Highlights
-
•
Growing cells incorporate thio-iodo-deoxyuridine and thio-bromo-deoxyuridine into DNA.
-
•
They are non-toxic but act as powerful UVA photosensitisers.
-
•
UVA lesions include DNA-protein and DNA–DNA crosslinks.
-
•
Singlet oxygen is involved in the formation of this potentially lethal damage.
-
•
Thio-halo-deoxynucleosides offer a potential selective therapeutic option.
Abstract
Photochemotherapy, the combination of a photosensitiser and ultraviolet (UV) or visible light, is an effective treatment for skin conditions including cancer. The high mutagenicity and non-selectivity of photochemotherapy regimes warrants the development of alternative approaches. We demonstrate that the thiopyrimidine nucleosides 5-bromo-4-thiodeoxyuridine (SBrdU) and 5-iodo-4-thiodeoxyuridine (SIdU) are incorporated into the DNA of cultured human and mouse cells where they synergistically sensitise killing by low doses of UVA radiation. The DNA halothiopyrimidine/UVA combinations induce DNA interstrand crosslinks, DNA-protein crosslinks, DNA strand breaks, nucleobase damage and lesions that resemble UV-induced pyrimidine(6-4)pyrimidone photoproducts. These are potentially lethal DNA lesions and cells defective in their repair are hypersensitive to killing by SBrdU/UVA and SIdU/UVA. DNA SIdU and SBrdU generate lethal DNA photodamage by partially distinct mechanisms that reflect the different photolabilities of their C–I and C–Br bonds. Although singlet oxygen is involved in photolesion formation, DNA SBrdU and SIdU photoactivation does not detectably increase DNA 8-oxoguanine levels. The absence of significant collateral damage to normal guanine suggests that UVA activation of DNA SIdU or SBrdU might offer a strategy to target hyperproliferative skin conditions that avoids the extensive formation of a known mutagenic DNA lesion.
1. Introduction
Many cancer therapies act by killing cells through the introduction of DNA damage. One of the major drawbacks of this therapeutic approach is its indiscriminate nature, resulting in potentially severe side effects including the development of therapy-induced cancer. Photochemotherapy partially obviates this problem by specifically targeting appropriately sensitised cancer cells with light. The combination of a photosensitiser and ultraviolet or visible radiation to induce synergistic cytotoxicity is effective for many skin conditions including psoriasis and cancer. Examples include psoralen plus UVA (PUVA) – for psoriasis and cutaneous T cell lymphoma – and photodynamic therapy (PDT) which is effective against head and neck and skin carcinomas (reviewed in [1]). Although these established approaches are effective, their high mutagenicity and association with cancer (PUVA) or pain and non-selectivity for cancer tissue (PDT) frequently result in unsatisfactory patient outcomes. Alternative therapies that combine enhanced selectivity for rapidly dividing cells and low radiation doses are clearly desirable.
Canonical DNA nucleobases absorb maximally around 260 nm and so have significant absorbance at both UVB (280–315 nm) and UVC (100–280 nm) wavelengths. UVB and UVC cause direct DNA damage that is lethal and potentially carcinogenic [2]. Their toxic effects are enhanced by the halopyrimidine deoxynucleosides 5-bromo-2’-deoxyuridine (BrdU) and 5-iodo-2’-deoxyuridine (IdU). These non-toxic ionising radiation and UVB/UVC sensitisers [3–5] are extensively used in various aspects of nucleic acid research. Both 5-halo-2’-deoxynucleosides absorb maximally at around 280 nm [6]. They are incorporated into the DNA of replicating cells where they interact synergistically with UVC or UVB to generate potentially lethal DNA strand-breaks [7], DNA interstrand crosslinks (ICLs) [8] and DNA-protein crosslinks (DPCs) [9].
In contrast to UVC and UVB, the less energetic, longer wavelength UVA (315–400 nm) is absorbed very poorly by canonical DNA bases and causes relatively little direct DNA damage [10]. DNA containing a UVA chromophore is, however, susceptible to damage by normally harmless UVA doses. Hoechst 33258 dye, a DNA-intercalating UVA chromophore, synergistically sensitises cells containing DNA BrdU or IdU to killing by UVA. The DNA damage spectrum induced by the Hoechst 33258/UVA combination in cells containing DNA BrdU or IdU is similar to that generated by UVB or UVC and includes strand breaks [11,12], base damage [13] and ICLs [14]. The potential clinical utility of the DNA binding bisbenzimidazoles like Hoechst 33258 is, however, limited by their genotoxicity and mutagenicity [15].
Modification of canonical nucleobases offers an alternative strategy to introduce a UVA chromophore into DNA. The presence of a sulphur atom shifts nucleobase absorbance maxima into the UVA region (approximately 340 nm). S4-thiothymine (S4T) and 6-thioguanine (6-TG) are UVA chromophores that can be extensively incorporated into cellular DNA where they sensitise to killing by normally harmless UVA doses. We have previously reported the synthesis of the UVA chromophores 5-bromo-4-thio-2’-deoxyuridine (SBrdU) [16] and 5-iodo-4-thio-2’-deoxyuridine (SIdU) [17]. Here we report that human cells can accumulate DNA SIdU and SBrdU. Non-toxic levels of these DNA-embedded UVA chromophores sensitise the formation of lethal DNA strand breaks, ICLs, DPCs and DNA base lesions by UVA and obviate the requirement for a DNA binding photosensitising dye.
2. Materials and methods
2.1. Chemicals
Synthesis of 5-bromo-4-thio-2’-deoxyuridine (SBrdU) and 5-iodo-4-thio-2’-deoxyuridine (SIdU) has been described previously [16,17]. 4-Thio-2’-deoxyuridine (SdU) was purchased from Carbosynth and 2’-deoxyuridine (dU) from Sigma.
2.2. Cell culture
Cells were obtained from Clare Hall Laboratories Cell Services. Wild type (F11.1) and ung−/− mouse embryonic fibroblasts (MEFs) [18] were grown in 50% DMEM/50% RPMI. MEFs from fancd2−/− and their WT counterpart mice [19], SV40 transformed human fibroblasts MRC5VA, XP12RO, XP129 [20] and HeLa were cultured in DMEM. All human cell lines were authenticated by STR profiling. Media were supplemented with 10% fetal calf serum. Survival was determined by MTT assays or by clonal survival. Cells were treated with nucleosides for 18 or 48 h, washed and UVA irradiated in PBS. For MMT assays, they were then seeded in normal medium into 96-well plates (500 cells/well) and the fraction of viable cells was assayed 7–10 days later. For clonal survival, treated cells were seeded into 6-well plates (300 cells/well) and colonies counted 10 days later. Each determination was performed in triplicate and experiments were repeated at least twice.
2.3. UVA irradiation
Cells were irradiated in PBS with a UVH 253 lamp (UV Light Technology, Ltd.) with a maximum output of 100 mW/cm2 at the source outlet. Emission wavelengths ranged from 320 to 400 nm with a maximum at 365 nm.
2.4. Analysis of photoproducts
2’-Deoxyribonucleosides were separated by HPLC on a Waters Symmetry C18 Reverse Phase column as described [21]. Thiodeoxyribonucleosides, which all maximally absorb at around 340 nm, were quantified by their absorbance at 342 nm (A342); dT, BrdU and dU were quantified by A260. To analyse photoproducts generated in vivo, MEFs were grown in the presence of 100 μM nucleoside for 48 h and UVA irradiated. Genomic DNA was extracted using the Wizard Genomic DNA purification kit (Promega). Purified DNA was digested to nucleosides with nuclease P1 followed by shrimp alkaline phosphatase as described [22]. SdU, BrdU and dU were identified by comparison to authentic standards.
2.5. FANCD2 immunoblotting
Cell extracts (50 μg protein) were separated on 3–8% Tris-Acetate gels (Invitrogen) and transferred to Immobilon-P membranes (Millipore), which were probed with anti-FANCD2 antibody (Novus Biologicals). Antigen-antibody complexes were detected by ECL blotting detection reagent (GE Healthcare).
2.6. Comet assay
Alkaline comet assays were performed as described [23] on HeLa cells that had been grown for 48 h in the presence of SIdU or SBrdU and UVA irradiated. Targets of the uracil (UNG) or OGG1 DNA glycosylases were revealed by washing cell lysates three times with glycosylase reaction buffer and then incubating them with UNG (Trevigen) (1 unit/50 μl buffer per slide for 1 h at 37 °C) or OGG1 (Trevigen) (2 units/50 μl buffer per slide for 1 h at 37 °C) before electrophoresis. Control slides were treated with buffer alone.
