Abstract
Parthenogenesis is the development of an oocyte without fertilization. Mammalian parthenogenetic (PG) embryos are not viable, but can develop into blastocysts from which embryonic stem cells (ESCs) have been derived in mouse and human. PG ESCs are frequently homozygous for alleles encoding major histocompatibility complex (MHC) molecules. MHC homozygosity permits much more efficient immune matching than MHC heterozygosity found in conventional ESCs, making PG ESCs a promising cell source for cell therapies requiring no or little immune suppression. However, findings of restricted differentiation and proliferation of PG cells in developmental chimeras have cast doubt on the potential of PG ESC derivatives for organ regeneration. To address this uncertainty, we determined whether PG ESC derivatives are effective in rescuing mice with lethal liver failure due to deficiency of fumarylacetoacetate hydrolase (Fah). In developmental chimeras generated by injecting wild-type PG ESCs into Fah-deficient blastocysts, PG ESCs differentiated into hepatocytes that could repopulate the liver, provide normal liver function, and facilitate long-term survival of adult mice. Moreover, after transplantation into adult Fah-deficient mice, PG ESC-derived hepatocytes efficiently engrafted and proliferated, leading to high-level liver repopulation. Our results show that—despite the absence of a paternal genome—PG ESCs can form therapeutically effective hepatocytes.
Keywords: liver regeneration, liver repopulation, liver cell therapy, parthenogenesis, embryonic stem cells, pluripotent stem cells
INTRODUCTION
Unlike other vertebrates [1, 2], mammals are not capable of parthenogenetic reproduction. Mammalian parthenogenetic (PG) embryos undergo early developmental demise due to imbalanced expression of imprinted genes [3, 4], and exhibit cell proliferation defects and restricted contribution to mesodermal and endodermal tissues when combined with normal embryos as chimeras [5–7]. However, in both mouse and human, PG embryonic stem cells (ESCs) can be readily derived from blastocysts developing after in vitro activation of unfertilized oocytes; these cells exhibit key characteristics of pluripotent stem cells, including multilineage in vitro differentiation potential [8, 9]. Frequent homozygosity at the major histocompatibility locus, permitting efficient immune matching, has raised interest in PG ESCs as a cell source for transplantation [10, 11]. The potential of PG ESCs for cell therapy is supported by recent studies showing engraftment and tissue-specific function of PG ESC derivatives in brain [12], blood [13], and heart [14]. These observations are at odds with previous findings of limited differentiation and proliferation potential of primary PG ESCs [6, 15–17], which has been attributed to differences in imprinted gene expression [17, 18]. To definitively determine the therapeutic potential of PG ESC derivatives, we investigated whether their differentiation and proliferation potential is sufficient for restoring the function of a vital organ. Specifically, we tested whether PG ESC-derived hepatocytes can prevent death from liver failure of mice with the liver disease fumarylacetoacetate hydrolase (Fah) deficiency [19]. For this, we used a rigorous experimental approach based on developmental chimeras and cell transplantation that demonstrated the liver regeneration potential of induced pluripotent stem cells [20]. Our results show that PG ESCs can differentiate into hepatocytes that are effective in therapeutic liver repopulation.
RESULTS AND DISCUSSION
Fah-deficient mice model the human disease tyrosinemia type I; these mice die from liver failure unless treated with 2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), a drug that inhibits accumulation of the toxic substrate of Fah in hepatocytes [21]. Wild-type hepatocytes rapidly expand in livers of Fah-deficient mice off NTBC due to a growth advantage over the damaged Fah-deficient cells, which eventually leads to near-complete liver repopulation [22]. This selective growth advantage is restricted to hepatocytes—it does not apply to immature liver progenitors. Therefore, Fah-deficient mice allow testing whether a stem cell type considered for liver cell therapy can differentiate into cells that replicate both function and proliferation of primary (normal; N) hepatocytes. To determine whether PG ESCs can give rise to such cells, we generated chimeras between PG ESCs and Fah-deficient mice. ESCs were derived by in vitro activation of oocytes with retention of the second polar body (PG) or by experimental exchange of the paternal genome of a zygote for a second zygotic maternal genome, producing gynogenetic (GG) ESCs that have the same developmental capacity as PG ESCs [23]. Blastocysts developing after in vivo fertilization—containing a paternal and maternal genome—were used to generate N ESCs used as controls. The different ESC types were injected into Fah-deficient blastocysts and chimeras were identified using ESC-specific markers (Table 1).
