Abstract
BACKGROUND
Biomarkers of cardiovascular stress have been associated with incident cardiovascular outcomes. Their relations with measures of subclinical atherosclerosis, as assessed by carotid intima-media thickness, have not been well described.
METHODS
Plasma growth differentiation factor-15 (GDF-15), soluble ST2 (sST2), and high-sensitivity troponin I (hsTnI) were measured in 3111 Framingham Offspring participants who also underwent carotid ultrasonography during the sixth examination (1995 – 1998, mean age 58 years, 54% women). Carotid measurements included maximal internal carotid artery (ICA) intima-media thickness (IMT), plaque presence (defined as ICA IMT > 1.5 mm), and mean common carotid artery IMT. Multivariable regressions for carotid measurements versus biomarkers were carried out using linear and logistic models; P < 0.0056 was deemed statistically significant.
RESULTS
Maximal ICA IMT was significantly associated with plasma GDF-15 (β-estimate 0.04 per 1 unit increase in log-GDF-15 SE 0.01, P < 0.0001). Similarly, the odds of having carotid plaque increased 33% (OR 1.33 per 1-unit increase in log-GDF-15, 95% CI 1.20-1.48, P < 0.0001). In contrast, there was no significant association of maximal ICA IMT or plaque presence with sST2 or hsTnI, and none of the three biomarkers was significantly associated with mean CCA IMT. GDF-15 was a stronger predictor of maximal ICA thickness and plaque presence compared with BNP and CRP when these conventional biomarkers were tested together.
CONCLUSION
Higher GDF-15 concentrations are associated with subclinical atherosclerosis, including maximal ICA IMT and carotid plaque presence. Future studies investigating the role of GDF-15 for screening and management of patients with subclinical atherosclerosis are warranted.
Keywords: carotid intima-media thickness, atherosclerosis, biomarkers
Carotid ultrasonography, via carotid intima-media thickness (IMT) assessment, has been well established not only as a surrogate of generalized atherosclerosis (1, 2) and linked with cardiovascular risk factors (3, 4), but also has been shown to be a predictor for myocardial infarction, stroke, coronary heart disease, and combined cardiovascular outcomes (5–7). In addition, carotid IMT has been shown to track subclinical atherosclerosis and help improve classification of cardiovascular risk in asymptomatic cohorts (4, 8).
Recently, biomarkers of cardiovascular stress, including growth differentiation factor-15 (GDF-15), soluble ST2 (sST2), and high-sensitivity troponin (hsTnI), have emerged as strong predictors of cardiovascular risk and outcomes in various community-based populations (9–12). The exact mechanism by which these biomarkers are linked to cardiovascular disease, particularly in asymptomatic, population-based cohorts are incompletely described to date.
Our group has previously demonstrated the longitudinal association of GDF-15, sST2, and hsTnI to incident cardiovascular outcomes in the community (11). Given that all three biomarkers previously related to incident cardiovascular disease, we hypothesized that these biomarkers would be associated cross-sectionally with measures of subclinical atherosclerosis, as assessed via carotid IMT of the common carotid artery and internal carotid artery. Further characterizing associations with subclinical cardiovascular disease measures may offer further insights into the role of these biomarkers in the development of atherosclerosis.
MATERIALS AND METHODS
Study Sample
The Framingham Offspring Study cohort, initiated in 1971 and comprising offspring (and their spouses) of the original Framingham cohort, underwent examinations every 4 – 8 years. The present investigation included participants who attended the sixth Offspring examination (1995 – 1998) and underwent carotid ultrasonography. From 3380 participants, 37 were excluded for missing carotid IMT data, 21 because of missing covariate data, 173 because of prevalent cardiovascular disease, 4 because of missing sST2 data, and 34 because of missing hsTnI data, leaving 3111 participants for analysis. The Institutional Review Board at Boston Medical Center approved the study protocol and all study participants provided written informed consent.
Risk Factor Assessment
Each examination included a comprehensive history and physical examination. Cardiovascular risk factors pertinent to our study included systolic blood pressure, diastolic blood pressure, antihypertensive drug treatment, diabetes (defined as fasting glucose ≥ 126 mg/dL (≥ 7 mmol/L) or the use of oral hypoglycemic medications or insulin, current smoking (defined as smoking at least 1 cigarette per day over the past year), total and HDL cholesterol concentrations (measured after an overnight fast), and body mass index.