DNA crosslinking was assayed by treatment with 15 Gy γ-radiation prior to UVA irradiation. Additionally, cells were washed three times with TE, pH 8.0 after lysis and then incubated with 1 mg/ml Proteinase K (Roche) in TE for 2 h at 37 °C. All comets were analysed using the Comet IV software.
2.7. Repair of UVC photoproducts
Cells were irradiated with 10 J/m2 UVC delivered from a UV Stratalinker 2400 (Stratagene). Immediately after irradiation, DNA was extracted using the QIAamp DNA Mini kit (Qiagen). Pyrimidine(6-4)pyrimidone (6-4 Py:Py) photoproducts were analysed by ELISA, using the 6-4 Py:Py photoproduct antibody (CosmoBio) according to the manufacturer’s instructions.
2.8. DNA protein crosslinks
DNA protein crosslinking was analysed using a modified protocol of the Wizard genomic DNA purification kit (Promega). Briefly, cells grown in the presence of SIdU or SBrdU for 48 h were UVA irradiated. Whole cell extracts were prepared, digested with RNAse A and incubated overnight at 50 °C with 100 μg/ml Proteinase K. Control extracts were incubated in the absence of Proteinase K. Residual protein was removed by precipitation and DNA recovered by isopropanol precipitation according to the manufacturer’s instructions. DNA concentrations were measured using a NanoDrop spectrophotometer (LabTech).
2.9. In vitro crosslinking
Oligodeoxynucleotides were obtained from atdbio.
5’-GAATCAGCCTGCACAGATACGAG-3’
5’-GAATCAGCCSGCACAGATACGAG-3’
5’-GAATCAGCTSGCACAGATACGAG-3’
5’-GAATCAGCGSTCACAGATACGAG-3’
S = SdU
Complementary strands that placed A or inosine opposite SdU were 5’end-radiolabelled with [32P] ATP (Perkin Elmer) using T4 polynucleotide kinase (New England Biolabs) and annealed in 50 mM Tris, pH 7.5, 100 mM NaCl. Duplex oligodeoxynucleotides were UVA irradiated (50 kJ/m2), heat denatured and the products analysed by electrophoresis on 16% polyacrylamide gels.
3. Results
3.1. UVA photosensitisation
SBrdU and SIdU are incorporated into DNA of growing cells and sensitise them to killing by UVA. Following growth of HeLa cells for 48 h in medium containing 100 μM SBrdU, the thionucleoside replaced between 0.045% and 0.06% of DNA thymidine in three independent experiments. Under the same conditions, SIdU substitution was between 0.025% and 0.033%. The approximately two-fold higher incorporation of SBrdU was maintained over a range of nucleoside concentrations. These levels of DNA substitution were not detectably toxic but caused significant UVA sensitivity (Fig. 1). Despite lower incorporation, SIdU was a more effective UVA photosensitiser than SBrdU. Photosensitisation required the pyrimidine thiol group and neither BrdU nor IdU potentiated UVA toxicity even at 10-fold higher levels of DNA substitution (Fig. 1). DNA SIdU and SBrdU were also UVA photosensitisers in the human HaCaT keratinocyte cell line (data not shown). The observed toxicity arises predominantly from incorporated SIdU or SBrdU rather than intracellular nucleoside/nucleotide pools and cell survival was largely unchanged by up to six-hours growth in normal medium to deplete unincorporated thionucleosides prior to UVA irradiation (Supplementary Fig. 1).
Fig. 1.
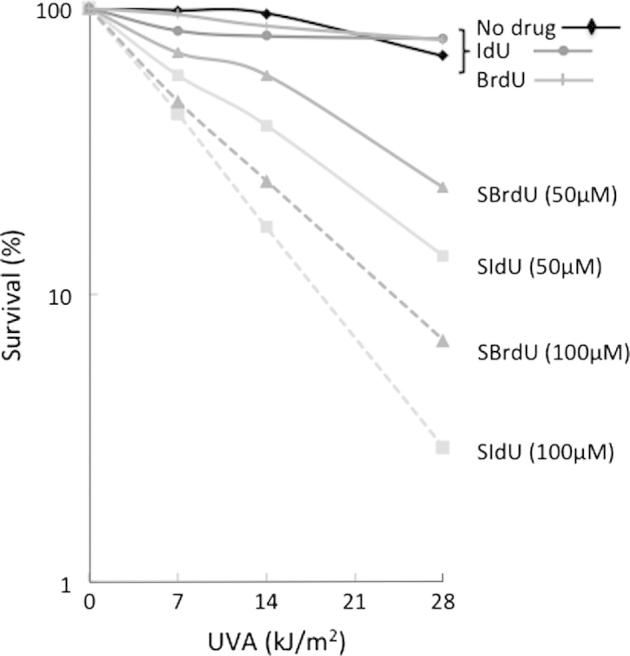
SIdU/UVA and SBrdU/UVA sensitivity. Survival: HeLa cells were grown in the presence of SIdU, SBrdU (50 or 100 μM), IdU (250 μM) or BrdU (125 μM) for 48 h and then UVA irradiated as indicated. Survival was assessed by colony formation. Because nucleoside incorporation varied somewhat between experiments resulting in different absolute survival values, a representative experiment (of three) is shown.
3.2. Potentially lethal DNA photoproducts
3.2.1. DNA–protein crosslinks (DPCs)
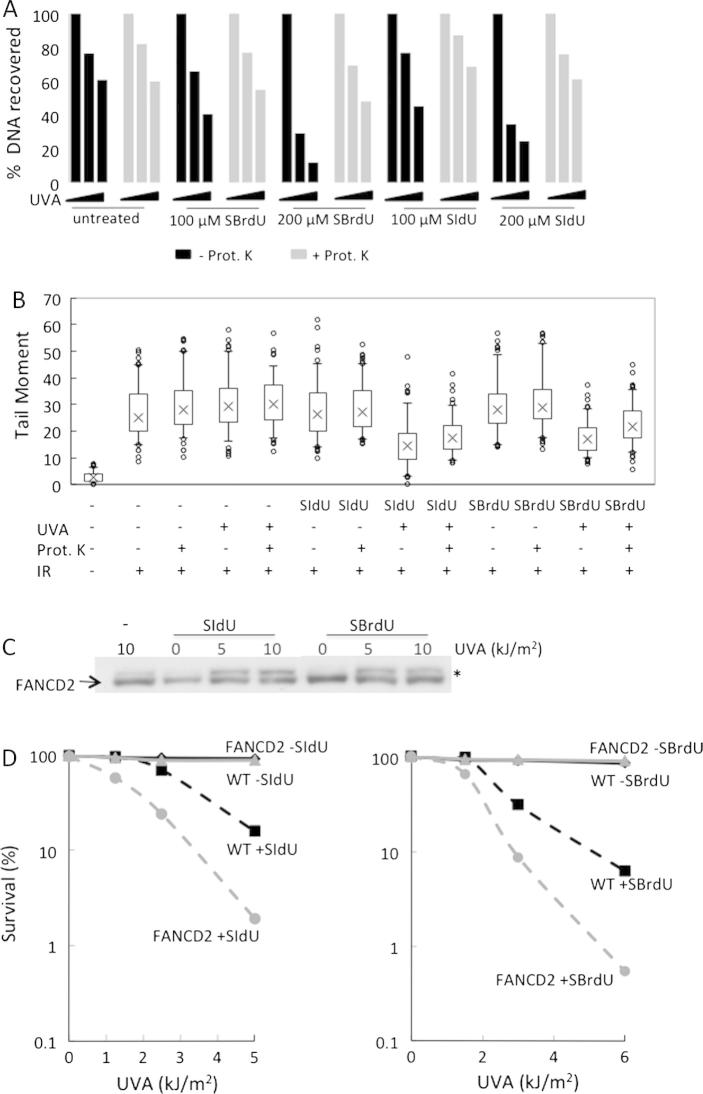
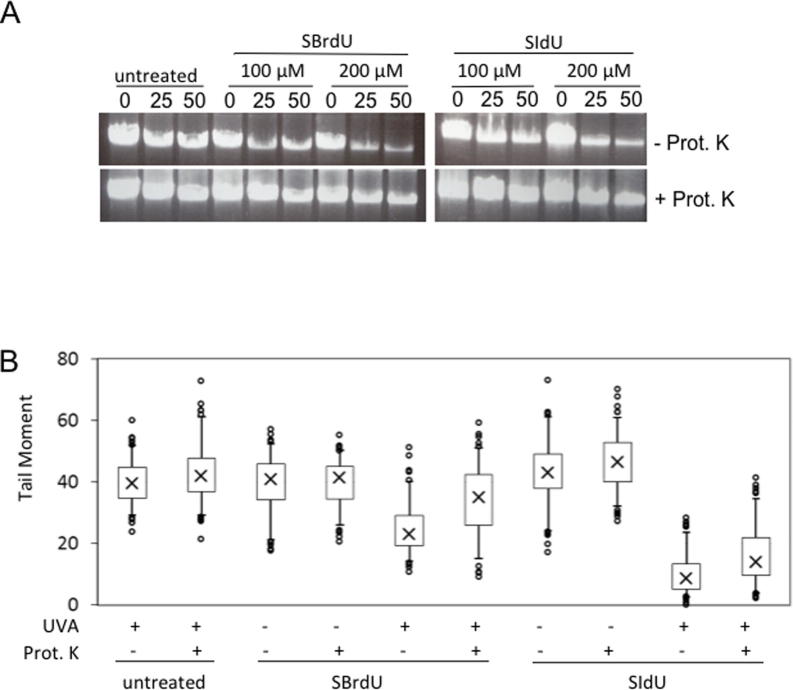
DNA-protein crosslinking by UVC irradiation of DNA containing BrdU or IdU is an established method to study nucleoprotein complexes [24]. DNA-protein crosslinks (DPCs) are particularly challenging to cellular DNA repair systems and are potentially lethal. DPC formation after SIdU/UVA or SBrdU/UVA treatment was investigated by a DNA purification protocol that includes a precipitation step at which DPCs co-precipitate with protein. This reduces DNA recovery. DNA recovery is restored if protease digestion precedes precipitation. Lysates prepared from SIdU/UVA- or SBrdU/UVA-treated cells were divided into equal parts one of which was extensively digested with Proteinase K. DNA was then precipitated. UVA irradiation reduced DNA yields from SIdU- or SBrdU-treated cells in a dose-dependent fashion (Supplementary Fig. 2, Fig. 2A). Proteinase K digestion reversed the DNA losses indicating the presence of DPCs. The formation of DPCs in SIdU/UVA and SBrdU/UVA cells was confirmed by comet assays (see below).