Table 1.
N, PG, and GG ESC contribution to skin, blood, and liver of chimeras.
| ESC type |
Mouse # | Skin (neonates, EGFP) |
Blood (neonates, Gpi-1) |
Liver (adults, Fah) |
|---|---|---|---|---|
| N | 1 | + | 10% | 90% |
| 5 | + | 10% | 90% | |
| 3, 4 | + | 25%/15% | ND | |
| 2, 6, 7 | − | <5% | ND | |
| PG | ||||
| 10 | NA | 10% | 90% | |
| 11 | NA | 20% | 30% | |
| 25 | NA | 60% | 90% | |
| 9 | NA | <5% | 0% | |
| 8 | NA | <5% | ND | |
| GG | ||||
| 21 | − | <5% | 20% | |
| 22 | + | 10% | 30% | |
| 12–20, 23, 24 | − | <5% | ND |
Chimeras were generated by injecting N, PG, or GG ESCs into Fah-deficient blastocysts. ESC contribution to skin and blood was determined by analysis of enhanced green fluorescent protein (EGFP) expression (only N and GG ESCs) and glucose phosphate isomerase 1 (Gpi-1) allele variants (see detailed description in Methods), respectively. ESC contribution to the liver was determined by Fah immunostaining. +: detected; −: not detected; NA: not applicable; ND: not determined.
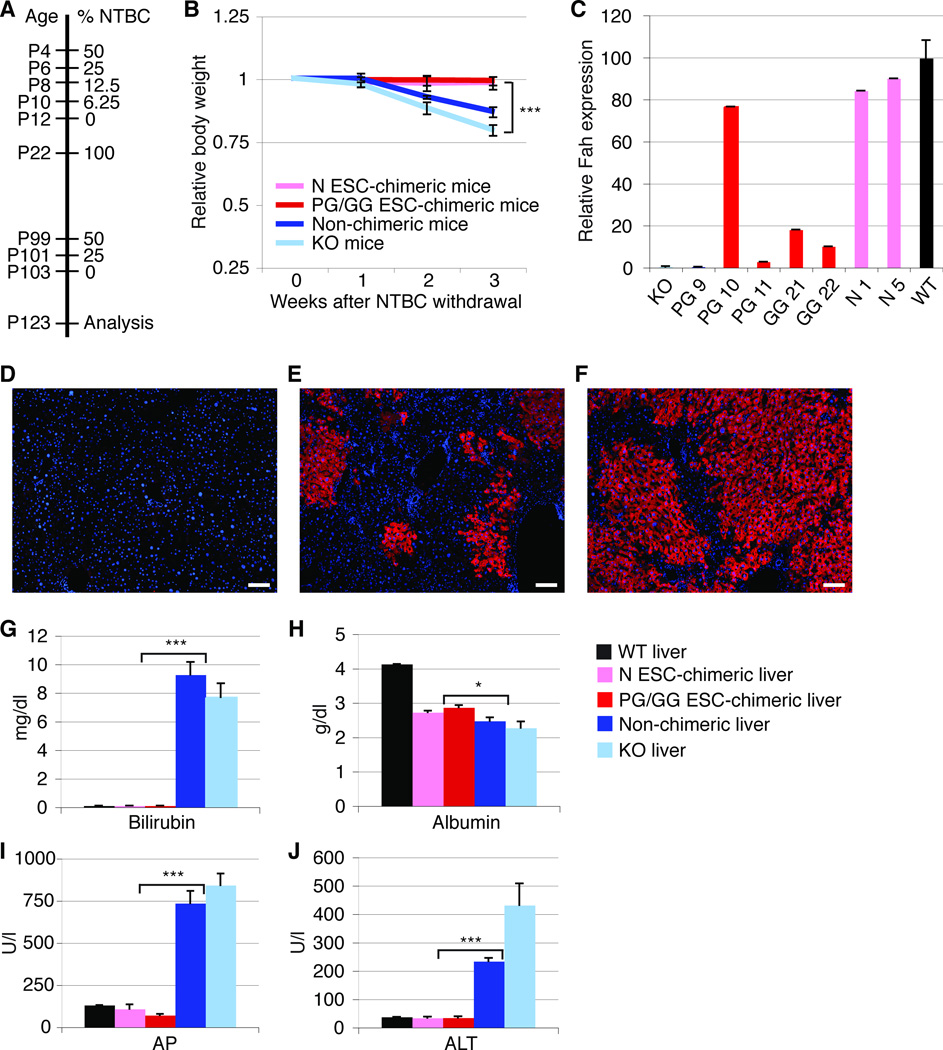
NTBC treatment of chimeric and non-chimeric mice was gradually reduced starting on postnatal day (P) 4 (Fig. 1A). After complete NTBC withdrawal on P12, all PG/GG- and N ESC-chimeric neonates showed normal weight gain, whereas all non-chimeric mice and additional age-matched Fah-deficient control mice rapidly lost weight and required reinstatement of NTBC to prevent death. After an additional round of NTBC withdrawal initiated after the mice reached adulthood (gradual withdrawal from P99 to P103; Fig. 1A), all chimeric mice maintained their body weight confirming NTBC independence (data not shown). In contrast, all non-chimeric and age-matched Fah-deficient mice lost on average 14% and 21% of their body weight by P123, respectively.
Figure 1.
Hepatocytes derived from PG/GG ESCs rescue Fah-deficient mice from liver failure. (A) NTBC withdrawal protocol. (B) Body weight curves of PG/GG- and N ESC-chimeric, non-chimeric, and Fah-deficient (KO) control mice off NTBC. The slightly higher weight loss observed in the KO mice as compared to non-chimeric mice is most likely due to differences in mouse strain