Abstract
Although oligodeoxynucleotides containing CpG motifs (CpG-ODN) are potent immune stimulators, the use of natural CpG-ODN—phosphodiester-backbone CpG—has been limited due to its instability by nuclease in vivo. The aim of this study is to investigate the anticancer efficiency of CpG-ODN capsulated using liposome, which enhances the stability of CpG-ODN. We formulated lipoplex, encapsulated natural CpG-ODN from Mycobacterium bovis with liposome, and tested its immune stimulatory activity in vitro and in vivo. The lipoplex induced a systemic innate immune response in vivo and stimulated dendritic cells, but not macrophages, to stimulate proinflammatory cytokines such as tumor necrosis factor alpha and interleukin-6 in vitro. As expected, the lipoplex effectively mediated the prolonged cancer-therapeutic activity against B16 melanoma, which was dependent on natural killer and CD8+ T cells. The therapeutic activity was observed after only intratumoral administration of lipoplex among several treatment routes. Intratumoral treatment of lipoplex significantly increased the populations of natural killer and CD8+ T cells and reduced regulatory CD4+ T cell recruitment, which was correlated with expression profiles of chemokines (CCL1, CCL3, CXCL1, CXCL10, and CCL22). The antitumor therapeutic effect of lipoplex was dependent on the altered lymphocyte population that might be developed by the profile of intratumoral chemokine expression.
Introduction
The innate immune system is an antigen-nonspecific defense mechanism that involves pattern recognition receptors (PRRs) recognizing pathogen-associated molecular patterns produced by microorganisms. Activation of toll-like receptors (TLRs), a representative PRRs family, enhances the immune response in various disease conditions, including cancer [1,2]. Activation of TLRs leads to the release of several mediators of inflammation from tissue macrophages and dendritic cells (DCs). These mediators influence the expression of DC chemokine receptors. These TLR-mediated events are necessary for the recruitment of immature DCs to disease sites and their return to the lymph nodes where they activate naive T cells. These chemokines also guide the activated T cells to the site of disease. Thus, chemokines are the bridge between immune cell activation and the tissue recruitment of lymph-node-generated, antigen-specific T cells [3].
Unmethylated oligodeoxynucleotides (ODNs) containing CpG motifs elicit innate immune responses and adaptive immune responses through binding to TLR9 [4]. In contrast to currently licensed adjuvants, CpG-ODN without antigen co-delivery can promote DC maturation, antigen presentation, and production of proinflammatory cytokines and chemokines. DCs maturated by CpG induce the production of interleukin (IL)-12, IL-18, and interferon (IFN)-γ and lead to enhanced T cell-, B cell-, and natural killer (NK) cell–mediated anti-tumor immune responses [5]. Indeed, CpG-ODN treatment without antigens has induced antitumor activity in clinical trials [6]. Although CpG-ODNs have strong immune stimulatory activities, CpG-ODN is not available for clinical use because the natural phosphodiester bond in CpG-ODN is too unstable to effectively stimulate the required immune response [7]. In addition, phosphorothionate-modified CpG-ODN (PS-ODN) is very stable but induces several side effects, including transient lymphoadenopathy, lymphoid follicle destruction, arthritis, and PS-ODN–specific immunoglobulin M ([8,9] in PS-ODN–treated mice in a CG sequence–dependent and backbone modification–dependent manner [10,11]. In a previous study, we constructed synthetic MB-ODN 4531, which originated from Mycobacterium bovis genomic DNA sequences, encapsulated with liposomes (lipoplex) to induce the optimal immune response without side effects and being degraded [12,13].
In this study, the immune stimulatory function and anti-cancer therapeutic effect of lipoplex were examined in vivo and in vitro. Lipoplex can promote a Th1-type immune response and strong inflammatory activity in DCs, but not in macrophages. Intratumoral (i.t.) administration of lipoplex into B16 melanoma-bearing mice induced anti-tumor activity by infiltration of T cells and NK cells and reduced the recruitment of regulatory T cells (Tregs). This antitumor activity was induced by stimulation of several inflammatory chemokines, which thereby altered the tumor microenvironment.
Materials and Methods
Lipoplex preparation
MB-ODN 4531 and its control, MB-ODN 4531GC, were obtained from Samchully Pharm and GenoTech, respectively. The MB-ODN 4531 originated from Mycobacterium tuberculosis and consists of 20 bases and 3 CpG motifs (underlined): AGCAGCGTTCGTGTCGGCCT. GC 4531 is a derivative of CpG 4531 with one of its CG sequences reversed to GC (underlined): AGCAGGCTTCGTGTCGG CCT. When necessary, fluorescent tags were conjugated to the 3′ end of each ODN. Lipoplex was generated as previously described [14]. Briefly, dioleylphosphatidyl ethanolamine (DOPE) and cholesteryl hemisuccinate (CHEMS) were mixed in ethanol at a molar ratio of 1:1, evaporated with nitrogen gas to make a solvent-free lipid film, and resuspended in a mixture containing equal volumes of water soluble CpG-ODN, followed by vigorous stirring at room temperature for 30 min. After adjusting the pH to 7.0, the Lipoplex solution was slightly sonicated for 30 s with a sonicator. We then filtered the solution with a 0.22 μm filter and freeze–thawed it three times with liquid nitrogen. For uptake assay on MB-ODN 4531, PE-Cy5 was conjugated to the MB-ODN 4531 and then the uptake of MB-ODN 4531 by cells was determined using flow cytometry.