Fig. 2.
Crosslink formation and cell sensitivity. (A) DNA-protein crosslinks: HeLa cells were grown for 48 h in the presence of SIdU or SBrdU as indicated and irradiated with 0, 25 or 50 kJ/m2 UVA. Cells were lysed immediately after irradiation. Equal amounts of lysate were incubated overnight at 50 °C in the presence (+Prot K) or absence (−Prot K) of Proteinase K. Proteins were then precipitated and discarded. DNA recovered from the supernatant was quantified. Data are expressed as a percentage of recovery from unirradiated cells and are representative of three independent determinations. (B) Crosslink formation – comet assay: HeLa cells grown in the presence of 100 μM SIdU or SBrdU for 48 h were UVA irradiated (20 kJ/m2). They were then γ-irradiated with 15 Gy and analysed by the alkaline comet assay either immediately after irradiation or following Proteinase K digestion as indicated. DNA breakage is expressed as comet tail moment. (C) FANCD2 activation: HeLa cells that had been treated for 24 h with 25 μM SIdU or SBrdU were irradiated with the indicated UVA doses. 2 h after irradiation, cell extracts were analysed by western blotting with FANCD2 antibody. The positions of FANCD2 and its monoubiquitinated form (∗) are indicated. (D) Sensitivity of Fanconi anemia defective cells: FancD2-proficient (WT) and -deficient (FANCD2) MEFs were grown for 18 h in the presence of 5 μM SIdU (left panel) or 10 μM SBrdU (right panel) to obtain similar levels of DNA substitution. Following UVA-irradiation at the indicated doses, survival was assessed by colony formation. A representative experiment (of three) is shown.
3.2.2. DNA interstrand crosslinks
UVC activation of DNA IdU or BrdU induces ICLs [8,9]. The alkaline comet assay detects ICLs by their reduced comet tail moments following γ-irradiation. In combination with protease digestion, this assay also detects the presence of DPCs. Fig. 2B shows that SIdU/UVA and SBrdU/UVA treatment reduced comet tail moments, consistent with the formation of ICLs and/or DPCs. Proteinase K digestion prior to electrophoresis increased the comet tail moment confirming that both SBrdU/UVA and SIdU/UVA induce DPCs. Protease digestion did not fully restore tail moments to untreated values indicating that both SBrdU/UVA and SIdU/UVA also induce ICLs.
Cells process ICLs by the Fanconia anemia (FA) pathway [25] and FA cells are exquisitely sensitive these lesions. ICLs trigger monoubiquitination of the FANCD2 protein – a key step in FA pathway activation. UVA irradiation of SIdU- or SBrdU-treated HeLa cells induced robust FANCD2 modification (Fig. 2C), confirming that both treatments produced ICLs. To determine whether ICLs contribute to SIdU and SBrdU phototoxicity, we examined the sensitivity of FANCD2-defective MEFs. Fig. 2D shows that FANCD2−/− cells are more sensitive than their FANCD2+/+ counterparts. These findings implicate ICLs in the cytotoxicity of SIdU/UVA and SBrdU/UVA.
3.2.3. DNA strand breaks and uracil DNA glycosylase substrates
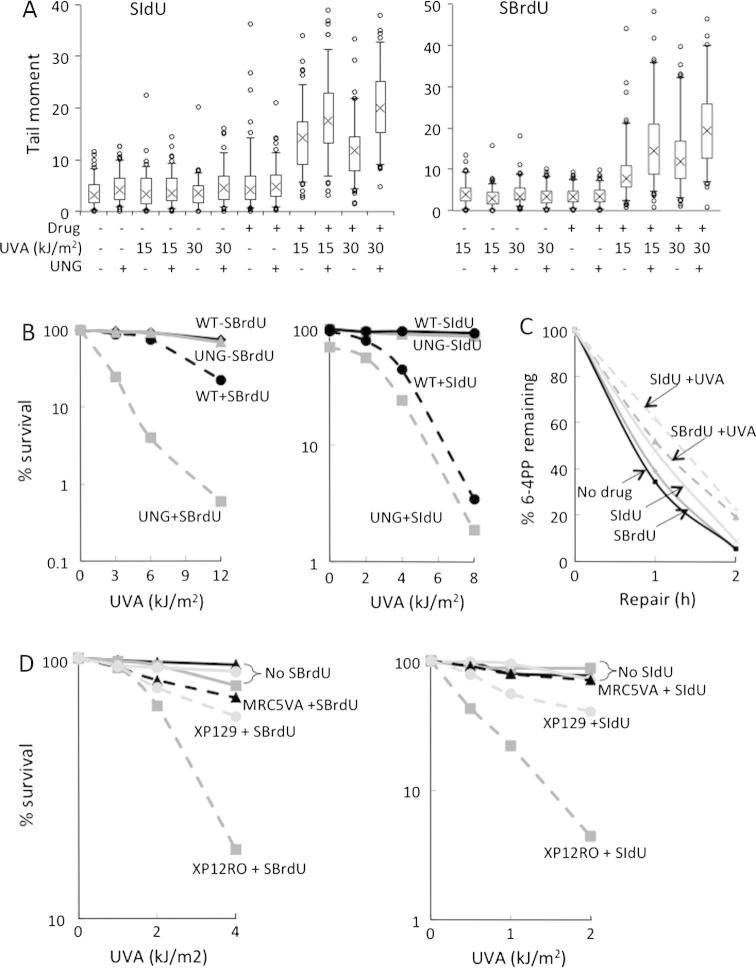
The alkaline comet assay was used to investigate the induction of single-strand DNA breaks and/or alkali-labile sites. Fig. 3A shows that both SIdU and SBrdU potentiated UVA-dependent increases in comet tail moment consistent with the induction of single-strand breaks/alkali-labile sites. Digestion with uracil DNA glycosylase (UNG) prior to electrophoresis caused a further increase in comet tail moment indicating that both treatments generate UNG substrates. No DNA breakage was detected when UNG digestion was combined with SIdU, SBrdU or UVA treatment alone, indicating that UNG substrates are UVA photoproducts of SIdU/SBrdU. In parallel experiments, pulse-field gel electrophoresis revealed that neither SIdU/UVA nor SBrdU/UVA induced detectable DNA double-strand breakage (data not shown).