background (129S4 vs. 129S4 X C57BL/6, respectively). (C) qRT-PCR shows Fah gene expression levels 3 weeks after NTBC withdrawal (except PG 11 that was on NTBC) relative to wild-type (WT) mice. (D–F) Fah immunostaining (red) on liver sections of a non-chimeric mouse (D), and of chimeric mice with ~20% (E) or ~90% (F) liver repopulation. Nuclei are stained with DAPI (blue). (G–J) Plots showing serum concentrations of bilirubin, albumin, alkaline phosphatase (AP), and alanine aminotransferase (ALT) in adult mice 3 weeks after NTBC withdrawal. Error bars represent average relative to initial body weight ± SE for 4 N ESC-chimeric, 3 PG/GG ESC-chimeric, 4 non-chimeric, and 8 KO mice in (B), additional 3 WT mice in (G–J), and 1 WT, 2 N ESC-chimeric, 4 PG/GG ESC-chimeric, 1 non-chimeric, and 1 KO mice in (C). *P < 0.05, ***P < 0.0001. Size bars = 100 µm.
Quantitative Reverse Transcription-PCR (qRT-PCR) and Fah immunostaining of liver samples obtained by 1/3 partial hepatectomy after the second round of NTBC withdrawal confirmed the absence of Fah-expressing cells in non-chimeric (PG 9) and Fah-deficient mice (Fig. 1C, D), whereas Fah-positive cells constituted ~20% to ~90% of livers of chimeric mice (Table 1, Fig. 1E, F). Immunostaining for major urinary protein (Mup), a protein that is only expressed in mature hepatocytes [24, 25], indicated that PG/GG ESC-derived hepatocytes were fully differentiated and functional (Supporting Information Fig. S1). Indeed, serum levels of markers of liver function, including bilirubin for metabolic function, albumin for synthetic function, alkaline phosphatase for intrahepatic cholestasis, and alanine aminotransferase for hepatocyte injury, were normal in PG/GG- and N ESC-chimeric mice, but indicated liver failure in non-chimeric and Fah-deficient mice (Fig. 1G–J). Two GG ESC-chimeric mice not used for subsequent experiments survived off NTBC for more than 1 year. These results show that PG/GG ESCs can differentiate into hepatocytes that resemble N ESC-derived hepatocytes in their ability to repopulate the livers of Fah-deficient mice and restore their liver function, thereby preventing death from liver failure.