Biomarker measurements
Blood samples were obtained from study participants in the morning following an overnight fast. The citrated plasma samples were centrifuged immediately and stored at −80° C until assayed. No freeze-thaw cycles were performed prior to the assays described below. Reference limits and assay characteristics have previously been described (11, 13, 14). In brief, a pre-commercial, automated electrochemiluminiscent immunoassay utilizing a Cobas e 411 analyzer (Roche Diagnostics) at a core laboratory (K.C. Wollert) was used to measure plasma GDF-15 concentrations. The assay had an intraassay coefficient of variation (CV) of 0.8% and interassay CV of 2.3% at low concentrations (1120 ng/L), and intra- and interassay CV of 1.1 and 1.0% at high concentrations (9031 nl/L). sST2 concentrations were measured with a high-sensitivity sandwich immunoassay (Presage™ ST2, Critical Diagnostics) at Critical Diagnostics. This assay had an interassay CV of 7.5% at low (25.6 ng/mL) and 6.0% at high (70.9 ng/mL) sST2 concentrations. Single-molecule counting technology (Erenna hsTnI, Singulex) was used for measurement of hsTnI at Singulex. The assay had an interassay CV of 10.0% at low (4.7 ng/L) and 7.7% at high (19.0 n/L) hsTnI concentrations. B-type natriuretic peptide (BNP) and C-reactive protein (CRP) concentrations were measured as previously described (15). In brief, BNP was measured using a high-sensitivity immunoradiometric assay at Shionogi (Shionogi, Osaka, Japan) with average interassay CV of 12.2%, and CRP was measured at the Framingham Heart Study core laboratory using a Dade Behring BN100 nephelometer with an average interassay CV of 2.2%.
Carotid ultrasonography
A single sonographer, certified by the Registry of Diagnostic Medical Sonographers, performed all carotid ultrasonography. Ultrasound image acquisition was obtained at the end of the R wave of an electrocardiogram to capture end-diastole. Using a standard 45° projection from vertical, data was acquired from images obtained at three levels: the common carotid artery (CCA), level of the carotid artery bulb, and proximal internal carotid artery (ICA). One additional image was taken of the carotid artery bulb and proximal ICA centered on the largest plaque. Intima-media thickness values were calculated from the intima-media interface lines drawn by a certified reader on each acquired image (4). Using a distance of approximately 0.5 cm below the carotid-artery bulb and measuring IMT over a 1 cm long segment (deemed not to have any plaque presence), the mean IMT of the CCA was determined. The largest IMT in either the left or right ICA from 1cm above the carotid sinus to the bulb determined the maximum ICA IMT. Maximum ICA IMT was also examined as a dichotomous variable, defined as plaque presence if ICA IMT > 1.5 mm. Replicate measurements in 37 participants was performed for assessment of reproducibility with Pearson correlation coefficients (between replicate measurements) for the mean CCA IMT and maximum ICA IMT, 0.94 and 0.76, respectively (4).
Statistical Analyses
All biomarkers and two carotid IMT outcomes (maximum ICA IMT, and mean CCA IMT) were natural-logarithmically transformed owing to their right-skewed distributions. In primary analyses, multivariable linear regression was used to examine the associations of maximum ICA IMT and mean CCA IMT with each biomarker (GDF-15, sST2, and hsTnI). Beta-estimates were expressed as relative change in IMT per 1-standard deviation (SD) change in log-transformed biomarker, and were back-transformed for interpretation. For the carotid outcome of plaque presence, multivariable logistic regression modeling was applied, with reported odds ratios per 1-SD change in log-transformed biomarker. Adjustment for age, sex, systolic blood pressure, hypertension treatment, total cholesterol, HDL cholesterol, diabetes, smoking, body mass index, and baseline estimated glomerular filtration rate was used in primary models. To account for testing of multiple biomarkers (n=3) and carotid measures (n=3), results for primary analyses were considered significant at a Bonferroni-corrected P-value threshold of 0.05/9 = 0.0056.
In secondary analyses, we tested effect modification by age and sex for associations of three biomarkers and carotid outcomes. In addition, we further adjusted multivariable models for CRP and BNP, which have previously been shown to be associated with carotid IMT measures (16, 17). A P-value threshold of 0.05 was deemed significant in secondary analyses. Analyses were conducted with SAS version 9.2.
RESULTS
The study sample contained 3111 Framingham Offspring cohort participants, 54% were female with a mean age of 58 years. Twenty-six percent were treated for hypertension, 15% were current smokers, and 9% had diabetes. Clinical, biomarker, and carotid measurements are shown in Table 1.
Table 1.