Cell culture and mice
Female C57BL/6 mice aged 8 weeks were obtained from Koatech. All mice were kept at 23°C±1°C with a 12 h light/dark cycle. They had free access to water and diet. All procedures were reviewed and approved by the Animal Ethical Committee of Gyeongsang National University. Murine melanoma B16 cell line was grown in Dulbecco's modified eagle media supplemented with 10% fetal bovine serum (FBS; Sigma), 100 U/mL penicillin, and 100 μg/mL streptomycin (LONZA). Elicited macrophages of the C57BL/6 mice were obtained after intraperitoneal (i.p.) injection of a 4% thioglycollate broth (1 mL; Sigma) [15]. Three days later, peritoneal exudate cells were isolated from the peritoneal cavity. Then, the cells were cultured for 2 h in culture medium, and the adherent cells were used as peritoneal macrophages. Bone marrow-derived dendritic cells (BMDCs) from the C57BL/6 mice were prepared from the tibia and the femur [16]. After red blood cell lysis, the bone marrow cells were counted and seeded at a density of 3×106 cells in petri dishes containing complete medium supplemented with granulocyte macrophage colony-stimulating factor (PeproTech) for 10 days. Fresh medium was added at day 4 and day 7, and the cells were harvested, washed, and plated again. Finally, the BMDCs were assessed using CD11c staining (>95%). The macrophages and DCs were cultured in RPMI1640 supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were gown at 37°C in a humidified atmosphere of 5% CO2.
Measurement of cytokines
The mice received i.p. injections of CpG 4531 or lipoplex (50 μg/200 μL for each) and the blood samples were taken from the retro-orbital sinus at 12 h. The macrophages and DCs were stimulated with 5 μg/mL of GC 4531, GC 4531 encapsulated with liposome (lipoplex GC 4531), CpG 4531, or lipoplex for 24 h. The cytokines from the blood sample or culture medium were analyzed using a cytometric bead array mouse inflammation kit or Mouse Th1/Th2 Cytokine kit (BD Biosciences).
Stimulation of OT-II mouse splenocytes
The spleen cells from the OT-II mouse were prepared by enzymatic digestion with collagenase (1 mg/mL; Sigma). The cell suspension was filtered and plated on tissue culture plates. After 2 h, the cells were stimulated with liposome, 5 μg/mL GC 4531, lipoplex GC 4531, CpG 4531, or lipoplex with or without 20 ng/mL of the OT-II OVA peptide (chicken OVA peptide 323-339 ISQAVHAAHAEINEAGR) and incubated for 48 h.
Tumor treatment and monitoring
The B16 tumor cells (1×106) were subcutaneously implanted into the mice. Subsequently, the mice received phosphate buffered saline (PBS) (50 μL), CpG 4531 (12.5 μg/ 50 μL), or lipoplex (12. 5 μg/50 μL) by i.t., intravenous (i.v.), intramuscular (i.m.), or i.p. injection on days 12, 15, and 18 after the initial implantation. The tumor volumes were estimated on days 12, 15, 18, 24, and 28 after the injection.
Lymphocyte depletion
The monoclonal anti-NK1.1 antibody (Ab; PK136) was used for NK cell depletion, the anti-CD4 Ab (GK 1.5) was used for CD4+ T cell depletion, and the anti-CD8 Ab (Yis 169.4.2) was used for CD8+ T cell depletion. The depletion was confirmed using flow cytometry. The mice were i.p. administrated with 250 μg of each Ab 3 times, at 12, 15, and 18 days after tumor injection. Lipoplex was administrated i.t. three times, on day 14, day 17, and day 20 after tumor injection. The tumor volumes were estimated on days 14, 17, and 20 after the injection.
Analysis of tumor-infiltrated lymphocytes
The B16 tumor-bearing mice were killed at 24 h after the final injection of CpG 4531 or lipoplex, and the tumors were resected. The chapped tissues were incubated with 5 mL of collagenase (1 mg/mL; Sigma) under shaking conditions for 20 min and filtered using a cell strainer (BD). The prepared samples were washed and incubated with red blood cell lysis buffer for 5 min. After the incubation, the cells were washed and stained with anti-CD4, anti-CD8, anti-CD45, and anti-NK1.1 Abs (BD) for 30 min. If necessary, the cells were washed and fixed with the Foxp3 staining kit (eBioscience) and stained with anti-Foxp3 Ab for 30 min.