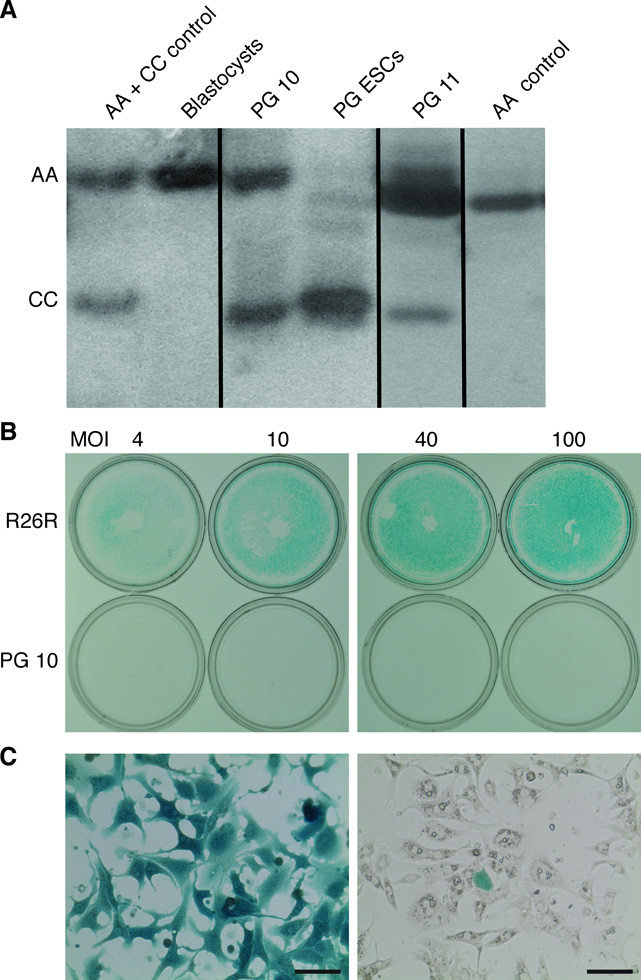
To ascertain that our finding of therapeutic efficacy of PG/GG ESCs was due to differentiation into hepatocytes, and not fusion of hematopoietic or other types of PG/GG ESC derivatives with blastocyst-derived hepatocytes [26, 27], we used 2 independent methods. Electrophoretic analysis of mouse strain-specific Gpi-1 isoforms showed that chimeric liver tissue contained the expected ESC- and blastocyst-specific Gpi-1 homodimers, but no heterodimers, which would have formed in fused cells (Fig. 2A). In addition, activation of a blastocyst-specific Rosa26 reporter (R26R) gene with a Cre recombinase-expressing adenovirus in hepatocytes isolated from a highly repopulated PG ESC-chimeric mouse showed the expected low frequency of blastocyst-derived hepatocytes (Fig. 2B). These results exclude cell fusion as the mechanism underlying the formation of PG/GG ESC-derived hepatocytes, which shows that PG/GG ESCs are intrinsically capable of full hepatocyte differentiation.
Figure 2.