Baseline characteristics of 3111 participants free of prevalent CVD
| Total sample (n=3111) | |
|---|---|
| Clinical characteristics | |
| Age, years | 58 (10) |
| Female sex, n (%) | 1687 (54) |
| Systolic blood pressure, mmHg | 128 (19) |
| Treatment for high blood pressure, n (%) | 809 (26) |
| Diabetes, n (%) | 274 (9) |
| Body mass index, kg/m2 | 27.9 (5.2) |
| Current smoker, n (%) | 470 (15) |
| Total cholesterol, mg/dL | 206 (40) |
| HDL, mg/dl | 51 (16) |
| Biomarkers (median, 25th – 75th percentile) | |
| GDF-15, ng/L | 1028 (809–1334) |
| sST2, ng/mL | 20.8 (16.6–26.0) |
| hs-TnI, ng/L | 1.3 (0.9–2.2) |
| Intima-media thickness (median, 25th–75th percentile) | |
| Maximum ICA IMT, mm | 1.11 (0.81–1.78) |
| Plaque presence (ICA IMT > 1.5 mm), n (%) | 1025 (33) |
| Mean CCA IMT, mm | 0.58 (0.51–0.66) |
HDL, high-density lipoprotein; GDF-15, growth differentiation factor-15; sST2, soluble ST2; hsTnI, high-sensitivity troponin I; CCA, common carotid artery; ICA, internal carotid artery.
Values are mean (standard deviation) unless otherwise indicated.
To convert cholesterol in mg/dL to mmol/L, multiply by 0.02586.
Associations of carotid measures with GDF-15, sST2, and hsTnI
In age- and sex-adjusted regression models, maximal ICA IMT was significantly associated with each biomarker. After multivariable adjustment (including age, sex, systolic blood pressure, hypertension treatment, total and HDL cholesterol, diabetes, smoking, body mass index) the association remained significant only for log-GDF-15 (β-estimate 0.04, SE 0.01, P < 0.0001, Table 2). Specifically, maximal ICA IMT increased 4% per 1-SD increase in log- GDF-15. Neither sST2 nor hsTnI were associated with maximal ICA IMT after multivariable adjustment.
Table 2.
Association of carotid measures with GDF-15, sST2, and hsTnI
| Outcome | Biomarker | Age- and sex-adjusted model |
Multivariable-adjusted modela, b |
||
|---|---|---|---|---|---|
|
Maximum ICA IMTd |
β estimate (SE)b | Pc | β estimate (SE)b | Pc | |
| GDF-15 | 0.070 (0.009) | <0.0001 | 0.04 (0.010) | <0.0001 | |
| sST2 | 0.025 (0.008) | 0.003 | 0.013 (0.008) | 0.10 | |
| hsTnI | 0.024 (0.008) | 0.004 | 0.02 (0.008) | 0.01 | |
|
Plaque Presence |
OR (95% CI)b | Pc | OR (95% CI)b | Pc | |
| GDF-15 | 1.48 (1.34–1.63) | <0.0001 | 1.33 (1.20–1.48) | <0.0001 | |
| sST2 | 1.15 (1.05–1.25) | 0.002 | 1.10 (1.00–1.20) | 0.05 | |
| hsTnI | 1.11 (1.02–1.21) | 0.02 | 1.09 (1.00–1.19) | 0.04 | |
|
Mean CCA IMTd |
β estimate (SE)b | Pc | β estimate (SE)b | Pc | |
| GDF-15 | 0.014 (0.004) | 0.0003 | 0.004 (0.004) | 0.30 | |
| sST2 | 0.009 (0.003) | 0.01 | 0.003 (0.003) | 0.31 | |
| hsTnI | 0.009 (0.003) | 0.007 | 0.007 (0.003) | 0.03 | |
GDF-15, growth differentiation factor-15; sST2, soluble ST2; hsTnI, high-sensitivity troponin I; CCA, common carotid artery; ICA, internal carotid artery
Adjusted for age, sex, systolic blood pressure, hypertension treatment, total cholesterol, high-density cholesterol, diabetes, smoking, body mass index, and estimated glomerular filtration rate
β estimate and OR reflects per 1-SD increase in log-transformed biomarker
Corrected P-value threshold P = 0.0056
Mean CCA and maximum ICA IMT were log-transformed;
In age- and sex-adjusted models, plaque presence (defined as ICA IMT > 1.5 mm) was significantly associated with GDF-15 and sST2. After multivariable adjustment, plaque presence remained significantly related to GDF-15 (odds ratio (OR) 1.33 per 1-SD increase in log-GDF-15, 95% CI 1.20-1.48, P < 0.0001, Table 2). Neither sST2 nor hsTnI were associated with plaque presence after multivariable adjustment.