Chemokine mRNA expression
The B16 tumor-bearing mice were killed at 24 h after the final injection of CpG 4531 or lipoplex, and the tumors were resected and weighed. Total RNA was isolated from tissues using a total RNA isolation solution (Riboex, GeneAll), and the cDNA was synthesized by using oligo-dT primers and reverse transcriptase (Fermentas). Real-time polymerase chain reaction (PCR) was performed using the SSoFast EvaGreen Supermix and CFX96 real-time detection system (Bio-Rad Laboratories). The following primers were used for amplification: CCL1, 5′-GGA TGT TGA CAG CAA GAG C-3′ and 5′-CAG GGG TTC ACC TTC TTC A-3′; CCl3, 5′-ATC ATG AAG GTC TCC ACC AC-3′ and 5′-TCT CAG GCA TTC AGT TCC AG-3′; CXCL1, 5′-CGC TTC TCT GTG CAG CGC-3′ and 5′-AAG CCT CGC GAC CAT TCT T-3′; CXCL10, 5′-GGA AGC CTC CCC ATC AGC AC-3′ and 5′-AGA GAG GCT CTC TGC TGT C-3′; CCL22, 5′-ATG TGA GGC CAA ATA GAC GAA-3′ and 5′-CTG GCA CTG TCA ATC CCT G-3′; GAPDH, 5′-CCA TCA CCA TCT TCC AGG AG-3′ and 5′-ACA GTC TTC TGG GTG GCA GT-3′.
Statistical analysis
Student's t-test was used. Error bars represent the standard error of the mean. A p value<0.05 was considered statistically significant.
Results
Lipoplex induced immune response through DCs
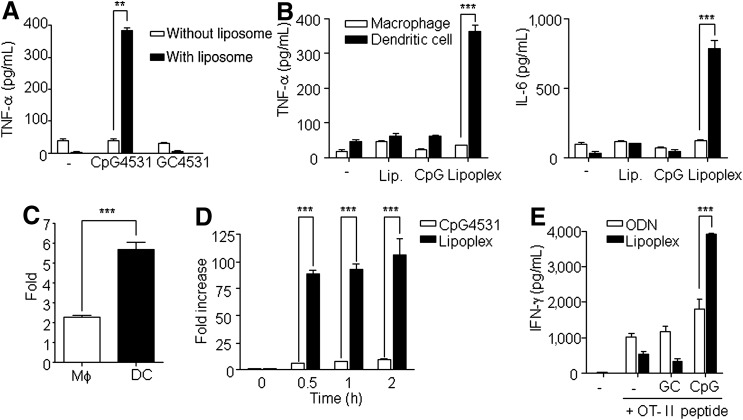
To test whether lipoplex effectively induces an immune response, the mice were administrated a single i.p. injection of naked CpG 4531, lipoplex, naked GC 4531, or lipoplex GC 4531. Elevated tumor necrosis factor alpha (TNF-α) production was observed in the lipoplex-treated mice, whereas CpG 4531, GC 4531, and lipoplex GC did not induce TNF-α production (Fig. 1A). To investigate which cells were activated by lipoplex, the peritoneal macrophages and BMDCs were treated with liposome, CpG 4531, or lipoplex to examine which innate cells are targeted by lipoplex. The production of TNF-α and IL-6 was significantly increased in the DCs treated with lipoplex, but not in the macrophages (Fig. 1B). These results might be due to the high lipoplex uptake efficiency of DCs compared with macrophages (Fig. 1C). Next, we examined the uptake efficacy of DCs to CpG 4531 and lipoplex. Lipoplex was taken up by the DCs within a short time, but the naked CpG 4531 was not (Fig. 1D).
FIG. 1.
Immune stimulatory activity of lipoplex. (A) Mice were intraperitoneally (i.p.) injected with naked CpG 4531, lipoplex, naked GC 4531, or lipoplex GC 4531 (50 μg/200 μL), and tumor necrosis factor (TNF-α) secretion was measured at 12 h post injection. **p<0.01. Data represent mean±standard error of the mean (SEM), n=5. (B) Macrophages and dendritic cells (DCs) were stimulated with 5 μg/mL of liposome, CpG 4531, or lipoplex for 24 h. The production levels of TNF-α and interleukin (IL)-6 were estimated from the culture medium. (C) Macrophages (Mφ) and DCs were incubated with PE-Cy5-conjugated lipoplex for 2 h and the intracellular uptake of lipoplex was estimated by fluorescence-activated cell sorting analysis. (D) The DCs were treated with PE-Cy5–conjugated CpG 4531 or lipoplex, and the intracellular uptake was determined at multiple incubation times. (E) Splenocytes of the OT-II Tg mouse were stimulated with liposomes, 5 μg/mL of GC 4531, lipoplex GC 4531, CpG 4531, or lipoplex in the presence or absence of 20 ng/mL of the OT-II OVA peptide, and incubated for 48 h. After incubation, the supernatant was stained with the cytometric bead array mouse Th1/Th2 kit. Data in B–E represent mean±SEM, n=3. ***p<0.001.
To test whether lipoplex could induce antigen-specific Th1 immune response, splenocytes of the OT-II Tg mouse were stimulated under the indicated conditions for 48 h (Fig. 1E). The results analyzing the antigen specific CD4+ T cell activation revealed that lipoplex induced a Th1-mediated immune response, which induced IFN-γ. The amounts of IFN-γ, which was mainly produced by the Th1 cells, were increased in the presence of lipoplex and the OT-II peptide complex. Comparatively, IL-4, which is produced by the Th2 cells, was not detected in the control or in the presence of lipoplex or the OT-II peptide complex (data not shown). Therefore, these data suggest that capsulation of CpG with liposome increased its ability to be taken up by DCs, which effectively induced immunostimulatory activity involving both innate and adaptive immunity.