PG ESC-derived cells do not acquire hepatocyte function by fusion with blastocyst-derived cells. (A) Electrophoretic analysis of mouse strain-specific isoforms of the dimeric Gpi-1 enzyme. Livers from PG ESC-chimeric mice (PG 10 and PG 11) contain a mixture of the Gpi-1 CC homodimer specific for PG ESCs, and of the Gpi-1 AA homodimer specific for Fah-deficient blastocyst-derived cells, but not the Gpi-1 AC heterodimer, thereby excluding cell fusion of PG ESC-derived cells with blastocyst-derived cells. Sections of non-contiguous parts of the same gel are separated by a black line. (B) Fusion-independent differentiation of PG ESC-derived cells into hepatocytes was confirmed by exclusion of a blastocyst-derived marker in hepatocytes isolated from a chimera. PG ESC-chimeric mice were generated using Fah-deficient blastocysts that also expressed a lacZ reporter (R26R). Hepatocytes isolated from a chimeric mouse (PG 10) exhibiting ~90% liver repopulation were transduced with a Cre-expressing adenovirus to test for the activation of lacZ expression, which would only occur if PG ESC-derived hepatocytes had acquired the R26R transgene by fusion with blastocyst-derived cells. At MOI 40 and 100, 100% of 400,000 R26R control hepatocytes stained positively in X-gal staining (blue), reflecting lacZ expression due to R26R activation. In contrast, less than 10% of 400,000 hepatocytes isolated from the PG ESC-chimeric mouse expressed lacZ, which is proportional to the blastocyst-derived cell population (lower row). (C) Higher magnification of the hepatocytes from the R26R control mouse (left panel) or from the PG ESC-chimeric mouse with ~90% liver repopulation, both at MOI 100 (right panel). Size bars = 50 µm.
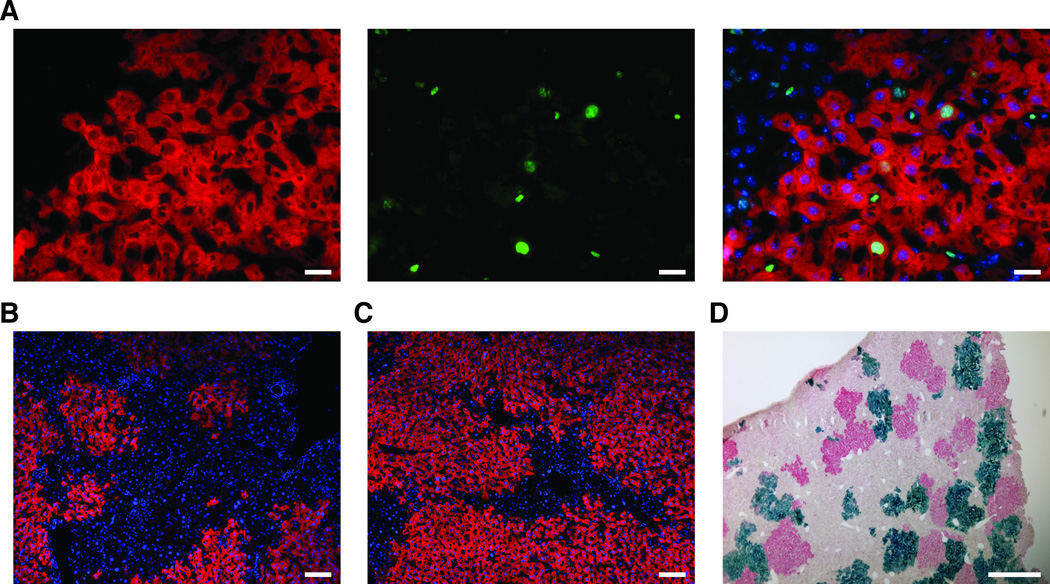
Next, we investigated the liver repopulation capacity of PG/GG ESC-derived hepatocytes in more detail. Using immunostaining for the pan-proliferation marker Ki67, we found that PG/GG ESC-derived hepatocytes proliferated in response to NTBC withdrawal not only in neonatal but also in adult mice (Fig. 3A). To determine whether this capacity was afforded by changes in genomic imprinting [17, 18], we performed bisulfite sequencing of the differentially methylated region (DMR) 2 of the maternally expressed insulin-like growth factor 2 receptor (Igf2r) gene, which has been suggested to inhibit hepatocyte proliferation in liver regeneration [28, 29]. We found similar levels of Igf2r DMR2 methylation in repopulating nodules comprised of PG/GG ESC-derived hepatocytes or N hepatocytes isolated by laser-capture microscopy (Supporting Information Fig. S2). As PG/GG ESCs are largely devoid of methylation at the DMR2 locus (Supporting Information Fig. S2), this finding can be explained by a selective growth advantage of a subset of PG/GG ESC derivatives with increased DMR2 methylation, which would reduce Igf2r expression.
