Marchini M, Landucci E, Gerace E, et al. Pharmacological effects of 3-iodothyronamine (T1AM) in mice include facilitation of memory acquisition and retention and reduction of pain threshold. Br J Pharmacol. 2013;168:354–362. doi: 10.1111/j.1476-5381.2012.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musilli C, De Siena G, Manni ME, Logli A, Landucci E, Zucchi R, et al. Histamine mediates behavioural and metabolic effects of 3-iodothyroacetic acid, an endogenous end product of thyroid hormone metabolism. Br J Pharmacol. 2014;171:3476–3484. doi: 10.1111/bph.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Hiura A. Four possible itching pathways related to the TRPV1 channel, histamine, PAR-2 and serotonin. Malays J Med Sci. 2013;20:5–12. [PMC free article] [PubMed] [Google Scholar]
- Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AC, Fantozzi R. The role of histamine in neurogenic inflammation. Br J Pharmacol. 2013;170:38–45. doi: 10.1111/bph.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Hachisuka J, Todd AJ. Spinal microcircuits and the regulation of itch. In: Cartens E, Akihama T, editors. Itch: Mechanisms and Treatment. Boca Raton, FL: CRC Press; 2014. , Chapter 20. In: (eds). [PubMed] [Google Scholar]
- Schelmz M. Sensitization for itch. In: Cartens E, Akihama T, editors. Itch: Mechanism and Treatment. Boca Raton, FL: CRC Press; 2014. , Chapter 26. In: (eds). [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain. 2011;152:2485–2494. doi: 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Zachrisson O, McNaughton PA. Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated by the neurotrophin GDNF. J Physiol. 2004;560:391–401. doi: 10.1113/jphysiol.2004.067462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JR, Bernhard JD. Willan's itch and other causes of pruritus in the elderly. Int J Dermatol. 2005;44:267–273. doi: 10.1111/j.1365-4632.2004.02553.x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71:225–235. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- Wiertelak EP, Roemer B, Maier SF, Watkins LR. Comparison of the effects of nucleus tractus solitarius and ventral medial medulla lesions on illness-induced and subcutaneous formalin-induced hyperalgesias. Brain Res. 1997;748:143–150. doi: 10.1016/s0006-8993(96)01289-9. [DOI] [PubMed] [Google Scholar]