Lipoplex induced anti-tumor activity
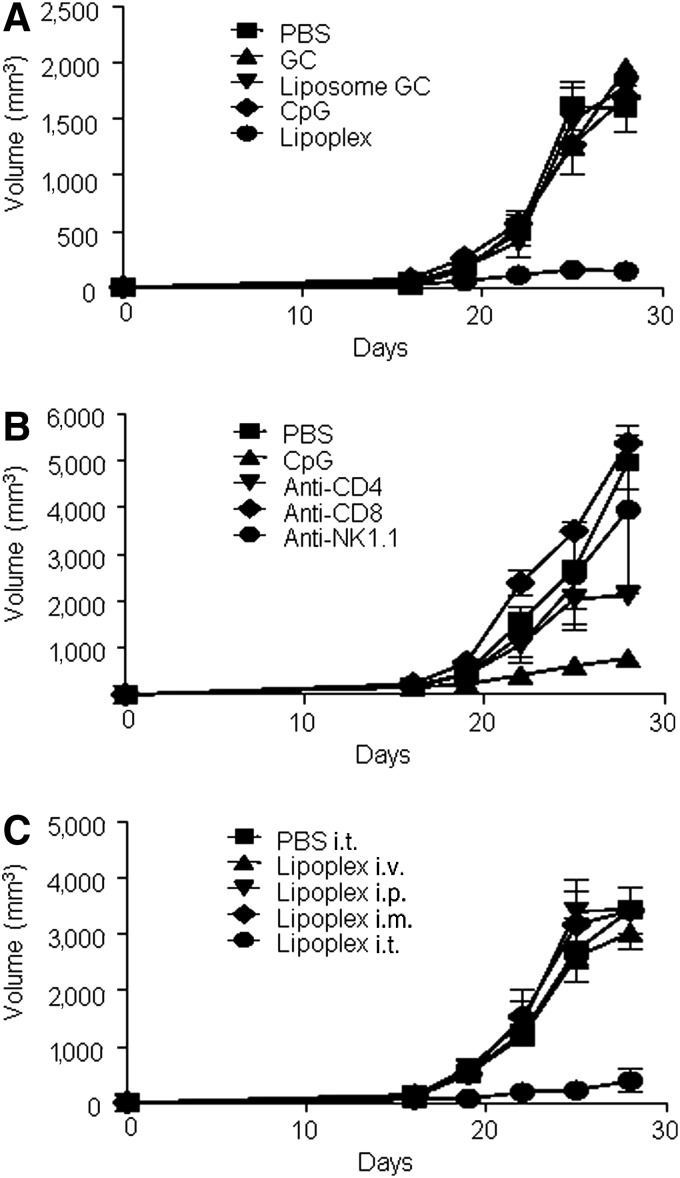
The aforementioned data showed that lipoplex induced the Th1 immune response, which promotes the cell-mediated immune response and cytotoxic T lymphocyte (CTL) response [17]. Because activated CTLs are critical components in the treatment of tumors, we examined the antitumor activity of lipoplex using B16 melanoma as a tumor model. Mice were s.c. implanted with B16 tumor cells. On days 12, 15, and 18 after implantation, PBS, CpG 4531, or lipoplex were i.t. injected. As expected, treatment with lipoplex attenuated the tumor growth (Fig. 2A). To test whether a specific lymphocyte population is necessary for lipoplex mediated-tumor therapy, anti-CD4 mAb, anti-CD8 mAb, and anti-NK mAb were used for the depletion of each lymphocyte in vivo. Treatment with each antibody caused a loss of 98% of each lymphocyte in peripheral blood cells (data not shown). Our results also revealed that NK and CD8+ T cells are essential for lipoplex-mediated tumor therapy (Fig. 2B). CpG-ODN induced different effects with respect to tumor growth inhibition depending on the route of administration [18]. Therefore, we explored the role of the administration route (i.p., i.t., i.m., and i.v.) on the anti-tumor effect of lipoplex. As shown in Fig. 2C, only i.t. injection of lipoplex induced an antitumor growth effect.
FIG. 2.
Antitumor activity of lipoplex. (A) Mice were subcutaneously implanted with B16 tumor cells and were then injected intratumorally (i.t.) with phosphate buffered saline (PBS), GC 4531 (GC), GC4531 liposome (liposome GC), CpG 4531, or lipoplex as described in the “Materials and Methods” section. Tumor size was estimated at multiple time points. (B) Anti-tumor activity of lipoplex was estimated in natural killer (NK), CD8+ T, or CD4+ T cell–depleted B16-bearing mice. (C) B16-implanted mice were injected with lipoplex at days 12, 15, and 18 via multiple routes. The tumor volumes were estimated at the indicated times. The values are shown as mean±SEM, n=5.