Fig. 3.
DNA strand breaks, UNG substrates and NER. (A) Comet assays: HeLa cells were treated with 100 μM SIdU (left panel) or SBrdU (right panel) for 48 h and UVA irradiated. DNA strand breaks and/or abasic sites were determined by single cell electrophoresis with or without digestion with recombinant UNG as indicated. (B) Sensitivity of ung−/− MEFs: UNG-proficient (WT) and -deficient (UNG) cells were grown in the presence of SBrdU (5 and 30 μM, respectively; left panel) or SIdU (10 and 60 μM; right panel) for 24 h to ensure equal levels of DNA substitution (0.025% for SBrdU,0.022% for SIdU). They were UVA irradiated and survival was determined 7 days later by MTT assay. (C) 6:4 Py:Py excision: HeLa cells grown in 200 μM SIdU or 300 μM SBrdU for 48 h were irradiated as indicated with 20 kJ/m2 UVA followed by 20 J/m2 UVC and returned to normal growth medium. DNA was extracted at the times indicated and 6-4 Py:Py photoproducts were quantified by ELISA. (D) Sensitivity of NER-defective cells: MRC5VA (NER-competent), XP12RO (XPA, NER-deficient) and XP129 (NER proficient XP ‘revertant’) cells were incubated with 120 μM SBrdU (left panel) or SIdU (right panel) for 72 h and UVA irradiated. Survival was measured by MTT assay 10 days later. In the absence of drug treatment, survival of all three cell lines was >90% at all UVA doses used. In the experiment shown (representative of two), DNA substitution by the three cell lines was similar at 0.035% of TdR for SBrdU and 0.02% for SIdU.
The contribution of the UNG-susceptible DNA lesions to SIdU/UVA and SBrdU/UVA toxicity was examined by comparing the sensitivity of UNG-proficient or -deficient MEFs. Fig. 3B shows that the UNG substrates generated by these treatments differ in their potential toxicity. UNG-deficient cells were more sensitive to SBrdU/UVA than their UNG proficient counterparts (Fig. 3B, left panel). In contrast, SIdU/UVA was equitoxic to both UNG-proficient and -deficient cells (Fig. 3B, right panel). This differential toxicity indicates that SBrdU/UVA induces potentially lethal lesions which are excised by UNG whereas UNG substrates produced by SIdU/UVA would not contribute significantly to toxicity.
3.2.4. Nucleotide excision repair substrates
SIdU and SBrdU are analogs of 4-thiothymidine (S4TdR) which generates thietane intrastrand crosslinks with an adjacent DNA thymine when activated by UVA [26,27]. Thietanes are intermediates in the formation of lesions that resemble canonical 6-4 Py:Py nucleotide excision repair (NER) substrates and that compete for removal by this pathway [27]. To examine the effect of photoactivated SIdU and SBrdU on NER, SIdU- or SBrdU-treated HeLa cells were irradiated with UVA and a low dose of UVC. The removal of the UVC-induced 6-4 Py:Pys was then analysed by ELISA. Fig. 3C shows that SIdU/UVA and SBrdU/UVA slightly inhibited NER. In untreated cells, 6-4 Py:Pys were excised with a half-life of approximately 60 min and only 5% remained after 2 h. SIdU/UVA or SBrdU/UVA treatment increased the half-life of 6-4 Py:Py photoproducts to around 75 min and 20% remained after 2 h. To examine whether SIdU/UVA and SBrdU/UVA induced competing NER substrates, we compared the sensitivity of NER wild-type (MRC5VA), NER-deficient XP (XP12RO) and XP12RO “revertant” XP129 cells. XP129 cells have regained the ability to repair 6-4 Py:Pys but not other potentially lethal UV photoproducts [20]. XP12RO cells were sensitive to both SIdU/UVA and SBrdU/UVA consistent with the introduction of potentially lethal NER substrates. In contrast, XP129 were almost as resistant as NER-proficient MRC5VA cells (Fig. 3D). The relative resistance of XP129 cells to both SIdU/UVA and SBrdU/UVA indicates that both treatments induce potentially lethal DNA photoproducts that resemble canonical UVC 6-4 Py:Py photoproducts and are excised by NER.
3.3. Collateral DNA damage and singlet oxygen
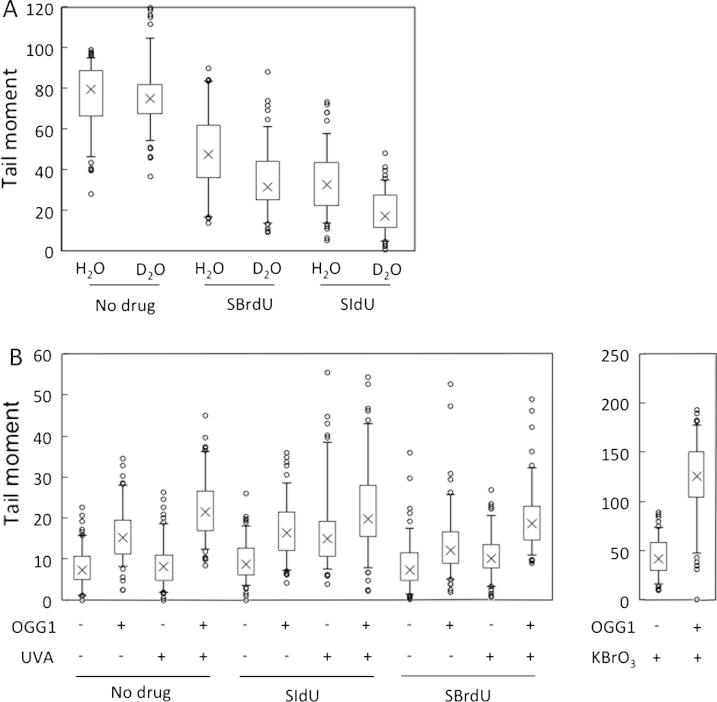
It is well established that UVA irradiation of cells generates singlet oxygen (1O2) via reactions that are enhanced by exogenous Type II photosensitisers (reviewed in [28]). To examine whether SBrdU and SIdU photosensitization involved a Type II component, SBrdU and SIdU treated cells were UVA irradiated in D2O which extends the half-life of 1O2 and increases the likelihood of reaction. The alkaline comet assay (Fig. 4A) indicated that irradiation in D2O caused a reduction in IR-induced comet tail moments. Since all samples were extensively Proteinase K digested prior to electrophoresis, these findings indicate that ICL formation by SIdU/UVA and SBrdU/UVA is at least partially dependent on 1O2.
Fig. 4.
Singlet oxygen production and oxidation of DNA. (A) Singlet oxygen and ICL formation: HeLa cells grown in the presence of 200 μM SIdU or SBrdU for 48 h were incubated for 1 h in PBS made in H2O or D2O and then irradiated with UVA (20 kJ/m2) followed by 15 Gy γ-rays. Analysis was by the alkaline comet assay following Proteinase K digestion. DNA breakage is expressed as comet tail moment. (B) Generation of 8-oxoguanine: HeLa cells were treated with 100 μM SBrdU or SIdU for 48 h and UVA irradiated. The presence of DNA 8-oxoguanine was revealed by single cell electrophoresis following digestion with recombinant OGG1 as indicated. Right panel: In a control experiment HeLa cells were treated with 10 mM KBrO3 for 1 h and analysed in the same way.
Alkaline comet assays with OGG-1 digestion were used to detect DNA 8-oxoguanine (8-oxoG), a common DNA lesion and a sensitive marker of oxidative DNA damage. Fig. 4C indicates that neither SBrdU/UVA nor SIdU/UVA measurably increased DNA 8-oxoG over the low levels induced by UVA irradiation alone. In control experiments, DNA 8-oxoG levels were significantly increased by treatment with KBrO3. Thus, although their effects are partially dependent on 1O2, highly toxic SBrdU/UVA and SIdU/UVA combinations do not produce widespread collateral damage in the form of oxidised guanine.
3.4. SIdU and SBrdU photoproducts – in vitro identification
Reverse phase HPLC was used to investigate the photochemical reactions of SIdU and SBrdU. Where appropriate, photoproducts were identified by comparison with authentic compounds.