Figure 3.
PG ESC-derived hepatocytes are capable of proliferation and liver repopulation after transplantation. (A) Co-immunostaining for Fah (red) and Ki67 (green) shows proliferating PG ESC-derived hepatocytes in the periphery of a repopulating nodule bordering on blastocyst-derived, Fah-negative hepatocytes. Merged image shows nuclei stained with DAPI (blue). (B,C) Fah immunostaining (red) shows nodules of PG ESC-derived hepatocytes repopulating livers of FRG mice to ~50% or ~90% 5 months after transplantation. Nuclei are stained with DAPI (blue). (D) Combined Fah immunostaining (red) and X-gal staining (blue) shows similar frequency and size of nodules derived from transplanted PG ESC-derived hepatocytes (red) and N hepatocytes (blue) in a representative liver section from an FRG mouse transplanted with equal numbers of these cells. Size bars = 50 µm (A), 100 µm (B,C) and 500 µm (D).
Finally, we assessed the capacity of PG/GG ESC-derived hepatocytes to replace diseased hepatocytes after transplantation into adult animals. We transplanted PG ESC-derived hepatocytes isolated from adult chimeras into Fah-deficient mice that were also immune deficient to avoid rejection (so-called FRG mice [30]), and intermittently withdrew NTBC until the animals were able to maintain their body weight off NTBC (data not shown). We found that PG ESC-derived hepatocytes isolated from an adult chimera (PG 10) repopulated the livers of recipient FRG mice between 50% and ~90% (Fig. 3B,C). Similarly, co-transplantation of equal numbers of PG ESC-derived hepatocytes (PG 25) and hepatocytes isolated from an age-matched N control mouse—a Rosa26 mouse so that N hepatocytes could be distinguished based on lacZ expression—into FRG mice produced liver-repopulating nodules of similar frequency and size (Fig. 3D). These results show that transplanted PG ESC-derived hepatocytes can efficiently engraft in the adult liver and afford near-complete liver repopulation of FRG mice, rendering them NTBC independent.
CONCLUSION
In this proof-of-principle study, we show that PG/GG ESCs can differentiate into hepatocytes whose function and proliferation are sufficient for therapeutic liver repopulation. Our study provides a reliable assessment of the potential of PG/GG ESCs for therapy of liver diseases for the following reasons: First, we investigated the hepatocyte differentiation potential of PG/GG ESCs in the context of the developing embryo, which excludes biases potentially introduced by current imperfect protocols for in vitro differentiation. Second, we evaluated function and proliferation of PG/GG ESC-derived hepatocytes in Fah-deficient mice, a rigorous model of liver failure, and did not simply rely on analysis of marker gene expression [31]. Our finding of normal liver function in mice with near-complete liver repopulation suggests that PG/GG ESC-derived hepatocytes function normally. Therefore, PG/GG ESC-derived hepatocytes should also be therapeutically effective in other liver diseases, but whether that is indeed the case needs to be specifically tested.
Surprisingly, our results reveal that the absence of a paternal genome in PG/GG ESCs has no apparent consequences for their ability to form hepatocytes, although it is likely that this finding is due to some level of epigenetic “normalization” such as Igfr2 DMR2 methylation. In accord with this idea, previous studies showed that manipulation of expression of a few imprinted genes rescues development of fetuses with only maternal genomes [32], or maternal duplication of large chromosomal imprinting clusters [33]. Irrespective of the underlying mechanism, if human PG/GG ESC-derived hepatocytes were similarly effective in mouse liver repopulation, they would have potential for human liver cell therapy.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Kimberly Jennings for help with bisulfite sequencing and N. Adrian Leu and Girish Rajgolikar for assistance with chimera generation.