Lipoplex enhanced the intratumoral infiltration of NK and CD8+ T cells
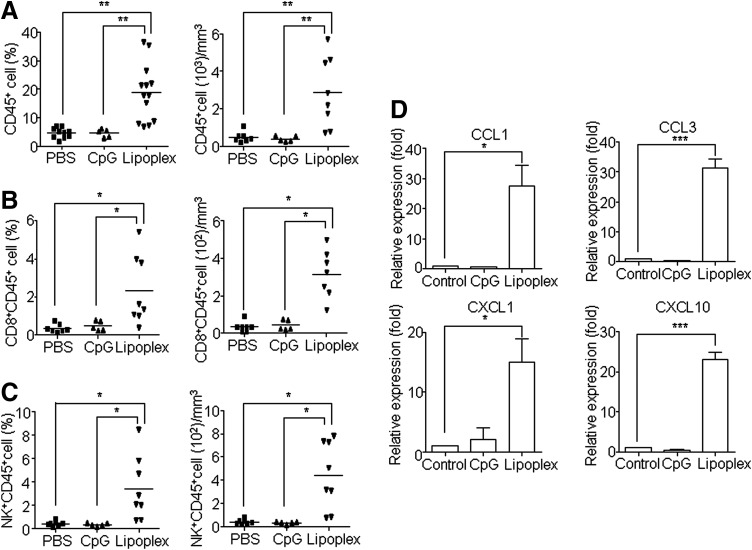
The previous results suggested that the local immune environment and NK and CD8+ T cells played important roles in the antitumor activity of lipoplex. Therefore, we analyzed the infiltration of lymphocytes into tumor tissue using flow cytometry. As expected, treatment with lipoplex, but not CpG 4531, induced the infiltration of CD45+ lymphocytes (Fig. 3A), CD8+ T (Fig. 3B), and NK cells (Fig. 3C). The recruitment of NK and CD8+ T cells into tumor tissues by lipoplex indicated that the expression of lymphocyte-attracting chemokines might be elevated in the tumor microenvironment. Therefore, we explored the effect of CpG4531 and lipoplex treatments on the mRNA expression profiles of various chemokines (CCL1, CCL3, CXCL1, and CXCL10) in the tumor tissue. These proinflammatory chemokines regulate intratumoral trafficking of leukocytes, including T cells and NK cells, and inhibit tumor growth [19–22]. As shown in Fig. 3D, the administration of lipoplex enhanced the expression of inflammatory chemokines.
FIG. 3.
Accumulation of CD8+ T and NK cells at the tumor site. B16 melanoma-bearing mice were administered i.t. CpG 4531 or lipoplex at days 12, 15, and 18, and the tumor was resected and sized on day 20. The tumor tissues were homogenized, treated with collagenase, filtered, and stained with CD45, CD8, and NK for flow cytometry analysis. The percentages of tumor-infiltrating CD45+ (A), CD8+CD45+ T (B), and NK+CD45+ (C) cells were calculated by gating the total living cells. B16 tumor–bearing mice were killed 48 h after the final injection of CpG 4531 or lipoplex. The tumors were resected and the gene expression levels of CCL1, CCl3, CXCL1, and CXCL10 were estimated (D). *p<0.05, **p<0.01, ***p<0.001. Data represent mean±SEM, n=5.
Lipoplex inhibited Treg trafficking into tumor tissue
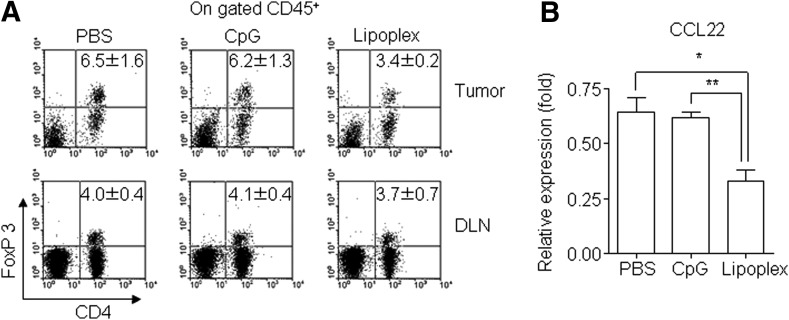
We further tested the ability of lipoplex to regulate the recruitment of Foxp3+ Tregs. As shown in Fig. 4A, the population of Foxp3+ Tregs in the tumor microenvironment was reduced by treatment of lipoplex, whereas there were no observed differences in the draining lymph node following treatment with PBS, CpG 4531, or lipoplex. These results suggest that lipoplex attenuated the recruitment of Foxp3+ Treg into tumor tissues. To determine whether the decreased Foxp3+ Treg accumulation at the tumor site was due to decreased levels of CCL22, which accumulates in Foxp3+ Treg cells [23], we performed real-time PCR for this chemokine. As shown in Fig. 4B, CCL22 expression was downregulated by lipoplex treatment.
FIG. 4.