3.4.1. SIdU
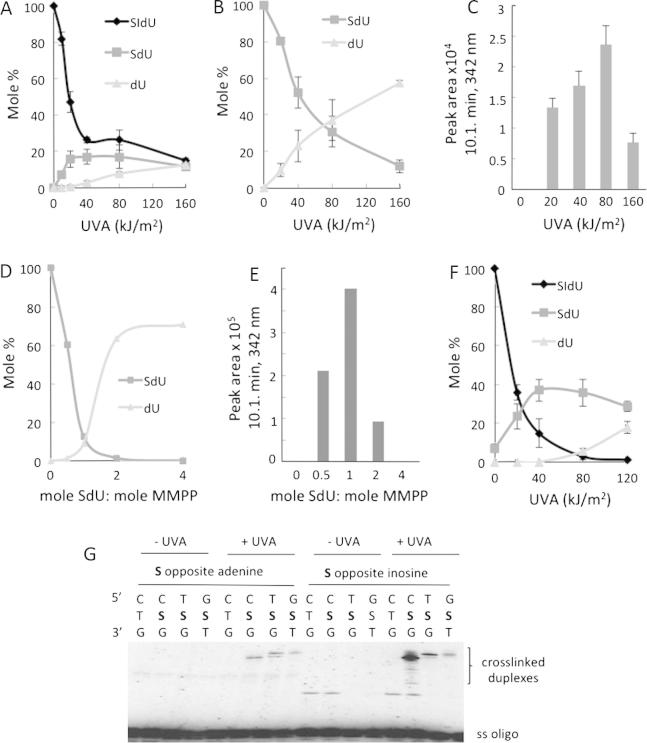
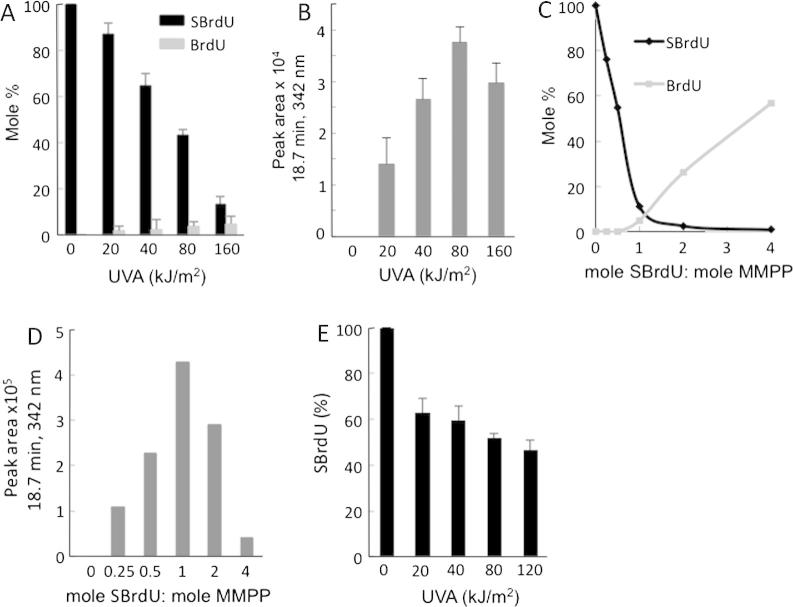
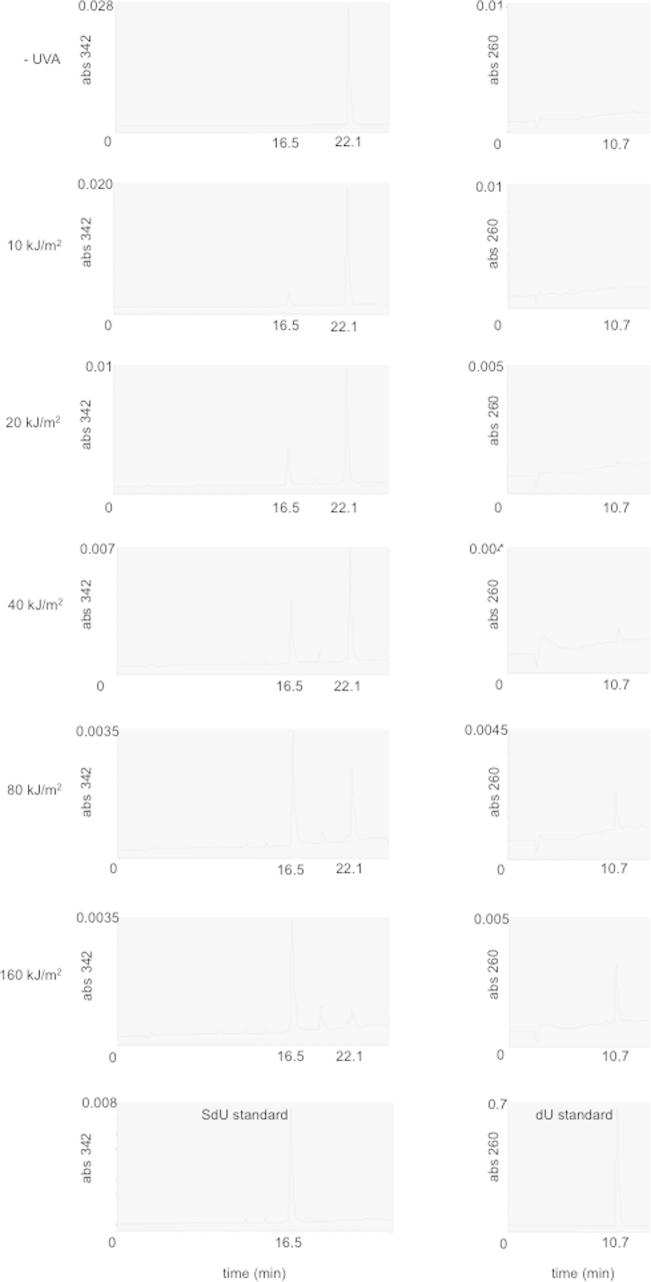
UVA irradiation of SIdU in aqueous solution destroyed the thionucleoside. Low UVA doses (20–40 kJ/m2) caused de-iodination of SIdU to SdU (Fig. 5A and Supplementary Fig. 3). SdU yields were not increased at higher UVA doses (40–160 kJ/m2). A second photoproduct, identified as dU was generated at higher UVA doses consistent with possible formation from SdU. Analysis of the irradiation products of authentic SdU confirmed this possibility. Fig. 5B shows that dU accounts for most (>60%) of the SdU destroyed by UVA. In addition to dU, a second photoproduct with absorbance at 342 nm eluted at 10.1 min. It was formed in a UVA dose-dependent fashion between 0 and 80 kJ/m2 but was destroyed at the highest UVA dose of 160 kJ/m2 (Fig. 5C). This photoproduct is a plausible intermediate in the conversion of SdU to dU.
Fig. 5.
UVA photoproducts of SIdU. (A–C) Photodestruction of SIdU: Aqueous solutions (100 μM) of SIdU (A) or SdU (B) were irradiated with the indicated doses of UVA. Photoproducts were analysed by reverse phase HPLC with 260 nm and 342 nm detection. Each product was quantified by comparison to authentic standards. Data are expressed as Mole% of the unirradiated input nucleoside. For the unidentified product SIdU irradiation product eluting at 10.1 min (panel C), values are expressed as A342 peak area (μV × s). Traces corresponding to (A) are shown in Supplementary Fig. 3. (D and E) MMPP oxidation: SdU (100 μM) in solution was treated with MMPP for 20 min at the indicated molar ratios and analysed by HPLC as above. (F) Photodestruction of DNA SIdU: HeLa cells that had been grown in 100 μM SIdU for 24 h and in which SIdU replaced approximately 0.03% of DNA TdR were UVA irradiated at the indicated doses. DNA was extracted, digested to nucleosides and analysed by HPLC as in A. (G) Crosslink formation in vitro – gel migration assay: Radiolabelled 23mer oligonucleotide duplexes containing a single SdU(S):A or: I base pair were irradiated with 50 kJ/m2 UVA. Products were heat denatured and resolved on a 16% polyacrylamide gel. The 5’ and 3’ bases flanking the SdU are shown above the corresponding lane in the gel image. The position of migration of crosslinked duplexes is indicated.