This work was supported by R01 DK080852 from the NIH (K. J. McLaughlin and H. Willenbring), a Cystinosis Research Foundation Fellowship (S. Espejel), the NRSA Hepatology Training Grant at UCSF (NIH T32 DK060414; G. Roll), and the UCSF Liver Center (NIH P30 DK026743; H. Willenbring).
Footnotes
Author contribution summary:
Silvia Espejel: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing
Sigrid Eckardt: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing
Jack Harbell: Collection and assembly of data, data analysis and interpretation
Garrett R. Roll: Collection and assembly of data, data analysis and interpretation
K. John McLaughlin: Conception and design, financial support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
Holger Willenbring: Conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript
The authors declare no conflict of interest.
REFERENCES
- 1.Booth W, Smith CF, Eskridge PH, et al. Facultative parthenogenesis discovered in wild vertebrates. Biol Lett. 2012;8:983–985. doi: 10.1098/rsbl.2012.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neaves WB, Baumann P. Unisexual reproduction among vertebrates. Trends Genet. 2011;27:81–88. doi: 10.1016/j.tig.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 3.McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984;37:179–183. doi: 10.1016/0092-8674(84)90313-1. [DOI] [PubMed] [Google Scholar]
- 4.Surani MA, Barton SC, Norris ML. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature. 1984;308:548–550. doi: 10.1038/308548a0. [DOI] [PubMed] [Google Scholar]
- 5.Fundele R, Norris ML, Barton SC, et al. Systematic elimination of parthenogenetic cells in mouse chimeras. Development. 1989;106:29–35. doi: 10.1242/dev.106.1.29. [DOI] [PubMed] [Google Scholar]
- 6.Fundele RH, Norris ML, Barton SC, et al. Temporal and spatial selection against parthenogenetic cells during development of fetal chimeras. Development. 1990;108:203–211. doi: 10.1242/dev.108.1.203. [DOI] [PubMed] [Google Scholar]
- 7.Bender R, Surani MA, Kothary R, et al. Tissue specific loss of proliferative capacity of parthenogenetic cells in fetal mouse chimeras. Roux’s Arch Dev Biol. 1995;204:436–443. doi: 10.1007/BF00360851. [DOI] [PubMed] [Google Scholar]
- 8.Robertson EJ, Kaufman MH, Bradley A, et al. Isolation, properties and karyotype analysis of pluripotent (EK) cell lines from normal and parthenogenetic embryos. Teratocarcinomal Stem Cells Cold Spring Harbor Conferences on Cell Proliferation. 1993;10:647–663. [Google Scholar]
- 9.Revazova ES, Turovets NA, Kochetkova OD, et al. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9:432–449. doi: 10.1089/clo.2007.0033. [DOI] [PubMed] [Google Scholar]
- 10.Kim K, Lerou P, Yabuuchi A, et al. Histocompatible embryonic stem cells by parthenogenesis. Science. 2007;315:482–486. doi: 10.1126/science.1133542. [DOI] [PubMed] [Google Scholar]
- 11.Revazova ES, Turovets NA, Kochetkova OD, et al. HLA homozygous stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2008;10:11–24. doi: 10.1089/clo.2007.0063. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Pernaute R, Studer L, Ferrari D, et al. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23:914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckardt S, Leu NA, Bradley HL, et al. Hematopoietic reconstitution with androgenetic and gynogenetic stem cells. Genes Dev. 2007;21:409–419. doi: 10.1101/gad.1524207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didie M, Christalla P, Rubart M, et al. Parthenogenetic stem cells for tissue-engineered heart repair. J Clin Invest. 2013;123:1285–1298. doi: 10.1172/JCI66854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen ND, Barton SC, Hilton K, et al. A functional analysis of imprinting in parthenogenetic embryonic stem cells. Development. 1994;120:1473–1482. doi: 10.1242/dev.120.6.1473. [DOI] [PubMed] [Google Scholar]
