Abstract
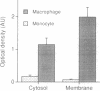
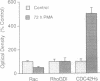
In a search for a model cell system that might allow studies of the function of the Rho-related GTPase CDC42Hs in human cells, we measured the content and distribution of CDC42Hs in monocytes that were differentiating into macrophages. The total content of this protein increased 5- to 6-fold in phorbol ester-treated human monocytic THP-1 and U-937 cells and increased 13-fold in normal human blood monocytes. Moreover, membrane-associated CDC42Hs in these cells increased 13-fold and 30-fold, respectively, while cytosolic CDC42Hs increased only 3- and 6-fold. Measurements made specifically in U-937 cells showed that the increase in membrane CDC42Hs correlated closely with an increase in cell spreading. The changes in CDC42Hs in U-937 cells probably depended on increased mRNA translation and/or decreased protein degradation, since no change in CDC42Hs mRNA could be detected. Finally, the changes in CDC42Hs were relatively specific, since contents of the CDC42Hs-binding protein Rho-GDI and the Rho-related protein Rac2 were unaffected and no change in CDC42Hs occurred when the cells were stimulated by agonists that induce monocytes to differentiate into nonadherent cells. These findings show that marked changes in content and distribution of CDC42Hs occur when monocytes differentiate into macrophages, suggesting that membrane CDC42Hs may play a role in cell spreading.
Full text
PDF

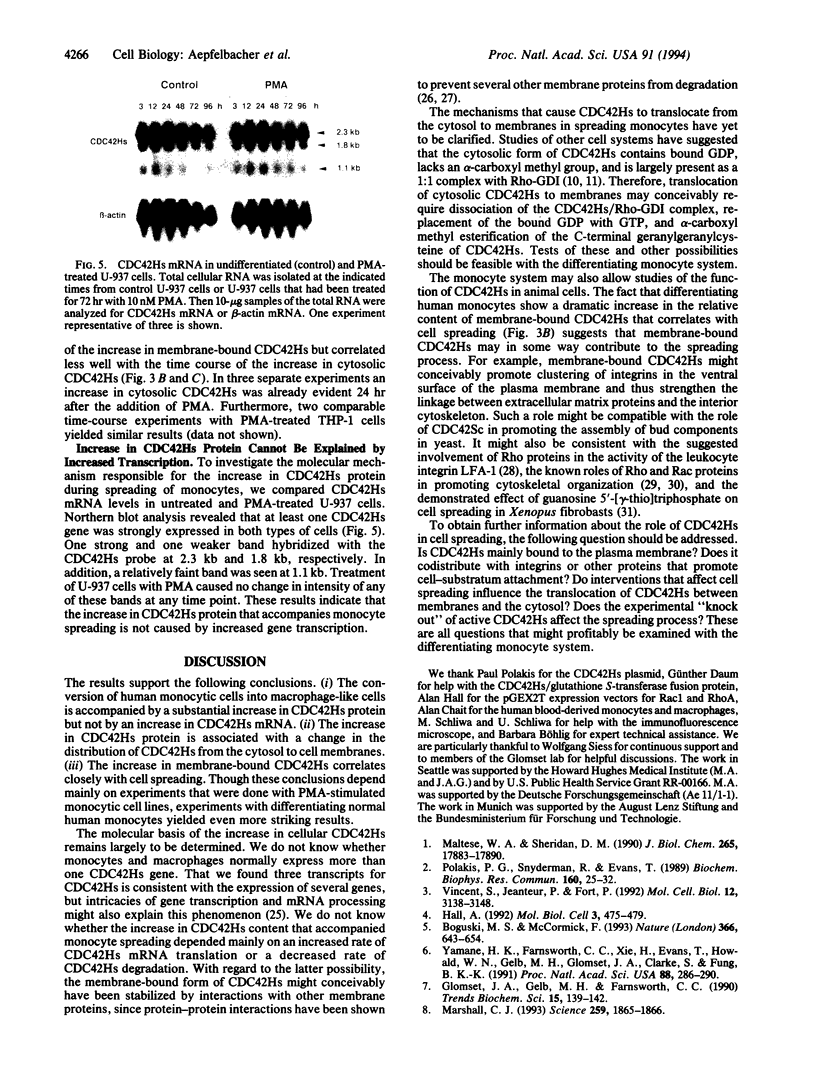

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991 Oct 17;353(6345):668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Adams A. E., Johnson D. I., Longnecker R. M., Sloat B. F., Pringle J. R. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990 Jul;111(1):131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund P. S., Jr GTP-stimulated carboxyl methylation of a soluble form of the GTP-binding protein G25K in brain. J Biol Chem. 1992 Sep 15;267(26):18432–18439. [PubMed] [Google Scholar]
- Boguski M. S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993 Dec 16;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Classon M., Henriksson M., Klein G., Sümegi J. The effect of myc proteins on SV40 replication in human lymphoid cells. Oncogene. 1990 Sep;5(9):1371–1376. [PubMed] [Google Scholar]
- Cox A. D., Der C. J. Protein prenylation: more than just glue? Curr Opin Cell Biol. 1992 Dec;4(6):1008–1016. doi: 10.1016/0955-0674(92)90133-w. [DOI] [PubMed] [Google Scholar]
- Finegold A. A., Johnson D. I., Farnsworth C. C., Gelb M. H., Judd S. R., Glomset J. A., Tamanoi F. Protein geranylgeranyltransferase of Saccharomyces cerevisiae is specific for Cys-Xaa-Xaa-Leu motif proteins and requires the CDC43 gene product but not the DPR1 gene product. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4448–4452. doi: 10.1073/pnas.88.10.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidano G., Bergui L., Schena M., Gaboli M., Cremona O., Marchisio P. C., Caligaris-Cappio F. Integrin distribution and cytoskeleton organization in normal and malignant monocytes. Leukemia. 1990 Oct;4(10):682–687. [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Hall A. Ras-related GTPases and the cytoskeleton. Mol Biol Cell. 1992 May;3(5):475–479. doi: 10.1091/mbc.3.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. J., Eva A., Evans T., Aaronson S. A., Cerione R. A. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991 Nov 28;354(6351):311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- Hass R., Bartels H., Topley N., Hadam M., Köhler L., Goppelt-Strübe M., Resch K. TPA-induced differentiation and adhesion of U937 cells: changes in ultrastructure, cytoskeletal organization and expression of cell surface antigens. Eur J Cell Biol. 1989 Apr;48(2):282–293. [PubMed] [Google Scholar]