To examine whether the 10.1 min eluting material was an oxidation product of SdU, SdU was treated in aqueous solution with magnesium monoperoxyphthalate (MMPP), a mild oxidising agent that selectively oxidises thiol-containing nucleobases [21]. The SdU/MMPP reaction was almost complete at equimolar nucleoside and oxidant concentrations (Fig. 5D) and generated a single product with A342 eluting at 10.1 min that was destroyed at higher MMPP concentrations (Fig. 5E). Its destruction was not accompanied by the formation of other A342-absorbing products but coincided with the formation of dU, which was detected by its A260. We conclude that the 10.1 min eluting product contains an oxidised thiol group and is an intermediate in the conversion of SdU to dU.
The photochemical properties of DNA SIdU were generally consistent with those of the free nucleoside. HPLC analysis of nucleoside digests of DNA extracted from irradiated SIdU-treated cells revealed that UVA also destroys DNA SIdU. Fig. 5F shows that 20 kJ/m2 UVA destroyed >60% of DNA SIdU and its disappearance was complete between 40 and 80 kJ/m2. At low UVA doses (20 kJ/m2) SdU accounted for about half of the DNA SIdU destroyed. SdU levels were not significantly increased by higher UVA doses, but above 80 kJ/m2 measurable amounts of dU were generated, most likely as an SdU photoproduct. The formation of DNA dU in these experiments is consistent with the observation (Fig. 3B) that UVA activation of DNA SIdU in intact cells generates low levels of a non-toxic substrate for the UNG DNA glycosylase.
This analysis identifies SdU as a plausible intermediate in the photochemical destruction of DNA SIdU. Since SdU can be built into synthetic oligonucleotides and ICLs are a significant photoproduct in SIdU/UVA treated cells, we examined whether DNA SdU could participate in UVA-mediated ICL formation in vitro. Oligonucleotide duplexes containing a single SdU paired with a complementary A were UVA irradiated and the products analysed by denaturing gel electrophoresis. We observed the SdU/UVA-dependent formation of slowly-migrating products with characteristics of crosslinked duplexes (Fig. 5G). Crosslinking was influenced by local sequence. ICL formation was more efficient when SdU was flanked by a 5’ pyrimidine and 3’G than when the flanking bases were 5’G/3’T. The amino group of the complementary A was dispensable for crosslinking and the replacement of A with a wobble-paired inosine even significantly enhanced ICL formation.
In summary, the formation of DNA dU and ICLs that we inferred from experiments with living cells can be recapitulated by UVA irradiation of SIdU or DNA SIdU in vitro. The formation of these photoproducts depends on an initial deiodination of SIdU.
3.4.2. SBrdU
UVA also destroyed SBrdU in aqueous solution (Fig. 6A and Supplementary Fig. 4). SBrdU disappearance coincided with the formation of a photoproduct with 342 nm absorbance and a retention time of 18.7 min (Fig. 6B). Higher UVA doses (160 kJ/m2) destroyed this photoproduct with the concomitant formation of low, but detectable levels of BrdU (Fig. 6A). Importantly, SdU was not detected indicating that UVA irradiation does not cause SBrdU debromination. The photoproduct eluting at 18.7 min was an oxidation product of SBrdU and it was generated by treatment with MMPP (Fig. 6C and D). Oxidation of SBrdU coincided with the formation of BrdU as the only detectable UV-absorbing product. At the highest MMPP concentration, BrdU accounted for ⩾60% of the initial SBrdU with a small residual amount of the intermediate.
Fig. 6.
Photooxidation of SBrdU. (A and B) Photodestruction: An aqueous solution (100 μM) of SBrdU was irradiated with the indicated doses of UVA. Photoproducts were separated by HPLC and monitored at 260 and 342 nm. Products were quantified by comparison to authentic standards and data are expressed as Mole% of the unirradiated SBrdU. For the unidentified SBrdU irradiation product eluting at 18.7 min (panel B), values are expressed as A342 peak area (μV × s). Traces corresponding to (A) are shown in Supplementary Fig. 4. (C and D) MMPP oxidation: An aqueous solution of SBrdU (100 μM) was treated with MMPP for 20 min at the indicated molar ratios and analysed by HPLC as above. (E) Photodestruction of DNA SBrdU: Cells that had been grown in 100 μM SBrdU for 24 h and in which SBrdU replaced approximately 0.06% of DNA TdR were UVA irradiated at the indicated doses. DNA was extracted, digested to nucleosides and analysed by HPLC as in A. As SBrdU was the only nucleoside detected at 342 nm, recovered SBrdU is expressed as percentage SBrdU in DNA from unirradiated cells.
The behaviour of DNA SBrdU differed significantly from that of the free nucleoside. In irradiated HeLa cells approximately 40% of DNA SBrdU was destroyed at the lowest UVA dose (20 kJ/m2) (Fig. 6E). The residual DNA SBrdU was relatively UVA resistant and approximately 50% remained even after 120 kJ/m2 UVA. SdU was not observed indicating that debromination occurred infrequently, if at all.
In summary, the in vitro photochemical properties of SIdU and SBrdU are consistent with the formation of DNA damage that we infer from in vivo experiments. In particular, they define possible routes for dU and ICL formation by DNA SIdU/UVA. The reactions of SBrdU/UVA are different and do not involve loss of the Br atom.
4. Discussion
The halopyrimidine 2’-deoxynucleosides BrdU and IdU are acknowledged ionising radiation and UVB sensitisers. In the presence of DNA-intercalated Hoescht 33258 dye they also sensitise the formation of lethal DNA damage by UVA [29,13]. We have previously characterised SIdU [17] and SBrdU [16]. Here we show that both cause lethal DNA photodamage in human cells. The presence of the pyrimidine thiol group obviates the need for a separate intercalated UVA chromophore and UVA irradiation of cells containing DNA-embedded halogenated thiopyrimidines generates potentially cytotoxic DNA lesions.
SBrdU was more extensively incorporated into DNA than SIdU but the latter was a more effective photosensitiser at equivalent levels of DNA substitution. The dissimilar effectiveness of DNA SIdU and SBrdU probably reflects the relative ease of dehalogenation. UVA-induced deiodination of SIdU is favoured in free solution and in DNA. The resulting thiouracil-5-yl radical can be converted to SdU, one of the photoproducts we identified. In DNA, thiouracil-5-yl radicals can abstract hydrogen from 2’-deoxyribose to generate single-strand breaks [6]. They may also react with nearby functional groups of DNA or proteins to form the ICLs and DPCs that are significant contributors to SIdU-induced photodamage. The C5–Br bond of SBrdU is more stable. Debromination is less favoured and SdU was not a detectable photoproduct of SBrdU either in free solution or in DNA. In this regard, UVA activation of DNA SBrdU differs significantly from UVB photosensitisation of DNA BrdU and UVA/dye/DNA BrdU reactions both of which generate DNA uracil [6,13].
The thiol group of the thionucleosides is also a potential oxidation target [30]. Selective oxidation of SdU and SBrdU thiol groups by MMPP yielded intermediates that retained 342 nm absorbance and underwent further oxidation to products that no longer absorbed UVA. These oxidised thiols are reactive and the UVA-induced generation of dU and BrdU from SdU and SBrdU, respectively, is consistent with the hydrolytic loss of an oxidised S4 atom. The yields of DNA dU and DNA SdU from cells treated with SIdU/UVA were not, however, stoichiometric. SdU levels were not related to UVA dose and its disappearance was not quantitatively accounted for by conversion to dU. We speculate that reactions between oxidised nucleobase thiols and functional groups of other DNA nucleobases or proteins is favoured over hydrolysis. These preferred reactions to generate ICLs and DPCs, are also consistent with our observation that BrdU is undetectable in DNA SBrdU/UVA treated cells.
Using cell lines with defined defects in DNA repair, we identified several potentially cytotoxic DNA SIdU and SBrdU photoproducts. These included ICLs and the hypersensitivity of FANCD2-deficient cells to both SBrdU/UVA and SIdU/UVA emphasizes the important contribution of ICLs to toxicity. ICLs have a high probability of being lethal. Their formation is advantageous in photochemotherapy and the therapeutic effectiveness of PUVA, for example, reflects efficient ICL induction. Significantly, FANCD2 monoubiquitination – an indicator that the cellular response to ICLs has been engaged – occurs following mild treatment with SIdU/UVA or SBrdU/UVA indicating that ICLs are likely to be abundant and a major contributor to lethality. Our experiments demonstrated that UVA photochemically crosslinks oligodeoxyribonucleotides via SdU, an identified DNA SIdU photoproduct. ICL formation was enhanced by a less stable structure conferred by a complementary inosine and by the presence of C flanking the complementary base. In folded tRNA, 4-thiouridine (SrU), a naturally-occurring minor base at the unpaired position 8, undergoes UVA-mediated crosslinking to cytosine 13 [31] and it seems plausible that SdU is an intermediate in UVA-mediated ICL formation from DNA SIdU.