- 16.Sturm KS, Berger CN, Zhou SX, et al. Unrestricted lineage differentiation of parthenogenetic ES cells. Dev Genes Evol. 1997;206:377–388. doi: 10.1007/s004270050067. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez L, Kozlov S, Piras G, et al. Paternal and maternal genomes confer opposite effects on proliferation, cell-cycle length, senescence, and tumor formation. Proc Natl Acad Sci U S A. 2003;100:13344–13349. doi: 10.1073/pnas.2234026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Chen Z, Liu Z, et al. Correlation of expression and methylation of imprinted genes with pluripotency of parthenogenetic embryonic stem cells. Hum Mol Genet. 2009;18:2177–2187. doi: 10.1093/hmg/ddp150. [DOI] [PubMed] [Google Scholar]
- 19.Grompe M, al-Dhalimy M, Finegold M, et al. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 1993;7:2298–2307. doi: 10.1101/gad.7.12a.2298. [DOI] [PubMed] [Google Scholar]
- 20.Espejel S, Roll GR, McLaughlin KJ, et al. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest. 2010;120:3120–3126. doi: 10.1172/JCI43267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grompe M, Lindstedt S, al-Dhalimy M, et al. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1995;10:453–460. doi: 10.1038/ng0895-453. [DOI] [PubMed] [Google Scholar]
- 22.Overturf K, Al-Dhalimy M, Tanguay R, et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 23.Surani MA, Barton SC. Development of gynogenetic eggs in the mouse: implications for parthenogenetic embryos. Science. 1983;222:1034–1036. doi: 10.1126/science.6648518. [DOI] [PubMed] [Google Scholar]
- 24.Derman E. Isolation of a cDNA clone for mouse urinary proteins: age- and sex-related expression of mouse urinary protein genes is transcriptionally controlled. Proc Natl Acad Sci U S A. 1981;78:5425–5429. doi: 10.1073/pnas.78.9.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malato Y, Naqvi S, Schurmann N, et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest. 2011;121:4850–4860. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willenbring H, Bailey AS, Foster M, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. 2004;10:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- 27.Okamura K, Asahina K, Fujimori H, et al. Generation of hybrid hepatocytes by cell fusion from monkey embryoid body cells in the injured mouse liver. Histochem Cell Biol. 2006;125:247–257. doi: 10.1007/s00418-005-0065-1. [DOI] [PubMed] [Google Scholar]
- 28.Jirtle RL, Carr BI, Scott CD. Modulation of insulin-like growth factor-II/mannose 6-phosphate receptors and transforming growth factor-beta 1 during liver regeneration. J Biol Chem. 1991;266:22444–22450. [PubMed] [Google Scholar]
- 29.Villevalois-Cam L, Rescan C, Gilot D, et al. The hepatocyte is a direct target for transforming-growth factor beta activation via the insulin-like growth factor II/mannose 6-phosphate receptor. J Hepatol. 2003;38:156–163. doi: 10.1016/s0168-8278(02)00378-1. [DOI] [PubMed] [Google Scholar]
- 30.Azuma H, Paulk N, Ranade A, et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox IJ, Strom SC. To be or not to be: generation of hepatocytes from cells outside the liver. Gastroenterology. 2008;134:878–881. doi: 10.1053/j.gastro.2008.01.065. [DOI] [PubMed] [Google Scholar]
- 32.Kawahara M, Wu Q, Takahashi N, et al. High-frequency generation of viable mice from engineered bi-maternal embryos. Nat Biotechnol. 2007;25:1045–1050. doi: 10.1038/nbt1331. [DOI] [PubMed] [Google Scholar]
- 33.Han L, Szabo PE, Mann JR. Postnatal survival of mice with maternal duplication of distal chromosome 7 induced by a Igf2/H19 imprinting control region lacking insulator function. PLoS Genet. 2010;6:e1000803. doi: 10.1371/journal.pgen.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.