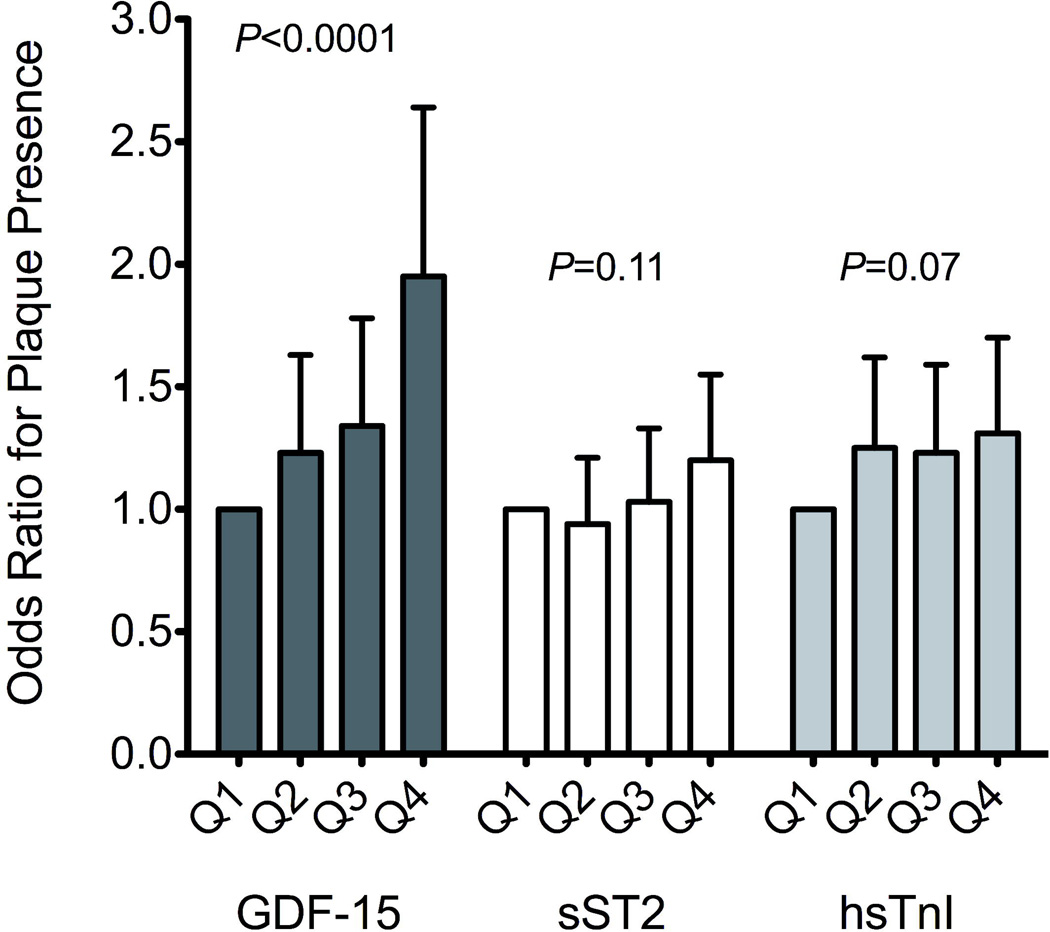
The risk of plaque presence across increasing quartiles of the 3 biomarkers, GDF-15, sST2, and hsTnI are displayed in Figure 1. Increasing quartiles of GDF-15 were associated with increasing risk of plaque in multivariable analyses (P for trend < 0.0001). Specifically, participants in the upper quartile of GDF-15 had a nearly two-fold increased odds of carotid plaque compared with the lowest quartile (OR 1.95, 95% CI 1.44-2.64, P < 0.0001).
Figure 1.
Multivariable-adjusted odds ratios for carotid plaque presence across quartiles of GDF-15, sST2, and hs-TnI. Error bars represent 95% CI.
The associations of the biomarkers, GDF-15, sST2, and hsTnI, were evaluated with the outcome mean CCA IMT (Table 2). GDF-15 but not sST2 or hsTnI were significantly associated with mean CCA IMT in age- and sex- adjusted models, and none of the three biomarkers were significantly associated after multivariable adjustment at the pre-specified statistical threshold of P=0.0056.
Comparison with established biomarkers
After further adjusting multivariable models for BNP and CRP, biomarkers that previously have been linked with carotid measures, GDF-15 remained a significant predictor of maximal ICA IMT, conferring a 1.04-fold increase in carotid ICA thickness per 1-SD increase of log-GDF-15 (P=0.0002). In contrast, BNP was not associated with maximal ICA IMT in the multimarker model (P=0.48), and CRP only nominally so (P=0.04). Both GDF-15 and CRP were significant predictors of plaque presence in multivariable-adjusted models, and the association with GDF-15 appeared more significant (OR per 1-SD unit increase in log-GDF-15 1.32; 95% CI, 1.18-1.47; P < 0.0001; OR per 1-SD unit increase in log-CRP 1.15; 95% CI, 1.04-1.27; P = 0.008) (Table 3).
Table 3.