Treatment of cells with SIdU/UVA or SBrdU/UVA generated lesions that are substrates for removal by UNG-mediated base excision repair. In the case of SBrdU/UVA, these substrates are potentially lethal. Because UNG has to discriminate DNA uracil from thymine, SBrdU photoproducts that retain the Br atom are unlikely to be recognised by UNG. This suggests that SBrdU must undergo debromination to generate a substrate for UNG. SBrdU debromination was below our level of detection by HPLC and so the precise nature of these potentially lethal DNA lesions remains to be determined. UNG−/− cells were not sensitive to SIdU/UVA, a treatment that generates DNA dU. This observation is consistent with the known properties of DNA dU. Rare incorporation of DNA uracil generates DNA dU:dA base pairs. These base pairs are well tolerated even if they are not excised and UNG-defective cells in which they persist, proliferate normally [18]. For the same reasons, SdU, another potential target for excision by UNG that also pairs well with adenine is unlikely to contribute to toxicity.
The SIdU/UVA and SBrdU/UVA hypersensitivity of NER-defective XP12RO cells is consistent with the formation of potentially cytotoxic intrastrand crosslinks that distort DNA in a similar fashion to UV-induced 6-4 Py:Py photoproducts [32,27]. This possibility is strengthened by the almost wild-type SIdU/UVA and SBrdU/UVA sensitivity of ‘revertant’ XP129 cells. In NER proficient cells, 6-4 Py:Py photoproducts are excised very rapidly and are generally considered to contribute less to the toxicity of UV radiation than other photoproducts. Rapid excision by NER will tend to minimise contribution of 6-4 Py:Py analogs to SIdU/UVA and SBrdU/UVA toxicity.
UVB radiation induces protein-tRNA crosslinking via SrU [33,31]. Our experiments show that an analogous reaction involving DNA SdU generated by UVA-induced deiodination of SIdU contributes to DPC formation. Although we cannot exclude the participation of rare debrominated forms, the properties of DNA SBrdU are consistent with the possible involvement of thiol oxidation in DPC formation. The products of MMPP-induced thiol oxidation of SIdU and SBrdU appeared identical to intermediates in their photochemical destruction. In addition, ICL yields were increased by irradiation in D2O, implicating 1O2 in ICL formation. Oxidised thiols, sulfinate and sulfonates are good leaving groups in nucleophilic substitution reactions and the UVA-induced generation of dU and BrdU from SdU and SBrdU, respectively, is consistent with oxidation and hydrolytic loss of the S4 atom. These oxidised forms of SIdU and SBrdU are plausible intermediates in ICL formation via reaction with functional groups in DNA-associated proteins. In view of the involvement of 1O2 in the photochemical effects of DNA SIdU and SBrdU, it is surprising that we did not observe significant levels of DNA 8-oxoG as collateral damage. Although we have not examined this point experimentally, we speculate that the photochemically-generated 1O2 might react preferentially with DNA SIdU or SBrdU and thereby attenuate the threat to DNA guanine.
UVA activation of DNA thiopyrimidines has therapeutic potential. S4TdR/UVA eradicated cancer in an orthotopic bladder carcinoma model in rats [34]. Nucleoside salvage enzymes like thymidine kinase are frequently upregulated in tumours suggesting that systemically administered SIdU or SBrdU may selectively target tumour cells. Indeed, radio-iodine-containing IdU preferentially localises to tumours in mice [35]. Exogenous nucleosides such as SIdU and SBrdU must compete with endogenous pools of thymidine nucleotides for incorporation into DNA. We note in this regard that treatment with the thymidylate synthase inhibitor tomudex (Raltitrexed) that reduces the size of intracellular thymidine pools, increases the DNA accumulation of S4TdR [34], the direct analog of SIdU and SBrdU. Co-treatment of SIdU or SBrdU with tomudex would offer a straightforward way to improve UVA sensitisation and therapeutic effectiveness. Thiopyrimidine phototoxicity is dependent on the induction of potentially lethal DNA damage and DPCs and ICLs are likely to be major contributors. These DNA lesions are challenging to cellular DNA repair systems and suggest that UVA photoactivation of DNA SIdU or SBrdU would be an effective therapeutic strategy.
Acknowledgements
This work was supported by Cancer Research UK. X.Z. gratefully acknowledges support from the Scientific Research Foundation for Returned Overseas Chinese Scholars, Ministry of Education, PR China. We are grateful to Clare Hall Laboratories Cell Services for provision of cell lines.
Appendix A. Supplementary material
Supplementary Figure 1.
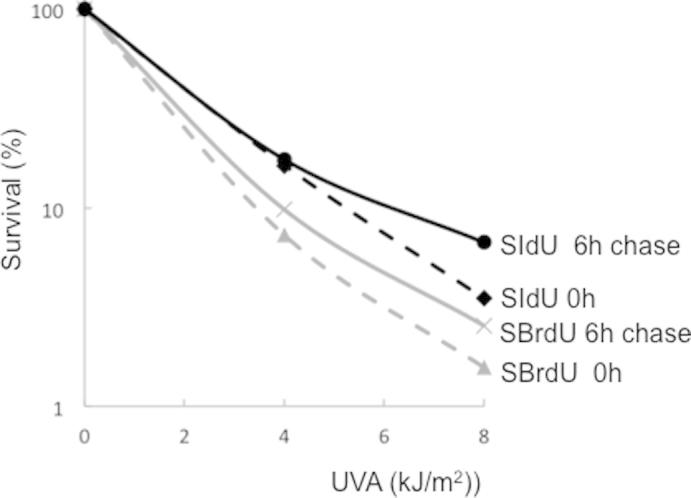
Nucleotide pool depletion by chase. HeLa cells were treated with 100 µM SIdU or 200 µM SBrdU for 48 h and UVA irradiated either immediately (0 h) or after incubation in normal growth medium (chase) for 6 h. Survival was determined by MTT assay.
Supplementary Figure 2.
DNA-Protein Crosslinks. (A) DNA recovery. Treatment-induced reduction in DNA yield is reversed by protease digestion. HeLa cells were grown for 48 h in the presence of SIdU or SBrdU as indicated and irradiated with 0, 25 or 50 kJ/m2 UVA. Cells were lysed immediately after irradiation. Equal amounts of lysate were incubated overnight at 50 ºC in the presence (+Prot K) or absence (−Prot K) of Proteinase K. Proteins were then precipitated and discarded. DNA recovered from the supernatant was analyzed by agarose gel electrophoresis. (B) Crosslink formation – comet assay. HeLa cells grown in the presence of 100 µM SIdU or SBrdU for 48 h were UVA irradiated (20 kJ/m2). They were then g-irradiated with 15 Gy and analysed by the alkaline comet assay either immediately after irradiation or following Proteinase K digestion as indicated. DNA breakage is expressed as comet tail moment. These experiments are repetitions of those shown in Fig. 2.
Supplementary Figure 3.
SdU and dU formation from UVA irradiated SIdU.
Supplementary Figure 4.
BrdU formation from UVA irradiated SBrdU.