Regulatory T cell (Treg) infiltration in tumor tissues. Tumor tissues were harvested from the B16 tumor–bearing mice at 2 days after the final injection of lipoplex. (A) Amount of CD45+CD4+FoxP3+ Tregs was estimated by flow cytometry. (B) Gene expression of CCL22 was determined by real-time polymerase chain reaction. *p<0.05; **p<0.01. Data represent mean±SEM, n=5.
Discussion
It has been well established that CpG motifs are recognized by the innate immune system via interaction with the PRR TLR9 [24,25]. The CpG motif promotes an immune response that activates immune cells, such as DCs, macrophages, NK cells, B cells, and T cells, and induces secretion of various cytokines and chemokines [26]. Although CpG-ODN has strong immunostimulatory activities, the natural phosphodiester bond CpG-ODN is not viable in vivo because it is rapidly degraded by nucleases [27,28]. To solve this problem, we constructed lipoplex that induces an optimal immune response with slower degradation rates compared to CpG-ODN [14]. In this study, we have shown that lipoplex induces the production of proinflammatory cytokines to a greater extent than CpG 4531. It is well established that encapsulating CpG-ODN in liposomes facilitates their uptake by macrophages and DCs and increases their immunostimulatory activity [29,30]. Our study suggests that DCs are the main target cells of lipoplex and that DCs stimulated by lipoplex produce greater amounts of cytokines such as TNF-α and IL-6 than macrophages. The liposomes used in this study were a mixture of the pH-sensitive liposomes DOPE and CHEMS [31]. Therefore, the DOPE:CHEMS formulations were stable at neutral and basic pH, but not at acidic pH [32]. Phagosomal pH in the DCs is >7, whereas the pH in macrophages is <6 [33]. Consequently, lipoplex might be unstable in macrophages and therefore unable to effectively stimulate macrophages compared with DCs.
Upon activation, DCs rapidly produce type 1 IFNs, which in turn activates NK cells, B cells, and T cells, thereby leading to the induction of innate and adaptive immune responses [34]. CD4+ T cells provoked with antigen-specific stimuli proliferate and differentiate into either the Th1 or Th2 subset. Systemic administration of CpG-ODN induces the Th1 cell immune response that inhibits the development of the Th2 type responses such as allergic asthma [35]. In our study, we revealed that lipoplex induced a greater Th1-mediated immune response with respect to antigen specific CD4+ T cell activation compared to CpG 4531. The Th1 immune response would be required for immunological memory of tumor-specific CTLs [36]. CTLs recognize tumor-associated antigens from tumor cells through uptake and processing by DCs and they subsequently induce tumor-specific immunity [37]. Indeed, we showed that lipoplex has the potential to inhibit tumor growth and that the anti-tumoral activity of lipoplex is associated with the presence of NK and CD8+ T cells. Although the lipoplex had a similar immune stimulatory effect to PS-ODN, usage of lipoplex would be available due to low side effect compared with that of PS-ODN [8–14].
The anti-tumor therapeutic effect of CpG-ODN is known to be dependent on injection route [18] because the absorption and accumulation rate varies with the administration site [38,39], and the cytokine production in tumor cells varies depending on the route used to inject liposome-capsulated CpGs [40]. In this context, we analyzed the role of the administration route in the anti-tumor activity of lipoplex. We revealed that only i.t. injection of lipoplex inhibited tumor growth, not i.p., i.v., or i.m. These results suggest that lipoplex activates a local immune response in the tumor environment. Indeed, we found that i.t. injection of lipoplex induced the recruitment of NK and CD8+ T cells at the tumor site, which was crucial for the anti-tumor activity of lipoplex.
Recently, chemokines have been utilized in tumor immunotherapy as a means of recruiting effector immune cells [41]. Tumor infiltrating lymphocytes produce chemokines, such as CCL1, CCL3, CXCL1, and CXCL10, which promote the migration of NK and CD8+ T cells to the tumor site [19–22,42]. These findings are in accordance with our observation that lipoplex induced infiltration of NK and CD8+ T cells into the tumor, thereby resulting in antitumor activity. A recent study showed that i.t injection of CpG-ODN induces differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells [43]. In addition to these functions of CPG-ODN, our data showed another important contribution of lipoplex in antitumor activity; we demonstrated the ability of lipoplex to downregulate CCL22 expression and reduce the amounts of Foxp3+ Tregs accumulated at the tumor site. CD4+CD25+ Tregs can potently inhibit CD8+ T and NK cell responses, which leads to tumor-specific CTL activity [44–46]. Therefore, the ability of lipoplex to inhibit the recruitment of Foxp3+ Tregs to the tumor site might contribute to tumor regression.
The selective delivery of anticancer drugs to tumor sites remains an important aim of cancer therapy. Many studies demonstrating the benefits of lipid-based delivery, including protection from the nucleic acid payload, improved pharmacokinetic characteristics, enhanced intracellular uptake, and enhanced delivery to target antigen-presenting cells of CpG-ODN [4]. Additionally our results further our understanding of lipid-based delivery systems by demonstrating that i.t. injection of lipoplex might be the optimal route of delivery for cancer therapy.