Association of GDF-15 and established biomarkers with carotid measures
| Modela | Maximum ICA IMT | Plaque presence | Mean CCA IMT | |||
|---|---|---|---|---|---|---|
| β estimate (SE)b | Pc | Odds ratio (95% CI)b | P | β estimate (SE)b | Pc | |
| Individual biomarker | ||||||
| GDF-15 | 0.040 (0.01) | <0.0001 | 1.33 (1.20–1.48) | <0.0001 | 0.004 (0.004) | 0.30 |
| Biomarker combination | ||||||
| GDF-15 | 0.037 (0.01) | 0.0002 | 1.32 (1.18–1.47) | <0.0001 | 0.004 (0.004) | 0.36 |
| BNP | 0.006 (0.009) | 0.48 | 0.98 (0.89–1.08) | 0.71 | 0.003 (0.004) | 0.33 |
| CRP | 0.019 (0.009) | 0.04 | 1.15 (1.04–1.27) | 0.008 | 0.002 (0.004) | 0.53 |
GDF-15, growth differentiation factor-15; BNP, B-type natriuretic peptide; CRP, C-reactive protein; CCA, common carotid artery; ICA, internal carotid artery
All models adjusted for age, sex, systolic blood pressure, hypertension treatment, total cholesterol, high-density cholesterol, diabetes, smoking, body mass index, and estimated glomerular filtration rate
β estimate and OR reflects per 1-SD increase in log-transformed biomarker
Corrected P-value threshold P = 0.0056
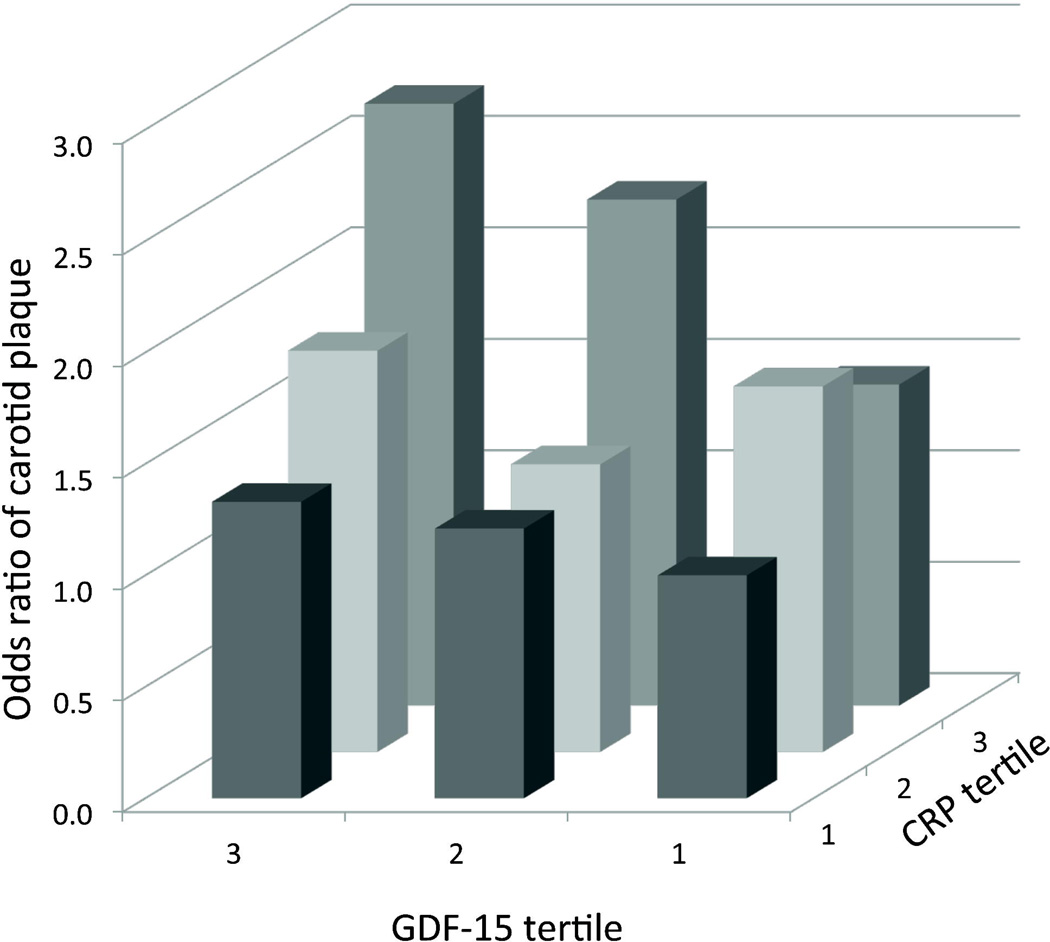
For a given CRP tertile, GDF-15 added further information with regards to plaque risk (Figure 2). For example, when examining individuals in the highest tertile of CRP, those in the lowest tertile of GDF-15 had a 1.44-fold increased odds of plaque, compared with a 2.7-fold increased odds in the highest GDF-15 tertile (with those in the lowest tertile for both biomarkers serving as the referent group).