- Malden L. T., Chait A., Raines E. W., Ross R. The influence of oxidatively modified low density lipoproteins on expression of platelet-derived growth factor by human monocyte-derived macrophages. J Biol Chem. 1991 Jul 25;266(21):13901–13907. [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M. Isoprenoid modification of G25K (Gp), a low molecular mass GTP-binding protein distinct from p21ras. J Biol Chem. 1990 Oct 15;265(29):17883–17890. [PubMed] [Google Scholar]
- Marshall C. J. Protein prenylation: a mediator of protein-protein interactions. Science. 1993 Mar 26;259(5103):1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Lindstrom J. Assembly in vivo of mouse muscle acetylcholine receptor: identification of an alpha subunit species that may be an assembly intermediate. Cell. 1983 Oct;34(3):747–757. doi: 10.1016/0092-8674(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Minami Y., Weissman A. M., Samelson L. E., Klausner R. D. Building a multichain receptor: synthesis, degradation, and assembly of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1987 May;84(9):2688–2692. doi: 10.1073/pnas.84.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S., Innis M. A., Clark R., McCormick F., Ullrich A., Polakis P. Molecular cloning and expression of a G25K cDNA, the human homolog of the yeast cell cycle gene CDC42. Mol Cell Biol. 1990 Nov;10(11):5977–5982. doi: 10.1128/mcb.10.11.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. G., Snyderman R., Evans T. Characterization of G25K, a GTP-binding protein containing a novel putative nucleotide binding domain. Biochem Biophys Res Commun. 1989 Apr 14;160(1):25–32. doi: 10.1016/0006-291x(89)91615-x. [DOI] [PubMed] [Google Scholar]
- Regazzi R., Kikuchi A., Takai Y., Wollheim C. B. The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem. 1992 Sep 5;267(25):17512–17519. [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Vetvicka V. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol. 1993 May;92(2):181–184. doi: 10.1111/j.1365-2249.1993.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjo K., Koland J. G., Hart M. J., Narasimhan V., Johnson D. I., Evans T., Cerione R. A. Molecular cloning of the gene for the human placental GTP-binding protein Gp (G25K): identification of this GTP-binding protein as the human homolog of the yeast cell-division-cycle protein CDC42. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9853–9857. doi: 10.1073/pnas.87.24.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Symons M. H., Mitchison T. J. A GTPase controls cell-substrate adhesion in Xenopus XTC fibroblasts. J Cell Biol. 1992 Sep;118(5):1235–1244. doi: 10.1083/jcb.118.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T., Sugie K., Hirata M., Morii N., Fukata J., Uchida A., Imura H., Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993 Mar;120(6):1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauti F., Siess W. Simple method of RNA isolation from human leucocytic cell lines. Nucleic Acids Res. 1993 Oct 11;21(20):4852–4853. doi: 10.1093/nar/21.20.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S., Jeanteur P., Fort P. Growth-regulated expression of rhoG, a new member of the ras homolog gene family. Mol Cell Biol. 1992 Jul;12(7):3138–3148. doi: 10.1128/mcb.12.7.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Evans T., Howald W. N., Gelb M. H., Glomset J. A., Clarke S., Fung B. K. Membrane-binding domain of the small G protein G25K contains an S-(all-trans-geranylgeranyl)cysteine methyl ester at its carboxyl terminus. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):286–290. doi: 10.1073/pnas.88.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]