In conclusion, our data suggests that administration of lipoplex inhibits tumor growth and induces infiltration of NK and CD8+ T cells to the tumor site by inducing proinflammatory chemokine expression, which alters the anti-tumor activity. Furthermore, i.t. injection of lipoplex inhibits the recruitment of Foxp3+ Tregs to the tumor site, and therefore, i.t. administration is the most efficient method for the application of lipoplex in tumor regression therapies.
Conclusions
CpG-ODN capsulated with liposome (lipolex) was a strong immune stimulator that activated dendritic cell in vitro and induced TNF-α in vivo. The anticancer activity of lipoplex was limited to i.t. administration route only, not other routes (i.p., i.v., i.m.). The activity was dependent on lymphocytes, NK and CD8+ T cells, and the lymphocytes were highly infiltrated in tumor tissue. Additionally, Treg infiltration was reduced by lipoplex administration. Chemokine expression patterns were correlated with intratumoral lymphocyte infiltration. Taken together, the antitumor therapeutic effect of lipoplex might be dependent on alterations to the chemokine environment in tumor tissue as well as the induction of innate and acquired immune responses.
Acknowledgment
This study is supported by next-generation biogreen21 (SSAC, PJ01107002), Rural Development Administration, Republic of Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Krieg AM. (2004). Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep 6:88–95 [DOI] [PubMed] [Google Scholar]
- 2.Murad YM. and Clay TM. (2009). CpG oligodeoxynucleotides as TLR9 agonists: therapeutic applications in cancer. BioDrugs 23:361–375 [DOI] [PubMed] [Google Scholar]
- 3.Luster AD. (2002). The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol 14:129–135 [DOI] [PubMed] [Google Scholar]
- 4.Wilson KD, de Jong SD. and Tam YK. (2009). Lipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacy. Adv Drug Deliv Rev 61:233–242 [DOI] [PubMed] [Google Scholar]
- 5.Jahrsdorfer B. and Weiner GJ. (2008). CpG oligodeoxynucleotides as immunotherapy in cancer. Update Cancer Ther 3:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pashenkov M, Goess G, Wagner C, Hormann M, Jandl T, Moser A, Britten CM, Smolle J, Koller S, et al. (2006). Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol 24:5716–5724 [DOI] [PubMed] [Google Scholar]
- 7.Zhao Q, Matson S, Herrera CJ, Fisher E, Yu H. and Krieg AM. (1993). Comparison of cellular binding and uptake of antisense phosphodiester, phosphorothioate, and mixed phosphorothioate and methylphosphonate oligonucleotides. Antisense Res Dev 3:53–66 [DOI] [PubMed] [Google Scholar]
- 8.Lipford GB, Sparwasser T, Zimmermann S, Heeg K. and Wagner H. (2000). CpG-DNA-mediated transient lymphadenopathy is associated with a state of Th1 predisposition to antigen-driven responses. J Immunol 165:1228–1235 [DOI] [PubMed] [Google Scholar]
- 9.Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, Zinkernagel R. and Aguzzi A. (2004). Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med 10:187–192 [DOI] [PubMed] [Google Scholar]
- 10.Zhao Q, Temsamani J, Iadarola PL, Jiang Z. and Agrawal S. (1996). Effect of different chemically modified oligodeoxynucleotides on immune stimulation. Biochem Pharmacol 51:173–182 [DOI] [PubMed] [Google Scholar]
- 11.Sparwasser T, Hultner L, Koch ES, Luz A, Lipford GB. and Wagner H. (1999). Immunostimulatory CpG-oligodeoxynucleotides cause extramedullary murine hemopoiesis. J Immunol 162:2368–2374 [PubMed] [Google Scholar]
- 12.Kim D, Rhee JW, Kwon S, Sohn WJ, Lee Y, Kim DW, Kim DS. and Kwon HJ. (2009). Immunostimulation and anti-DNA antibody production by backbone modified CpG-DNA. Biochem Biophys Res Commun 379:362–367 [DOI] [PubMed] [Google Scholar]
- 13.Lee KW, Jung J, Lee Y, Kim TY, Choi SY, Park J, Kim DS. and Kwon HJ. (2006). Immunostimulatory oligodeoxynucleotide isolated from genome wide screening of Mycobacterium bovis chromosomal DNA. Mol Immunol 43:2107–2118 [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Kwon S, Rhee JW, Kim KD, Kim YE, Park CS, Choi MJ, Suh JG, Kim DS, Lee Y. and Kwon HJ. (2011). Production of antibodies with peptide-CpG-DNA-liposome complex without carriers. BMC Immunol 12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies JQ. and Gordon S. (2005). Isolation and culture of murine macrophages. Methods Mol Biol 290:91–103 [DOI] [PubMed] [Google Scholar]
- 16.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N. and Schuler G. (1999). An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223:77–92 [DOI] [PubMed] [Google Scholar]
- 17.Wieder T, Braumuller H, Kneilling M, Pichler B. and Rocken M. (2008). T cell-mediated help against tumors. Cell Cycle 7:2974–2977 [DOI] [PubMed] [Google Scholar]
- 18.Lou Y, Liu C, Lizee G, Peng W, Xu C, Ye Y, Rabinovich BA, Hailemichael Y, Gelbard A, Zhou D, Overwijk WW. and Hwu P. (2011). Antitumor activity mediated by CpG: the route of administration is critical. J Immunother 34:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhawan P. and Richmond A. (2002). Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol 72:9–18 [PMC free article] [PubMed] [Google Scholar]