Figure 2.
Multivariable-adjusted odds ratios for carotid plaque presence across increasing tertiles of GDF and CRP. Referent group for odds ratio was GDF-15 tertile 1 and CRP tertile 1.
DISCUSSION
GDF-15, sST2, and hsTnI, have recently emerged as predictors of cardiovascular outcomes in both patients with existing cardiovascular disease, as well as in the community-dwelling population. The present investigation extends these findings and supports the concept that GDF-15 is strongly associated with subclinical atherosclerosis as measured by carotid ultrasonography, even before clinical cardiovascular disease is recognized. Moreover, GDF-15 was associated with carotid plaque independent of established biomarkers of cardiovascular risk, CRP and BNP.
GDF-15 is a divergent member of the transforming growth factor β cytokine family (18) that is upregulated in response to stressors including in macrophages exposed to oxidized LDL in atherosclerotic carotid arteries (19). It is expressed in several cell types, including macrophages, vascular smooth muscle cells, and endothelial cells in response to oxidative, inflammatory, or metabolic stressors (19–21).
Specific to atherosclerosis, GDF-15 has shown predictive abilities of coronary heart disease mortality and composite outcomes in stable and acute coronary syndromes in patients with prevalent cardiovascular risk factors (22–25). In community cohorts, GDF-15 has conferred prognostic abilities for composite cardiovascular outcomes as well as hard mortality outcomes (9–11, 26). Our findings support the concept that GDF-15 is in fact associated with subclinical atherosclerosis as assessed by maximal ICA IMT as well as the presence of carotid plaque.
In contrast to the strong association of GDF-15 with maximal ICA IMT, we found no such association with mean CCA IMT. Previous studies suggest that maximum ICA IMT and mean CCA IMT may reflect different underlying process, the former related to localized atherosclerotic plaque (27), and the latter representing diffuse arterial wall hypertrophy (28). Interestingly, maximal ICA and plaque presence, but not mean CCA IMT improved risk classification for incident cardiovascular disease in a previous study (29). Our findings suggest that GDF-15 is associated with focal atherosclerosis, rather than diffuse inflammatory processes and arterial thickening. Similar results were found in an older community-based cohort, where GDF-15 was associated with carotid plaque but not IMT (10). Our results extend these findings to a population more than a decade younger than the previous study, without known prevalent cardiovascular disease, and directly compare GDF-15 to other emerging and established biomarkers.
We found that the association of GDF-15 and carotid plaque is independent of CRP, a marker of inflammation. Notably, in multi-marker models with CRP and BNP, the association of GDF-15 with maximum ICA IMT and carotid plaque was more robust than either BNP or CRP. This suggests that GDF-15 may reflect an orthogonal pathway associated with cardiovascular disease, the mechanism of which remains unclear. Experimental studies suggest anti-thrombotic and anti-platelet effects (30). However, in vascular tissue studies, there is growing evidence that GDF-15 may promote increased inflammation and atherosclerosis progression, a process that may involve p53 pathway activation (31, 32). Whether GDF-15 is a mediator of cardiovascular disease or upregulated in response to cardiovascular injury remains unclear.
In comparison to GDF-15, sST2 and hsTnI exhibited no significant associations with carotid measures in our study. Soluble ST2, a member of the interleukin-1 cytokine family, has been shown to serve as a decoy receptor, binding IL-33 – a cytokine shown to have protective effects in the cardiovascular system (33, 34). In atherosclerosis, IL-33 administration in murine models of atherosclerosis led to significantly smaller atherosclerotic plaques while sST2 administration led to larger aortic sinus atherosclerotic plaques (35). Although sST2 has shown associations with cardiovascular outcomes in acute coronary syndrome (36, 37), more recent studies have not shown sST2 to be predictive of atherosclerotic plaque in both patients with carotid atherosclerotic disease (38) as well as patients free of vascular disease (39).
There are several limitations to the present study that merit discussion. Ours is an observational cross-sectional study, and causal inferences cannot be drawn. It is also important to note that carotid IMT may incompletely capture the complex process of atherosclerosis in multiple vascular beds (40), and interval carotid IMT measurements may confer more specificity in this process and relation with atherosclerotic outcomes. The use of our Bonferroni corrected P-value threshold may have been too conservative, given that two of the carotid measures (ICA IMT and plaque presence) are not independent of one another. However, even when using a less conservative P-value threshold, the main results did not differ materially. Lastly, our population was predominantly white, limiting generalizability to other ethnic groups.