References
- 1.Honigsmann H. Phototherapy. J. Invest. Dermatol. 2013;133:E18–20. doi: 10.1038/skinbio.2013.180. [DOI] [PubMed] [Google Scholar]
- 2.Ghissassi F.E., Baan R., Straif K., Grosse Y., Secretan B., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., Cogliano V., on behalf of the WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens – Part D: Radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- 3.Dewey W.C., Humphrey R.M. Increase in radiosensitivity to ionizing radiation related to replacement of thymine in mammalian cells with 5-bromodeoxyuridine. Radiat. Res. 1965;26:538–553. [PubMed] [Google Scholar]
- 4.Cleaver J. Repair replication and degradation of bromouracil-substituted DNA in mammalian cells after irradiation with ultraviolet light. Biophys. J. 1968;8:775–790. doi: 10.1016/S0006-3495(68)86520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling L.L., Ward J.F. Radiosensitization of Chinese hamster cells by bromodeoxyuridine substitution of thymidine: enhancement of radiation-induced toxicity and DNA strand break production by monofilar and bifilar substitution. Radiat. Res. 1990;121:76–83. [PubMed] [Google Scholar]
- 6.Hutchinson F. The lesions produced by ultraviolet light in DNA containing 5-bromouracil. Q. Rev. Biophys. 1973;6:201–246. doi: 10.1017/s0033583500001141. [DOI] [PubMed] [Google Scholar]
- 7.Krasin F., Hutchinson F. Strand breaks and alkali-labile bonds induced by ultraviolet light in DNA with 5-bromouracil in vivo. Biophys. J. 1978;24:657–664. doi: 10.1016/S0006-3495(78)85411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wojcik A., Bochenek A., Lankoff A., Lisowska H., Padjas A., Szumiel I., vonSonntag C., Obe G. DNA interstrand crosslinks are induced in cells prelabelled with 5-bromo-2’-deoxyuridine and exposed to UVC radiation. J. Photohem. Photobiol. B. 2006;84:15–20. doi: 10.1016/j.jphotobiol.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Meisenheimer K.M., Koch T.H. Photocross-linking of nucleic acids to associated proteins. Crit. Rev. Biochem. Mol. Biol. 1997;32:101–140. doi: 10.3109/10409239709108550. [DOI] [PubMed] [Google Scholar]
- 10.Cadet J., Douki T. Oxidatively generated damage to DNA by UVA radiation in cells and human skin. J. Invest. Dermatol. 2011;131:1005–1007. doi: 10.1038/jid.2011.51. [DOI] [PubMed] [Google Scholar]
- 11.Limoli C.L., Ward J.F. A new method for introducing double-strand breaks into cellular DNA. Radiat. Res. 1993;134:160–169. [PubMed] [Google Scholar]
- 12.Limoli C.L., Ward J.F. Response of bromodeoxyuridine-substituted Chinese hamster cells to UVA light exposure in the presence of Hoechst dye 33258: survival and DNA repair studies. Radiat. Res. 1994;138:312–319. [PubMed] [Google Scholar]
- 13.Limoli C.L., Wu C.C., Milligan J.R., Ward J.F. Photochemical production of uracil quantified in bromodeoxyuridine-substituted SV40 DNA by uracil DNA glycosylase and a lysyl-tyrosyl-lysine tripeptide. Mutagenesis. 1997;12:443–447. doi: 10.1093/mutage/12.6.443. [DOI] [PubMed] [Google Scholar]
- 14.Zeng Y., Wang Y. Sequence-dependent formation of interstrand crosslink products from the UVB irradiation of duplex DNA containing a 5-bromo-2’-deoxyuridine or 5-bromo-2’-deoxycytidine. Nucleic Acids Res. 2006;34:6521–6529. doi: 10.1093/nar/gkl892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner P.R., Denny W.A. The mutagenic properties of DNA minor groove binding ligands. Mutat. Res. 1996;355:141–169. doi: 10.1016/0027-5107(96)00027-9. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y.-Z., Zhang X., Wu H.-C., Massey A., Karran P. 4-thio-5-bromo-2’-deoxyuridine: chemical synthesis and therapeutic potential of UVA-induced DNA damage. Bioorg. Med. Chem. Lett. 2004;14:995–997. doi: 10.1016/j.bmcl.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X., Xu Y.-Z. NMR and UV studies of 4-thio-2’-deoxyuridine and its derivatives. Molecules. 2011;16:5655–5664. doi: 10.3390/molecules16075655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsen H., Rosewell I., Robins P., Skelbred C.F., Andersen S., Slupphaug G., Daly G., Krokan H.E., Barnes D.E. Uracil-DNA glycosylase (UNG)-deficient mice reveal a primary role of the enzyme during DNA replication. Mol. Cell. 2000;5:1059–1065. doi: 10.1016/s1097-2765(00)80271-3. [DOI] [PubMed] [Google Scholar]
- 19.Houghtaling S., Timmers C., Noll M., Finegold M.J., Jones S.N., Meyn M.S., Grompe M. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev. 2003;17:2021–2035. doi: 10.1101/gad.1103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDowell M.L., Nguyen T., Cleaver J.E. A single-site mutation in the XPAC gene alters photoproduct recognition. Mutagenesis. 1993;8:155–161. doi: 10.1093/mutage/8.2.155. [DOI] [PubMed] [Google Scholar]
- 21.Ren X., Li F., Jeffs G., Zhang X., Xu Y.-Z., Karran P. Guanine sulphinate is a major stable product of photochemical oxidation of DNA 6-thioguanine by UVA irradiation. Nucleic Acids Res. 2010;38:1832–1840. doi: 10.1093/nar/gkp1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brem R., Li F., Karran P. Reactive oxygen species generated by thiopurine/UVA cause irreparable transcription-blocking DNA lesions. Nucleic Acids Res. 2009;37:1951–1961. doi: 10.1093/nar/gkp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brem R., Li F., Montaner B., Reelfs O., Karran P. DNA breakage and cell cycle checkpoint abrogation induced by a therapeutic thiopurine and UVA radiation. Oncogene. 2010;29:3953–3963. doi: 10.1038/onc.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis M.C., Hicke B.J., Uhlenbeck O.C., Cech T.R., Koch T.H. Photocrosslinking of 5-iodouracil-substituted RNA and DNA to proteins. Science. 1993;262:1255–1257. doi: 10.1126/science.7694369. [DOI] [PubMed] [Google Scholar]
- 25.Deans A.J., West S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer. 2011;11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly B.A., Newman P.C. Synthesis and properties of oligonucleotides containing 4-thiothymine, 5-methyl-2-pyrimidinone-1-b-D(2’-deoxyriboside) and 2-thiothymine. Nucleic Acids Res. 1989;17:4957–4974. doi: 10.1093/nar/17.13.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reelfs O., Macpherson P., Ren X., Xu Y.-Z., Karran P., Young A. Identification of potentially cytotoxic lesions induced by UVA photoactivation of DNA 4-thiothymidine in human cells. Nucleic Acids Res. 2011;39:9620–9632. doi: 10.1093/nar/gkr674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cadet J., Douki T., Ravanat J.-L. Oxidatively generated damage to cellular DNA by UVB and UVA. Photochem. Photobiol. 2014;91:140–155. doi: 10.1111/php.12368. [DOI] [PubMed] [Google Scholar]
- 29.Limoli C.L., Ward J.F. Photochemical production of double-strand breaks in cellular DNA. Mutagenesis. 1995;10:453–456. doi: 10.1093/mutage/10.5.453. [DOI] [PubMed] [Google Scholar]
- 30.Y-Z Xu. Reactive DNA: 6-methylsulphoxypurine used for site-specific and chemical cross-linking with cysteine and its peptide. Tetrahedron. 1998;54:187–196. [Google Scholar]
- 31.Favre A., Saintomé C., Fourrey J.-L., Clivio P., Laugaa P. Thionucleobases as intrinsic photoaffinity probes of nucleic acid structure and nucleic acid-protein interactions. J. Photochem. Photobiol. B Biol. 1998;42:109–124. doi: 10.1016/s1011-1344(97)00116-4. [DOI] [PubMed] [Google Scholar]
- 32.Warren M.A., Murray J.B., Connolly B.A. Synthesis and characterisation of oligodeoxynucleotides containing thio analogues of (6-4) pyrimidine-pyrimidone photo-dimers. J. Mol. Biol. 1998;279:89–100. doi: 10.1006/jmbi.1998.1719. [DOI] [PubMed] [Google Scholar]
- 33.McGregor A., Rao M.V., Duckworth G., Stockley P.G., Connolly B.A. Preparation of oligoribonucleotides containing 4-thiouridine using Fpmp chemistry. Photocrosslinking to RNA binding proteins using 350 nm irradiation. Nucleic Acids Res. 1996;24:3173–3180. doi: 10.1093/nar/24.16.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pridgeon S.W., Heer R., Taylor G.A., Newell D.R., O’Toole K., Robinson M., Xu Y.-Z., Karran P., Boddy A.V. Thiothymidine combined with UVA as a potential novel therapy for bladder cancer. Br. J. Cancer. 2011;104:1869–1876. doi: 10.1038/bjc.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgenroth A., Deisenhofer S., Glatting G., Kunkel F.H., Dinger C., Zlatopolskiy B., Vogg A.T., Kull T., Reske S.N. Preferential tumor targeting and selective tumor cell cytotoxicity of 5-[131/125I] iodo-4’-thio-2’-deoxyuridine. Clin. Cancer Res. 2008;14:7311–7319. doi: 10.1158/1078-0432.CCR-08-0907. [DOI] [PubMed] [Google Scholar]