- 20.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE. and Luster AD. (2002). IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol 168:3195–3204 [DOI] [PubMed] [Google Scholar]
- 21.Hoelzinger DB, Smith SE, Mirza N, Dominguez AL, Manrique SZ. and Lustgarten J. (2010). Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses. J Immunol 184:6833–6842 [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Li YY, Matsushima K, Baba T. and Mukaida N. (2008). CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. J Immunol 181:6384–6393 [DOI] [PubMed] [Google Scholar]
- 23.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H. and Fujii H. (2008). CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer 122:2286–2293 [DOI] [PubMed] [Google Scholar]
- 24.Takeshita F, Leifer CA, Gursel I, Ishii KJ, Takeshita S, Gursel M. and Klinman DM. (2001). Cutting edge: Role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol 167:3555–3558 [DOI] [PubMed] [Google Scholar]
- 25.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K. and Akira S. (2000). A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745 [DOI] [PubMed] [Google Scholar]
- 26.Krieg AM. (2002). CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20:709–760 [DOI] [PubMed] [Google Scholar]
- 27.Kurreck J. (2003). Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem 270:1628–1644 [DOI] [PubMed] [Google Scholar]
- 28.Shi F. and Hoekstra D. (2004). Effective intracellular delivery of oligonucleotides in order to make sense of antisense. J Control Release 97:189–209 [DOI] [PubMed] [Google Scholar]
- 29.Gursel I, Gursel M, Ishii KJ. and Klinman DM. (2001). Sterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of CpG oligonucleotides. J Immunol 167:3324–3328 [DOI] [PubMed] [Google Scholar]
- 30.Mutwiri GK, Nichani AK, Babiuk S. and Babiuk LA. (2004). Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides. J Control Release 97:1–17 [DOI] [PubMed] [Google Scholar]
- 31.Simoes S, Slepushkin V, Duzgunes N. and Pedroso de Lima MC. (2001). On the mechanisms of internalization and intracellular delivery mediated by pH-sensitive liposomes. Biochim Biophys Acta 1515:23–37 [DOI] [PubMed] [Google Scholar]
- 32.Chu CJ, Dijkstra J, Lai MZ, Hong K. and Szoka FC. (1990). Efficiency of cytoplasmic delivery by pH-sensitive liposomes to cells in culture. Pharm Res 7:824–834 [DOI] [PubMed] [Google Scholar]
- 33.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G. and Amigorena S. (2006). NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126:205–218 [DOI] [PubMed] [Google Scholar]
- 34.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V. and Banchereau J. (2003). Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19:225–234 [DOI] [PubMed] [Google Scholar]
- 35.Klinman DM, Kamstrup S, Verthelyi D, Gursel I, Ishii KJ, Takeshita F. and Gursel M. (2000). Activation of the innate immune system by CpG oligodeoxynucleotides: immunoprotective activity and safety. Springer Semin Immunopathol 22:173–183 [DOI] [PubMed] [Google Scholar]
- 36.Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, Ohta A, Koda T. and Nishimura S. (2000). The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol 46Suppl, S52–S61 [DOI] [PubMed] [Google Scholar]
- 37.Knutson KL. and Disis ML. (2005). Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 54:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leeds JM, Henry SP, Geary R, Burckin T. and Levin AA. (2000). Comparison of the pharmacokinetics of subcutaneous and intravenous administration of a phosphorothioate oligodeoxynucleotide in cynomolgus monkeys. Antisense Nucleic Acid Drug Dev 10:435–441 [DOI] [PubMed] [Google Scholar]
- 39.von Beust BR, Johansen P, Smith KA, Bot A, Storni T. and Kundig TM. (2005). Improving the therapeutic index of CpG oligodeoxynucleotides by intralymphatic administration. Eur J Immunol 35:1869–1876 [DOI] [PubMed] [Google Scholar]
- 40.Kuramoto Y, Nishikawa M, Hyoudou K, Yamashita F. and Hashida M. (2006). Inhibition of peritoneal dissemination of tumor cells by single dosing of phosphodiester CpG oligonucleotide/cationic liposome complex. J Control Release 115:226–233 [DOI] [PubMed] [Google Scholar]
- 41.Dubinett SM, Lee JM, Sharma S. and Mule JJ. (2010). Chemokines: can effector cells be redirected to the site of the tumor? Cancer J 16:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M. and Gajewski TF. (2009). Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 69:3077–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirota Y, Shirota H. and Klinman DM. (2012). Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol 188:1592–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI. and Hayakawa Y. (2006). CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 176:1582–1587 [DOI] [PubMed] [Google Scholar]
- 45.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H. and Khazaie K. (2005). Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci (USA) 102:419–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Kostareli E, Suffner J, Garbi N. and Hammerling GJ. (2010). Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol 40:3325–3335 [DOI] [PubMed] [Google Scholar]