In summary, we found that GDF-15 was associated with subclinical atherosclerosis, including higher maximum ICA IMT and the presence of carotid plaque in an ostensibly healthy community-based population without prevalent cardiovascular disease. This association was independent of other established biomarkers including CRP and BNP. Future studies are warranted to elucidate the potential use of GDF-15 for screening and management of patients with subclinical atherosclerosis.
ACKNOWLEDGMENTS
This work was partially supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195). Dr. Januzzi is partially supported by the DeSanctis Clinical Scholar Endowment. Dr. Cheng is supported by NIH grant R00- HL107642 and an award from the Ellison Foundation. Dr. Wollert was supported by the German Ministry of Education and Research (BMBF, BioChancePlus). Dr. Ho was supported by NIH grant K23-HL116780, and a Boston University School of Medicine Department of Medicine Career Investment Award. Measurement of sST2 was performed by Critical Diagnostics, Inc. GDF-15 assays were provided by Roche Diagnostics, Inc. Measurement of hsTnI was performed by Singulex, Inc.
Abbreviations
- BNP
B-type natriuretic peptide
- CCA
common carotid artery
- CI
confidence interval
- CRP
C-reactive protein
- GDF-15
growth differentiation factor-15
- hsTnI
high-sensitivity troponin I
- ICA
internal carotid artery
- IMT
intima-media thickness
- OR
odds ratio
- SD
standard deviation
- sST2
soluble ST2
Footnotes
Statement of competing financial interests: Dr. Januzzi has received research grant funding from Roche Diagnostics and Critical Diagnostics, and has served on the advisory board for Critical Diagnostics. Drs. Wollert and Kempf are named as co-inventors on a patent for the use of GDF-15 for cardiovascular applications, and have a contract with Roche Diagnostics for the development of the GDF-15 assay. Drs. Wang and Ho served in an advisory role at the 2014 ST2 Consensus panel, sponsored by Critical Diagnostics.
REFERENCES
- 1.Bots ML, Hofman A, Grobbee DE. Common carotid intima-media thickness and lower extremity arterial atherosclerosis. The Rotterdam Study. Arterioscler Thromb. 1994;14:1885–1891. doi: 10.1161/01.atv.14.12.1885. [DOI] [PubMed] [Google Scholar]
- 2.Bots ML, Witteman JC, Grobbee DE. Carotid intima-media wall thickness in elderly women with and without atherosclerosis of the abdominal aorta. Atherosclerosis. 1993;102:99–105. doi: 10.1016/0021-9150(93)90088-c. [DOI] [PubMed] [Google Scholar]
- 3.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by b-mode ultrasound in populations: Associations with cardiovascular risk factors in the aric study. Am J Epidemiol. 1991;134:250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 4.Polak JF, Pencina MJ, Meisner A, Pencina KM, Brown LS, Wolf PA, D'Agostino RB., Sr. Associations of carotid artery intima-media thickness (IMT) with risk factors and prevalent cardiovascular disease: Comparison of mean common carotid artery imt with maximum internal carotid artery imt. J Ultrasound Med. 2010;29:1759–1768. doi: 10.7863/jum.2010.29.12.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 6.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 7.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr. Carotidartery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 8.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The atherosclerosis risk in communities (ARIC) study; 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 9.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: The Rancho Bernardo Study. Circulation. 2011;123:2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: Results from the prospective investigation of the vasculature in uppsala seniors (PIVUS) study. Eur Heart J. 2009;30:2346–2353. doi: 10.1093/eurheartj/ehp261. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, et al. Prognostic utility of novel biomarkers of cardiovascular stress: The Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin t using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho JE, Hwang SJ, Wollert KC, Larson MG, Cheng S, Kempf T, et al. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin Chem. 2013;59:1613–1620. doi: 10.1373/clinchem.2013.205716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, et al. Distribution and clinical correlates of the interleukin receptor family member soluble st2 in the framingham heart study. Clin Chem. 2012;58:1673–1681. doi: 10.1373/clinchem.2012.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 16.Thakore AH, Guo CY, Larson MG, Corey D, Wang TJ, Vasan RS, et al. Association of multiple inflammatory markers with carotid intimal medial thickness and stenosis (from the Framingham Heart Study) Am J Cardiol. 2007;99:1598–1602. doi: 10.1016/j.amjcard.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Zhou W, Ni Z, Yu Z, Shi B, Wang Q. Brain natriuretic peptide is related to carotid plaques and predicts atherosclerosis in pre-dialysis patients with chronic kidney disease. Eur J Intern Med. 2012;23:539–544. doi: 10.1016/j.ejim.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxldl-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 20.Bermúdez B, López S, Pacheco YM, Villar J, Muriana FJG, Hoheisel JD, et al. Influence of postprandial triglyceride-rich lipoproteins on lipid-mediated gene expression in smooth muscle cells of the human coronary artery. Cardiovasc Res. 2008;79:294–303. doi: 10.1093/cvr/cvn082. [DOI] [PubMed] [Google Scholar]
- 21.Secchiero P, Corallini F, Gonelli A, Dell'Eva R, Vitale M, Capitani S, et al. Antiangiogenic activity of the mdm2 antagonist nutlin-3. Circ Res. 2007;100:61–69. doi: 10.1161/01.RES.0000253975.76198.ff. [DOI] [PubMed] [Google Scholar]
- 22.Bonaca MP, Morrow DA, Braunwald E, Cannon CP, Jiang S, Breher S, et al. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: Observations from prove it-timi 22. Arterioscler Thromb Vasc Biol. 2011;31:203–210. doi: 10.1161/ATVBAHA.110.213512. [DOI] [PubMed] [Google Scholar]
- 23.Eggers KM, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Jantzen F, et al. Growth-differentiation factor-15 for long-term risk prediction in patients stabilized after an episode of non-st-segment-elevation acute coronary syndrome. Circ Cardiovasc Genet. 2010;3:88–96. doi: 10.1161/CIRCGENETICS.109.877456. [DOI] [PubMed] [Google Scholar]
- 24.Kempf T, Sinning J-M, Quint A, Bickel C, Sinning C, Wild PS, et al. Growth-differentiation factor-15 for risk stratification in patients with stable and unstable coronary heart disease: Results from the atherogene study. Circ Cardiovasc Genet. 2009;2:286–292. doi: 10.1161/CIRCGENETICS.108.824870. [DOI] [PubMed] [Google Scholar]
- 25.Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, et al. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non st-elevation acute coronary syndrome. Circulation. 2007;116:1540–1548. doi: 10.1161/CIRCULATIONAHA.107.697714. [DOI] [PubMed] [Google Scholar]
- 26.Rohatgi A, Patel P, Das SR, Ayers CR, Khera A, Martinez-Rumayor A, et al. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: Observations from the Dallas Heart Study. Clin Chem. 2012;58:172–182. doi: 10.1373/clinchem.2011.171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalager S, Paaske WP, Kristensen IB, Laurberg JM, Falk E. Artery-related differences in atherosclerosis expression: Implications for atherogenesis and dynamics in intima-media thickness. Stroke. 2007;38:2698–2705. doi: 10.1161/STROKEAHA.107.486480. [DOI] [PubMed] [Google Scholar]
- 28.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 29.Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB., Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossaint J, Vestweber D, Zarbock A. Gdf-15 prevents platelet integrin activation and thrombus formation. J Thromb Haemost. 2013;11:335–344. doi: 10.1111/jth.12100. [DOI] [PubMed] [Google Scholar]
- 31.Bonaterra GA, Zugel S, Thogersen J, Walter SA, Haberkorn U, Strelau J, Kinscherf R. Growth differentiation factor-15 deficiency inhibits atherosclerosis progression by regulating interleukin-6-dependent inflammatory response to vascular injury. J Am Heart Assoc. 2012;1:e002550. doi: 10.1161/JAHA.112.002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, Filipovic Z, Brown D, Breit SN, Vassilev LT. Macrophage inhibitory cytokine-1: A novel biomarker for p53 pathway activation. Mol Cancer Ther. 2003;2:1023–1029. [PubMed] [Google Scholar]
- 33.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. Il-33 and st2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2:684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 35.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, et al. Il-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008;117:1936–1944. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 38.Willems S, Quax PH, de Borst GJ, de Vries JP, Moll FL, de Kleijn DP, et al. Soluble st2 levels are not associated with secondary cardiovascular events and vulnerable plaque phenotype in patients with carotid artery stenosis. Atherosclerosis. 2013;231:48–53. doi: 10.1016/j.atherosclerosis.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Miller AM, Purves D, McConnachie A, Asquith DL, Batty GD, Burns H, et al. Soluble ST2 associates with diabetes but not established cardiovascular risk factors: A new inflammatory pathway of relevance to diabetes? PLoS One. 2012;7:e47830. doi: 10.1371/journal.pone.0047830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bots ML, Baldassarre D, Simon A, de Groot E, O'Leary DH, Riley W, et al. Carotid intima-media thickness and coronary atherosclerosis: Weak or strong relations? Eur Heart J. 2007;28:398–406. doi: 10.1093/eurheartj/ehl482. [DOI] [PubMed] [Google Scholar]